Abstract
It is still uncertain whether the final kissing balloon technique (FKBT) is mandatory after crossover stenting for the left main coronary artery (LMCA). Assessing Optimal Percutaneous Coronary Intervention for LMCA (AOI-LMCA) registry, a 6-center retrospective registry, enrolled 1809 consecutive patients for LMCA stenting in Japan. In the present analysis, 5-year clinical outcomes were compared between non-FKBT (n = 160) and FKBT (n = 578) groups in patients treated with crossover stenting with drug-eluting stents from the LMCA to the left anterior descending artery. Propensity score-matched analysis was also performed in 160 patient pairs. In the entire study population as well as in the propensity-matched population, the cumulative 5-year incidence of the primary outcome measure (target lesion revascularization: TLR) was not significantly different between the FKBT and non-FKBT groups (10.7 versus 14.3%, P = 0.49, and 11.8 versus 14.3%, P = 0.53, respectively). In the sensitivity analysis by the multivariable Cox proportional hazard model, the effect of FKBT relative to non-FKBT for TLR remained insignificant (adjusted HR 0.89, 95% CI 0.47–1.69, P = 0.72). Regarding the TLR location, there were no significant differences in the cumulative incidences of TLR for LMCA-only, for the main branch, and for the side branch between the 2 groups (2.2 versus 1.3%, P = 0.93, 11.8 versus 9.1%, P = 0.71, and 8.2 versus 7.6%, P = 0.82, respectively). FKBT after a 1-stent strategy for LMCA crossover stenting did not affect TLR and other clinical outcomes during 5-year follow-up.
Clinical Trial Registration: Assessing Optimal Percutaneous Coronary Intervention for Left Main Coronary Artery Stenting Registry (AOI LMCA Stenting Registry). http://www.umin.ac.jp/ctr/index/htm/. Unique Identifier: UMIN000014706.
Electronic supplementary material
The online version of this article (10.1007/s12928-018-0522-0) contains supplementary material, which is available to authorized users.
Keywords: Left main coronary artery, Kissing balloon technique, One-stent strategy
Introduction
The current international guidelines have recommended coronary artery bypass grafting (CABG) as a class 1 indication in patients with left main coronary artery (LMCA) disease [1–3]. Percutaneous coronary intervention (PCI) has been more and more frequently performed in patients with LMCA disease with a low-moderate SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score as an alternative to CABG, due to improvements of drug-eluting stents (DES) and advancing technique [4, 5]. A 1-stent strategy is currently considered a standard stenting strategy for LMCA bifurcation lesions, because a 2-stent strategy is associated with higher rates of adverse events such as target lesion revascularization (TLR), and stent thrombosis (ST) [6, 7]. However, the role of final kissing balloon technique (FKBT) after crossover stenting for the main branch is controversial for lesions at any bifurcation [8]. Particularly, the effects of FKBT for LMCA disease have not been adequately assessed in previous reports [9–13]. Therefore, we sought to compare the long-term clinical outcomes between the 2 groups of patients with and without FKBT after crossover DES stenting from LMCA to the left anterior descending artery (LAD), using data from a large multicenter registry in Japan.
Methods
Study design and patient population
The AOI-LMCA (Assessing Optimal percutaneous coronary Intervention for Left Main Coronary Artery) stenting registry is a retrospective, multicenter registry that enrolled 1809 consecutive patients who underwent LMCA stenting with bare-metal stents or DES in 6 Japanese hospitals experienced with LMCA stenting between November 2004 and December 2012. The protocol and details of patient enrollment have been described elsewhere [6].
The current study population comprised 738 patients treated with crossover DES stenting as a 1-stent strategy from LMCA to LAD. Five-year clinical outcomes were compared between the non-FKBT group (N = 160) and the FKBT group (N = 578) after excluding those patients who had ST-elevation myocardial infarction (MI) with cardiogenic shock, or LMCA–left circumflex coronary artery (LCX) crossover stenting (Fig. 1).
Fig. 1.
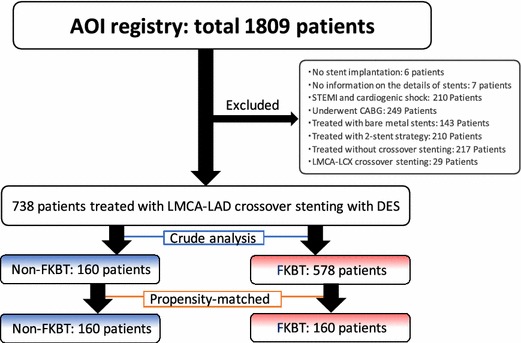
Study flow chart. AOI assessing optimal percutaneous coronary intervention for left main coronary artery stenting, CABG coronary artery bypass grafting, DES drug-eluting stents, FKBT final kissing balloon technique, LAD left anterior descending coronary artery, LCX left circumflex coronary artery, LMCA left main coronary artery, STEMI ST-segment elevation myocardial infarction
Stenting strategies and use of FKBT were left to the discretion of the operators in 5 of 6 participating centers, except for one center in which culotte was the default bifurcation stenting strategy and FKBT after crossover stenting was mandatory. The Medina classification was used to assess each bifurcation lesion, and true bifurcation was defined as Medina classification (1, 1, 1), (1, 0, 1), or (0, 1, 1) [14]. TLR-main branch was defined as TLR from the LMCA to the LAD, and TLR-side branch was defined as TLR involving the LCX ostium only. After the procedure, aspirin (100 mg/day) and ticlopidine (200 mg/day) or clopidogrel (75 mg/day) were to be prescribed in all patients. The recommended duration of dual antiplatelet therapy (DAPT) was at least 8–12 months after DES implantation. Actual DAPT duration in each patient was determined by each cardiologist based on the institutional protocol. Follow-up data collection was performed by a review of medical records and follow-up contact with patients and/or their relatives. The study protocol was approved by the institutional review board in each participating center. Written informed consent from each patient was waived in this retrospective study, because we used clinical information obtained in the routine clinical practice and no patients refused to participate in the study when contacted for follow-up.
Endpoints
The primary outcome measure in the present analysis was TLR at 5-year. Secondary outcome measures included all-cause death, cardiac death, sudden death, MI, definite or probable ST, stroke, any coronary revascularization, or major adverse cardiovascular events (MACE: cardiac death, MI, or TLR) at 5-year. Definitions for each endpoint have been previously described in detail [6].
Statistical analysis
Categorical variables are presented as counts and percentages, and compared using Chi-squared or Fisher’s exact tests. Continuous variables are expressed as mean ± standard deviation or medians with interquartile range. Continuous variables were compared using Student’s t test or the Wilcoxon rank-sum test based on distributions. The cumulative incidence of each endpoint was estimated using the Kaplan–Meier method, and the curves of the 2 groups were compared by the log-rank test.
To adjust the potential confounding for the choice of FKBT, we evaluated the effects of FKBT strategy relative to non-FKBT strategy in a propensity score-matched population. A logistic regression model was used to develop propensity scores for the choice of FKBT strategy with 15 independent variables relevant to the decision regarding FKBT strategy listed in Tables 1 and 2. The propensity score was then calculated by summing up all coefficient multiples for the corresponding variables (Supplemental Table). To create the propensity score-matched cohort, patients without FKBT were matched to those with FKBT using a 1:1 greedy matching technique [15]. The cumulative incidences of clinical events were compared between FKBT and non-FKBT strategies in the propensity score-matched cohort.
Table 1.
Baseline patient characteristics in the entire study population and in the propensity-matched population: FKBT versus non-FKBT
| Entire study population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| FKBT (n = 578) | Non-FKBT (n = 160) | P value | FKBT (n = 160) | Non-FKBT (n = 160) | P value | |
| Age (years) | 72 ± 10 | 73 ± 11 | 0.34 | 74 ± 9 | 73 ± 11 | 0.52 |
| Age ≥ 80 yearsa,b | 141 (24%) | 48 (30%) | 0.15 | 44 (28%) | 48 (30%) | 0.62 |
| Male | 441 (76%) | 117 (73%) | 0.41 | 120 (75%) | 117 (73%) | 0.7 |
| Hypertension | 436 (75%) | 131 (82%) | 0.09 | 115 (72%) | 131 (82%) | 0.03 |
| Diabetes mellitusa,b | 258 (45%) | 78 (49%) | 0.36 | 67 (42%) | 78 (49%) | 0.22 |
| Insulin-treated diabetes | 62 (11%) | 23 (14%) | 0.2 | 14 (9%) | 23 (14%) | 0.12 |
| Dyslipidemia | 328 (57%) | 106 (66%) | 0.03 | 91 (57%) | 106 (66%) | 0.09 |
| Current smoker | 80 (14%) | 32 (20%) | 0.06 | 28 (18%) | 32 (20%) | 0.57 |
| eGFR (mL/min/1.73 m2) | 60.8 ± 23.1 | 58.6 ± 24.3 | 0.31 | 58.7 ± 21.4 | 58.6 ± 24.3 | 0.96 |
| Hemodialysisa,b | 26 (4.5%) | 14 (8.8%) | 0.036 | 11 (6.9%) | 14 (8.8%) | 0.53 |
| eGFR < 60 mL/min/1.73 m2 and non-hemodialysisa,b | 229 (40%) | 68 (43%) | 0.51 | 66 (41%) | 68 (43%) | 0.82 |
| Previous PCIa | 286 (50%) | 75 (47%) | 0.56 | 76 (48%) | 75 (47%) | 0.91 |
| Previous myocardial infarction | 183 (32%) | 46 (29%) | 0.48 | 42 (26%) | 46 (29%) | 0.62 |
| Previous heart failurea,b | 72 (13%) | 18 (11%) | 0.68 | 22 (14%) | 18 (11%) | 0.5 |
| Malignancya | 58 (10%) | 19 (12%) | 0.5 | 12 (7.5%) | 19 (12%) | 0.19 |
| Strokea | 80 (14%) | 22 (14%) | 0.98 | 23 (14%) | 22 (14%) | 0.87 |
| Peripheral vascular diseasea | 64 (11%) | 36 (23%) | <0.0001 | 21 (13%) | 36 (23%) | 0.03 |
| Euro score | 4.2 ± 2.4 | 3.9 ± 2.5 | 0.34 | 5.1 ± 3.0 | 5.5 ± 3.5 | 0.34 |
| Clinical presentation | 0.7 | 0.57 | ||||
| Stable angina pectoris | 469 (81%) | 132 (83%) | 128 (80%) | 132 (83%) | ||
| UAP/NSTEMIa,b | 109 (19%) | 28 (18%) | 32 (20%) | 28 (18%) | ||
| Decompensated heart failure | 38 (6.6%) | 12 (7.6%) | 0.66 | 8 (5.0%) | 12 (7.6%) | 0.34 |
| Medication | ||||||
| Aspirin | 565 (98%) | 155 (98%) | 0.74 | 157 (98%) | 155 (98%) | 0.7 |
| Thienopyridine | 559 (97%) | 155 (98%) | 0.77 | 157 (98%) | 155 (98%) | 0.7 |
| Warfarin | 42 (7.3%) | 11 (6.9%) | 0.87 | 16 (10.0%) | 11 (6.9%) | 0.32 |
| Statinsa | 410 (71%) | 107 (67%) | 0.32 | 111 (69%) | 107 (67%) | 0.63 |
| β-Blockersa | 161 (28%) | 48 (30%) | 0.59 | 43 (27%) | 48 (30%) | 0.54 |
| ACE-I/ARBa | 337 (58%) | 95 (59%) | 0.81 | 88 (55%) | 95 (59%) | 0.43 |
| Proton pump inhibitors | 272 (47%) | 61 (38%) | 0.047 | 69 (43%) | 61 (38%) | 0.39 |
| H2-blocker | 83 (14%) | 23 (15%) | 0.99 | 22 (14%) | 23 (15%) | 0.85 |
| Time perioda,b | 0.04 | 0.01 | ||||
| Wave 1: 2004–2006 (bare-metal stent period) | 88 (15%) | 37 (23%) | 37 (23%) | 27 (17%) | ||
| Wave 2: 2007–2009 (G1-DES period) | 213 (37%) | 47 (29%) | 44 (28%) | 69 (43%) | ||
| Wave 3: 2010–2012 (G2-DES period) | 277 (48%) | 76 (48%) | 79 (49%) | 64 (40%) | ||
| Institutea,b | <0.0001 | 0.9 | ||||
| 1 | 39 (6.7%) | 23 (14%) | 22 (14%) | 23 (14%) | ||
| 2 | 63 (11%) | 53 (33%) | 50 (31%) | 53 (33%) | ||
| 3 | 349 (60%) | 22 (14%) | 22 (14%) | 22 (14%) | ||
| 4 | 94 (16%) | 50 (31%) | 56 (35%) | 50 (31%) | ||
| 5 | 25 (4.3%) | 8 (5.0%) | 5 (3.1%) | 8 (5.0%) | ||
| 6 | 8 (1.4%) | 4 (2.5%) | 5 (3.1%) | 4 (2.5%) | ||
eGFR estimated glomerular filtration rate, PCI percutaneous coronary intervention, UAP unstable angina pectoris, NSTEMI non-ST-segment elevation myocardial infarction, ACE-I angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, G1-DES first-generation drug-eluting stent, G2-DES second-generation drug-eluting stent
aPotential independent risk-adjusting variables selected for Cox proportional hazards models
bDaggers indicate the variables selected for propensity score matching
Table 2.
Baseline lesion and procedural characteristics in the entire study population and in the propensity-matched population: non-FKBT versus FKBT
| Entire study population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| FKBT (n = 578) | Non-FKBT (n = 160) | P value | FKBT (n = 160) | Non-FKBT (n = 160) | P value | |
| Lesion characteristic | ||||||
| CTO in the RCAa,b | 75 (13%) | 20 (13%) | 0.87 | 18 (11%) | 20 (13%) | 0.73 |
| SYNTAX score | 26.0 ± 9.4 | 27.2 ± 9.7 | 0.13 | 25.6 ± 8.8 | 27.3 ± 9.7 | 0.1 |
| Bifurcation lesion | 539 (93%) | 146 (91%) | 0.39 | 142 (89%) | 146 (91%) | 0.46 |
| True bifurcationa,b | 206 (36%) | 59 (37%) | 0.77 | 62 (39%) | 59 (37%) | 0.73 |
| Extent of coronary artery disease | 0.13 | |||||
| Left main only | 43 (7%) | 13 (8%) | 15 (9.4%) | 13 (8.1%) | ||
| Left main + 1 vessel | 222 (38%) | 47 (29%) | 54 (34%) | 47 (29%) | ||
| Left main + 2 vessels | 212 (37%) | 62 (39%) | 65 (41%) | 62 (39%) | ||
| Left main + 3 vessels | 101 (18%) | 38 (24%) | 26 (16%) | 38 (24%) | ||
| Multi-vessel (left main + ≥ 2 vessels)a,b | 313 (54%) | 100 (63%) | 0.06 | 91 (57%) | 100 (63%) | 0.31 |
| Medina classification | 0.03 | 0.28 | ||||
| (1, 0, 0) | 76 (13%) | 19 (12%) | 23 (14%) | 19 (12%) | ||
| (0, 1, 0) | 31 (5.4%) | 7 (4.4%) | 7 (4.4%) | 7 (4.4%) | ||
| (0, 0, 1) | 3 (0.5%) | 6 (3.8%) | 0 (0%) | 6 (3.8%) | ||
| (1, 1, 0) | 221 (38%) | 52 (33%) | 50 (31%) | 55 (34%) | ||
| (1, 0, 1) | 21 (3.6%) | 3 (1.9%) | 6 (3.8%) | 3 (1.9%) | ||
| (0, 1, 1) | 5 (0.9%) | 4 (2.5%) | 3 (1.9%) | 5 (3.1%) | ||
| (1, 1, 1) | 169 (29%) | 50 (31%) | 53 (33%) | 51 (32%) | ||
| True trifurcation | 71 (13%) | 27 (17%) | 0.15 | 23 (15%) | 27 (17%) | 0.57 |
| Calcified lesiona,b | 91 (16%) | 18 (11%) | 0.16 | 18 (11%) | 18 (11%) | 1.0 |
| In-stent restenosis lesion | 15 (2.6%) | 4 (2.5%) | 0.94 | 4 (2.5%) | 4 (2.5%) | 0.99 |
| Procedural characteristic | ||||||
| Arterial access site | 0.04 | 0.90 | ||||
| Femoral | 393 (68%) | 122 (76%) | 123 (77%) | 122 (76%) | ||
| Radial or brachial | 185 (32%) | 38 (24%) | 37 (23%) | 38 (24%) | ||
| Use of mechanical support | ||||||
| IABP | 31 (5.4%) | 10 (6.2%) | 0.67 | 16 (10.0%) | 10 (6.3%) | 0.22 |
| PCPS | 2 (0.3%) | 1 (0.6%) | 0.63 | 1 (0.6%) | 1 (0.6%) | 1.0 |
| Use of rotablator | 42 (7.3%) | 17 (11%) | 0.17 | 11 (6.9%) | 17 (11%) | 0.24 |
| Stent types | 0.23 | 0.13 | ||||
| G1-DES | 342 (59%) | 103 (64%) | 90 (56%) | 103 (64%) | ||
| G2-DESa,b | 236 (41%) | 57 (36%) | 70 (44%) | 57 (36%) | ||
| Use of intracoronary imaging modalities | ||||||
| IVUSa,b | 409 (71%) | 134 (84%) | 0.001 | 127 (79%) | 134 (84%) | 0.31 |
| OCT | 24 (4.2%) | 6 (3.8%) | 0.82 | 15 (9.4%) | 6 (3.8%) | 0.04 |
| None | 145 (25%) | 20 (13%) | 0.001 | 18 (11%) | 20 (13%) | 0.73 |
| Proximal optimization technique | 119 (21%) | 13 (8.1%) | 0.001 | 26 (16%) | 13 (8.1%) | 0.03 |
| Number of stents per lesion | 1.3 ± 0.5 | 1.5 ± 0.7 | 0.001 | 1.3 ± 0.6 | 1.5 ± 0.7 | 0.07 |
| Stent size (MV) (mm) | 3.5 ± 0.7 | 3.5 ± 0.6 | 0.61 | 3.6 ± 0.6 | 3.5 ± 0.6 | 0.32 |
| Stent size (MV) ≥ 3.5 mma,b | 354 (61%) | 104 (65%) | 0.39 | 111 (69%) | 104 (65%) | 0.41 |
| Stent length (MV) (mm) | 26.7 ± 12.6 | 26.1 ± 13.2 | 0.57 | 26.0 ± 13.1 | 26.1 ± 13.2 | 0.09 |
| Stent length (MV) ≥ 30 mm | 141 (24%) | 38 (24%) | 0.87 | 32 (20%) | 38 (24%) | 0.42 |
| Final balloon size (MV) (mm) | 3.5 ± 0.6 | 3.7 ± 0.6 | 0.003 | 3.5 ± 0.6 | 3.7 ± 0.6 | 0.004 |
| Maximum balloon size (SV) (mm) | 2.5 ± 0.6 | – | – | 2.4 ± 0.5 | – | – |
CTO chronic total occlusion, RCA right coronary artery, SYNTAX SYNergy between PCI with TAXus and Cardiac Surgery, IABP intra-aortic balloon pumping, PCPS percutaneous cardiopulmonary support, IVUS intravascular ultrasound, OCT optical coherence tomography, MV main vessel, SV side vessel. Other abbreviations are the same as in Table 1
aPotential independent risk-adjusting variables selected for Cox proportional hazards models
bDaggers indicate independent model for variables selected for propensity score matching
As a sensitivity analysis, the effects of FKBT strategy relative to non-FKBT strategy were evaluated in the entire study population using the multivariable Cox proportional hazard models, and were expressed as hazard ratios (HRs) with 95% CI. We included 22 clinically relevant factors listed in Tables 1 and 2 as the risk-adjusting variables. Proportional hazard assumptions for the variables were assessed on plots of log (time) versus log (log [survival]) stratified by each variable and were verified as acceptable for all variables. As treatment strategies and other related factors changed over time, 3 periods were defined based on the dominant stent types; bare-metal stent period: 2004–2006; first-generation DES (G1-DES) period: 2007–2009; and second-generation DES (G2-DES) period: 2010–2012. The period was used as a stratification variable.
Two physicians (K. Nishida and M. Toyofuku) and a statistician (T. Morimoto) conducted all the statistical analyses using SPSS version 24 (SPSS, Chicago, IL), JMP version 10.0 (SAS Institute, Cary, NC) and SAS version 9.2 (SAS Institute). All reported P values are 2-sided, and P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Baseline characteristics were mostly similar between the FKBT and non-FKBT groups in the entire study population, except for the higher prevalence of hemodialysis and peripheral vascular disease in the non-FKBT group. The prevalence of FKBT was significantly different across centers (Table 1). In terms of lesion and procedural characteristics, the prevalence of true bifurcation lesions and mean SYNTAX scores did not differ significantly between the 2 groups (Table 2). Femoral artery was the dominant access site, and G1-DES was implanted in approximately two-thirds of cases without any significant differences between the 2 groups. Patients in the non-FKBT group had significantly higher prevalence of intravascular ultrasound (IVUS) use, as well as lower prevalence of proximal optimization technique, greater number of stents, and larger final balloon size.
In the propensity-matched population of 160 pairs, baseline characteristics were well balanced except for the prevalence of hypertension, peripheral vascular disease, optical coherence tomography (OCT) use, proximal optimization technique, and final balloon size (Tables 1, 2).
Clinical outcomes: FKBT versus non-FKBT
Median duration of follow-up after the procedure was 3.8 (interquartile range: 2.2–5.3) years. Overall, 83.4% of patients in this study underwent follow-up coronary artery angiography regardless of the presence of symptoms. In the entire study population, the cumulative 5-year incidence of the primary outcome measure (TLR) was not significantly different between the FKBT and non-FKBT groups (10.7 versus 14.3%, P = 0.49) (Table 3, and Fig. 2). Regarding the TLR location, there were no significant differences in the cumulative incidences of TLR for LMCA-only, for the main branch, and for the side branch between the 2 groups (2.2 versus 1.3%, P = 0.93, 11.8 versus 9.1%, P = 0.71, and 8.2 versus 7.6%, P = 0.82, respectively) (Table 3). In the propensity-matched population, the cumulative 5-year incidence of TLR was also not significantly different between the FKBT and non-FKBT groups (11.8 versus 14.3%, P = 0.53) (Table 3, and Fig. 2). In the sensitivity analysis by the multivariable Cox proportional hazard model, the effect of FKBT relative to non-FKBT for TLR remained insignificant (adjusted HR 0.89, 95% CI 0.47–1.69, P = 0.72) (Table 4). Cumulative 5-year incidences of the secondary outcome measures including MACE were also not significantly differences between the FKBT and non-FKBT groups both in the entire study population and in the propensity-matched population (Table 3 and Fig. 3). The effects of FKBT relative to non-FKBT for the secondary outcome measures were also not significant (Table 4).
Table 3.
Five-year clinical outcomes in the entire study population and in the propensity-matched population: non-FKBT and FKBT
| Entire study population | Propensity-matched population | |||||
|---|---|---|---|---|---|---|
| Patients with at least 1 event (cumulative 5-year incidence, %) | Patients with at least 1 event (cumulative 5-year incidence, %) | |||||
| FKBT (n = 578) | Non-FKBT (n = 160) | P value | FKBT (n = 160) | Non-FKBT (n = 160) | P value | |
| TLR | 59 (10.7) | 17 (14.3) | 0.49 | 17 (11.8) | 17 (14.3) | 0.53 |
| TLR-LMCA only | 7 (1.3) | 2 (2.2) | 0.93 | 1 (0.6) | 2 (2.2) | 0.56 |
| TLR-main branch | 54 (9.1) | 15 (11.8) | 0.71 | 14 (10.4) | 15 (12.5) | 0.5 |
| TLR-side branch | 41 (7.6) | 11 (8.2) | 0.82 | 10 (6.7) | 11 (8.2) | 0.59 |
| All-cause death | 98 (19.9) | 36 (23.1) | 0.23 | 29 (21.2) | 36 (23.1) | 0.6 |
| Cardiac death | 30 (6.3) | 18 (9.1) | 0.14 | 11 (8.1) | 18 (9.1) | 0.68 |
| Sudden death | 9 (1.9) | 5 (2.0) | 0.61 | 2 (1.5) | 5 (2.0) | 0.64 |
| Myocardial infarction | 12 (2.6) | 8 (6.4) | 0.06 | 6 (4.1) | 8 (6.6) | 0.57 |
| Definite or probable stent thrombosis | 2 (0.3) | 1 (0.6) | 0.62 | 2 (1.3) | 1 (0.6) | 0.57 |
| Stroke | 25 (5.0) | 6 (4.5) | 0.87 | 7 (4.2) | 6 (4.5) | 0.75 |
| Ischemic | 21 (4.2) | 3 (2.2) | 0.51 | 5 (2.8) | 3 (2.2) | 0.99 |
| Hemorrhagic | 4 (0.8) | 3 (2.2) | 0.16 | 2 (1.4) | 3 (2.2) | 0.64 |
| Any coronary revascularization | 160 (29.9) | 42 (34.1) | 0.75 | 39 (26.4) | 42 (34.1) | 0.24 |
| MACE | 92 (17.0) | 34 (21.3) | 0.24 | 27 (18.7) | 34 (21.3) | 0.45 |
The number of patients with at least 1 event was counted through the entire follow-up period, while the cumulative incidence was truncated at 5 years
P values estimated by the log-rank test
MACE major adverse cardiac events, TLR target lesion revascularization
Fig. 2.
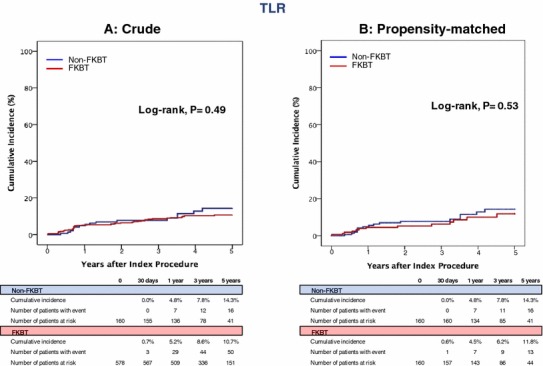
Kaplan–Meier curves for TLR in the entire study population and in the propensity-matched population: FKBT versus Non-FKBT. FKBT final kissing balloon technique, TLR target lesion revascularization
Table 4.
Effects of FKBT relative to non-FKBT for clinical outcomes in the crude population
| Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
|---|---|---|---|---|
| TLR | 0.82 (0.47–1.4) | 0.49 | 0.89 (0.47–1.69) | 0.72 |
| All-cause death | 0.78 (0.51–1.18) | 0.24 | 0.69 (0.43–1.12) | 0.14 |
| Cardiac death | 0.6 (0.31–1.19) | 0.15 | 0.41 (0.18–0.94) | 0.03 |
| Sudden death | 0.71 (0.19–2.67) | 0.61 | 0.31 (0.06–1.56) | 0.15 |
| Myocardial infarction | 0.42 (0.16–1.01) | 0.07 | 0.55 (0.17–1.79) | 0.32 |
| Definite or probable stent thrombosis | N/A | N/A | N/A | N/A |
| Stroke | 0.93 (0.37–2.3) | 0.87 | 0.89 (0.32–2.46) | 0.82 |
| Ischemic | 1.5 (0.44–5.12) | 0.52 | 1.15 (0.29–4.5) | 0.84 |
| Hemorrhagic | 0.36(0.08–1.59) | 0.18 | 0.71 (0.12–4.29) | 0.71 |
| Any coronary revascularization | 0.95 (0.67–1.34) | 0.75 | 0.81 (0.55–1.2) | 0.3 |
| MACE | 0.78 (0.51–1.19) | 0.25 | 0.64 (0.39–1.06) | 0.08 |
Effect of FKBT relative to non-FKBT is expressed as a hazard ratio with the 95% confidence interval by Cox proportional hazard models
CI confidence interval, HR hazard ratio, N/A not assessed. Other abbreviations are the same as in Table 3
Fig. 3.
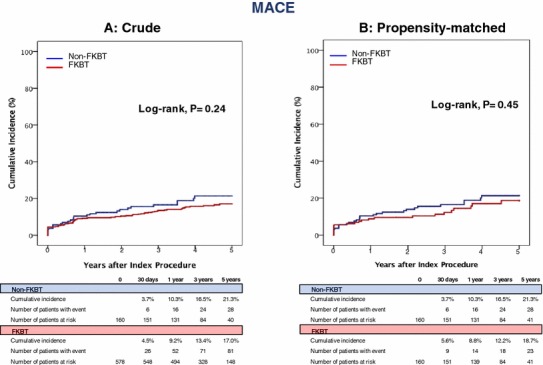
Kaplan–Meier curves for MACE in the entire study population and in the propensity-matched population: FKBT versus Non-FKBT. FKBT final kissing balloon technique, MACE major adverse cardiac events
Discussion
The main findings of the present study from a large multicenter registry in Japan were the followings; (1) FKBT after a 1-stent strategy for LMCA crossover stenting did not affect TLR and other clinical outcomes during 5-year follow-up; (2) FKBT also did not affect the location of TLR (Fig. 3).
FKBT has been widely performed at any bifurcation on the expectations of optimizing stent apposition at the main branch, ameliorating the side branch ostial narrowing caused by carina shift, reducing strut protrusion into the side branch ostium, and maintaining access to the side branch [16]. In contrast, FKBT might be associated with injury of the side branch ostium potentially leading to restenosis, stent deformation related to over-dilation of the stent proximal to the side branch, and strut mal-apposition due to inadequate rewiring position [17, 18]. FKBT is generally advocated after a two-stent strategy for any bifurcation [19, 20]. However, the clinical benefits of routine FKBT after a 1-stent strategy appear uncertain at any bifurcation lesion according to the several previous studies [8–11]. The role of FKBT at LMCA bifurcation might be markedly different from that at any other bifurcation, because LMCA bifurcation involves LCX, which is often a large vessel and supplies a large myocardial territory. FKBT would be justified in cases of hemodynamically significant ostial LCX stenosis or flow impairment of LCX after crossover stenting. However, it remains uncertain whether FKBT should be performed in cases of preserved LCX flow or without significant stenosis at ostial LCX ostium after LMCA crossover stenting.
Crossover stenting for the LMCA as a 1-stent strategy is forced to jail the LCX ostium by stent struts. There were concerns on the possibility that formation of neointima and thrombus at the jailed strut with compromise of LCX flow may increase the risk of ST or TLR [21]. A OCT study noted that FKBT may reduce the frequency of uncovered struts and subclinical thrombus at the side branch orifice, although FKBT did not decrease MACE and TLR in this small cohort [22]. The COBIS II registry, which included LMCA lesions in 26% of cases, demonstrated that a 1-stent technique with FKBT for any bifurcation lesions was associated with favorable long-term clinical outcomes, whereas a 1-stent strategy with FKBT for LMCA was not associated with better MACE outcomes compared with a 1-stent strategy without FKBT [11]. Furthermore, two recent single center registries found that midterm clinical outcomes were not significantly different regardless of FKBT after crossover stenting of the LMCA [12, 13]. As in the previous reports, the present multicenter study with longer follow-up, with a higher frequency of FKBT, and with TLR as the primary outcome measure did not demonstrate superiority of FKBT strategy over non-FKBT strategy.
Interestingly, the frequency of FKBT after crossover stenting for the LMCA in the present study was higher (78%) than those reported in previous studies (32–35%) [11, 12]. Reflecting the operator preferences, there is a wide variation across countries and institutions, regarding the performance of FKBT after crossover stenting for LMCA. The reason for the high frequency of FKBT in our registry might be that FKBT has been mainly performed not only for decreased coronary blood flow, but also for opening the jailed strut with FKBT to maintain access to the LCX for future PCI. The rationale for this “prophylactic” FKBT would be a concern about stent deformation and restenosis caused by balloon dilatation for a stable jailed strut of the LCX ostium and for stable LMCA in cases that need TVR for LCX in the future.
Despite concerns of increased TLR rate for the proximal LMCA regarding polymer damage, strut deformation, and asymmetry of the proximal stent by the hugging balloon, which potentially decrease tissue concentrations of the eluted drug, TLR rate for the proximal LMCA did not differ significantly regardless of FKBT in the present study [23]. The frequency of TLR for LCX involving the ostium was similar with or without FKBT in the present study, despite concerns about balloon injury or incomplete stent apposition with FKBT. Therefore, FKBT would not be harmful in terms of midterm outcomes in this study. One of the reasons for these neutral results might be that FKBT was not harmful despite its potential risks, because the frequency of IVUS use was higher than in previous reports [9–12]. Guidance with IVUS or OCT is useful particularly for the LMCA for confirming side branch distal rewiring before FKBT and determination of stent and balloon sizes [18, 24]. However, FKBT often needs a higher volume of contrast, longer procedure time, and more devices than no FKBT [9, 12]. Based on our present results, FKBT may not be mandatory in cases without flow limitation after stenting. Further and longer term validation study would be required for this issue.
Limitations
The present study has several important limitations. First, this registry was conducted as a nonrandomized and retrospective observational study. Therefore, we performed propensity-matched analysis to adjust for the potential confounders. Nevertheless, we could not deny the presence of unmeasured confounders and selection bias. Second, we did not assess the anatomic assessments such as the extent of myocardial territory and of previous myocardial infarction or diameter of LCX, and the degree of stenosis at the LCX ostium after crossover stenting, which might be closely related to the decision whether to perform FKBT. Particularly, significance of LCX territory or viability may affect the clinical outcome. Additionally, patients treated with 2-stent strategy for bailout subsequent to FKBT was excluded in this study cohort, who might have been benefitted from LCX intervention. Third, physiological assessment such as fractional flow reserve was not adequately analyzed, although angiography often overestimate the stenosis of the LCX ostium compared with functional assessment [25]. Fourth, the frequency of follow-up coronary artery angiography was high compared to previous reports, which might have increased the incidence of angiography-driven TLR. Fifth, the sample size was not large enough to evaluate those clinical outcomes such as ST, MI, and cardiac death. Indeed, the risk for MI and cardiac death favored FKBT, although we could not draw definitive conclusions. Finally, G1-DES were used in a large proportion of patients. Use of newer generation DES has been shown to improve clinical outcomes after LMCA stenting [4, 5].
Conclusion
FKBT after a 1-stent strategy for LMCA crossover stenting did not affect TLR and other clinical outcomes during 5-year follow-up.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the members of the catheterization laboratories and physicians of the participating centers.
Compliance with ethical standards
Conflict of interest
Takeshi Kimura serves as an advisory board member for Abbott Vascular. The other authors declare that they have no conflict of interest.
References
- 1.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016;375:2223–2235. doi: 10.1056/NEJMoa1610227. [DOI] [PubMed] [Google Scholar]
- 5.Mäkikallio T, Holm NR, Lindsay M, Spence MS, Erglis A, Menown IB, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388:2743–2752. doi: 10.1016/S0140-6736(16)32052-9. [DOI] [PubMed] [Google Scholar]
- 6.Ohya M, Kadota K, Toyofuku M, Morimoto T, Higami H, Fuku Y, et al. Long-term outcomes after stent implantation for left main coronary artery (from the Multicenter Assessing Optimal Percutaneous Coronary Intervention for Left Main Coronary Artery Stenting Registry) Am J Cardiol. 2017;119:355–364. doi: 10.1016/j.amjcard.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Takagi K, Naganuma T, Chieffo A, Fujino Y, Latib A, Tahara S, et al. Comparison between 1- and 2-stent strategies in unprotected distal left main disease: the Milan and New-Tokyo Registry. Circ Cardiovasc Interv. 2016;9:e003359. doi: 10.1161/CIRCINTERVENTIONS.116.003359. [DOI] [PubMed] [Google Scholar]
- 8.Liu G, Ke X, Huang ZB, Wang LC, Huang ZN, Guo Y, et al. Final kissing balloon inflation for coronary bifurcation lesions treated with single-stent technique: a meta-analysis. Herz. 2017 doi: 10.1007/s00059-017-4647-1. [DOI] [PubMed] [Google Scholar]
- 9.Niemelä M, Kervinen K, Erglis A, Holm NR, Maeng M, Christiansen EH, et al. Randomized comparison of final kissing balloon dilatation versus no final kissing balloon dilatation in patients with coronary bifurcation lesions treated with main vessel stenting: the Nordic-Baltic Bifurcation Study III. Circulation. 2011;123:79–86. doi: 10.1161/CIRCULATIONAHA.110.966879. [DOI] [PubMed] [Google Scholar]
- 10.Gwon HC, Hahn JY, Koo BK, Song YB, Choi SH, Choi JH, et al. Final kissing ballooning and long-term clinical outcomes in coronary bifurcation lesions treated with 1-stent technique: results from the COBIS registry. Heart. 2012;98:225–231. doi: 10.1136/heartjnl-2011-300322. [DOI] [PubMed] [Google Scholar]
- 11.Yu CW, Yang JH, Song YB, Hahn JY, Choi SH, Choi JH, et al. Long-term clinical outcomes of final kissing ballooning in coronary bifurcation lesions treated with the 1-stent technique: results from the COBIS II Registry (Korean Coronary Bifurcation Stenting Registry) JACC Cardiovasc Interv. 2015;8:1297–1307. doi: 10.1016/j.jcin.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Xu B, Yang YJ, Qiao SB, Wu YJ, Chen T, et al. Effect of final kissing balloon dilatation after one-stent technique at left-main bifurcation: a single center data. Chin Med J. 2015;128:733–739. doi: 10.4103/0366-6999.152468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn JM, Lee PH, Park DW, Kang SJ, Lee SW, Kim YH, et al. Benefit of final kissing balloon inflation mandatory after simple crossover stenting for left main bifurcation narrowing. Am J Cardiol. 2017;119:528–534. doi: 10.1016/j.amjcard.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Lassen JF, Holm NR, Banning A, Burzotta F, Lefèvre T, Chieffo A, et al. Percutaneous coronary intervention for coronary bifurcation disease: 11th consensus document from the European Bifurcation Club. EuroIntervention. 2016;12:38–46. doi: 10.4244/EIJV12I1A7. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sgueglia GA, Chevalier B. Kissing balloon inflation in percutaneous coronary interventions. JACC Cardiovasc Interv. 2012;5:803–811. doi: 10.1016/j.jcin.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Rahman S, Leesar T, Cilingiroglu M, Effat M, Arif I, Helmy T, et al. Impact of kissing balloon inflation on the main vessel stent volume, area, and symmetry after side-branch dilation in patients with coronary bifurcation lesions: a serial volumetric intravascular ultrasound study. JACC Cardiovasc Interv. 2013;6:923–931. doi: 10.1016/j.jcin.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Okamura T, Nagoshi R, Fujimura T, Murasato Y, Yamawaki M, Ono S, et al. Impact of guidewire recrossing point into stent jailed side branch for optimal kissing balloon dilatation: core lab 3D optical coherence tomography analysis. EuroIntervention. 2018;13:e1785–e1793. doi: 10.4244/EIJ-D-17-00591. [DOI] [PubMed] [Google Scholar]
- 19.Hoye A, Iakovou I, Ge L, van Mieghem CA, Ong AT, Cosgrave J, et al. Long-term outcomes after stenting of bifurcation lesions with the “crush” technique: predictors of an adverse outcome. J Am Coll Cardiol. 2006;47:1949–1958. doi: 10.1016/j.jacc.2005.11.083. [DOI] [PubMed] [Google Scholar]
- 20.Adriaenssens T, Byrne RA, Dibra A, Iijima R, Mehilli J, Bruskina O, et al. Culotte stenting technique in coronary bifurcation disease: angiographic follow-up using dedicated quantitative coronary angiographic analysis and 12-month clinical outcomes. Eur Heart J. 2008;29:2868–2876. doi: 10.1093/eurheartj/ehn512. [DOI] [PubMed] [Google Scholar]
- 21.Nakazawa G, Yazdani SK, Finn AV, Vorpahl M, Kolodgie FD, Virmani R. Pathological findings at bifurcation lesions: the impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J Am Coll Cardiol. 2010;55:1679–1687. doi: 10.1016/j.jacc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Hariki H, Shinke T, Otake H, Shite J, Nakagawa M, Inoue T, et al. Potential benefit of final kissing balloon inflation after single stenting for the treatment of bifurcation lesions–insights from optical coherence tomography observations. Circ J. 2013;77:1193–1201. doi: 10.1253/circj.CJ-12-0848. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Lee JH, Roh JH, Ahn JM, Yoon SH, Park DW, et al. Randomized comparisons between different stenting approaches for bifurcation coronary lesions with or without side branch stenosis. JACC Cardiovasc Interv. 2015;8:550–560. doi: 10.1016/j.jcin.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Andell P, Karlsson S, Mohammad MA, Götberg M, James S, Jensen J, et al. Intravascular ultrasound guidance is associated with better outcome in patients undergoing unprotected left main coronary artery stenting compared with angiography guidance alone. Circ Cardiovasc Interv. 2017;10:e004813. doi: 10.1161/CIRCINTERVENTIONS.116.004813. [DOI] [PubMed] [Google Scholar]
- 25.Koo BK, Waseda K, Kang HJ, Kim HS, Nam CW, Hur SH, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3:113–119. doi: 10.1161/CIRCINTERVENTIONS.109.887406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


