Abstract
The red blood cell distribution width (RDW) is a simple and inexpensive laboratory parameter that can be linked to oxidative stress, inflammation and microvascular flow resistance. For this research, we performed a large-sample case-control study to describe the relationships between the RDW and primary angle-closure glaucoma (PACG). A total of 1191 PACG patients (422 males and 769 females), who were divided into mild, moderate and severe PACG groups, and 982 healthy controls (344 males and 638 females) were recruited between January 2008 and June 2018. Detailed eye and physical examinations were performed for each subject. Based on the laboratory results, the mean RDW was significantly higher (p < 0.001) in the PACG group (13.01 ± 0.82%) than in the control group (12.65 ± 0.53%). Moreover, the mean RDW level was lower (p < 0.05) in the mild PACG group than in the moderate and severe PACG groups. The Pearson correlation analyses showed significant positive correlations between the mean deviation and the RDW (r = 0.141, p < 0.001) and the intraocular pressure and the RDW (r = 0.085, p = 0.004). After adjusting for the confounding factors, the logistic regression analyses indicated that the odds ratio for the PACG group was 2.318 (p < 0.001, 95% confidence interval 1.997, 2.690) when compared to the control group. Additionally, an increased RDW was associated with the PACG severity, and this trend was also observed in the gender and age subgroups. In summary, the results of our study showed that an elevated RDW was associated with PACG and its severity. If future studies confirm this relationship, the use of an RDW assessment may help to predict the PACG severity in each patient in order to better customise effective prevention treatments.
Keywords: Primary angle-closure glaucoma, Red blood cell distribution width, Patient stratification, Recommendation, Laboratory medicine, Oxidative stress, Inflammation, Endothelial dysfunction, Individualised patient profile, Predictive preventive personalised medicine
Introduction
Primary glaucoma is one of the most common causes of irreversible blindness worldwide [1], and the rapid global increase in glaucoma patients has placed a huge burden on their caregivers and communities [2]. Primary angle-closure glaucoma (PACG) is the most common type of glaucoma diagnosed in China [2]. Although the underlying mechanisms leading to glaucomatous atrophy in PACG cases are not fully understood, several mechanisms have been suggested. For example, inflammation [3], microangiopathy [4–9] and oxidative stress [10, 11] are all currently being discussed in terms of the leading factors causing PACG, which can damage the optic nerve and lead to the progressive loss of retinal ganglion cells and their axons.
The red blood cell distribution width (RDW) is a simple and inexpensive laboratory parameter that reflects the variability in the size of the circulating erythrocytes [12]. The RDW shows the heterogeneity degree of the erythrocyte volume, and an elevated RDW means that some of the red blood cells (RBCs) are relatively too large or too small. An increased RDW mirrors a profound deregulation in the erythrocyte homeostasis involving both impaired erythropoiesis and abnormal RBC survival. This may be attributed to a variety of underlying metabolic abnormalities, such as oxidative stress, inflammation, a poor nutritional status and telomere length shortening [12]. In addition, increased RDWs have been related to underlying oxidative stress, inflammation and microvascular flow resistance [12, 13]. For example, Lippi et al. [14] suggested that the RDW is associated with increased high-sensitivity C-reactive protein levels and erythrocyte sedimentation rates. Semba et al. [15] reported that the total serum carotenoid and selenium levels, which are part of the antioxidant defence system in humans, were significantly associated with the RDW. Moreover, Akpinar et al. [13] showed that the RDW is an independent predictor of a slow coronary blood flow. As described above, the inflammatory response, microangiopathy and oxidative stress all play pivotal roles in PACG.
Unfortunately, data concerning the RDW and its association with PACG is lacking. However, determining the RDW in PACG patients may lead to a more efficient way to predict the disease severity that is better tailored to the individual patient [16]. For this reason, we conducted a large-sample cross-sectional investigation designed to evaluate the associations between the RDW and the PACG risk and severity.
Methods
This study was approved by the ethical committee of the Taishan Medical University Affiliated Hospital and the ethical committee of the Taian City Central Hospital in Shandong, China. In addition, this study was performed according to the tenets of the Declaration of Helsinki, and written consent was obtained from each patient before using their clinical data for these research purposes.
A retrospective medical chart review of PACG patients who were recruited consecutively from the Taishan Medical University Affiliated Hospital and the Taian Central Hospital was performed. A group of healthy controls was also recruited from those individuals who participated in yearly health screenings during the study period. All of the participants were Chinese.
If both eyes were affected in the PACG patients, only the right eye of each patient was included in the study. PACG was diagnosed when the presence of angle closure with glaucomatous optic neuropathy was detected. Angle closure was determined in those eyes in which at least 180° of the posterior pigmented trabecular meshwork was not visible during the gonioscopic examination in the primary position of the gaze with no indentation. Glaucomatous optic neuropathy was determined based on one or more of the following: The neuroretinal rim had a vertical cup-to-disc ratio (VCDR) of ≥ 0.7, the between-eye VCDR asymmetry was > 0.2 and the focal notching of the neuroretinal rim had a visual field defect (which is suggestive for glaucoma) [17]. Visual field tests were performed using automated perimetry (Octopus; Haag-Streit AG, Koeniz, Switzerland). After considering the learning curve of the visual field tests, the results of the first two tests were excluded. Only the reliable (a false positive/negative under 15% and a reliability factor under 15%) and compatible visual field results were included in the data analysis.
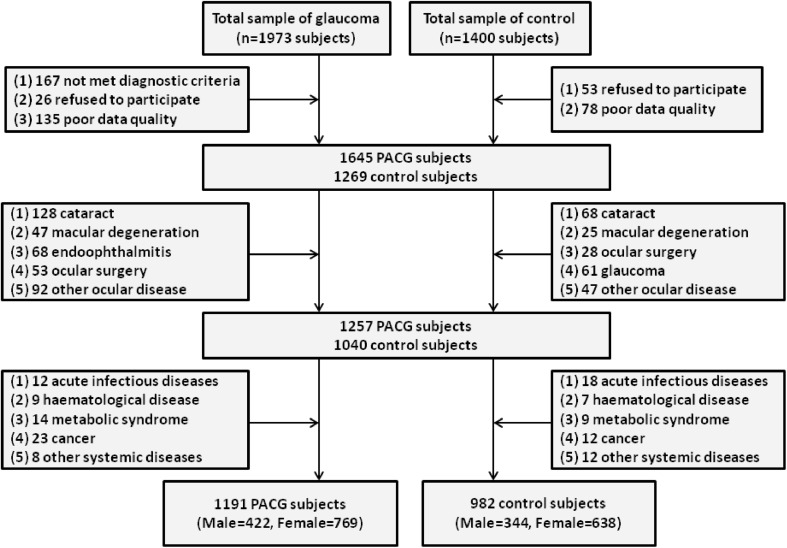
The patients who had histories of any other secondary glaucomas, previous eye surgeries, and other eye diseases were excluded from this study. Moreover, those patients with any systemic diseases, such as acute infectious diseases, haematological diseases, metabolic syndrome, kidney diseases and cancer, were also excluded. In total, 1973 patients who visited the Department of Ophthalmology between 1 January 2008 and 1 September 2018 were enrolled in this study as PACG subjects. Later, 782 of them were excluded. For the healthy controls, 1400 individuals who participated in yearly health screenings during the study period were consecutively recruited. Later, 418 of them were excluded. For an overview of the study subjects, see the study cohort flow diagram in Fig. 1.
Fig. 1.
The study cohort flow diagram
All of the subjects involved in this study underwent standardised ophthalmic examinations that consisted of a slit-lamp examination (Haag-Streit AG, Bern, Switzerland), an intraocular pressure (IOP) measurement using Goldmann applanation tonometry (Haag-Streit AG, Koeniz, Switzerland) and a gonioscopic evaluation. A-scan ultrasound biometry (Ultrasonic, Exton, PA, USA) was used to measure the axial length, anterior chamber depth and central corneal thickness. In addition, each study subject underwent a medical examination, including liver function, renal function, blood pressure, heart rate [18] and body temperature assessments. The demographic information of each study subject was also collected, including the age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), diabetes mellitus history and hypertension history.
The patients’ blood samples were taken before they underwent treatment, and they were collected in ethylenediaminetetraacetic acid tubes in order to prevent blood coagulation. A 2-ml blood sample was used within 0.5 h after the blood collection to perform a complete blood count, including the RBC, white blood cell (WBC), RDW and haemoglobin (HG) values, using an automated haematology analyser (Sysmex Corporation, Tokyo, Japan). Then, another 4-ml blood sample, which was obtained in the morning after the subject had fasted for 8 h, was used for the biochemical measurements. The aspartate transaminase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) and creatinine (Cr) serum levels were measured using a commercially available kit (Roche Diagnostics GmbH, Mannheim, Germany).
The statistical analysis was performed using IBM SPSS Statistics for Windows (Version 20.0; IBM Corp., Armonk, NY, USA). The results are presented as the mean ± standard deviation. The chi-squared test was used for the categorical variables, while the independent Student’s t test and the one-way analysis of variance were used to compare the participants’ characteristics among the groups. Logistic regression analyses were performed to identify the associations between the RDW levels and the PACG severity and risk. A two-sided p value of < 0.05 was considered to be statistically significant.
Results
A total of 1191 (422 males and 769 females) PACG patients and 982 (344 males and 638 females) healthy controls were recruited for this study. There were no significant differences between the PACG and control groups (p > 0.05) with regard to the mean age, gender, BMI, SBP, SDP, diabetes status, hypertension status, smoking status and drinking status. The baseline demographic and ocular parameters of the study subjects are shown in Table 1.
Table 1.
Characteristics of subjects with PACG
| PACG group (n = 1191) | Control group (n = 982) | t value | p value | |
|---|---|---|---|---|
| Age (years) | 63.54 ± 10.77 | 63.08 ± 10.63 | 1.005 | 0.315 |
| Gender (male/female) | 422/769 | 344/638 | 0.038 | 0.845 |
| BMI (kg/m2) | 22.77 ± 3.27 | 22.92 ± 3.27 | 0.931 | 0.352 |
| SBP (mm Hg) | 130.40 ± 15.77 | 130.10 ± 15.57 | 0.437 | 0.662 |
| SDP (mm Hg) | 75.07 ± 9.33 | 75.17 ± 9.36 | 0.248 | 0.804 |
| Smoking (yes/no) | 298/893 | 265/717 | 1.082 | 0.298 |
| Drinking (yes/no) | 366/825 | 323/659 | 1.161 | 0.281 |
| Diabetes (yes/no) | 109/1082 | 94/888 | 0.112 | 0.738 |
| Hypertension (yes/no) | 358/833 | 292/690 | 0.027 | 0.870 |
| IOP (mm Hg) | 30.92 ± 11.83 | 13.67 ± 2.68 | 20.361 | < 0.001 |
| VCDR | 0.59 ± 0.25 | – | – | – |
| CCT (μm) | 546.54 ± 49.23 | – | – | – |
| ACD (mm) | 1.87 ± 0.56 | – | – | – |
| AL (mm) | 22.40 ± 1.25 | – | – | – |
| MD (dB) | 13.57 ± 8.21 | – | – | – |
| MS (dB) | 13.32 ± 8.71 | – | – | – |
Data are expressed as mean ± standard deviation (SD). The independent Student’s t test and χ2 tests were used
PACG primary angle-closure glaucoma, BMI body mass index, SBP systolic blood pressure, SDP diastolic blood pressure, IOP intraocular pressure, VCDR vertical cup-disc ratio, CCT central corneal thickness, ACD anterior chamber depth, AL axial length, MD visual fields mean deviation, MS visual fields mean sensitivity
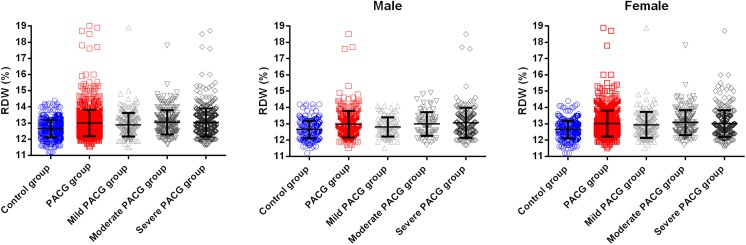
Table 2 shows the mean RDW, WBC, RBC, HG, AST, ALT, BUN and Cr values in the PACG and control groups. A comparison of these results showed that the mean RDW was significantly higher (p < 0.001) in the PACG group (13.01 ± 0.82%) than in the healthy control group (12.65 ± 0.53%) (Fig. 2). The PACG and control subjects were also categorised into female and male subgroups, followed by another categorisation into subgroups based on the age (< 50, 40–50, 50–60 and > 70 years old). In all of the gender and age subgroups, the mean RDW was significantly higher (p < 0.05) in the PACG group when compared to the control group (Table 3 and Fig. 2).
Table 2.
Comparison of RDW and other blood parameters between PACG and control subjects
| PACG group (n = 1191) | Control group (n = 982) | t value | p value | |
|---|---|---|---|---|
| RDW (%) | 13.01 ± 0.82 | 12.65 ± 0.53 | 12.162 | < 0.001 |
| WBC (109/l) | 6.22 ± 1.76 | 6.19 ± 1.74 | 0.468 | 0.640 |
| RBC (1012/l) | 4.41 ± 1.68 | 4.38 ± 1.42 | 0.361 | 0.718 |
| HG (g/l) | 131.40 ± 13.91 | 133.43 ± 12.62 | 3.569 | < 0.001 |
| AST (U/l) | 20.22 ± 8.21 | 20.00 ± 8.03 | 0.646 | 0.518 |
| ALT (U/l) | 22.64 ± 14.15 | 22.80 ± 15.00 | 0.248 | 0.804 |
| BUN (mmol/l) | 5.83 ± 1.72 | 5.76 ± 1.66 | 1.031 | 0.303 |
| Cr (umol/l) | 69.53 ± 16.95 | 68.83 ± 16.17 | 0.983 | 0.326 |
Data are expressed as mean ± standard deviation (SD). The independent Student’s t test was used
PACG primary angle-closure glaucoma, Cr creatinine, BUN urea nitrogen, AST aspartate transaminase, ALT alanine aminotransferase, HG haemoglobin, RBC red blood cell, WBC white blood cell, RDW red blood cell distribution width
Fig. 2.
Comparison of red blood cell distribution width level in the PACG group (including mild, moderate and severe) and in the control group. Each data point represents one subject. The top of the box plot represents the mean, and the bar of each box represents the standard deviation
Table 3.
Comparison of RDW between PACG and control subjects, stratified according to age and gender
| PACG group | Control group | t value | p value | |
|---|---|---|---|---|
| Male | 12.96 ± 0.84 | 12.65 ± 0.53 | 6.349 | < 0.001 |
| < 50 | 12.63 ± 0.61 | 12.34 ± 0.52 | 2.250 | 0.027 |
| 40–50 | 13.08 ± 1.07 | 12.67 ± 0.56 | 3.287 | 0.001 |
| 50–60 | 12.96 ± 0.82 | 12.65 ± 0.50 | 4.002 | < 0.001 |
| > 70 | 13.00 ± 0.68 | 12.75 ± 0.53 | 2.951 | 0.004 |
| Female | 13.02 ± 0.81 | 12.65 ± 0.52 | 10.452 | < 0.001 |
| < 50 | 13.17 ± 1.08 | 12.62 ± 0.55 | 3.640 | < 0.001 |
| 40–50 | 12.96 ± 0.88 | 12.56 ± 0.56 | 5.066 | < 0.001 |
| 50–60 | 12.94 ± 0.63 | 12.64 ± 0.50 | 6.015 | < 0.001 |
| > 70 | 13.14 ± 0.83 | 12.75 ± 0.53 | 5.804 | < 0.001 |
Data are expressed as mean ± standard deviation (SD). The independent Student’s t test was used
PACG primary angle-closure glaucoma, RDW red blood cell distribution width
Pearson correlation analyses were performed to identify the associations between the IOP and the mean deviation (MD) and the RDW. A significant positive correlation was found between the MD and the RDW (r = 0.141, p < 0.001) and the IOP and the RDW (r = 0.085, p = 0.004). A partial correlation analysis was also performed to identify the associations between the RDW and MD while adjusting for the IOP. A significant positive correlation was found between the MD and the RDW (r = 0.116, p < 0.001).
Logistic regression analyses were performed to identify the associations between the RDW and the PACG risk in the PACG and control groups using three models (A, B and C) (Table 4). In model A, the odds ratio (OR) (after being adjusted for the age and sex) for the PACG subjects was 2.318 [p < 0.001, 95% confidence interval (CI) 1.997, 2.690] when compared to the control subjects. These results did not change after adjusting the data for the BMI, SBP, SDP, smoking status, drinking status, diabetes status and hypertension status in model B (OR = 2.139, p = 0.001, 95% CI 1.369, 3.340) or after adjusting the data for the WBC, RBC, HG, AST, ALT, BUN and Cr values in model C (OR = 2.116, p = 0.002, 95% CI 1.319, 3.397).
Table 4.
Logistic regression analysis of the association between RDW with PACG
| Model A | Model B | Model C | ||||
|---|---|---|---|---|---|---|
| OR | p (95% CI) | OR | p (95% CI) | OR | p (95% CI) | |
| RDW (%) | 2.318 | < 0.001 (1.997–2.690) | 2.139 | 0.001 (1.369–3.340) | 2.116 | 0.002 (1.319–3.397) |
| Age (years) | 0.998 | 0.706 (0.990–1.007) | 1.005 | 0.709 (0.980–1.030) | 1.003 | 0.830 (0.977–1.029) |
| Gender | 0.956 | 0.626 (0.796–1.147) | 0.892 | 0.655 (0.542–1.470) | 0.891 | 0.830 (0.461–1.721) |
| BMI (kg/m2) | 1.003 | 0.931 (0.929–1.084) | 1.003 | 0.940 (0.925–1.088) | ||
| SBP (mm Hg) | 0.998 | 0.811 (0.979–1.016) | 0.997 | 0.794 (0.979–1.017) | ||
| SDP (mm Hg) | 0.999 | 0.964 (0.971–1.029) | 1.000 | 0.982 (0.972–1.030) | ||
| Diabetes | 1.161 | 0.637 (0.625–2.155) | 1.159 | 0.647 (0.617–2.174) | ||
| Hypertension | 1.023 | 0.940 (0.566–1.847) | 1.001 | 0.998 (0.545–1.836) | ||
| WBC (109/l) | 0.998 | 0.983 (0.862–1.157) | ||||
| RBC (1012/l) | 0.886 | 0.890 (0.159–4.930) | ||||
| HG (g/l) | 1.000 | 0.994 (0.948–1.055) | ||||
| AST (U/l) | 1.008 | 0.669 (0.973–1.043) | ||||
| ALT (U/l) | 0.996 | 0.709 (0.974–1.018) | ||||
| BUN (mmol/l) | 0.981 | 0.805 (0.840–1.145) | ||||
| Cr (umol/l) | 1.005 | 0.537 (0.989–1.021) | ||||
Model A: adjusted for age and sex
Model B: adjusted for Model A covariates plus BMI, SBP, SDP, Smoking, Drinking, Diabetes, and Hypertension
Model C: adjusted for Model B covariates plus WBC, RBC, HG, AST, ALT, BUN, and Cr
PACG primary angle-closure glaucoma, RDW red blood cell distribution width, BMI body mass index, SBP systolic blood pressure, SDP diastolic blood pressure, Cr creatinine, BUN urea nitrogen, AST aspartate transaminase, ALT alanine aminotransferase, HG haemoglobin, RBC red blood cell, WBC white blood cell
Based on the visual field MDs, the PACG subjects were categorised into three subgroups of different disease severity levels: 259 were classified as mild (MD ≤ 6 dB), 261 as moderate (6 dB < MD ≤ 12 dB) and 671 as severe (MD > 12 dB). The comparisons of the RDWs and the ocular parameters of the PACG patients (stratified according to severity) are shown in Table 5. The mean RDW was lower in the mild PACG group (12.89 ± 0.74%) when compared to the moderate (13.05 ± 0.75%) and severe (13.03 ± 0.86%) groups, and these comparisons were significant (p = 0.042) (Fig. 2).
Table 5.
Comparison of RDW and ocular biometry in subjects with PACG, stratified according to glaucoma severity
| Mild (n = 259) | Moderate (n = 261) | Severe (n = 671) | p value | |
|---|---|---|---|---|
| Age (years) | 60.11 ± 10.28 | 64.84 ± 9.20 | 64.45 ± 10.98 | < 0.001a,b |
| Gender (male/female) | 89/170 | 79/182 | 254/417 | 0.414 |
| RDW (%) | 12.89 ± 0.74 | 13.05 ± 0.75 | 13.03 ± 0.86 | 0.042a,b |
| IOP (mm Hg) | 26.34 ± 7.45 | 27.17 ± 9.01 | 33.58 ± 12.62 | < 0.001a,b,c |
| VCDR | 0.43 ± 0.15 | 0.49 ± 0.18 | 0.72 ± 0.25 | < 0.01a,b,c |
| CCT (μm) | 542.09 ± 45.36 | 549.75 ± 54.97 | 545.63 ± 44.42 | 0.256 |
| ACD (mm) | 1.85 ± 0.28 | 1.83 ± 0.42 | 1.89 ± 0.70 | 0.422 |
| AL (mm) | 22.22 ± 1.05 | 22.25 ± 1.00 | 22.51 ± 1.30 | 0.002a,c |
| MD (dB) | 3.28 ± 1.65 | 8.79 ± 1.78 | 21.08 ± 5.30 | < 0.001a,b,c |
| MS (dB) | 23.70 ± 2.15 | 18.39 ± 2.53 | 6.71 ± 4.87 | < 0.001a,b,c |
Data are expressed as mean ± standard deviation (SD). One-way ANOVA was used
PACG primary angle-closure glaucoma, RDW red blood cell distribution width, IOP intraocular pressure, VCDR vertical cup-disc ratio, CCT central corneal thickness, AL axial length, ACD anterior chamber depth, MD mean deviation values for the visual field, MS mean sensitivity values for the visual field
ap < 0.05 for the difference between mild PACG and severe PACG (one-way ANOVA with the LSD post hoc test)
bp < 0.05 for the difference between mild PACG and moderate PACG (one-way ANOVA with the LSD post hoc test)
cp < 0.05 for the difference between moderate PACG and severe PACG (one-way ANOVA with the LSD post hoc test)
Logistic regression analyses were performed to identify the associations between the RDW and the PACG severities using three models (A, B and C) (Table 6). In model A, an increased RDW (after being adjusted for the age and sex) was found to be associated with the PACG severity in the PACG patients. These results did not change after adjusting the data for the BMI, SBP, SDP, smoking status, drinking status, diabetes status and hypertension status in model B, or after adjusting the data for the WBC, RBC, HG, AST, ALT, BUN and Cr values in model C. Moreover, similar results were found in the male and female subgroups.
Table 6.
Logistic regression analysis of the association between RDW and severity of PACG
| Model A | Model B | Model C | |||||
|---|---|---|---|---|---|---|---|
| Severity | OR | p (95% CI) | OR | p (95% CI) | OR | p (95% CI) | |
| PACG | Mild (reference) | 1.0 | 1.0 | 1.0 | |||
| Moderate | 1.336 | 0.021 (1.046–1.708) | 1.327 | 0.027 (1.033–1.704) | 1.276 | 0.091 (0.962–1.693) | |
| Severe | 1.249 | 0.030 (1.022–1.527) | 1.253 | 0.029 (1.024–1.532) | 1.253 | 0.045 (1.005–1.561) | |
| Male | Mild (reference) | 1.0 | 1.0 | 1.0 | |||
| Moderate | 1.520 | 0.082 (0.948–2.435) | 1.521 | 0.088 (0.939–2.464) | 1.265 | 0.406 (0.727–2.200) | |
| Severe | 1.538 | 0.025 (1.055–2.242) | 1.554 | 0.023 (1.062–2.276) | 1.451 | 0.070 (0.970–2.169) | |
| Female | Mild (reference) | 1.0 | 1.0 | 1.0 | |||
| Moderate | 1.410 | 0.027 (1.041–1.911) | 1.393 | 0.036 (1.021–1.910) | 1.507 | 0.020 (1.067–2.129) | |
| Severe | 1.308 | 0.044 (1.007–1.699) | 1.321 | 0.039 (1.015–1.720) | 1.535 | 0.009 (1.114–2.116) | |
Model A: adjusted for age and sex
Model B: adjusted for Model A covariates plus BMI, SBP, SDP, smoking, drinking, diabetes and hypertension
Model C: adjusted for Model B covariates plus WBC, RBC, HG, AST, ALT, BUN and Cr
PACG primary angle-closure glaucoma, RDW red blood cell distribution width
Discussion
PACG is a common cause of blindness, and its complexity has long plagued clinicians [19]. Because of its chronic nature, the progression of PACG brings with it severe living, economic and psychological burdens to the patients, and most importantly, a decrease in vision that affects their quality of life [20–22]. Therefore, it is essential to identify the factors that initiate and contribute to the pathogenesis and progression of PACG. The aim of the present study was to investigate the associations between the RDW and PACG while taking potential confounders into consideration. In this study, we showed that the mean RDW was significantly higher (p < 0.001) in PACG patients when compared to the healthy controls and that the mean RDW was lower in the mild PACG group when compared to the moderate and severe groups. Moreover, the logistic regression analyses suggested that an increased RDW is a risk factor for PACG and that it is associated with the PACG severity. Similar results were observed in the gender and age subgroups. To the best of our knowledge, such an association has not yet been demonstrated by other studies. Overall, our findings indicate that an elevated RDW has important associations with the PACG risk and severity.
Previous studies have demonstrated that a higher RDW is strongly associated with the all-cause mortality risk. One of these studies predicted increased cardiovascular mortality in a large community-based sample [23]. In another study, Tonelli et al. reported that the participants with RDWs in the highest quartile had an adjusted hazard ratio for death of 1.78 (95% CI 1.28, 2.47) when compared to those in the lowest quartile. Higher RDWs have also been associated with increased risks of coronary deaths/nonfatal myocardial infarctions, new symptomatic heart failure and strokes [24]. In addition, Montagnana et al. reviewed a larger number of previous studies, and they affirmed that the RDW could be useful because it adds prognostic information to oncological disease cases [25]. Moreover, Garofoli et al. reported that high RDWs were risk indicators in critically ill newborns [26]. Because of the potential roles of inflammation, endothelial dysfunction and oxidative stress in the development of these diseases, mediators reflecting these processes, such as the RDW, might be associated with the all-cause mortality risk. As such, the association between the RDW and glaucoma has been suggested to be mediated through oxidative stress [27], chronic inflammation [28] and endothelial dysfunction [29].
The RDW can be used as a simple, rapid and reliable parameter that reflects the size variability of the circulating RBCs. RDW measurements do not require additional blood samples, and they are performed routinely with the complete blood count, which is frequently required for clinical case management [26]. In this study, after adjusting for the confounding factors, an increased RDW was found to be a PACG risk factor, and it was associated with the PACG severity. However, the specific biological mechanisms that substantiate the association between the RDW and PACG remain unknown. In this case, we sup
pose that the RDW may be an independent PACG risk factor in that it represents a marker of concomitant disorders, such as an underlying inflammatory state, endothelial dysfunction and oxidative stress damage. In addition, because chronic inflammation plays an important role in the pathogenesis of glaucoma, it has been suggested that multiple inflammatory cytokines are significantly involved in PACG, such as C-reactive protein, interleukin (IL)-8, eotaxin, interferon gamma-induced protein (IP)-10, IL-9, IL-17, tumour necrosis factor-alpha and macrophage inflammatory protein-1-beta [30]. For example, Duvesh et al. [30] reported significantly higher IL-8, eotaxin, IP-10 (p < 0.001) and macrophage inflammatory protein-1-beta levels in the aqueous humour of chronic PACG patients when compared to the controls. Li et al. [3] suggested that the mean neutrophil, neutrophil to lymphocyte ratio and WBC values were higher in the PACG patients than in the healthy controls and that they were the lowest in the mild PACG group, followed by the moderate and severe groups. It is worth noting that a state of inflammation has been regarded as an important RDW determinant [31].
Another important factor regarding the PACG pathogenesis is oxidative stress. Goyal et al. [32] found that the superoxide dismutase and glutathione peroxidase activities were significantly higher in the aqueous humour of PACG patients and that the aqueous humour also had significantly lower vitamin C and E levels. In another study, it was shown that the total antioxidant status concentration had a statistically significantly lower pattern among glaucoma subjects [33]. Under normal circumstances, reactive oxygen species are converted by superoxide dismutase to H2O2, which, in turn, is reduced to water by glutathione peroxidase or catalase. Any reactive oxygen species that escape this system are available to react with other RBC constituents, which leads to impaired erythropoiesis and abnormal RBC survival [34]. Impaired erythropoiesis and abnormal RBC survival increase the RDW. As such, it has been suggested that oxidative stress may reduce the erythrocyte lifespan and increase their haemolysis vulnerability [14]. For this reason, oxidative stress damage has also been regarded as an important RDW determinant [12].
There has been a gradual accumulation of evidence suggesting that peripheral vascular endothelial dysfunction and abnormal ocular blood flow may play important roles in the PACG pathophysiology [35–37]. For instance, Alattar et al. [38] showed that the RDW correlates with the diastolic vascular and vascular endothelial dysfunction markers [13]. Interestingly, several studies have reported that the vessel density in the optic nerve head region was decreased in PACG patients. For example, Rao et al. [39] found that the vessel density and structural measurements were significantly lower (p < 0.05) in the PACG patients when compared to the healthy controls. Another study also reported that the average peripapillary vessel density was significantly lower in the PACG patients (52.7%) when compared to the healthy controls (60.8%) [40]. We also considered the fact that the RDW may be an independent factor in vascular function. It has been hypothesised that the chronic inflammatory state, which is common in patients with cardiovascular disorders, may also play a role in increasing the anisocytosis degree in patients with this condition [12]. Therefore, the RDW may be a cause of PACG. Decreased RBC deformability among patients with higher RDWs impairs the blood flow through the microcirculation, resulting in the diminution of the oxygen supply at the tissue level. A greater variation in the erythrocyte volume would increase the blood viscosity and, concomitantly, impair the blood flow through the microcirculation, thus triggering or amplifying the adverse consequences of a pre-existing vascular occlusion [12]. Overall, in conjunction with the results of previous studies, we hypothesised that the RDW may be an independent risk factor and a marker of PACG.
There was a significant positive correlation between the MD and the RDW in this study. We postulate that using the RDW as a simple, rapid, inexpensive and reliable parameter may be an important future tool for the prediction of the PACG severity. Limited data is available in the literature with regard to the RDW as a risk factor or a simple epiphenomenon of an underlying biological or metabolic imbalance. Therefore, the following two considerations arise [34]:
The RDW may be a PACG biomarker. Inflammation, microangiopathy and oxidative stress are currently being discussed as leading factors causing PACG, which causes damage to the optic nerve and leads to a progressive loss of retinal ganglion cells and their axons. Oxidative stress, microangiopathy and inflammation can lead to impaired erythropoiesis and abnormal RBC survival. Impaired erythropoiesis and abnormal RBC survival cause an RDW increase. Thus, the RDW increases with the disease onset, and the RDW in PACG patients may reflect the disease severity.
The RDW may be a cause of PACG. Decreased RBC deformability among patients with higher RDW values impairs the blood flow through the microcirculation, resulting in the diminution of the oxygen supply at the tissue level. A greater variation in the erythrocyte volume would increase the blood viscosity and, concomitantly, impair the blood flow through the microcirculation, thus triggering or amplifying the adverse consequences of a pre-existing vascular occlusion and accelerating optic nerve injury.
This study did have several limitations. First, a cross-sectional design was used, and as such, we cannot directly conclude that an RDW increase is a risk factor for PACG development. For this reason, future longitudinal studies should also evaluate the value of the RDW in detecting PACG progression. Second, those PACG subjects with acute infectious diseases were excluded, whereas chronic subclinical inflammation in PACG patients is usually more difficult to detect through a medical screening. This may have led to a bias in the results.
Conclusions and expert recommendations
The field of blood analysis for an earlier diagnosis of glaucoma and other ocular diseases has been undergoing a ‘seachange’ [41]. We found that an increased RDW was associated with the PACG risk and severity in PACG patients. Inflammation, endothelial dysfunction and oxidative stress, which are reflected by the RDW, are currently being discussed as leading factors in the cause of PACG. Moreover, an increased RDW is an indicator of the all-cause mortality risk. Contextually, it makes good sense to stratify PAGC patients according to the comorbidities noted in their patient records. Thus, the RDW, as a simple, rapid, inexpensive and reliable parameter, may be an important future tool for a prediction of PACG severity. This paper contributes to the paradigm shift from reactive medicine to predictive, preventive and personalised medicine (PPPM), and it conforms with the PPPM concepts presented in the 2012 European Association for Predictive, Preventive and Personalised Medicine (EPMA) white paper and the 2016 EPMA position paper [16, 42]. This measure may also serve to improve personalised medicine for patients as a step forward in realising PPPM strategies in the near future.
Authors’ contributions
Q C, B Z and YJ L designed the study; BZ, MY W and YJ L contributed to the patient recruitment and collected the data; XY C, D L, XQ J and JH T performed the statistical analysis; Q C wrote the manuscript. All authors read and approved the final manuscript.
Funding information
This work was supported by the National Natural Science Foundation of China (No. 31500148).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Written informed consent for the use of any clinical data in research was obtained for all patients. All individuals were informed about the purposes of the study and have signed their consent for publishing the data.
Ethical approval
All the patient investigations conformed to the principles outlined in the Declaration of Helsinki, and the study was approved by the ethical committee of the Affiliated Hospital of Taishan Medical University and the ethical committee of the Taian City Central Hospital, Shandong, China. All the patients were informed about the purposes of the study and have signed their “consent of the patient.” This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiang Chen and Bin Zhao contributed equally to this work.
References
- 1.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet Lond Engl. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Song P, Wang J, Bucan K, Theodoratou E, Rudan I, Chan KY. National and subnational prevalence and burden of glaucoma in China: a systematic analysis. J Glob Health. 2017;7:020705. doi: 10.7189/jogh.07.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Cao W, Han J, Tang B, Sun X. The diagnostic value of white blood cell, neutrophil, neutrophil-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio in patients with primary angle closure glaucoma. Oncotarget. 2017;8:68984–68995. doi: 10.18632/oncotarget.16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Wu C, Liu L, Jia Y, Zhang Y, Zhang Y, Zhang H, Zhong Y, Huang D. Optical coherence tomography angiography of the Peripapillary retina in primary angle-closure glaucoma. Am J Ophthalmol. 2017;182:194–200. doi: 10.1016/j.ajo.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binggeli T, Schoetzau A, Konieczka K. In glaucoma patients, low blood pressure is accompanied by vascular dysregulation. EPMA J. 2018;9:387–391. doi: 10.1007/s13167-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mustur D, Vahedian Z, Bovet J, Mozaffarieh M. Retinal venous pressure measurements in patients with Flammer syndrome and metabolic syndrome. EPMA J. 2017;8:339–344. doi: 10.1007/s13167-017-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. EPMA J. 2017;8:75–97. doi: 10.1007/s13167-017-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abegão Pinto L, Willekens K, Van Keer K, Shibesh A, Molenberghs G, Vandewalle E, Stalmans I. Ocular blood flow in glaucoma—the Leuven eye study. Acta Ophthalmol. 2016;94:592–598. doi: 10.1111/aos.12962. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Higashide T, Udagawa S, Takeshima S, Sakaguchi K, Nitta K. Sugiyama, K. J Glaucoma: The relationship between interocular asymmetry of visual field defects and optic nerve head blood flow in patients with glaucoma; 2018. [DOI] [PubMed] [Google Scholar]
- 10.Hondur G, Göktas E, Yang X, Al-Aswad L, Auran JD, Blumberg DM, Cioffi GA, Liebmann JM, Suh LH, Trief D, et al. Oxidative stress-related molecular biomarker candidates for glaucoma. Invest Ophthalmol Vis Sci. 2017;58:4078–4088. doi: 10.1167/iovs.17-22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura A, Namekata K, Guo X, Noro T, Harada C, Harada T. Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxidative Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 13.Akpinar I, Sayin MR, Gursoy YC, Aktop Z, Karabag T, Kucuk E, Sen N, Aydin M, Kiran S, Buyukuysal MC, Haznedaroglu IC. Plateletcrit and red cell distribution width are independent predictors of the slow coronary flow phenomenon. J Cardiol. 2014;63:112–118. doi: 10.1016/j.jjcc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 15.Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s health and aging study I. Clin Nutr Edinb Scotl. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. [DOI] [PMC free article] [PubMed]
- 17.Verma S, Nongpiur ME, Atalay E, Wei X, Husain R, Goh D, Perera SA, Aung T. Visual field progression in patients with primary angle-closure glaucoma using pointwise linear regression analysis. Ophthalmology. 2017;124:1065–1071. doi: 10.1016/j.ophtha.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Kurysheva NI, Ryabova TY, Shlapak VN. Heart rate variability: the comparison between high tension and normal tension glaucoma. EPMA J. 2018;9:35–45. doi: 10.1007/s13167-017-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Dai Y, Chen Y, Yu D-Y, Cringle SJ, Chen J, Kong X, Wang X, Jiang C. Primary angle closure glaucoma: what we know and what we don’t know. Prog Retin Eye Res. 2017;57:26–45. doi: 10.1016/j.preteyeres.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi G, Otori Y, Urashima M, Kuwayama Y. Quality of life improvement committee evaluation of quality of life in Japanese glaucoma patients and its relationship with visual function. J Glaucoma. 2016;25:e150–e156. doi: 10.1097/IJG.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 21.Sabel BA, Wang J, Cárdenas-Morales L, Faiq M, Heim C. Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. 2018;9:133–160. doi: 10.1007/s13167-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vahedian Z, Fakhraie G, Bovet J, Mozaffarieh M. Nutritional recommendations for individuals with Flammer syndrome. EPMA J. 2017;8:187–195. doi: 10.1007/s13167-017-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajuddin SM, Nalls MA, Zonderman AB, Evans MK. Association of red cell distribution width with all-cause and cardiovascular-specific mortality in African American and white adults: a prospective cohort study. J Transl Med. 2017;15:208. doi: 10.1186/s12967-017-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, for the Cholesterol and Recurrent Events (CARE) Trial investigators relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 25.Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med. 2016;4:399. doi: 10.21037/atm.2016.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garofoli F, Ciardelli L, Mazzucchelli I, Borghesi A, Angelini M, Bollani L, Genini E, Manzoni P, Paolillo P, Tinelli C, et al. The red cell distribution width (RDW): value and role in preterm, IUGR (intrauterine growth restricted), full-term infants. Hematol Amst Neth. 2014;19:365–369. doi: 10.1179/1607845413Y.0000000141. [DOI] [PubMed] [Google Scholar]
- 27.Chidlow G, Wood JPM, Casson RJ. Investigations into hypoxia and oxidative stress at the optic nerve head in a rat model of glaucoma. Front Neurosci. 2017;11:478. doi: 10.3389/fnins.2017.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Chen S, Liu Y, Huang W, Li X, Zhang X. Inflammatory cytokine profiles in eyes with primary angle-closure glaucoma. Biosci Rep. 2018;38:BSR20181411. doi: 10.1042/BSR20181411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Zhang A, Cao W, Sun X. Elevated plasma endothelin-1 levels in normal tension glaucoma and primary open-angle glaucoma: a meta-analysis. J Ophthalmol. 2016;2016:2678017. doi: 10.1155/2016/2678017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duvesh R, Puthuran G, Srinivasan K, Rengaraj V, Krishnadas SR, Rajendrababu S, Balakrishnan V, Ramulu P, Sundaresan P. Multiplex cytokine analysis of aqueous humor from the patients with chronic primary angle closure glaucoma. Curr Eye Res. 2017;42:1608–1613. doi: 10.1080/02713683.2017.1362003. [DOI] [PubMed] [Google Scholar]
- 31.Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, Bandinelli S, Phillips CS, Yu B, Connelly S, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- 33.Benoist d’Azy C, Pereira B, Chiambaretta F, Dutheil F. Oxidative and anti-oxidative stress markers in chronic glaucoma: a systematic review and meta-analysis. PLoS One. 2016;11:e0166915. doi: 10.1371/journal.pone.0166915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Su W-W, Cheng S-T, Ho W-J, Tsay P-K, Wu S-C, Chang SHL. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115:1173–1178.e1. doi: 10.1016/j.ophtha.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Hafez AS, Bizzarro RLG, Rivard M, Lesk MR. Changes in optic nerve head blood flow after therapeutic intraocular pressure reduction in glaucoma patients and ocular hypertensives. Ophthalmology. 2003;110:201–210. doi: 10.1016/S0161-6420(02)01716-5. [DOI] [PubMed] [Google Scholar]
- 37.Cherecheanu AP, Garhofer G, Schmidl D, Werkmeister R, Schmetterer L. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr Opin Pharmacol. 2013;13:36–42. doi: 10.1016/j.coph.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alattar FT, Imran NB, Patel P, Usmani S, Shamoon FE. Red cell distribution width (RDW) correlates with markers of diastolic dysfunction in patients with impaired left ventricular systolic function. Int J Cardiol Heart Vasc. 2016;10:13–16. doi: 10.1016/j.ijcha.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao HL, Pradhan ZS, Weinreb RN, Riyazuddin M, Dasari S, Venugopal JP, Puttaiah NK, Rao DAS, Devi S, Mansouri K, Webers CAB. Vessel density and structural measurements of optical coherence tomography in primary angle closure and primary angle closure glaucoma. Am J Ophthalmol. 2017;177:106–115. doi: 10.1016/j.ajo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Rao HL, Kadambi SV, Weinreb RN, Puttaiah NK, Pradhan ZS, Rao DAS, Kumar RS, Webers CAB, Shetty R. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2017;101:1066–1070. doi: 10.1136/bjophthalmol-2016-309377. [DOI] [PubMed] [Google Scholar]
- 41.Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15. doi: 10.1186/s13167-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]