Abstract
Objective
In the era of fast track surgery, early and accurately estimating whether postoperative length of stay (p-LOS) will be prolonged after lung cancer surgery is very important, both for patient’s discharge planning and hospital bed management. Pulmonary function tests (PFTs) are very valuable routine examinations which should not be underutilized before lung cancer surgery. Thus, this study aimed to establish an accurate but simple prediction tool, based on PFTs, for achieving a personalized prediction of prolonged p-LOS in patients following lung resection.
Methods
The medical information of 1257 patients undergoing lung cancer surgery were retrospectively reviewed and served as the training set. p-LOS exceeding the third quartile value was considered prolonged. Using logistic regression analyses, potential predictors of prolonged p-LOS were identified among various preoperative factors containing PFTs and intraoperative factors. A nomogram was constructed and subjected to internal and external validation.
Results
Five independent risk factors for prolonged p-LOS were identified, including older age, being male, and ratio of residual volume to total lung capacity (RV/TLC) ≥ 45.0% which is the only modifiable risk factor, more invasive surgical approach, and surgical type. The nomogram comprised of these five predictors exhibited sufficient predictive accuracy, with the area under the receiver operating characteristic curve (AUC) of 0.76 [95% confidence interval (CI) 0.73–0.79] in the internal validation. Also its predictive performance remained fine in the external validation, with the AUC of 0.70 (95% CI 0.60–0.79). The calibration curves showed satisfactory agreements between the model predicted probability and the actually observed probability.
Conclusions
Preoperative amelioration of RV/TLC may prevent lung cancer patients from unnecessary prolonged p-LOS. The integrated nomogram we developed could provide personalized risk prediction of prolonged p-LOS. This prediction tool may help patients perceive expected hospital stays and enable clinicians to achieve better bed management after lung cancer surgery.
Electronic supplementary material
The online version of this article (10.1007/s13167-019-00168-z) contains supplementary material, which is available to authorized users.
Keywords: Length of stay, Lung cancer, Surgery, Pulmonary function tests, Prediction model, Nomogram, Advanced healthcare, Individualized patient profile, Hospitalization, Economic burden, Risk assessment, Predictive preventive personalized medicine
Introduction
With modern medicine moving towards the era of valuing economics and efficiency, speeding up hospital bed turnover rate and carrying out a planned hospital bed management are becoming more and more necessary. Lung cancer, the most prevalent malignancy worldwide, has consumed large medical resources and imposed enormous economic burdens [1, 2]. Despite recent progress in cancer radiotherapy and immunotherapy, nowadays, surgical resection remains the mainstream for lung cancer curative treatment [3]. Over the past few decades, with the popularity of low-dose computed tomography, a growing number of lung cancer patients could be diagnosed at early stage, leading to a significantly increased volume of lung cancer surgery [4]. Therefore, there is a mounting demand to allocate perioperative resources efficiently and economically, such as hospital beds, for lung cancer patients following surgical treatment. Postoperative length of stay (p-LOS) is a frequently used metric which may reflect the surgical skill, quality of care, and medical resources utilization level [5, 6]. Prolonged p-LOS not only increases hospitalization cost but also implies increased risk of hospital acquired infection [7]. Therefore, it is of great clinical and financial significance to early find and fix potential risk factors for prolonged p-LOS during the perioperative period of lung cancer surgery.
It is recommended that all lung cancer patients who are candidates for surgery should have pulmonary function tests (PFTs), because the parameters of PFTs are very useful for lung cancer preoperative risk assessments [8, 9]. More than that, in recent years, emerging studies suggested that valuable PFT parameters might also play a role in predicting prolonged p-LOS following lung resection: Some suggested inspiratory capacity (IC) and some suggested forced expiratory volume in 1 s (FEV1) or diffusion capacity for carbon monoxide (DLCO) [10, 11]. Currently, it is still worthy to unveil more optimal or novel predictors among these various PFTs for better prediction of prolonged p-LOS after lung cancer surgery.
To date, in the research field of lung cancer surgery, although some variables, such as age, surgical types, and surgical approaches [12–14], have been well recognized as determinants of p-LOS, few studies integrated them so as to comprehensively assess a patient’s specific risk of prolonged p-LOS, let alone combined PFT parameters. A nomogram is an easy pictorial representation of a scoring model, with a user-friendly interface and preferred predictive performance [15]. It consists of several scale axes, and each scale axis represents a determinant variable of the clinical outcome. By integrating a patient’s diverse determinant variables, a nomogram can tell a personalized probability for quantitatively predicting the incidence of the outcome [16]. Thus, in this study, we selected a large population of surgically treated lung cancer patients as the participants and mainly aimed to (i) identify novel risk predictors of prolonged p-LOS among PFTs, hoping to remedy and prevent unwanted prolonged hospitalization; and (ii) for the first time, integrate the PFTs predictors with the other determinant predictors of p-LOS and construct a comprehensive nomogram for providing simple and precise personalized prediction.
Methods
Study population
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. Between 2012 and 2013, a total of 1293 consecutive patients, who had complete medical information, underwent lung resection for primary lung cancer at Zhongshan Hospital of Fudan University. As the previous study recommended [6], we excluded 36 patients who died or developed major postoperative complications such as persistent air leak, bronchial fistula, or acute respiratory distress syndrome. The reasons for this exclusion were that this was a small proportion of the population and it was highly variable in p-LOS values, which may not be suitable for prediction. And, their p-LOS were actually closely determined by postoperative complications themselves, such as the complication types, severities, and following treatments in practice. Hence, in the end, a total of 1257 patients were enrolled as the training set and analyzed for developing the prediction model. In order to assess the model predictive performance independently, another 173 patients who had lung cancer surgery between January and April 2014 were also enrolled as the prospective external validation set.
Data collection
Days of p-LOS were calculated from the date of surgery to the date of discharge. A p-LOS exceeding the third quartile value of the study population was defined as prolonged p-LOS [17]. Demographic and clinical variables potentially associated with p-LOS were extracted from the institutional electronic medical records. Preoperative variables collected included age, gender, health insurance, smoking status, and presenting respiratory symptoms (cough, sputum, bloody sputum, or hemoptysis). Preoperative PFTs were also collected, including FEV1, ratio of FEV1 to predicted values (FEV1 % predicted), forced vital capacity (FVC), ratio of FVC to predicted values (FVC % predicted), ratio of residual volume to total lung capacity (RV/TLC), and DLCO. According to the criteria of Global Initiative for Chronic Obstructive Lung Disease (GOLD) [18], patients with a FEV1/FVC ratio less than 70% would be diagnosed as chronic obstructive pulmonary disease (COPD). Two main intraoperative variables, surgical approach including open or video-assisted thoracic surgery (VATS) and surgical type including sub-lobectomy, lobectomy, or pneumonectomy, were also collected because they are commonly considered closely related to p-LOS.
Statistical analysis and nomogram
All statistical analyses and graphs were conducted in SPSS for Windows (Version 17.0, Chicago, IL, USA) and R software (Version 3.5.0, https://www.r-project.org/). Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR), while categorical variables were expressed as frequencies with percentages. Patient’s demographic and clinical characteristics were compared between the training set and the validation set using chi-square test, Student’s t test, or Mann-Whitney U test, as appropriate. In the training set, univariate logistic regression analysis was conducted to screen the variables associated with prolonged p-LOS, and the magnitude of the association was measured by odds ratio (OR) with 95% confidence interval (CI). By selecting the variables with univariate P < 0.05, a stepwise multivariate logistic regression analysis was conducted to identify independent risk factors for prediction of prolonged p-LOS. The fitness of the logistic regression model was measured by the Hosmer-Lemeshow test, of which P > 0.05 indicates that the model was in a good fit. In order to test a linear trend of the OR, P value for trend was calculated by entering the median value of each category into the regression analyses in a continuous variable way.
Based on the results of multivariate logistic regression analysis, a nomogram was constructed using R software with the rms package as described previously [19, 20]. In order to validate the predictive performance, the nomogram was applied to 1000 bootstrap resamples in the training set for internal validation, and to the prospective validation set for external validation. Receiver operating characteristic (ROC) curves were depicted to assess the nomogram discriminative ability, with calculating the area under the curve (AUC). AUC varies from 0.5 to 1.0 and higher value indicates better discriminative ability. Calibration curves were also depicted to visually assess how close the nomogram predicted risk is to the actual risk. All reported P values were two-tailed, and P < 0.05 was considered statistically significant.
Results
Patient’s baseline characteristics
The patient’s baseline demographic and clinical characteristics were summarized in detail as seen in Table 1. The average p-LOS of the validation set (6.1 days) were slightly shorter than that of the training set (6.8 days), and this result can be also observed from the perspective of median p-LOS (5 days vs 6 days). A significantly increased rate of VATS approach was found in the validation set (80.3%) when compared with that in the training set (66.2%). Except surgical approach, there was no more statistically significant difference between the training set and the validation set. The third quartile values of p-LOS in the both sets were 7 days; thus, patients with a p-LOS of 8 days or more in this study were regarded as the patients with prolonged p-LOS.
Table 1.
Baseline demographic and clinical characteristics of the patients
| Variable | Training set, n = 1257 | Validation set, n = 173 | P value |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 60.6 ± 9.7 | 61.3 ± 8.9 | 0.365 |
| Median (IQR) | 61 (55–68) | 62 (56.5–68) | |
| Gender | |||
| Female | 582 (46.3%) | 77 (44.5%) | |
| Male | 675 (53.7%) | 96 (55.5%) | 0.658 |
| Health insurance | |||
| Yes | 408 (32.5%) | 54 (31.2%) | |
| No | 849 (67.5%) | 119 (68.8%) | 0.743 |
| Smoking status | |||
| Never or ever | 986 (78.4%) | 126 (72.8%) | |
| Current | 271 (21.6%) | 47 (27.2%) | 0.096 |
| Respiratory symptoms | |||
| No | 784 (62.4%) | 114 (65.9%) | |
| Yes | 473 (37.6%) | 59 (34.1%) | 0.368 |
| FEV1 (L) | |||
| Mean ± SD | 2.34 ± 0.60 | 2.30 ± 0.66 | 0.458 |
| Median (IQR) | 2.29 (1.93–2.71) | 2.21 (1.79–2.70) | |
| FEV1 % predicted (%) | |||
| Mean ± SD | 89.1 ± 18.5 | 86.9 ± 17.9 | 0.143 |
| Median (IQR) | 89.4 (77.1–101.2) | 87.1 (74.6–101.3) | |
| FVC (L) | |||
| Mean ± SD | 3.04 ± 0.74 | 3.02 ± 0.83 | 0.764 |
| Median (IQR) | 2.94 (2.52–3.51) | 2.91 (2.38–3.59) | |
| FVC % predicted (%) | |||
| Mean ± SD | 91.1 ± 14.9 | 89.1 ± 15.6 | 0.108 |
| Median (IQR) | 90.7 (81.2–100.4) | 88.6 (78.3–100.3) | |
| FEV1/FVC < 70% (COPD) | |||
| No | 1026 (81.6%) | 138 (79.8%) | |
| Yes | 231 (18.4%) | 35 (20.2%) | 0.557 |
| RV/TLC (%) | |||
| Mean ± SD | 37.7 ± 7.8 | 38.1 ± 7.7 | 0.481 |
| Median (IQR) | 37.5 (33.3–42.6) | 38.5 (34.2–43.1) | |
| DLCO | |||
| Mean ± SD | 18.3 ± 5.3 | 18.0 ± 5.1 | 0.492 |
| Median (IQR) | 18.3 (15.2–21.6) | 17.8 (15.2–20.5) | |
| Surgical approach | |||
| VATS | 832 (66.2%) | 139 (80.3%) | |
| Open | 425 (33.8%) | 34 (19.7%) | < 0.001* |
| Surgical type | |||
| Sub-lobectomy | 147 (11.7%) | 30 (17.3%) | |
| Lobectomy | 1085 (86.3%) | 142 (82.1%) | |
| Pneumonectomy | 25 (2.0%) | 1 (0.6%) | 0.052 |
| p-LOS (day) | |||
| Mean ± SD | 6.8 ± 2.5 | 6.1 ± 2.8 | 0.002* |
| Median (IQR) | 6 (5–7) | 5 (5–7) | < 0.001* |
| Normal (≤ 7 days) | 947 (75.3%) | 146 (84.4%) | |
| Prolonged (> 7 days) | 310 (24.7%) | 27 (15.6%) | 0.009* |
SD standard deviation, IQR interquartile range, FEV1 forced expiratory volume in one second, FVC forced vital capacity, COPD chronic obstructive pulmonary disease, RV/TLC ratio of residual volume to total lung capacity, DLCO diffusion capacity for carbon monoxide, VATS video-assisted thoracic surgery, p-LOS postoperative length of stay
*Statistically significant
Factors associated with prolonged p-LOS in the training set
For the 1257 patients in the training set, all demographic and clinical variables listed in Table 1 were preliminarily subjected to a univariate logistic regression analysis and the results are presented in Table 2. It was quite evident that age was significantly associated with prolonged p-LOS: Patients in the age groups from 50 to 59, 60 to 69, and 70 or over 70 years all had a higher probability of prolonged p-LOS than patients aged under 50 years. The crude risk of prolonged p-LOS progressively increased for every 10-year-old (about per SD) increase (P value for trend < 0.001). Compared to patients with normal p-LOS, those with prolonged p-LOS were more likely to be males (73.2% vs 47.3%), current smokers (32.3% vs 18.1%), and presenting respiratory symptoms (50.6% vs 33.4%). However, patient’s status of health insurance was found to be not statistically associated with p-LOS in this study (data shown in Supplementary table). Among the preoperative PFTs, except FVC which had no association with p-LOS (data shown in Supplementary table), all other PFTs parameters, including FEV1, FEV1 % predicted, FVC % predicted, FEV1/FVC < 70% (coexisting COPD), RV/TLC, and DLCO, were significantly associated with p-LOS in different magnitudes. It was noteworthy that FEV1 % predicted exhibited a well linear association with p-LOS: With FEV1 % predicted decreasing, the crude risk of prolonged p-LOS was gradually increasing (P value for trend < 0.001). Compared to patients with normal p-LOS, patients with prolonged p-LOS were more frequently found to have COPD (29.0% vs 14.9%). Meanwhile, RV/TLC ≥ 45.0%, generally regarded as an indicator of moderate emphysema, was also demonstrated to be associated with prolonged p-LOS. It was confirmed that two intraoperative factors, open surgery and more invasive surgical type, were both strongly associated with higher probability of prolonged p-LOS than their respective counterparts.
Table 2.
Univariate logistic regression analysis of factors potentially associated with prolonged p-LOS in the training set
| Variable | Normal p-LOS, n = 947 | Prolonged p-LOS, n = 310 | Crude OR (95% CI) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| < 50 | 144 (15.2%) | 16 (5.2%) | 1.0 (reference) | |
| 50–59 | 313 (33.1%) | 87 (28.1%) | 2.50 (1.42–4.42) | 0.002* |
| 60–69 | 349 (36.9%) | 124 (40.0%) | 3.20 (1.84–5.57) | < 0.001* |
| ≥ 70 | 141 (14.9%) | 83 (26.8%) | 5.30 (2.96–9.49) | < 0.001* |
| P value for trend | < 0.001* | |||
| Gender | ||||
| Female | 499 (52.7%) | 83 (26.8%) | 1.0 (reference) | |
| Male | 448 (47.3%) | 227 (73.2%) | 3.05 (2.30–4.04) | < 0.001* |
| Smoking status | ||||
| Never or ever | 776 (81.9%) | 210 (67.7%) | 1.0 (reference) | |
| Current | 171 (18.1%) | 100 (32.3%) | 2.16 (1.62–2.89) | < 0.001* |
| Respiratory symptoms | ||||
| No | 631 (66.6%) | 153 (49.4%) | 1.0 (reference) | |
| Yes | 316 (33.4%) | 157 (50.6%) | 2.05 (1.58–2.66) | < 0.001* |
| FEV1 (L) | ||||
| ≥ 3.00 | 137 (14.5%) | 34 (11.0%) | 1.0 (reference) | |
| 2.30–2.99 | 338 (35.7%) | 110 (35.5%) | 1.31 (0.85–2.02) | 0.220 |
| 1.60–2.29 | 395 (41.7%) | 128 (41.3%) | 1.31 (0.85–2.00) | 0.219 |
| < 1.60 | 77 (8.1%) | 38 (12.3%) | 1.99 (1.16–3.41) | 0.013* |
| P value for trend | 0.037* | |||
| FEV1 % predicted | ||||
| ≥ 100.0% | 287 (30.3%) | 53 (17.1%) | 1.0 (reference) | |
| 90.0–99.9% | 218 (23.0%) | 62 (20.0%) | 1.54 (1.03–2.31) | 0.037* |
| 80.0–89.9% | 194 (20.5%) | 62 (20.0%) | 1.73 (1.15–2.61) | 0.009* |
| 70.0–79.9% | 135 (14.3%) | 70 (22.6%) | 2.81 (1.86–4.24) | < 0.001* |
| < 70.0% | 113 (11.9%) | 63 (20.3%) | 3.02 (1.97–4.62) | < 0.001* |
| P value for trend | < 0.001* | |||
| FVC % predicted | ||||
| ≥ 100.0% | 262 (27.7%) | 65 (21.0%) | 1.0 (reference) | |
| 90.0–99.9% | 263 (27.8%) | 72 (23.2%) | 1.10 (0.76–1.61) | 0.608 |
| 80.0–89.9% | 232 (24.5%) | 85 (27.4%) | 1.48 (1.02–2.13) | 0.038* |
| < 80.0% | 190 (20.1%) | 88 (28.4%) | 1.87 (1.29–2.71) | 0.001* |
| P value for trend | < 0.001* | |||
| FEV1/FVC < 70% (COPD) | ||||
| No | 806 (85.1%) | 220 (71.0%) | 1.0 (reference) | |
| Yes | 141 (14.9%) | 90 (29.0%) | 2.34 (1.73–3.17) | < 0.001* |
| RV/TLC | ||||
| < 35.0% | 342 (36.1%) | 89 (28.7%) | 1.0 (reference) | |
| 35.0–44.9% | 483 (51.0%) | 150 (48.4%) | 1.19 (0.89–1.61) | 0.243 |
| ≥ 45.0% | 122 (12.9%) | 71 (22.9%) | 2.24 (1.54–3.25) | < 0.001* |
| P value for trend | < 0.001* | |||
| DLCO | ||||
| ≥ 23.0 | 173 (18.3%) | 37 (13.9%) | 1.0 (reference) | |
| 18.0–22.9 | 344 (36.3%) | 100 (32.3%) | 1.17 (0.78–1.75) | 0.444 |
| 13.0–17.9 | 316 (33.4%) | 113 (36.5%) | 1.44 (0.97–2.14) | 0.073 |
| < 13.0 | 114 (12.0%) | 60 (17.4%) | 1.91 (1.20–3.03) | 0.007* |
| P value for trend | 0.003* | |||
| Surgical approach | ||||
| VATS | 701 (74.0%) | 131 (42.3%) | 1.0 (reference) | |
| Open | 246 (26.0%) | 179 (57.7%) | 3.89 (2.98–5.09) | < 0.001* |
| Surgical type | ||||
| Sub-lobectomy | 129 (13.6%) | 18 (5.8%) | 1.0 (reference) | |
| Lobectomy | 813 (85.9%) | 272 (87.7%) | 2.40 (1.44–4.00) | 0.001* |
| Pneumonectomy | 5 (0.5%) | 20 (6.5%) | 28.67 (9.57–85.87) | < 0.001* |
Only variables with statistical significance (P < 0.05) in the univariate analysis are shown in the table
p-LOS postoperative length of stay, OR odds ratio, CI confidence interval, FEV1 forced expiratory volume in 1s, FVC forced vital capacity, COPD chronic obstructive pulmonary disease, RV/TLC ratio of residual volume to total lung capacity, DLCO diffusion capacity for carbon monoxide, VATS video-assisted thoracic surgery
*Statistically significant
All factors with statistical significance in the above univariate analyses were picked out for multivariate analysis, and the results are presented in Table 3. After adjustment, age was still a major risk factor for prolonged p-LOS, with a significant trend of older age leading to higher risk (OR = 2.11, 2.76, and 4.92 respectively for each increased age category, P value for trend < 0.001). Being male remained an important risk factor for prolonged p-LOS (OR = 2.27, 95% CI = 1.68–3.08). Only PFT of RV/TLC ≥ 45.0% was found to be an independent risk factor for prolonged p-LOS (OR = 1.92, 95% CI = 1.24–2.97). Definitely, open surgery (OR = 3.03, 95% CI = 2.25–4.09) and more invasive surgical types, especially pneumonectomy (OR = 14.63, 95% CI = 4.51–47.47), conferred a very high risk of developing prolonged p-LOS on patients following lung cancer resection.
Table 3.
Multivariate logistic regression analysis of independent risk factors associated with prolonged p-LOS in the training set
| Intercept and variable | Regression coefficient | Adjusted OR (95% CI) | P value |
|---|---|---|---|
| Intercept | − 3.77 | < 0.001* | |
| Age (years) | |||
| < 50 | 1.0 (reference) | ||
| 50–59 | 0.74 | 2.11 (1.13–3.92) | 0.019* |
| 60-69 | 1.02 | 2.76 (1.48–5.16) | 0.001* |
| ≥ 70 | 1.59 | 4.92 (2.54–9.53) | < 0.001* |
| P value for trend | < 0.001* | ||
| Gender | |||
| Female | 1.0 (reference) | ||
| Male | 0.82 | 2.27 (1.68–3.08) | < 0.001* |
| RV/TLC | |||
| < 35.0% | 1.0 (reference) | ||
| 35.0–44.9% | 0.02 | 1.02 (0.73–1.43) | 0.911 |
| ≥ 45.0% | 0.65 | 1.92 (1.24–2.97) | 0.004* |
| P value for trend | 0.060 | ||
| Surgical approach | |||
| VATS | 1.0 (reference) | ||
| Open | 1.11 | 3.03 (2.25–4.09) | < 0.001* |
| Surgical type | |||
| Sub-lobectomy | 1.0 (reference) | ||
| Lobectomy | 0.62 | 1.87 (1.08–3.24) | 0.027* |
| Pneumonectomy | 2.68 | 14.63 (4.51–47.47) | < 0.001* |
p-LOS postoperative length of stay, OR odds ratio, CI confidence interval, RV/TLC ratio of residual volume to total lung capacity, VATS video-assisted thoracic surgery
*Statistically significant
The nomogram and its performance
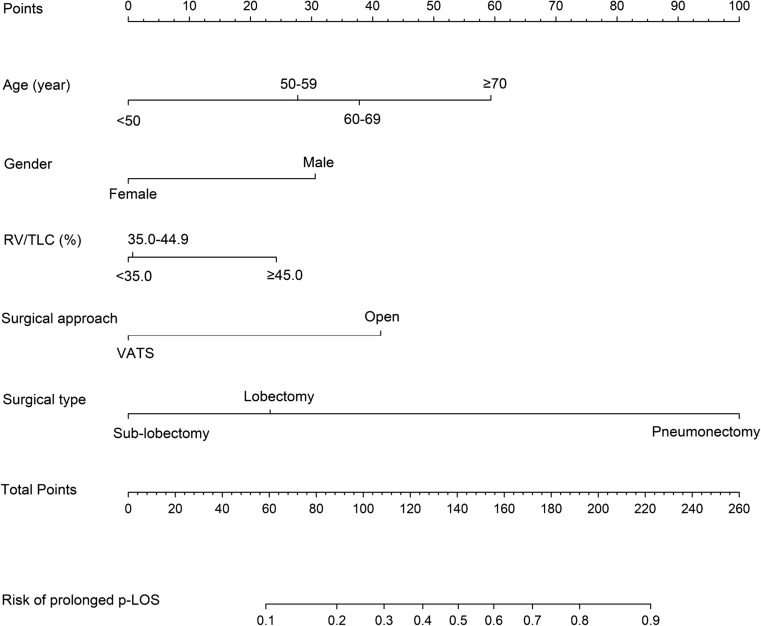
As shown in Fig. 1, a graphic nomogram was constructed by incorporating the five independent risk factors as predictors of prolonged p-LOS. After setting pneumonectomy, the value with the largest regression coefficient, as 100 points on the top Points scale for reference, each value of the remaining predictors was assigned a point on the Points scale according to their respective effect size reflected by the regression coefficient. For a specific patient, a total point was calculated by summing each point derived from his/her age, gender, RV/TLC, surgical approach, and surgical type. The location of the total point was vertically projected onto the bottom scale, thus easily obtaining an individualized risk estimation of prolonged p-LOS.
Fig. 1.
The nomogram for predicting prolonged p-LOS following lung cancer resection. Instructions: to estimate a patient’s probability of prolonged p-LOS, locate the patient’s value on each variable axis. Draw a vertical line from that value to the top Points scale for determining how many points are assigned by that variable value. Then, the points from the each variable value are summed. Locate the sum on the Total Points scale and vertically project it onto the bottom axis, thus obtaining a personalized risk of prolonged p-LOS. p-LOS postoperative length of stay, RV/TLC ratio of residual volume to total lung capacity, VATS video-assisted thoracic surgery
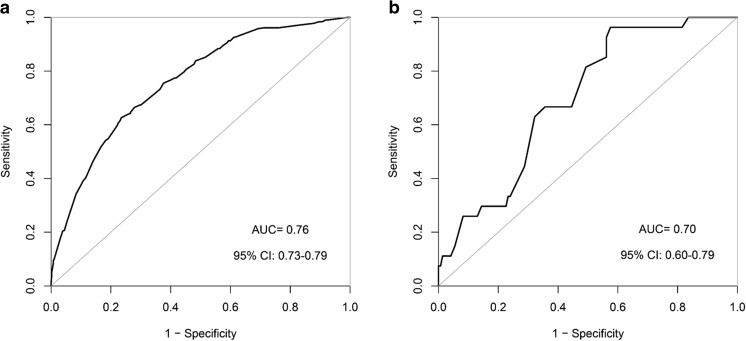
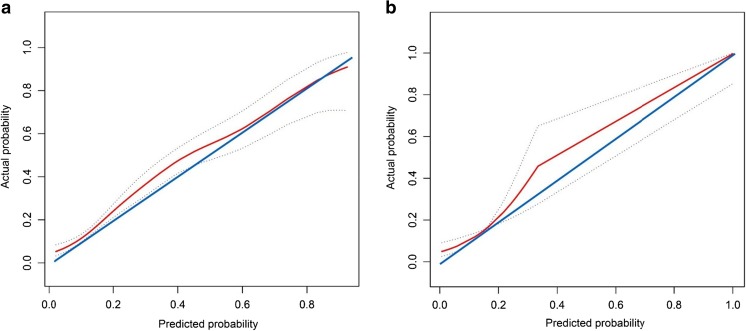
As seen in Fig. 2, the ROC curves demonstrated that our nomogram not only hold a very good discriminative ability in the internal validation (AUC = 0.76, 95% CI 0.73–0.79) but also remained a good discriminative ability in the external validation (AUC = 0.70, 95% CI 0.60–0.79). The P value of the Hosmer-Lemeshow test for this model was 0.672, indicating that the model was well fitted. As shown in Fig. 3, the calibration curves visually told that our nomogram predicted risk was close to the actually observed risk. Although some slight underestimation may exist, it should be allowed and practically noted. There were good agreements between our nomogram prediction and the actual observation of prolonged p-LOS risk, both in the internal and external validation.
Fig. 2.
The receiver operating characteristic (ROC) curves showing the nomogram discriminative ability. a The area under the curve (AUC) in the internal validation is 0.76, with the 95% CI 0.73–0.79. b The AUC in the external validation is 0.70, with the 95% CI 0.60–0.79
Fig. 3.
The calibration curves showing how close the nomogram predicted probability is to the actual probability regarding predicting prolonged p-LOS. a In the internal validation. b In the external validation. X-axis is the nomogram-predicted probability; Y-axis is the actually observed probability. The blue line represents a perfect prediction that the predicted risk is exactly the observed risk. The red line represents the nomogram performance, with the bilateral dotted lines representing its 95% confidence interval. The red line above or below the blue line represents underestimation or overestimation, respectively. The closer the red line is to the blue line, the better prediction of the nomogram holds
Discussion
In the present study, we collected 12 preoperative variables (containing seven PFTs parameters) and two main intraoperative variables for screening potential predictors of prolonged p-LOS following lung cancer resection. As expected, most of these variables demonstrated a well association with prolonged p-LOS in the univariate analysis. Age acted as a significant patient’s factor influencing p-LOS, and several studies indeed proved that lung cancer patients older than 70 or 75 years were more likely to suffer prolonged p-LOS [6, 17, 21]. Nevertheless, data of our study further indicated that the risk of prolonged p-LOS began to go up even for patients older than 50 years, continued to rise for patients older than 60 years, and dramatically elevated for patients older than 70 years. There was an obvious uptrend of prolonged p-LOS risk along with increase in age. Consistent with some previous studies [12, 22], our study also successfully identified that gender was an independent risk factor for prolonged p-LOS: Male patients would be more susceptible to prolonged p-LOS than females following lung cancer surgery. Presenting respiratory symptoms represents the chief complaint of many lung cancer patients. It is suggested that lung cancer patients admitted to hospital with respiratory symptoms may be distinct in certain clinical characteristics [23]. In our study, although patients with prolonged p-LOS more frequently presented respiratory symptoms preoperatively, respiratory symptoms seemed not to affect p-LOS independently. Whether lung cancer patients must quit smoking prior to surgery has long been in dispute [24]. The latest evidence suggested that current smoking before lung cancer surgery would not actually increase the risk of postoperative pulmonary complications [25]. Our study further revealed that, even for general lung cancer patients without incidence of postoperative complications, current smoking status was not responsible for prolonged p-LOS either. Thus, our finding provides more evidence to support the proposal that there is no deliberate need to quit smoking just before scheduled lung cancer surgery.
Among the seven PFT parameters in the univariate analysis, it is quite attractive that each hierarchical FEV1 % predicted exerted an inverse association with prolonged p-LOS risk: Patients with lower FEV1 % predicted were usually exposed to higher risk of encountering prolonged p-LOS. However, in the following multivariate analysis, FEV1 % predicted was found to fail to independently predict prolonged p-LOS. Although FEV1 % predicted an essential risk evaluation parameter and hold good performance in predicting lung cancer postoperative complications, our study suggested that it might not suit for predicting prolonged p-LOS in postoperative uncomplicated lung cancer patients. COPD is a common respiratory disease which is featured by progressive lung function decline and persistent airflow obstruction [26]. It should be noted that lung cancer patients undergoing surgery would sometimes have COPD as a comorbidity, which significantly impacts lung cancer perioperative management [27, 28]. In this study, compared to RV/TLC ≥ 45% (another indictor which may also reflect patients existing obstructive ventilation dysfunction), COPD, namely FEV1/FVC < 70%, appeared to be inferior in predicting prolonged p-LOS. Our study uncovered that only RV/TLC ≥ 45% was the most powerful parameter for independently determining p-LOS among these various PFTs. Coincidentally, study from Matsuo and colleagues [10] suggested that decreased IC can be served as the best risk predictor of prolonged p-LOS after lung resection. Their result was well supportive to our present finding, because IC and RV are generally in a reciprocal relationship to each other: A patient with a decreased IC generally holds an increased RV.
Besides the novel identified risk predictor of prolonged p-LOS (RV/TLC ≥ 45%), what the most commendable in this study was that we first integrated these existing risk predictors into a superior prediction tool called nomogram. Resorting to this tool, we can comprehensively predict a lung cancer patient’s personalized risk of prolonged p-LOS. In the following validation analyses, whether internal or external, our nomogram outputted both sufficient accuracy and satisfactory consistency in predicting prolonged p-LOS. Another advantage of our nomogram was that it is very simple and economical: Accurate predictions were made by just using five dominant risk predictors of prolonged p-LOS; thus, it would be easy to use in clinical practice. Additionally, in our study, the retained PFT predictor—RV/TLC—is percent value rather than absolute value, which would be relatively less influenced by individual weight or height when compared to those PFTs of absolute value. Obviously, for a specific lung cancer patients, her/his age, gender, RV/TLC, surgical approach, and type are all quite objective and could be immediately known once the surgical plan is decided. So, our nomogram could easily achieve a precise personalized prediction of prolonged p-LOS just during the preoperative period, with better efficiency and is more economy-friendly.
Strikingly, besides the sufficient predictive and personalized significance our nomogram displayed above, our present findings also carried clinical preventive significance, which fully expressed the advanced healthcare concept of predictive, preventive, and personalized medicine (PPPM) [29]. Compared to age, gender, surgical approach, and surgical type, preoperative RV/TLC ≥ 45% is the only modifiable risk factor identified for prolonged p-LOS. Therefore, for patients undergoing lung cancer surgery, we clinicians must note whether patients have a RV/TLC ≥ 45% and normalize it preoperatively. Only by this way can we avoid an unnecessary increased risk of prolonged p-LOS. Many studies have reported that preoperative pulmonary rehabilitation (high intensity training and exercising program), even in short-term, can markedly improve patient’s PFTs [30–32]. So, we suggested that future preoperative pulmonary rehabilitation should be recommended to patients with the need to ameliorate RV/TLC, thus preventing them from an unnecessary prolonged p-LOS.
Study limitations
Despite the strengths described above, there were inevitably some limitations in our study: (i) The enrolled population of the external validation set seemed to be not abundant, thus shaping a relatively steep ROC curve in the external validation. Our developed nomogram might get even higher external discriminate ability, measured by AUC, if there were more participants in the external validation set. (ii) Although our study has included as many perioperative variables as possible, some other important perioperative variables known for influencing lung cancer p-LOS were still missing in our present database, such as preoperative American Society of Anesthesiologists Classification or other non-respiratory comorbidities [6, 21]. On the other hand, it must be prudently done if we want to additionally include these variables, because this conduct would either improve the model predictive ability or incur an unwanted nomogram complexity. (iii) Our present results were yielded from one large tertiary hospital. It has been reported that the absolute days of p-LOS vary from hospital to hospital [33, 34]. Although our nomogram exhibited good predictive performance within our institution, whether it can be extended to other hospitals remains to be a question. So, when other hospitals apply this nomogram, they should first survey an overall institutional p-LOS and calculate the third quartile to determine their own threshold of prolonged p-LOS. Then, they can resort to the five risk predictors and the nomogram we recommended in order to harvest a personalized risk prediction and evaluate its accuracy. All of the above suggested that our present constructed nomogram is promising and deserves to be further explored in future clinical work and research.
Conclusions and recommendations
In summary, the present study, in a large simple size, yielded results substantially exhibiting predictive, preventive, and personalized implications in the perioperative management of lung cancer surgery. Firstly, the predictive implication was that we identified five main risk predictors (age, gender, RV/TLC, surgical approach, and type) for prolonged p-LOS. By recognizing these risk predictors in daily clinical practice, we can infer which patients are at an increased risk of prolonged p-LOS in advance. Secondly, the preventive implication was that the newly identified RV/TLC ≥ 45% was proved to be an optimal marker to impact hospitalization. Considering that RV/TLC is a well modifiable risk factor, preoperative interventions aiming at ameliorating RV/TLC, such as short-term high intensity pulmonary rehabilitation, might prevent some lung cancer patients from an unwanted prolonged p-LOS. Thirdly, the personalized implication was that we integrated all the five risk predictors into a user-friendly prediction tool called nomogram. By means of this nomogram, we can determine the individualized risk of prolonged p-LOS for a specific patient via comprehensively taking his/her age, gender, RV/TLC, surgical approach, and type into consideration.
The present results rooting in the advanced healthcare concept of PPPM [35, 36] can reliably answer a patient’s counseling of how much the probability he/she would be in prolonged hospitalization. As a result, the patients can then prepare themselves both psychologically and financially before lung cancer surgery, as well as appropriately schedule discharge planning. If the patient was identified as high-risk individual for prolonged p-LOS because of aberrant RV/TLC, he or she may escape from the risk through clinicians-provided RV/TLC correction, instead of passively or unawarely suffering in the traditional way. On the other hand, based on this prediction tool, clinicians can achieve a more well-organized hospital bed management and better clinical pathway management for lung cancer patients following surgery. Clearly, this optimized management calls for a multi-disciplinary team, namely a close collaboration of thoracic surgeons, pulmonologists, and nurses, which is exactly conformed to the PPPM-proposed principle that multi-professional consideration and consolidation [37]. This present study is another step forward to PPPM-guided cancer management, which is initiated and encouraged by EPMA [38, 39].
Electronic supplementary material
(DOCX 15 kb)
Abbreviations
EPMA European Association for Predictive, Preventive and Personalised Medicine
PPPM predictive, preventive and personalized medicine
p-LOS postoperative length of stay
PFTs pulmonary function tests
IC inspiratory capacity
FEV1 forced expiratory volume in 1 s
DLCO diffusion capacity for carbon monoxide
FVC forced vital capacity
COPD chronic obstructive pulmonary disease
RV/TLC ratio of residual volume to total lung capacity
VATS video-assisted thoracic surgery
SD standard deviation
IQR interquartile range
ROC receiver operating characteristic
AUC area under the receiver operating characteristic curve
OR odds ratio
CI confidence interval
Authors’ contributions
Hu Xiang-Lin contributed to the study conception and design, data analysis, interpretation of the data, and drafting the manuscript. Yang Dong, Xu Song-Tao, Song Yuan-Lin, and Bai Chun-Xue contributed to the interpretation of the data and critical revision of the manuscript. Hu Xiang-Lin, Xu Song-Tao, Luo Jin-Long, Wang Xiao-Cen, Hou Dong-Ni, Zhang Xiao-Min, and Bao Chen contributed to the collection of the data. All authors read and approved the final manuscript.
Funding
This work was supported by State’s Key Project of Research and Development Plan in China (2017YFC1310602, 2017YFC1310600).
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval and consent for participation
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University, Shanghai, 200032, China. Informed consent for participation was obtained in this study. All procedures performed in the study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang-Lin Hu and Song-Tao Xu contributed equally to this work.
References
- 1.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal A, Lewison G, Idir S, Peters M, Aldige C, Boerckel W, Boyle P, Trimble EL, Roe P, Sethi T, Fox J, Sullivan R. The state of lung cancer research: a global analysis. J Thorac Oncol. 2016;11(7):1040–1050. doi: 10.1016/j.jtho.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JP, Datta I, Hunt JD, Stefan K, Ball CG, Dixon E, Grondin SC. The impact of computed tomographic screening for lung cancer on the thoracic surgery workforce. Ann Thorac Surg. 2014;98(2):447–452. doi: 10.1016/j.athoracsur.2014.04.076. [DOI] [PubMed] [Google Scholar]
- 5.Farjah F, Lou F, Rusch VW, Rizk NP. The quality metric prolonged length of stay misses clinically important adverse events. Ann Thorac Surg. 2012;94(3):881–7; 887-8. doi: 10.1016/j.athoracsur.2012.04.082. [DOI] [PubMed] [Google Scholar]
- 6.Giambrone GP, Smith MC, Wu X, Gaber-Baylis LK, Bhat AU, Zabih R, Altorki NK, Fleischut PM, Stiles BM. Variability in length of stay after uncomplicated pulmonary lobectomy: is length of stay a quality metric or a patient metric? Eur J Cardiothorac Surg. 2016;49(4):e65–e67. doi: 10.1093/ejcts/ezv476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khullar OV, Fernandez FG, Perez S, Knechtle W, Pickens A, Sancheti MS, Force SD. Time is money: hospital costs associated with video-assisted thoracoscopic surgery lobectomies. Ann Thorac Surg. 2016;102(3):940–947. doi: 10.1016/j.athoracsur.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Choi H, Mazzone P. Preoperative evaluation of the patient with lung cancer being considered for lung resection. Curr Opin Anaesthesiol. 2015;28(1):18–25. doi: 10.1097/ACO.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo M, Hashimoto N, Usami N, Imaizumi K, Wakai K, Kawabe T, Yokoi K, Hasegawa Y. Inspiratory capacity as a preoperative assessment of patients undergoing thoracic surgery. Interact Cardiovasc Thorac Surg. 2012;14(5):560–564. doi: 10.1093/icvts/ivr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almquist D, Khanal N, Smith L, Ganti AK. Preoperative pulmonary function tests (PFTs) and outcomes from resected early stage non-small cell lung cancer (NSCLC) Anticancer Res. 2018;38(5):2903–2907. doi: 10.21873/anticanres.12537. [DOI] [PubMed] [Google Scholar]
- 12.Jawitz OK, Wang Z, Boffa DJ, Detterbeck FC, Blasberg JD, Kim AW. The differential impact of preoperative comorbidity on perioperative outcomes following thoracoscopic and open lobectomies. Eur J Cardiothorac Surg. 2017;51(1):169–174. doi: 10.1093/ejcts/ezw239. [DOI] [PubMed] [Google Scholar]
- 13.Sancheti MS, Chihara RK, Perez SD, Khullar OV, Fernandez FG, Pickens A, Force SD. Hospitalization costs after surgery in high-risk patients with early stage lung cancer. Ann Thorac Surg. 2018;105(1):263–270. doi: 10.1016/j.athoracsur.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Farjah F, Backhus LM, Varghese TK, Mulligan MS, Cheng AM, Alfonso-Cristancho R, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg. 2014;98(1):191–196. doi: 10.1016/j.athoracsur.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Shariat SF, Capitanio U, Jeldres C, Karakiewicz PI. Can nomograms be superior to other prediction tools? BJU Int. 2009;103(4):492–495. doi: 10.1111/j.1464-410X.2008.08073.x. [DOI] [PubMed] [Google Scholar]
- 16.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDevitt J, Kelly M, Comber H, Kelleher T, Dwane F, Sharp L. A population-based study of hospital length of stay and emergency readmission following surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44(4):e253–e259. doi: 10.1093/ejcts/ezt389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DMG, López Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 19.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Kattan MW. Drawing nomograms with R: applications to categorical outcome and survival data. Ann Transl Med. 2017;5(10):211. doi: 10.21037/atm.2017.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLuzio MR, Keshava HB, Wang Z, Boffa DJ, Detterbeck FC, Kim AW. A model for predicting prolonged length of stay in patients undergoing anatomical lung resection: a National Surgical Quality Improvement Program (NSQIP) database study. Interact Cardiovasc Thorac Surg. 2016;23(2):208–215. doi: 10.1093/icvts/ivw090. [DOI] [PubMed] [Google Scholar]
- 22.Rosen JE, Hancock JG, Kim AW, Detterbeck FC, Boffa DJ. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg. 2014;98(6):1953–1960. doi: 10.1016/j.athoracsur.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Hu XL, Xu ST, Wang XC, Hou DN, Chen CC, Song YL, Yang D. Prevalence of and risk factors for presenting initial respiratory symptoms in patients undergoing surgery for lung cancer. J Cancer. 2018;9(19):3515–3521. doi: 10.7150/jca.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raviv S, Hawkins KA, DeCamp MM, Jr, Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med. 2011;183(9):1138–1146. doi: 10.1164/rccm.201008-1274CI. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez M, Gómez-Hernandez MT, Novoa N, Jiménez MF, Aranda JL, Varela G. Refraining from smoking shortly before lobectomy has no influence on the risk of pulmonary complications: a case-control study on a matched population. Eur J Cardiothorac Surg. 2017;51(3):498–503. doi: 10.1093/ejcts/ezw359. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, Yao W, Zhi R, Wei L, He B, Zhang X, Yang C, Li Y, Li F, du J, Gui J, Hu B, Bai C, Huang P, Chen G, Xu Y, Wang C, Liang B, Li Y, Hu G, Tan H, Ye X, Ma X, Chen Y, Hu X, Tian J, Zhu X, Shi Z, du X, Li M, Liu S, Yu R, Zhao J, Ma Q, Xie C, Li X, Chen T, Lin Y, Zeng L, Ye C, Ye W, Luo X, Zeng L, Yu S, Guan WJ, Ran P. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 27.Hu XL, Xu ST, Wang XC, Hou DN, Chen CC, Yang D, Song YL. Status of coexisting chronic obstructive pulmonary disease and its clinicopathological features in patients undergoing lung cancer surgery: a cross-sectional study of 3,006 cases. J Thorac Dis. 2018;10(4):2403–2411. doi: 10.21037/jtd.2018.03.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto N, Matsuzaki A, Okada Y, Imai N, Iwano S, Wakai K, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014;14(14). 10.1186/1471-2466-14-14. [DOI] [PMC free article] [PubMed]
- 29.Golubnitschaja O. Time for new guidelines in advanced healthcare: the mission of The EPMA Journal to promote an integrative view in predictive, preventive and personalized medicine. EPMA J. 2012;3(1):5. doi: 10.1186/1878-5085-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293–296. doi: 10.1093/ejcts/ezs257. [DOI] [PubMed] [Google Scholar]
- 31.Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, Tracey M, Rocco G. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. 2013;44(4):e260–e265. doi: 10.1093/ejcts/ezt375. [DOI] [PubMed] [Google Scholar]
- 32.Gao K, Yu PM, Su JH, He CQ, Liu LX, Zhou YB, Pu Q, Che GW. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. 2015;6(4):443–449. doi: 10.1111/1759-7714.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otake H, Yasunaga H, Horiguchi H, Matsutani N, Matsuda S, Ohe K. Impact of hospital volume on chest tube duration, length of stay, and mortality after lobectomy. Ann Thorac Surg. 2011;92(3):1069–1074. doi: 10.1016/j.athoracsur.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 34.von Meyenfeldt EM, Marres GMH, van Thiel E, Damhuis RAM. Variation in length of hospital stay after lung cancer surgery in the Netherlands. Eur J Cardiothorac Surg. 2018;54(3):560–564. doi: 10.1093/ejcts/ezy074. [DOI] [PubMed] [Google Scholar]
- 35.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. 10.1186/1878-5085-3-14 [DOI] [PMC free article] [PubMed]
- 36.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016 Oct 25;7(23). 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed]
- 37.Golubnitschaja O, Costigliola V, Grech G. EPMA world congress: traditional forum in predictive, preventive and personalised medicine for multi-professional consideration and consolidation. EPMA J. 2017;8:1–54. doi: 10.1007/s13167-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, Vittadini G, Desiderio DM. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssens JP, Schuster K, Voss A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018;9(2):113–123. doi: 10.1007/s13167-018-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.