Abstract
Lifespan and metabolic health are influenced by dietary nutrients. Recent studies show that a reduced protein intake or low-protein/high-carbohydrate diet plays a critical role in longevity/metabolic health. Additionally, specific amino acids (AAs), including methionine or branched-chain AAs (BCAAs), are associated with the regulation of lifespan/ageing and metabolism through multiple mechanisms. Therefore, methionine or BCAAs restriction may lead to the benefits on longevity/metabolic health. Moreover, epidemiological studies show that a high intake of animal protein, particularly red meat, which contains high levels of methionine and BCAAs, may be related to the promotion of age-related diseases. Therefore, a low animal protein diet, particularly a diet low in red meat, may provide health benefits. However, malnutrition, including sarcopenia/frailty due to inadequate protein intake, is harmful to longevity/metabolic health. Therefore, further study is necessary to elucidate the specific restriction levels of individual AAs that are most effective for longevity/metabolic health in humans.
Keywords: Low protein intake, Low-protein/high-carbohydrate diet, Methionine, Branched-chain amino acids, Red meat, Longevity, Metabolic health
Abbreviations: mTORC1, mechanistic target of rapamycin complex 1; LPD, low protein diet; LPHC, low protein/high carbohydrate; Met, methionine; Leu, leucine; Arg, arginine; BCAAs, branched-chain amino acids; MetR, methionine restriction; SAM, S-adenosylmethionine; Gnmt, glycine-N-methyltransferase; GH/IGF-1, growth hormone/insulin-like growth factor-1; ROS, reactive oxygen species; EAAs, essential amino acids; NEAAs, nonessential amino acids; Cys, cysteine; tRNA, transfer ribonucleic acid; GCN2, general amino acid control nonderepressible-2; elF2α, eukaryotic initiation factor 2α; ATF, activating transcriptional factor; FGF21, fibroblast growth factor 21; TSP, transsulfuration pathway; CBS, cystathionine β-synthase; CGL, cystathionine γ-lyase; NUPR1, nuclear protein 1; H2S, hydrogen sulphate; Metyl-PP2A, methylated-phosphatase 2A; CASTOR1, cytosolic arginine sensor for mTORC1 subunit 1; GATOR1 and 2, GAP activity towards the Rags 1 and 2; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NF-κB, nuclear factor-κ B; DASH, Dietary Approaches to Stop Hypertension
1. Introduction
All organisms adapt and respond to the sources of nutrients that are available in the environment. Cellular activities, such as the regulation of metabolism, growth and ageing, are modulated by a network of nutrients and nutrient-sensing pathways. Dietary interventions, including calorie restriction (CR), dietary restriction (DR), and protein restriction (PR), have been investigated for their effects on longevity or the prevention of age-related diseases through their effects on metabolic health. CR without malnutrition has been shown to extend the lifespan and improve metabolic health in organisms [1]. However, recent evidence indicates that the quantity, source and amino acid (AA) composition of proteins are more strongly associated with longevity and metabolic health than CR [2]. The macronutrient balance of diets, including low protein/high carbohydrate (LPHC) diets, has been shown to have the greatest significant impact on longevity and metabolic health [[3], [4], [5]]. Additionally, epidemiological studies indicate that the quantity of protein intake; the protein sources, including animal or plant protein; and the intake of red meat or processed meat may affect mortality and lead to a wide range of diseases, including cancer, cardiovascular disease (CVD) and chronic kidney disease (CKD) [[6], [7], [8], [9]]. Moreover, the restriction of specific AAs, such as branched-chain AAs (BCAAs) or methionine, promotes longevity and metabolic health, which possibly mediates the benefits of PR [4,[10], [11], [12], [13]]. In this review, we focus on the physiological and molecular mechanisms underlying the promotion of ageing and age-related metabolic impairment induced by amino acids and their metabolites and discuss the current understanding of the effects of reduced dietary protein intake on longevity and metabolic health.
2. Mechanisms underlying the roles of AAs in longevity and metabolic health
Recent findings have revealed that essential AAs (EAAs), such as BCAAs and methionine, are involved in the regulation of the ageing process, longevity and metabolic health through multiple physiological and molecular mechanisms. In particular, the mechanisms underlying the role of methionine in the regulation of ageing have been widely studied.
2.1. Mechanistic target of rapamycin complex1 (mTORC1) and autophagy
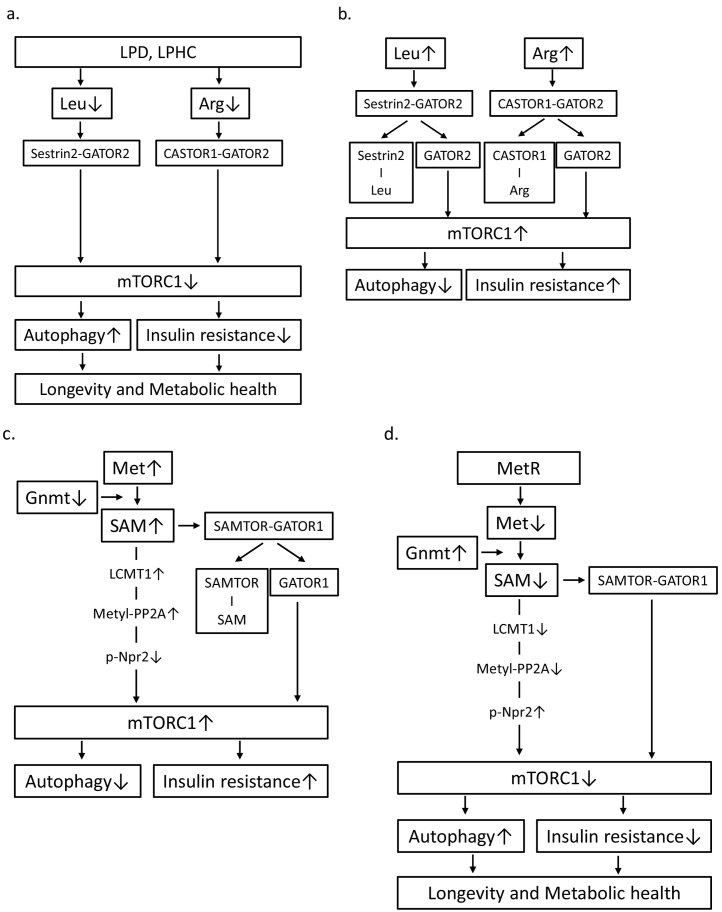
mTORC1, a subunit of mTOR, is a serine/threonine kinase that acts as a central regulator of cell growth and metabolism in response to nutrients and growth factors [14]. mTORC1 is activated by various factors, including AAs [14,15] and is the primary modulator of protein, lipid, and nucleotide synthesis; autophagy; and insulin signalling [14]. The pharmacological inhibition of mTORC1 by rapamycin has been shown to extend the lifespan and exert beneficial effects on a set of ageing-related traits in mice, indicating that mTORC1 may be related to lifespan regulation [[16], [17], [18], [19], [20]]. Nutritional interventions, such as a LPD, also suppress mTORC1 because AAs promote mTORC1 activation [15]. Several pathways responsible for sensing the AA levels in the regulation of mTORC1 have been identified. Long-term AA deprivation induces Sestrin production through the activation of activating transcription factor4 (ATF4). Under conditions of low leucine levels, Sestrin-2 interacts with and inhibits GAP activity towards Rags2 (GATOR2), a GTPase-activating protein of RagA/B and a positive regulator of mTORC1, leading to the suppression of mTORC1 [21](Fig. 1a). Leucine binds to Sestrin-2 and induces its dissociation from GATOR2, resulting in mTORC1 activation (Fig. 1b). Thus, Sestrin-2 is a leucine sensor in the mTORC1 pathway [21]. Similarly, arginine activates mTORC1 by preventing the interaction between the cytosolic arginine sensor of the mTORC1 subunit (CASTOR)1 and GATOR2 [22](Fig. 1b), and a low arginine level suppresses mTORC1 via the interaction of CASTOR1 and GATOR2 (Fig. 1b). Thus, since the presence of certain AAs activates mTORC1, the restriction of protein/AAs suppresses mTORC1.
Fig. 1.
(a) Under conditions of reduced levels of leucine or arginine as a result of a LPD or a LPHC diet, Sestrin-2 or CASTOR1 interacts with and inhibits GATOR2, which is a positive regulator of mTORC1, leading to the suppression of mTORC1 activation. The suppression of mTORC1 is associated with the induction of autophagy and improvement of insulin resistance, resulting in longevity and metabolic health. (b) Leucine or arginine binds Sestrin-2 or CASTOR1 and induces their dissociation from GATOR2. GATOR2 contributes to mTORC1 activation, leading to the suppression of autophagy and induction of insulin resistance. (c) An increased level of SAM due to a reduction in Gnmt activity leads to mTORC1 activation through two pathways. LCMT1 activation by SAM induces increased levels of metylated-PP2A. Metylated-PP2A dephosphorylates p-Npr2 (a negative regulator of mTOR), resulting in mTORC1 activation. SAM binds SAMTOR and induces its dissociation from GATOR1. GATOR1 contributes to mTORC1 activation, leading to the suppression of autophagy and induction of insulin resistance. (d) MetR or Gnmt activation leads to the suppression of mTORC1 via reduced levels of SAM.
Autophagy, including macroautophagy and selective autophagy, is a lysosomal degradation pathway and plays a crucial role in the removal of protein aggregates and damaged or excess organelles, including mitochondria, to maintain intracellular homeostasis [23]. Autophagy may protect cells against various age-related stress conditions, including hypoxia, endoplasmic reticulum stress and oxidative stress. AAs regulate autophagy through mTORC1 activity. Zhang et al. reported that hepatic mTOR activation by BCAAs inhibited lipid-induced hepatic autophagy and caused hepatic lipotoxicity [24]. Previously, we also demonstrated that a LPD exerts a reno-protective effect by inducing autophagy via the suppression of mTORC1 in rats with type 2 diabetes mellitus (T2DM)/obesity [25].
2.2. Glycine N-methyltransferase (Gnmt) and S-adenosylmethionine (SAM) metabolism
Methionine is converted to SAM in a reaction catalysed by the enzyme methionine adenosyl transferase, and SAM is converted to S-adenosylhomocysteine by Gnmt, a key SAM metabolism enzyme that maintains intracellular SAM levels [26]. Recent reports have shown that SAM, rather than methionine, may be the main contributor to methionine restriction (MetR)-induced lifespan extension. Obata et al. demonstrated that enhancing SAM catabolism by activating Gnmt extends the lifespan in Drosophila [27]. Notably, SAM levels are higher in old flies, even though Gnmt is transcriptionally induced in a fork head box O (FOXO)-dependent manner [27]. However, overexpression of Gnmt suppresses the age-dependent increase in SAM and extends the lifespan.
How is the accumulation of SAM related to ageing? Nonnitrogen starvation-induced autophagy is inhibited by high intracellular concentrations of methionine and SAM [28]. The methylation of the catalytic subunit of protein phosphatase2A (PP2A) by Ppm1(leucine carboxyl methyltransferase 1 (LCMT1) in mammals) is responsive to SAM concentrations [28,29](Fig. 1c). Methylated-PP2A can dephosphorylate Npr2, a component of a negative regulatory complex of mTORC1, promoting autophagy [28,29] (Fig. 1c). Additionally, SAMTOR has been identified as a SAM sensor that links methionine to mTORC1 signalling [30]. SAMTOR inhibits mTORC1 signalling by interacting with GAP activity towards Rag1 (GATOR1). The administration of SAM disrupts the SAMTOR-GATOR1 complex by directly binding SAMTOR, leading to a dissociation of the complex and resulting in mTORC1 activation (Fig. 1c), while reduced SAM levels promote the association between SAMTOR and GATOR1, leading to the inhibition of mTORC1 (Fig. 1d).
2.3. Fibroblast growth factor 21 (FGF21)
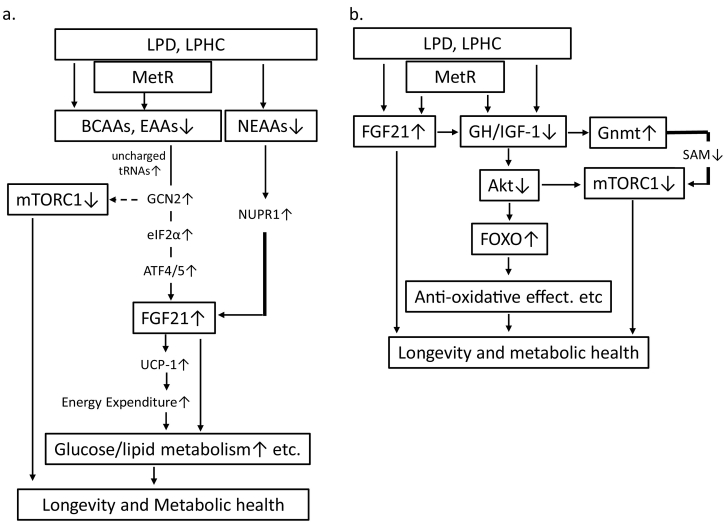
FGF21 is recognized as an endocrine signalling factor in PR and is associated with lifespan extension, metabolic control and organ protection [[31], [32], [33]]. Laeger et al. demonstrated that the serum levels of FGF21 specifically increase upon exposure to a LPD regardless of the overall caloric intake in both rodents and humans [31]. The reduced consumption of dietary protein and reduced delivery of AAs to the liver activates GCN2 (general AA control nonderepressible-2) and leads to increased eIF2α (eukaryotic initiation factor-2α) phosphorylation and ATF4/5 activation (Fig. 2a). ATF4/5 binds AA responsive elements within the FGF21 promoter, leading to increased production of FGF21 in the liver and increased circulating FGF21 [33] (Fig. 2a). The increased circulating levels of FGF21 play a role in the regulation of glucose/lipid homeostasis, mitochondrial activity, ketogenesis and energy expenditure (EE), which could be expected to be beneficial for age-related health. Additionally, GCN2, which phosphorylates eIF2α, senses the absence of one or more AAs by directly binding uncharged cognate transfer RNAs, thereby suppressing mTORC1 activity [34,35] (Fig. 2a). Our previous data indicated that a LPD induced continuous high levels of FGF21, which might be associated with improved glucose/lipid metabolism and body/fat weight in rats with T2DM/obesity [25,36]. Maida et al. showed that dietary protein/AA dilution promotes metabolic health in mice and humans with T2DM at least partially through a select nonessential AA (NEAA) insufficiency–activated liver nuclear protein1 (NUPR1)-FGF21 axis [37] (Fig. 2a). A LPD also enhances EE by increasing sympathetic flux via β-adrenergic receptor signalling to brown adipose tissue (BAT) with the consequent upregulation of uncoupling protein-1 (UCP-1) expression [[38], [39], [40]] and increased FGF21-mediated thermogenesis [33]. Additionally, PR increases UCP-1 and promotes the browning of white adipose tissue, and these effects require FGF21 [33]. Moreover, UCP-1 is required for FGF21-mediated improvements in glucose tolerance [41]. Our data showed that a LPD-induced increase in FGF21 may be related to the overexpression of UCP-1 in BAT, which is accompanied by improvement in glucose intolerance and dyslipidaemia in rats with T2DM/obesity [36]. However, Maida et al. showed that the dietary protein dilution-mediated improvement in glucose homeostasis was independent of UCP-1 [37]. Moreover, similar effects of a LPD on FGF21-induced regulation of metabolism have been reported in response to dietary MetR [42,43].
Fig. 2.
(a) Reduction in AAs (EAAs, BCAAs, and Met) induces uncharged tRNA and activates GCN2, eIF2α and ATF4/5, resulting in increased FGF21 production. FGF21 production is also increased due to the reduced levels of NEAAs via NUPR1. FGF21 improves glucose metabolism through UCP-1-dependent increases in energy expenditure or the UCP-1-independent pathway. GCN2 also suppresses mTORC1 activation. (b) LPD, LPHC or MetR-induced reduction in GH/IGF-1 signalling suppresses mTORC1 activation and FOXO activation via the suppression of Akt. FOXO activation leads to anti-ageing effects, such as anti-oxidation. FGF21 inhibits GH/IGF-1 signalling, and the reduction in GH/IGF-1 is related to the restoration of Gnmt activation.
2.4. Growth hormone/insulin-like growth factor-1 (GH/IGF-1) signalling
Reduced GH/IGF-1 signalling is linked to survival duration and decreased incidence of cancer and T2DM in humans [44,45]. Reducing IGF-1 signalling suppresses the ageing process through the activation of FOXOs and mTORC1 inhibition, which occur as a result of Akt inactivation (Fig. 2b). PR or restriction of particular AAs such as methionine, may explain part of the effects of CR on longevity and disease risk because PR and AA restriction can sufficiently reduce IGF-1 levels and cancer incidence and extend the lifespan in model organisms independently of calorie intake [46]. Additionally, FGF21 exerts its effect on lifespan extension by suppressing the GH/IGF-1 signalling pathway [33,47] (Fig. 2b). Moreover, methionine interacts with the GH/IGF-1 pathway to extend the lifespan and improve metabolic health in mice [48,49], and GH/IGF-1 represses Gnmt activity [50,51] (Fig. 2b). Therefore, reduced methionine intake may be linked to mTORC1 suppression by decreasing the GH/IGF-1-mediated Gnmt activation of SAM (Fig. 2b).
2.5. Hydrogen sulphate (H2S)
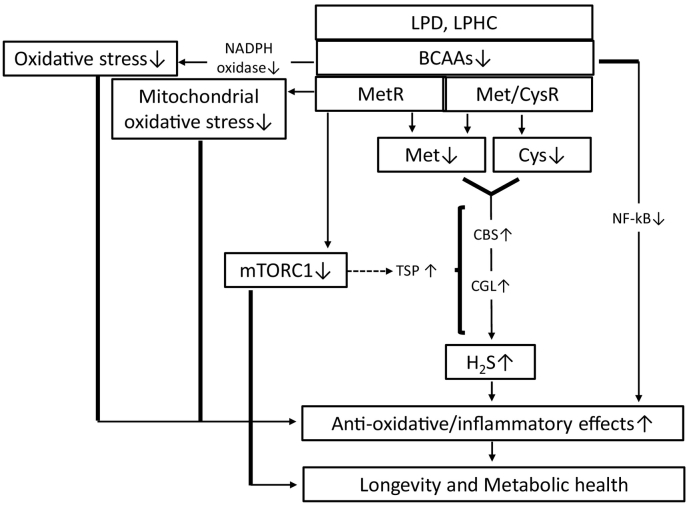
H2S has been identified as the third endogenous signalling gas following nitric oxide and carbon monoxide. H2S is produced endogenously by organisms, including mammals, via the transsulfuration pathway (TSP) via two key enzymes, i.e., cystathionine β-synthase and cystathionine γ-lyase [52]. H2S can readily diffuse through tissues and has pleiotropic and beneficial effects at the cellular, tissue and organismal levels with the potential to contribute to stress resistance by exerting positive effects, including anti-oxidative/anti-inflammatory effects [53]. The restriction of dietary sulfur-containing AAs (SAAs), including methionine, leads to stress resistance and longevity by increasing TSP-mediated H2S production (Fig. 3). Hine et al. demonstrated that 50% of the DR-induced liver protective effects against ischaemic reperfusion injury are abolished by the addition of SAAs, including methionine and cysteine, through the suppression of DR-induced H2S production [54]. Additionally, adult mice subjected to long-term MetR for 4 months in addition to fasting every other day or 20–30%DR for 6 weeks exhibited increased H2S production capacity in liver and kidney extracts compared to control mice fed a complete diet ad libitum (AL) [54]. Moreover, in fruit flies, maximal H2S production capacity was observed in whole-body extracts of flies subjected to varying levels of DR and MetR [54], which are correlated with the maximal lifespan extension [55]. In C. elegans and S. cerevisiae, lifespan extension or chronological lifespan extension was observed in a H2S production-dependent manner [54]. The effect of DR on longevity is mediated through SAA restriction, leading to increased endogenous H2S production via increased TSP activity, while the addition of specific SAAs and mTORC1 activation inhibits TSP and the H2S production pathway (Fig. 3).
Fig. 3.
Restriction of sulfur-containing AAs, such as Met or Cys, increases H2S production through TSP pathway activation, including the activation of CBS and CGL. H2S exerts anti-oxidative/anti-inflammatory effects. A LP/LPHC diet or MetR suppresses mTORC1, which leads to TSP pathway activation, and reduces the overproduction of ROS from mitochondria. BCAA restriction suppresses oxidative stress and inflammation through inhibition of NADPH oxidase and NF-κB.
2.6. Oxidative stress and inflammation
Mitochondria are recognized as major source of reactive oxygen species (ROS), and the oxidative damage associated with mitochondria is involved in mitochondrial dysfunction and cellular ageing. Previous reports have shown the effects of CR, PR and MetR on oxidative stress, particularly mitochondrial oxidative stress (Fig. 3). A 40%PR diet for 6–7 weeks without CR also decreases mitochondrial ROS (MtROS) production, specifically in complex I, in the rat liver [56]. Additionally, both 80% and 40% MetR without CR for 6–7 weeks decreased MtROS generation in the rat heart and liver [57] and brain, kidney, liver and heart [[58], [59], [60]], respectively, which is similar to the results observed for CR and PR. BCAAs also cause oxidative stress and inflammation in peripheral blood mononuclear cells [61] and endothelial cells [62] via the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, nuclear factor-κB (NF-κB) and mTORC1.
3. Relationships among dietary protein intake, longevity and metabolic health
3.1. Basic studies
Recent evidence suggests that the macronutrient balance and single nutrients, such as protein, play a more crucial role in longevity and metabolic health than total calorie intake. The significance of the macronutrient balance for longevity and metabolic health is shown by the Geometric Framework for Nutrition, which was developed to evaluate the relationship between diet and outcomes such as lifespan across a broad landscape of macronutrient and energy intakes [3]. In Drosophila melanogaster, the Queensland fruit fly or cricket, LPHC diets result in lifespan extension [[63], [64], [65], [66]] (Table 1), while a high proportion of dietary protein shortens the lifespan. In mice, Solon-Biet et al. demonstrated that LPHC diets, but not high-protein and/or diluted diets that reduce calorie intake, are associated with longevity and metabolic health, including lower blood pressure, improved glucose tolerance, higher levels of high-density lipoprotein, and reduced levels of low-density lipoprotein and triglycerides [4]. In the aforementioned study, over their lifetime, mice had AL access to 1 of 25diets differing in protein (5–60%), fat (16–75%), carbohydrate (16–75%), and energy (8(Low), 13(Medium), or 17(High) kJ/g of food) contents. The maximum lifespan was observed in mice fed medium calorie food with 5% protein/75% carbohydrate (Table 1). Additionally, mice fed a short-term (8 weeks) LPHC (5%protein) diet exhibited benefits in glucose/lipid metabolism that were similar to those observed in mice exposed to a 40%CR [5]. Additionally, LPHC (5%protein) diets are interestingly associated with increased dendritic spines in dentate gyrus neurons and improved performance in the Barnes maze and novel object recognition test, and the effects are similar to those associated with 20%CR [67]. To stabilize protein intake, mice fed a LPD exhibit compensatory increased energy intake; however, the LPD exerted beneficial effects on metabolic health due to increased EE [68].
Table 1.
Relationships among dietary protein intake, longevity and metabolic health.
| Effect of a LPHC diet on lifespan extension |
Metabolic benefits of a LPHC diet or low protein intake | Ref. | |
|---|---|---|---|
| Ratio or proportion of protein to carbohydrate in food for lifespan extension | |||
| Drosophila melanogaster | Increased | Not determined | [63] |
| P:C ratio = 1:16 | |||
| Drosophila melanogaster | Increased | Not determined | [64] |
| P:C ratio = 1:16 | |||
| Queensland fruit flies | Increased | Not determined | [65] |
| P:C ratio = 1:21 | |||
| Crickets | Increased | Not determined | [66] |
| P:C ratio = 1:3 (Male) P:C ratio = 1:8 (Female) | |||
| Mice | Increased | Insulin sensitivity↑, BP↓, HDL-C↑, TG↓, LDL-C↓, mTOR↓(liver), plasma BCAAs↓, mitochondrial function↑ | [3] |
| 5% protein/75% carbohydrate, energy 13 kJ/g food | |||
| The effect of LP intake on mortality |
Metabolic benefits of low protein intake | Ref. | |
|---|---|---|---|
| Proportion of protein intake for reduced mortality | |||
| Humans | Aged 50 years or older: Diabetes-related mortality↓ | Aged 50–65 years: Serum IGF-1↓ in LP intake group compared to IGF-1 in the HP intake group | [6] |
| Aged 50–65 years: All-cause mortality↓, cancer-related mortality↓, LP intake is more effective than MP or HP intake. | |||
| Aged 66 years or older: No change in serum IGF-1 levels between LP and HP intake groups. | |||
| Aged 66 years or older: All-cause mortality↑, Cancer mortality↓ HP intake is more effective than LP intake. |
3.2. Human studies
Levine et al. investigated the relationship between the level of protein intake and all-cause, cancer- and diabetes-related mortality in a major nationally representative study of nutrition involving a United States population (6381 individuals aged 50 years and over) [6]. The results were analysed using Cox proportional hazard models and revealed that both the moderate protein (MP;10–19% of calories from protein) and high protein (HP; ≥20% of calories from protein) intake groups had higher risks of diabetes-related mortality than the participants in the low protein(LP; <10% of calories from protein) group (Table 1). Among those aged 50–65 years, higher protein levels were linked to significantly increased risks of all-cause and cancer-related mortality (Table 1). In this age range, the HP intake group exhibited a 74% increase in their relative risk of all-cause mortality and were >4-fold likely to die of cancer than those in the LP group. Additionally, the higher risks of all-cause and cancer-related mortality in the HP intake group compared to those in the LP intake group were further increased among those who also had high levels of IGF-1 [6] (Table 1). However, among those aged 66 years and older, the HP diet was associated with the opposite effect on all-cause and cancer-related mortality (Table 1). Compared to those in the LP group, the participants in the HP and MP groups exhibited a 28% and 21% reduction in all-cause mortality, respectively. Additionally, compared to those in the LP group, HP consumption resulted in a 60% reduction in cancer mortality. Thus, LP intake during middle age may be beneficial for the prevention of cancer and improvement of overall mortality. However, among elderly people, avoiding LP intake or consuming adequate dietary protein may be important to prevent sarcopenia and frailty, thus potentially preventing an increase in all-cause mortality.
Interestingly, on the Japanese island of Okinawa, many people exhibit increased longevity, and the centenarian population is five times larger than that in other developed nations [69]. The CVD and cancer death rates in Okinawa were found to be only 60–70% of those in other regions of Japan on average, and the all-cause mortality rate among 60- to 64-year-olds was only half that of other Japanese populations. Based on the 1972 Japan National Nutrition Survey, Kagawa et al. reported that the Okinawan adult population had a low caloric intake (83% of the Japanese average) and documented that the anthropometric and morbidity data of older Okinawans were consistent with CR [70]. Therefore, CR may be associated with the longevity observed in Okinawa [71]. In addition to CR, many factors, including food, genes and physical activity, contribute to longevity. The types of foods included in the traditional Okinawan diet, which includes a high intake of green leafy and yellow root vegetables, sweet potatoes (as a dietary staple), and soy (as the principle protein) supplemented with small amounts of fish and meat, are adequate in most nutrients [72,73]. The energy obtained from the Okinawan diet is derived from 9%protein and 85%carbohydrates [74]. Interestingly, the Okinawan values of dietary protein and the protein to carbohydrate ratio (1:10) are very low and are remarkably similar to those found to optimize the lifespan in recent animal studies investigating ageing.
4. Relationships among protein sources, longevity and metabolic health
The protein source, including animal or plant protein, may be more important for mortality risk than the level of protein intake. The associations between animal and plant protein intake and the risk of mortality were examined by a prospective US cohort study involving 131,342 participants and 32 follow-up years [7]. Animal protein intake was related to a higher risk of mortality, particularly CVD mortality. In contrast, higher plant protein intake was associated with lower all-cause mortality. The substitution of animal protein from a variety of food sources, particularly processed red meat, with plant protein was associated with a lower risk of mortality, indicating that the protein source is important for long-term health.
As described above, compared to LP intake in middle-aged humans, HP intake is associated with increased all-cause mortality and cancer- and diabetes-related mortality [6]. However, after controlling for the percent of calories from animal protein, the association between the level of protein intake and all-cause and cancer-related mortality was eliminated or significantly reduced, suggesting that animal protein mediates a significant portion of those relationships. In contrast, after controlling for the effect of plant protein, there was no change in the association between protein intake and mortality, indicating not only that high levels of animal proteins promote mortality but also that plant proteins have a protective effect.
Among animal proteins, the consumption of red meat and processed meat is associated with the risk of developing chronic diseases, including CVD, CKD, cancer and diabetes [8,9]. A meta-analysis indicated that a high consumption of red meat tends to increase the risk of CVD mortality and cancer and that a high consumption of processed meat significantly increases the risk of cancer and CVD mortality and diabetes [8]. Red meat is an important dietary source of EAAs and micronutrients, including vitamins, iron and zinc, that perform many beneficial functions. However, a high intake of red meat and processed meat results in an increased intake of saturated fat, cholesterol, iron, salt, and phosphate; oxidative stress/inflammation; elevation of by-products of protein or AA digestion by the gut microbiota, such as trimethylamine n-oxide or indoxyl sulfate; acid load; and protein/AA load, which are possibly associated with increased risks of CVD mortality and CKD.
5. The impact of specific AAs on longevity and metabolic health
5.1. Role of BCAAs
Increased circulating BCAA levels or excess BCAAs may be harmful for longevity and metabolic health. Solon-Biet et al. indicated that BCAA levels were the lowest following LPHC diets, and these levels were correlated with dietary treatments resulting in lifespan extension and improved metabolic health in mice, as described above [4]. In a randomized controlled trial, a moderate PR (7–9%protein) diet improved markers of metabolic health in humans, and mice fed a reduced BCAA diet exhibited improved glucose tolerance and body composition, which were equivalent to those observed following a PR diet [12]. Reducing dietary BCAAs also leads to improvements in Western diet-induced obesity and glucose intolerance in mice [10]. Additionally, the supplementation of BCAAs abolishes the effect of PR on glucose metabolism and induces inflammation in visceral adipose tissue in mice [10]. In epidemiological studies, there is a positive relationship between increased circulating BCAA levels and insulin resistance in obese and diabetic patients [[75], [76], [77]] and CVD patients [[78], [79], [80], [81]]. Additionally, the increased circulating BCAAs are possibly due to abnormal BCAA metabolism associated with obesity resulting in an accumulation of toxic BCAA metabolites that, in turn, trigger mitochondrial dysfunction, which is associated with insulin resistance and T2DM [82].
However, BCAAs also have beneficial effects on health and are associated with mitochondrial function. BCAA supplementation increases the average lifespan of mice by increasing mitochondrial biogenesis and reducing oxidative stress in cardiac and skeletal muscles [83]. Several clinical studies have also shown that BCAA supplements reduce sarcopenia in elderly people and exert beneficial effects on body fat and glucose metabolism, possibly by increasing mitochondrial biogenesis and muscle function [84].
Thus, the high intake of BCAAs due to excessive food intake in obese people is harmful in terms of insulin resistance and T2DM. However, a low level of BCAA intake in elderly people is also harmful in terms of sarcopenia. Therefore, the appropriate intake of BCAAs for individuals is necessary to maintain longevity and metabolic health.
5.2. Role of methionine and SAM
Dietary MetR has been demonstrated to extend the lifespan of organisms ranging from yeast to rodents such as mice and rats [48,[85], [86], [87]]. As described above, Met is directly involved in promoting the ageing process through multiple mechanisms. Metabolically, MetR also decreases adiposity but acts through a paradoxical increase in both energy intake and EE in rodents [49,88,89]. The increase in EE compensates for the increased energy intake and effectively limits fat deposition. Moreover, MetR increases metabolic flexibility and overall insulin sensitivity and improves lipid metabolism while decreasing systemic inflammation [42,88,90]. Plaisance et al. investigated the effects of MetR (2 mg methionine/kg body weight (BW)/day) in humans for 16 weeks by exploring the EE, body composition and metabolism of individuals who were fed a MetR diet compared to the same parameters in obese adults with metabolic syndrome who were fed a control diet (33 mg methionine/kg BW/day) [91]. Although insulin sensitivity improved and EE was unaffected in both groups, MetR produced a significant increase in fat oxidation and a reduction in intrahepatic lipid content [91]. Additionally, Virtanen et al. reported that the relative risk of an acute coronary event in individuals with a high methionine intake (>2.2 g methionine/day) was higher than that of individuals with a low methionine intake (<1.7 g methionine/day) in a prospective cohort study (1981 men, aged 42–60 years at baseline, average 14.0 years of follow-up) [92].
Additionally, plasma SAM concentrations were associated with higher fasting insulin levels, homeostasis model assessment of insulin resistance and tumour necrosis factor-α in a cross-sectional study involving 118 subjects with metabolic syndrome [93]. Another report demonstrated that plasma SAM, but not methionine, is independently associated with fat mass and truncal adiposity in a cross-sectional study involving 610 elderly people [94], while overfeeding increases serum SAM in proportion to the fat mass gained [95]. Thus, increased SAM related to overfeeding or metabolic dysfunction may be associated with whole body metabolic impairment.
6. Conclusions, future prospective and outstanding questions
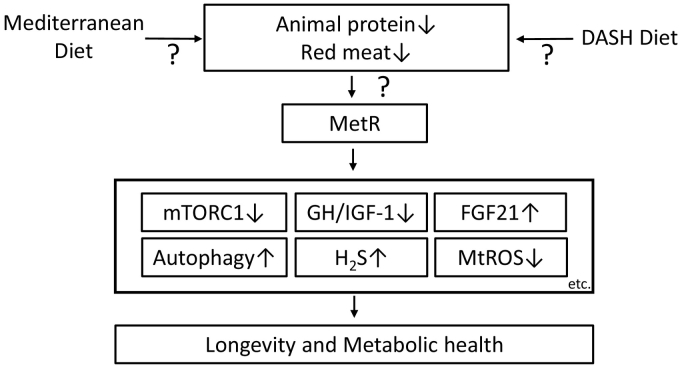
We presented the quantity, source and specific AA compositions of proteins, including the levels of BCAAs and methionine, that are associated with longevity and metabolic health. Among dietary interventions, MetR may be a candidate intervention for longevity and metabolic health (Fig. 4). Food sources of animal protein, such as beef, lamb, fish, pork and eggs, contain higher levels of methionine than plant food sources, including nuts, seeds, legumes, cereals, vegetables and fruits [96]. Therefore, an individual may need to eat less animal-based food to achieve MetR. For example, the Mediterranean diet [97] or the Dietary Approaches to Stop Hypertension (DASH) diet [98] may be useful for decreasing the consumption of animal protein, particularly red meat (Fig. 4). However, red meat is an important dietary source of micronutrients, including vitamins, iron and zinc; therefore, an appropriate intake is necessary to avoid malnutrition.
Fig. 4.
MetR may be a candidate dietary intervention for longevity and metabolic health through its effects that are exerted via multiple mechanisms. A Mediterranean diet or the DASH diet may be useful for reducing the consumption of animal protein, particularly red meat, to achieve MetR.
This review focuses on the detrimental effects of proteins; however, reduced protein intake does not decrease the potentially negative effects of certain types of carbohydrates and fats. Clinical studies comparing lifespan, mortality and metabolic health between groups randomly assigned to either LP or HP diets or specific AA restriction diets are necessary to identify diets that minimize the burden on the population while maximizing the protective effects. However, it is difficult to perform such randomized clinical trials; therefore, additional detailed epidemiological studies may be necessary. Furthermore, elucidating the detailed mechanism underlying the effect of protein or specific AA restriction on longevity and metabolic health could guide the development of novel therapies replacing dietary interventions.
7. Search strategy and selection criteria
Data for this review were collected through PubMed. The following search terms were used: low protein diet, low protein and high carbohydrate diet, protein dilution, Geometric Framework for Nutrition, methionine restriction, branched-chain amino acid, red meat, longevity, lifespan extension, mTORC1, FGF21, oxidative stress, IGF-1, SAM, Gnmt, H2S. Only articles published in English were included.
Acknowledgements/Funding
This work was financially supported by a Grant-in-Aid for Scientific Research (C) (KAKENHI) from the Japan Society for the Promotion of Science (24591218) awarded to MK. Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Kyowa Hakko Kirin, Taisho Toyama Pharmaceutical Co. and Ono Pharmaceutical Co. contributed to establishing the Division of Anticipatory Molecular Food Science and Technology. The authors declare that there are no conflicts of interest associated with this manuscript. The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author contributions
MK and DK contributed to the writing and drafting of the article. YO, IM and DK contributed to the discussion of the review. All authors revised the manuscript critically for important intellectual content and approved the final version to be published. MK and DK are responsible for the integrity of the work as a whole.
Contributor Information
Munehiro Kitada, Email: kitta@kanazawa-med.ac.jp.
Daisuke Koya, Email: koya0516@kanazawa-med.ac.jp.
References
- 1.Fontana L., Partridge L., Longo V.D. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa S., Lagisz M., Hector K.L., Spencer H.G. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11(3):401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- 3.Simpson S.J., Le Couteur D.G., James D.E., George J., Gunton J.E., Solon-Biet S.M. The geometric framework for nutrition as a tool in precision medicine. Nutr Healthy Aging. 2017;4(3):217–226. doi: 10.3233/NHA-170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solon-Biet S.M., McMahon A.C., Ballard J.W., Ruohonen K., Wu L.E., Cogger V.C. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solon-Biet S.M., Mitchell S.J., Coogan S.C., Cogger V.C., Gokarn R., McMahon A.C. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep. 2015;11(10):1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine M.E., Suarez J.A., Brandhorst S., Balasubramanian P., Cheng C.W., Madia F. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453–1463. doi: 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolk A. Potential health hazards of eating red meat. J Intern Med. 2017;281(2):106–122. doi: 10.1111/joim.12543. [DOI] [PubMed] [Google Scholar]
- 9.Lew Q.J., Jafar T.H., Koh H.W., Jin A., Chow K.Y., Yuan J.M. Red meat intake and risk of ESRD. J Am Soc Nephrol. 2017;28(1):304–312. doi: 10.1681/ASN.2016030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings N.E., Williams E.M., Kasza I., Konon E.N., Schaid M.D., Schmidt B.A. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–645. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu W.C., VanHoosier E., Elks C.M., Grant R.W. Long-term effects of dietary protein and branched-chain amino acids on metabolism and inflammation in mice. Nutrients. 2018;10(7):E918. doi: 10.3390/nu10070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee B.C., Kaya A., Gladyshev V.N. Methionine restriction and life-span control. Ann N Y Acad Sci. 2016;1363:116–124. doi: 10.1111/nyas.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J., Guan K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21(1):63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 15.Jewell J.L., Russell R.C., Guan K.L. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14(3):133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anisimov V.N., Zabezhinski M.A., Popovich I.G., Piskunova T.S., Semenchenko A.V., Tyndyk M.L. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 18.Miller R.A., Harrison D.E., Astle C.M., Fernandez E., Flurkey K., Han M. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson J.E., Burmeister L., Brooks S.V., Chan C.C., Friedline S., Harrison D.E. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Bokov A., Gelfond J., Soto V., Ikeno Y., Hubbard G. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfson R.L., Chantranupong L., Saxton R.A., Shen K., Scaria S.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxton R.A., Chantranupong L., Knockenhauer K.E., Schwartz T.U., Sabatini D.M. Mechanism of arginine sensing by CASTOR1 upstream of mTORC1. Nature. 2016;536(7615):229–233. doi: 10.1038/nature19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F., Zhao S., Yan W., Xia Y., Chen X., Wang W. Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy. EBioMedicine. 2016;13:157–167. doi: 10.1016/j.ebiom.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitada M., Ogura Y., Suzuki T., Sen S., Lee S.M., Kanasaki K. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia. 2016;59(6):1307–1317. doi: 10.1007/s00125-016-3925-4. [DOI] [PubMed] [Google Scholar]
- 26.Mato J.M., Lu S.C. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45(5):1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 27.Obata F., Miura M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat Commun. 2015;6(2015):8332. doi: 10.1038/ncomms9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutter B.M., Wu X., Laxman S., Tu B.P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell. 2013;154(2):403–415. doi: 10.1016/j.cell.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laxman S., Sutter B.M., Tu B.P. Methionine is a signal of amino acid sufficiency that inhibits autophagy through the methylation of PP2A. Autophagy. 2014;10(2):386–387. doi: 10.4161/auto.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X., Orozco J.M., Saxton R.A., Condon K.J., Liu G.Y., Krawczyk P.A. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science. 2017;358(6364):813–818. doi: 10.1126/science.aao3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solon-Biet S.M., Cogger V.C., Pulpitel T., Heblinski M., Wahl D., McMahon A.C. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24(4):555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Xie Y., Berglund E.D., Coate K.C., He T.T., Katafuchi T. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1(2012):e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallinetti J., Harputlugil E., Mitchell J.R. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449(1):1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao F., Huang Z., Li H., Yu J., Wang C., Chen S. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60(3):746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitada M., Ogura Y., Suzuki T., Monno I., Kanasaki K., Watanabe A. A low-protein diet exerts a beneficial effect on diabetic status and prevents diabetic nephropathy in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Nutr Metab (Lond) 2018;15(20):20. doi: 10.1186/s12986-018-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maida A., Zota A., Sjoberg K.A., Schumacher J., Sijmonsma T.P., Pfenninger A. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest. 2016;126(9):3263–3278. doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothwell N.J., Stock M.J. Influence of carbohydrate and fat intake on diet-induced thermogenesis and brown fat activity in rats fed low protein diets. J Nutr. 1987;117(10):1721–1726. doi: 10.1093/jn/117.10.1721. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell N.J., Stock M.J., Tyzbir R.S. Mechanisms of thermogenesis induced by low protein diets. Metabolism. 1983;32(3):257–261. doi: 10.1016/0026-0495(83)90190-7. [DOI] [PubMed] [Google Scholar]
- 40.Aparecida de Franca S., Dos Santos M.P., Garofalo M.A., Navegantes L.C., Kettelhut Ido C., Lopes C.F. Low protein diet changes the energetic balance and sympathetic activity in brown adipose tissue of growing rats. Nutrition. 2009;25(11−12):1186–1192. doi: 10.1016/j.nut.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Kwon M.M., O'Dwyer S.M., Baker R.K., Covey S.D., Kieffer T.J. FGF21-mediated improvements in glucose clearance require uncoupling protein 1. Cell Rep. 2015;13(8):1521–1527. doi: 10.1016/j.celrep.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Stone K.P., Wanders D., Orgeron M., Cortez C.C., Gettys T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63(11):3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lees E.K., Krol E., Grant L., Shearer K., Wyse C., Moncur E. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13(5):817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milman S., Atzmon G., Huffman D.M., Wan J., Crandall J.P., Cohen P. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renehan A.G., Zwahlen M., Minder C., O'Dwyer S.T., Shalet S.M., Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 46.Takenaka A., Oki N., Takahashi S.I., Noguchi T. Dietary restriction of single essential amino acids reduces plasma insulin-like growth factor-I (IGF-I) but does not affect plasma IGF-binding protein-1 in rats. J Nutr. 2000;130(12):2910–2914. doi: 10.1093/jn/130.12.2910. [DOI] [PubMed] [Google Scholar]
- 47.Inagaki T., Lin V.Y., Goetz R., Mohammadi M., Mangelsdorf D.J., Kliewer S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8(1):77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller R.A., Buehner G., Chang Y., Harper J.M., Sigler R., Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4(3):119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malloy V.L., Krajcik R.A., Bailey S.J., Hristopoulos G., Plummer J.D., Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 50.Brown-Borg H.M., Rakoczy S.G., Uthus E.O. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126(3):389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Aida K., Tawata M., Negishi M., Onaya T. Mouse glycine N-methyltransferase is sexually dimorphic and regulated by growth hormone. Horm Metab Res. 1997;29(12):646–649. doi: 10.1055/s-2007-978982. [DOI] [PubMed] [Google Scholar]
- 52.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 53.Li Z., Polhemus D.J., Lefer D.J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res. 2018;123(5):590–600. doi: 10.1161/CIRCRESAHA.118.311134. [DOI] [PubMed] [Google Scholar]
- 54.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1–2):132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valerio A., D'Antona G., Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging (Albany NY) 2011;3(5):464–478. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanz A., Caro P., Barja G. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J Bioenerg Biomembr. 2004;36(6):545–552. doi: 10.1007/s10863-004-9001-7. [DOI] [PubMed] [Google Scholar]
- 57.Sanz A., Caro P., Ayala V., Portero-Otin M., Pamplona R., Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20(8):1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 58.Caro P., Gomez J., Sanchez I., Naudi A., Ayala V., López-Torres M. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009;12(6):421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- 59.Caro P., Gomez J., Lopez-Torres M., Sanchez I., Naudi A., Jove M. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008;9(3):183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez-Roman I., Gomez A., Perez I., Sanchez C., Suarez H., Naudí A. Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology. 2012;13(4):399–411. doi: 10.1007/s10522-012-9384-5. [DOI] [PubMed] [Google Scholar]
- 61.Zhenyukh O., Civantos E., Ruiz-Ortega M., Sanchez M.S., Vazquez C., Peiró C. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med. 2017;104:165–177. doi: 10.1016/j.freeradbiomed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 62.Zhenyukh O., Gonzalez-Amor M., Rodrigues-Diez R.R., Esteban V., Ruiz-Ortega M., Salaices M. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22(10):4948–4962. doi: 10.1111/jcmm.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W., Taylor P.W. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen K., McClure C., Priest N.K., Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell. 2015;14(4):605–615. doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fanson B.G., Weldon C.W., Pérez-Staples D., Simpson S.J., Taylor P.W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8(5):514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 66.Harrison S.J., Raubenheimer D., Simpson S.J., Godin J.G., Bertram S.M. Towards a synthesis of frameworks in nutritional ecology: interacting effects of protein, carbohydrate and phosphorus on field cricket fitness. Proc Biol Sci. 2014;281 doi: 10.1098/rspb.2014.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wahl D., Solon-Biet S.M., Wang Q.P., Wali J.A., Pulpitel T., Clark X. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. 2018;25(8):2234–2243. doi: 10.1016/j.celrep.2018.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pezeshki A., Zapata R.C., Singh A., Yee N.J., Chelikani P.K. Low protein diets produce divergent effects on energy balance. Sci Rep. 2016;6 doi: 10.1038/srep25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willcox D.C., Willcox B.J., Hsueh W.C., Suzuki M. Genetic determinants of exceptional human longevity: insights from the Okinawa centenarian study. Age (Dordr) 2006;28(4):313–332. doi: 10.1007/s11357-006-9020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagawa Y. Impact of westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7(2):205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- 71.Willcox D.C., Willcox B.J., Todoriki H., Curb J.D., Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7(3):173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki M., Wilcox B.J., Wilcox C.D. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10(2):165–171. doi: 10.1111/j.1440-6047.2001.00219.x. 2001. [DOI] [PubMed] [Google Scholar]
- 73.Willcox D.C., Willcox B.J., Todoriki H., Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;Suppl:500S–516S. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- 74.Le Couteur D.G., Solon-Biet S.M., Wahl D., Cogger V.C., Willcox B.J., Willcox D.C. New horizons: dietary protein, ageing and the Okinawan ratio. Age Ageing. 2016;45(4):443–447. doi: 10.1093/ageing/afw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao X., Han Q., Liu Y., Sun C., Gang X., Wang G. The relationship between branched-chain amino acid related metabolomic signature and insulin resistance: a systematic review. J Diabetes Res. 2016;2016 doi: 10.1155/2016/2794591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flores-Guerrero J.L., Oste M.C.J., Kieneker L.M., Gruppen E.G., Wolak-Dinsmore J., Otvos J.D. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. J Clin Med. 2018;7(12):E513. doi: 10.3390/jcm7120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruiz-Canela M., Toledo E., Clish C.B., Hruby A., Liang L., Salas-Salvadó J. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem. 2016;62(4):582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah S.H., Bain J.R., Muehlbauer M.J., Stevens R.D., Crosslin D.R., Haynes C. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 80.Magnusson M., Lewis G.D., Ericson U., Orho-Melander M., Hedblad B., Engström G. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhattacharya S., Granger C.B., Craig D., Haynes C., Bain J., Stevens R.D. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232(1):191–196. doi: 10.1016/j.atherosclerosis.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lynch C.J., Adams S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D'Antona G., Ragni M., Cardile A., Tedesco L., Dossena M., Bruttini F. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12(4):362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 84.Yoon M.S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients. 2016;8(7):405. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee B.C., Kaya A., Ma S., Kim G., Gerashchenko M.V., Yim S.H. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nat Commun. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson J.E., Johnson F.B. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orentreich N., Matias J.R., DeFelice A., Zimmerman J.A. Low methionine ingestion by rats extends life span. J Nutr. 1993;123(2):269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 88.Hasek B.E., Stewart L.K., Henagan T.M., Boudreau A., Lenard N.R., Black C. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R728–R739. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plaisance E.P., Henagan T.M., Echlin H., Boudreau A., Hill K.L., Lenard N.R. Role of beta-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R740–R750. doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasek B.E., Boudreau A., Shin J., Feng D., Hulver M., Van N.T. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes. 2013;62(10):3362–3372. doi: 10.2337/db13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plaisance E.P., Greenway F.L., Boudreau A., Hill K.L., Johnson W.D., Gettys T.W. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E836–E840. doi: 10.1210/jc.2010-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Virtanen J.K., Voutilainen S., Rissanen T.H., Happonen P., Mursu J., Laukkanen J.A. High dietary methionine intake increases the risk of acute coronary events in middle-aged men. Nutr Metab Cardiovasc Dis. 2006;16(2):113–120. doi: 10.1016/j.numecd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Lind M.V., Lauritzen L., Vestergaard H., Hansen T., Pedersen O., Kristensen M. One-carbon metabolism markers are associated with cardiometabolic risk factors. Nutr Metab Cardiovasc Dis. 2018;28(4):402–410. doi: 10.1016/j.numecd.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Elshorbagy A.K., Nijpels G., Valdivia-Garcia M., Stehouwer C.D., Ocke M., Refsum H. S-adenosylmethionine is associated with fat mass and truncal adiposity in older adults. J Nutr. 2013;143(12):1982–1988. doi: 10.3945/jn.113.179192. [DOI] [PubMed] [Google Scholar]
- 95.Elshorbagy A.K., Jerneren F., Samocha-Bonet D., Refsum H., Heilbronn L.K. Serum S-adenosylmethionine, but not methionine, increases in response to overfeeding in humans. Nutr Diabetes. 2016;6:e192. doi: 10.1038/nutd.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ables G.P., Johnson J.E. Pleiotropic responses to methionine restriction. Exp Gerontol. 2017;94:83–88. doi: 10.1016/j.exger.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 97.Willett W.C., Sacks F., Trichopoulou A., Drescher G., Ferro-Luzzi A., Helsing E. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 Suppl):1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 98.Vogt T.M., Appel L.J., Obarzanek E.V.A., Moore T.J., Vollmer W.M., Svetkey L.P. Dietary approaches to stop hypertension: rationale, design, and methods. DASH collaborative research group. J Am Diet Assoc. 1999;99:S12–S18. doi: 10.1016/s0002-8223(99)00411-3. [DOI] [PubMed] [Google Scholar]