Abstract
Background
Leucine‐rich kinase 2 (LRRK2)‐linked Parkinson's disease (PD) is clinically indistinguishable from idiopathic PD (IPD). A pleiotropic neuropathology has been recognized but the majority of studies in LRRK2 p.G2019S patients reveal Lewy‐type synucleinopathy as its principal histological substrate. To date no in vivo biomarkers of synucleinopathy have been found in LRRK2 mutation carriers.
Objectives
We used real‐time quaking‐induced conversion (RT‐QuIC) technique to assess the presence of alpha‐synuclein (a‐syn) aggregates in cerebrospinal fluid (CSF) of LRRK2 p.G2019S carriers.
Methods
CSF samples of 51 subjects were analyzed: 15 LRRK2 p.G2019S PD, 10 IPD, 16 LRRK2 p.G2019S nonmanifesting carriers (NMC) and 10 healthy controls. The presence of parkinsonism and prodromal symptoms was assessed in all study subjects.
Results
Forty percent (n = 6) LRRK2‐PD, and 18.8% (n = 3) LRRK2‐NMC had a positive a‐syn RT‐QuIC response. RT‐QuIC detected IPD with 90% sensitivity and 80% specificity. No clinical differences were detected between LRRK2‐PD patients with positive and negative RT‐QuIC. A positive RT‐QuIC result in LRRK2‐NMC occurred in a higher proportion of subjects meeting the Movement Disorder Society research criteria for prodromal PD.
Interpretation
RT‐QuIC detects a‐syn aggregation in CSF in a significant number of patients with LRRK2‐PD, but less frequently than in IPD. A small percentage of LRRK2‐NMC tested also positive. If appropriately validated in long‐term studies with large number of mutation carriers, and hopefully, postmortem or in vivo confirmation of histopathology, RT‐QuIC could contribute to the selection of candidates to receive disease modifying drugs, in particular treatments targeting a‐syn deposition.
Introduction
Pathogenic mutations in the leucine‐rich repeat kinase 2 (LRRK2) gene are one of the most frequent causes of inherited Parkinson's disease (PD).1, 2 Among the different mutations in this gene, p.G2019S is the most frequently found in the population worldwide accounting for 1% of sporadic and 4% of familial PD patients.2 LRRK2‐linked PD (LRRK2‐PD) presents with an autosomal‐dominant inheritance pattern with incomplete penetrance. The cumulative risk of developing PD in LRRK2 p.G2019S cases is age dependant and varies widely depending on the studies, from 25% to 42.5% at age 80 years3, 4 and currently is not possible to identify which subjects will develop the disease. The clinical picture of LRRK2‐PD is largely indistinguishable from that of idiopathic PD (IPD).2, 5 Aggregated alpha‐synuclein (a‐syn) in the form of Lewy‐type pathology6 in the nervous tissue, similar to that encountered in IPD, has been reported to occur in a majority of LRRK2‐PD p.G2019S cases, but LRRK2‐PD can have a pleiotropic neuropathological substrate.6, 7, 8, 9, 10, 11 Kalia and colleagues have suggested that the occurrence of nonmotor symptoms such as cognitive impairment/dementia, and anxiety is related to an underlying Lewy‐type synucleinopathy (LTS) in LRRK2‐PD p.G2019S cases,9 however, it is currently not possible to reliably identify these cases in vivo.
Seeking for evidence of abnormal a‐syn aggregation in different body tissues and fluids of patients with suspected synucleinopathy has been one of the main strategies in PD biomarkers research during the last years.12, 13, 14, 15 Recently, an assay to detect a‐syn oligomers in cerebrospinal fluid (CSF), the real‐time quaking‐induced conversion (RT‐QuIC) test, has proven to identify PD patients with high sensitivity and specificity.16 This technique, previously used as a diagnostic tool in diseases caused by prion proteins, is based on the capacity of misfolded a‐syn to induce the aggregation of monomeric species of a‐syn.17, 18 Fairfoul and colleagues demonstrated in CSF of pathologically confirmed patients with Lewy body disease a positivity of the test in 92% of cases, whereas none of the controls were positive. A validation cohort of clinically diagnosed PD subjects resulted in a sensitivity of the test of 95% and a specificity of 100%. Shahnawaz and colleagues have equally demonstrated high sensitivity and specificity of a similar assay, based on aggregated a‐syn amplification in the CSF, for the detection of PD subjects.19 In the study by Fairfoul et al. three subjects with idiopathic rapid eye movement sleep behavior disorder (RBD), considered a prodromal stage of PD20 also presented abnormal aggregation of a‐syn in CSF, suggesting that this test could be used as a marker of premotor PD.
The objective of our study was to assess the presence of a‐syn aggregation in CSF of LRRK2 mutation carriers using RT‐QuIC. Detection of such aggregates could be indicative of the presence of an ongoing synucleinopathy. Patients with manifest LRRK2‐PD and a positive test could eventually be candidates to receive disease modifying drugs, in particular treatments directed against a‐syn. In LRRK2 nonmanifesting carriers (NMC) the identification of a‐syn in CSF may possible identify subjects who will develop the motor syndrome of PD.
Methods
Patient groups and clinical assessment
This study was carried out in the Neurology Service at the Hospital Clinic of Barcelona, in collaboration with the National CJD Research & Surveillance Unit in Western General Hospital, University of Edinburgh. The local ethics committee approved all clinical protocols and written informed consent was obtained from all the participants prior to enrollment.
We analyzed 51 CSF samples from LRRK2‐PD (n = 15) and LRRK2‐NMC (n = 16) carrying the p.G2019S mutation, IPD (n = 10) and HC (n = 10). IPD and LRRK2‐PD subjects were identified and recruited in the Movement Disorders Unit from the Hospital Clínic of Barcelona and collaborating centres of the Barcelona LRRK2 study group. LRRK2‐NMC were identified among the relatives of LRRK2‐PD subjects. All LRRK2 mutation carriers (n = 31) of our cohort belong to 21 different families. None of the 15 LRRK2‐PD cases are related. Among the 16 LRRK2‐NMC, eight subjects are relative to six LRRK‐PD, and eight subjects are not and belong to six different families. Genetic analysis was performed as previously described.21 DNA was extracted from peripheral blood following standard procedures. Genotyping of the G2019S mutation was performed using the predesigned TaqMan assay C‐63498123‐10 SNP rs34637584 and the R1441G/C/H mutation using a commercial TaqMan assay. Genotyping was performed on a StepOnePlus Real‐time PCR System (Life Tech. Inc.). CSF samples of 10 HC subjects were obtained for comparative purposes among subjects undergoing a surgical procedure in our institution that required an epidural anesthesia.
All the participants of this study were comprehensively assessed for demographic and clinical characteristics. Patients with IPD and LRRK2‐PD were diagnosed clinically according to UK PD Society Brain Bank criteria22 and recruited between May 2012 and October 2017. At the time of inclusion to the study, none of the LRRK2‐NMC and none of the healthy controls presented signs of parkinsonism. The severity of parkinsonism was assessed with the Movement Disorders Society (MDS) sponsored revision of the Unified Parkinson's Disease Rating Scale part III (MDS‐UPDRS‐III),23 the Hoehn and Yahr (H&Y) stage,24 and the Schwab and England scale (S&E)25 Nonmotor symptoms were assessed using the MDS‐UPDRS part I and specific validated questionnaires for each symptom. For evaluation of depression the Geriatric Depression Scale‐1526 or the Beck Depression Inventory‐II27 were used; dysautonomic symptoms were evaluated with the Scale for Autonomic Function (SCOPA‐AUT)28; olfaction was assessed using the Spanish version of the University of Pennsylvania Smell Test (UPSIT).29 The presence of RBD was based on the RBD screening questionnaire30 and the Epworth Sleepiness Scale31 was used to evaluate somnolence.
All LRRK2‐NMC consented to undergo a dopamine transporter (DaT) SPECT (123I‐ioflupane) in less than 30 days from the lumbar puncture. SPECT images were acquired in a dual‐headed gamma camera (E‐Cam, Siemens), 64 images/head, with a matrix of 64 × 64. SPECT images were classified visually by the local nuclear medicine team of the hospital nuclear medicine physicians as normal when a symmetric intense tracer uptake in striatum both caudate nucleus and putamen was observed. If a unilateral or bilateral reduction was observed in the striatal tracer uptake, the DAT‐SPECT was considered as abnormal.32 We also calculated the likelihood ratio and posttest probability for conversion to manifest PD for each LRRK2‐NMC, based on the MDS research criteria for prodromal PD as described elsewhere.33, 34 We included the available risk markers (age, sex, genetic status, and habits) and prodromal markers (subtle motor and nonmotor symptoms with the previously published cutoffs and DaT SPECT results) for each subject. LRRK2 carriers have been followed up prospectively and 12 of 16 NMC have undergone additional clinical evaluations during 2018.
CSF collection and handling
CSF collection protocol has been described elsewhere.35 Briefly, all the participants underwent the lumbar puncture after 8‐hour fast. Lumbar puncture was performed using 20 or 22G needle, after local anesthesia. CSF was collected into siliconized polypropilene tubes. 15–20 mL of CSF were collected at room temperature, centrifuged at 2000g for 10 min, then transferred to 1.5 mL precooled siliconized polypropilene aliquot tubes and immediately freezed on dry ice. The frozen aliquots were stored at −80°C, until March 2018, when thawed, aliquoted into coded 0.5–1 mL siliconized polypropilene tubes, refrozen and transferred on dry ice from Barcelona to the University of Edinburgh (Scotland, UK) for the study described here.
Real‐time quaking‐induced aggregation for alpha‐synuclein
The CSF samples were analyzed and reported without prior knowledge of the clinical diagnosis and genetic status of the donor. The RT‐QuIC reaction buffer (RB) was composed of 100 mmol/L phosphate buffer (pH 8.2), 10 μmol/L Thioflavin T (ThT) and 0.1 mg/mL human recombinant full‐length (1–140aa) a‐syn (Stratech, Cambridge, UK). Each well of a black 96‐well plate with a clear bottom (Nalgene Nunc International, Fisher Scientific Ltd, UK) contained 98, 90, or 85 μL RB (depending on volume of seed added) and 37 ± 3 mg of 0.5 mm zirconium/silica beads (Thistle Scientific Ltd, Glasgow, UK). Reactions were seeded with 2 μL of working strength brain homogenate, 15 μL of undiluted CSF to a final reaction volume of 100 μL. The plates were sealed with a plate sealer film (Fisher Scientific Ltd, UK) and incubated in a BMG OPTIMA FluoSTAR plate reader at 30°C for 120 h with intermittent shaking cycles: double orbital with 1 min shake (200 rpm), 14 min rest. ThT fluorescence measurements (450 nm excitation and 480 nm emission) were taken every 15 min. Each sample was run in duplicate. A positive response was defined as a relative fluorescence unit (rfu) value of >2SD above the mean of the negative controls at 120 h in both of the CSF duplicates. If only one of two CSF sample replicates gave a positive response, the RT‐QuIC analysis of the CSF samples was repeated in quadruplicate. A positive response in two or more of the replicates was considered positive. If only one of the replicates was positive, the RT‐QuIC was considered to be negative. The final fluorescence value was the mean fluorescence value taken at 120 h. The maximal fluorescence value was the highest mean fluorescent value seen during the a‐syn RT‐QuIC analytical run of 120 h. A measure of the lag‐phase was taken to be the time it took to get to 50% aggregation as measured by a rfu value of 44,000.
Statistical analysis
Qualitative variables were described by absolute and relative frequencies (%) and analyzed by the Fisher exact test. Quantitative variables were described by median and interquartile range (25th and 75th percentiles) and analyzed using the Kruskal–Wallis test for overall comparisons and the Mann–Whitney U test for pairwise comparisons.
Results
Compared to IPD, LRRK2‐PD subjects were younger (55 [48–64] years vs. 63.5 [57.5–70] years; P = 0.007), age at onset of motor symptoms was earlier (49 [44–57] years vs. 57.5 [50–62]; P = 0.03) and disease duration was shorter (5 [3–6] vs. 8 [5.5–8]; P = 0.01). There were no differences in the motor features, nonmotor symptoms or disease severity between LRRK2‐PD and IPD. The LRRK2‐NMC group was younger than the control group (51.5 [38.5–64] vs. 68.5 [59.5–72.2]; P = 0.01).
After decoding the CSF samples, the results showed that nine of 10 subjects in the IPD group and two of 10 in the control group were positive for RT‐QuIC reaction, obtaining a sensitivity of the test of a 90%, and a specificity of 80%. Six of 15 LRRK2‐PD subjects had positive results resulting in a sensitivity of 40% and three of 16 LRRK2‐NMC (18.8%) also tested positive (Table 1). Of the six LRRK2 patients that were positive, three had lag‐phases and final fluorescence values that were indistinguishable from the majority of the IPD group with the remaining three patients having lag‐phases that were longer and final fluorescence value that were less than the majority of the IPD group (Fig. 1). None of the LRRK2 mutation carriers with a positive RT‐QuIC result were related. Two of the three LRRK2‐NMC with a positive RT‐QuIC had LRRK2‐PD relatives with a negative RT‐QuIC result.
Table 1.
Prevalence of CSF positive a‐syn RT‐QuIC test, lag‐phase and final fluorescence values in IPD, LRRK2‐PD, LRRK2‐NMC, and healthy controls
| Subject group (n) | Positive RT‐QuIC [% (n)] | Lag‐phase in positive RT‐QuIC (hours)b | Final fluorescence in positive RT‐QuIC (rfua)b |
|---|---|---|---|
| IPD (10) | 90 (9) | 132 ± 20 | 60955 ± 7685 |
| LRRK2‐PD (15) | 40 (6) | 151 ± 33 | 50402 ± 13862 |
| LRRK2‐NMC (16) | 18.8 (3) | 125 ± 51 | 41228 ± 21852 |
| HC (10) | 20 (2) | 120 ± 40 | 49016 ± 17578 |
CSF, cerebrospinal fluid. IPD, idiopathic Parkinson's disease. LRRK2‐PD, Leucine‐rich repeat kinase 2 Parkinson's disease; LRRK2‐NMC, Leucine‐rich repeat kinase 2 nonmanifesting carriers; HC, Healthy controls.
rfu, relative fluorescence unit.
Mean ± SD.
Figure 1.
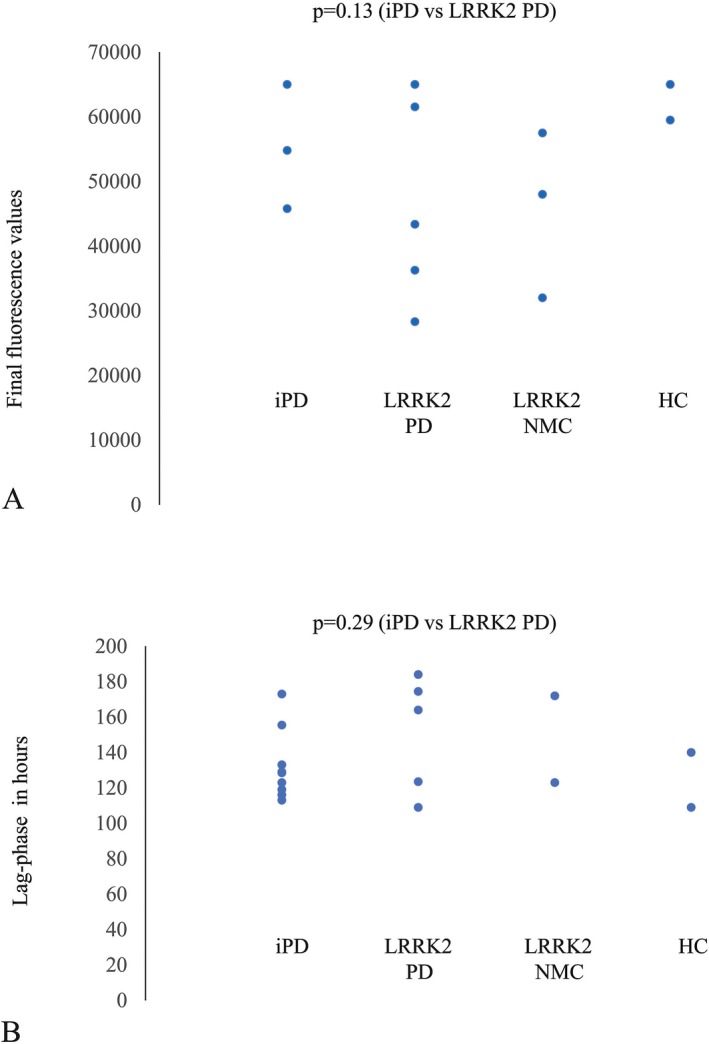
Final fluorescence values (A) and Lag‐phase (B) of positive a‐syn RT‐QuIC subjects in IPD, LRRK2‐PD, LRRK2‐NMC and healthy controls (HC). All but two of the IPD patients with positive RT‐QuIC had final fluorescence values of 65,000, with the remaining two patients having values of 54,801 and 45,792, respectively. The seven IPD that had final fluorescent values of 65,000 are represented by a single point on Figure 1A. Among the LRRK2‐PD patients with positive RT‐QuIC two patients had a final fluorescence value of 65000 and appear as a single point on Figure 1A. One LRRK2‐PD patient and one LRRK2‐NMC with positive RT‐QuIC had unmeasurable lag‐phases as the final fluorescence values were less than 44,000 in both duplicates and are not represented in Figure 1B.
The medical records of the IPD subject with a negative result were subsequently reviewed. This subject's motor symptoms started at the age of 50 years, with asymmetric rest tremor and bradykinesia. However, he has atypical features like poor levodopa response, absence of motor fluctuations or levodopa‐induced dyskinesias, and development of prominent lateralized trunkal dystonia (Pisa syndrome). He has bilaterally reduced striatal DaT binding and a normal brain magnetic resonance. One of the two control subjects with positive RT‐QuIC reaction has developed an action tremor of the hands and was diagnosed of having an action tremor of unknown cause 2 years after his participation in the study, and presents now with cognitive complains. The rest of control subjects have not developed symptoms or signs suggestive of a neurodegenerative disease.
No statistically significant differences were observed on MDS‐UPDRS part I, II, and III scores, H&Y stage, S&E and UPSIT scores between LRRK2‐PD with positive or negative RT‐QuIC tests (Table 2) and LRRK2‐NMC with positive and negative RT‐QuIC result (Table 3). Two LRRK2‐NMC, both with a negative RT‐QuIC result, reported to have vivid dreams and mobilization while sleeping, but no video‐polisomnography was performed. Five of the 16 LRRK2‐NMC had abnormal UPSIT scores after adjusting for age and gender; of these two had positive and three negative RT‐QuIC results. Two of three subjects with positive RT‐QuIC result (66.7%) had an abnormal DaT‐SPECT, whereas only two of 13 (15.4%) who were negative had altered striatal DaT binding (Table 3). Three NMC, all with an altered DaT SPECT met MDS criteria for probable prodromal PD (>80% of threshold); two had positive a‐syn RT‐QuIC result.
Table 2.
Nonmotor symptoms of LRRK2 Parkinson's disease patients
| Sex | Positive RT‐QuIC | Negative RT‐QuIC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | M | M | M | M | M | F | F | M | F | F | F | F | M | F | |
| Age | 51 | 38 | 51 | 59 | 50 | 64 | 45 | 44 | 55 | 68 | 68 | 57 | 48 | 64 | 60 |
| Cognitive impairmenta | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Depressiona | 2 | 0 | 1 | 0 | 3 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Urinary problemsa | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Constipation problemsa | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 3 |
| REM sleep behavior disorderb | No | No | No | No | No | Yes | No | No | No | No | No | No | Yes | No | No |
| Hyposmiac | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
MDS, International Parkinson's disease and Movement Disorders Society.
International Parkinson's disease and Movement Disorders Society Unified Parkinson's Disease Rating Scale part I scores: 0 = normal; 1 = slight, 2 = mild, 3 = moderate, 4 = severe (Goetz et al. 2008).
Based on the REM sleep behavior questionnaire (Stiasny‐Kolster et al. 2007).
Based on the University of Pennsylvania Smell Test normative values (Doty et al. 1995).
Table 3.
Clinical characteristics and MDS prodromal criteria likelihood ratio of LRRK2 nonmanifesting carriers
| Sex | Positive RT‐QuIC | Negative RT‐QuIC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | M | M | M | F | M | M | F | M | M | F | F | M | M | |
| Age | 64 | 47 | 52 | 40 | 64 | 38 | 61 | 27 | 71 | 60 | 28 | 37 | 43 | 51 | 68 | 72 |
| Cognitive impairmenta | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Depressiona | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Bladder symptomsa | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipationa | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| RBDb | No | No | No | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No |
| Hyposmiac | Yes | No | Yes | No | No | No | No | Yes | No | Yes | No | No | No | No | Yes | No |
| Abnormal DaT‐SPECT | Yes | No | Yes | Yes | No | No | No | No | Yes | No | No | No | No | No | No | No |
| Meets criteria for prodromal PDd | Yes | No | Yes | No | No | No | No | No | Yes | No | No | No | No | No | No | No |
| LR for probable prodromal PD PD† | 947.6 | 2.9 | 1076.8 | 127.3 | 2.3 | 2.9 | 1.3 | 29.5 | 289.3 | 16.2 | 0.74 | 0.74 | 9.2 | 5.42 | 174.7 | 5.2 |
RBD, REM sleep behavior disorder; MDS, International Parkinson's disease and Movement Disorders Society; PD, Parkinson's disease; LR, likelihood ratio.
International Parkinson's disease and Movement Disorders Society Unified Parkinson's Disease Rating Scale part I scores: 0 = normal; 1 = slight, 2 = mild, 3 = moderate, 4 = severe (Goetz et al. 2008).
Based on the REM sleep behavior questionnaire (Stiasny‐Kolster et al. 2007).
Based on the University of Pennsylvania Smell Test normative values (Doty et al. 1995).
The subject meets the probability threshold of >80% for probable prodromal PD based on the MDS research criteria for prodromal PD (Berg et al. 2015, Mirelman et al. 2018).
Total likelihood ratio based on the MDS Research Criteria for prodromal PD (Berg et al. 2015, Mirelman et al. 2018). Missing values were not imputed (LR = 1).
Twelve of 16 LRRK2‐NMC, including the three positive RT‐QuiC cases, were clinically re‐evaluated during 2018, after a mean of 36.2 months (s = 21.6 months) of follow‐up, and none of them has motor symptoms suggestive of clinical PD. Only one LRRK2‐NMC has mild bradykinesia on the evaluation after 31 months. This subject has negative RT‐QuIC, but an abnormal nigrostriatal function on the DaT‐SPECT at the time of CSF collection, and meets the criteria for probable prodromal PD.
Discussion
Our study confirms that a‐syn aggregation in CSF using RTQuIC identifies IPD with high specificity (90%) and sensitivity (80%). Positive a‐syn aggregation in the CSF occurred in a significant number of patients with LRRK2‐PD p.G2019S but less frequently than in patients with IPD.
Based on our results in IPD and previously published data referred above16 it seems safe to presume that our LRRK2‐PD subjects with a positive RTQuIC result have an underlying LTS. The percentage of positive subjects among the LRRK2‐PD group is lower than in IPD and lower than expected since a majority of LRRK2 p.G2019S cases are thought to have LTS.6 This lower percentage of positive RT‐QuIC in the LRRK2 cases could reflect the reported neuropathological variability in LRRK2‐PD p.G2019S. RT‐QuIC negative cases may not have LTS but an alternative neuropathological substrate. Nonspecific degeneration of the substantia nigra without abnormal protein deposits has been described in LRRK2‐PD p.G2019S and still in other cases ubiquitin or tau inclusions constitute the only abnormality.36, 37, 38, 39 Studies with larger cohorts of prospective brains of LRRK2 mutation carriers have not been performed and the current assumption that the majority of LRRK2 p.G2019S have a synucleinopathy is based on limited case reports and screens from LB specimens in brain banks,8 being possibly confounded by a selection bias.
It is also possible that CSF a‐syn seeds in LRRK2‐PD patients differ from those with IPD in their potency to induce fibril formation and this could be altering the RT‐QuIC result. In our LRRK2‐PD cases with a positive RT‐QuIC, three subjects had longer lag‐time phases and lesser final fluorescence values compared to IPD (Fig. 1) supporting this hypothesis. Mamais et al.40 have shown distinct biochemical properties of aggregated a‐syn in LRRK2‐PD p.G2019S compared to IPD, detecting a lower percentage of highly insoluble a‐syn species in LRRK2‐PD brain homogenates. Diversity in the structure and self‐aggregation potency of a‐syn could contribute to variability in RT‐QuIC quantification and even account for a negative result in some cases. Another possible explanation for a negative test in the presence of LTS could be that the negative LRRK2‐PD cases presented less a‐syn burden, insufficient to induce significant aggregation. So far, though, the studied LRRK2‐PD brains with LTS follow the staging scheme of Braak,41 and in studies comparing LRRK2‐PD to IPD histopathology no differences in Lewy body and neurite distribution have been observed.40
In LRRK2‐NMC RT‐QuIC CSF positivity occurred in three of the 15 (18.8%) subjects suggesting an ongoing synucleinopathy in these cases. Two LRRK2‐NMC with a positive result had a relative with manifest LRRK‐PD who had a negative RT‐QuIC result. Variable neuropathological substrates, even in the same kindreds as has been well documented to occur in cases with other pathogenic LRRK2 mutations,42 could explain this finding. A decline in DaT‐SPECT (123I‐ioflupane) binding has been previously suggested to be a useful tool to predict phenoconversion in LRRK2‐NMC.43 In our study two of three (66.7%) LRRK2‐NMC with a positive RTQuIC had an abnormal DaT‐SPECT result, whereas this occurred only in two of 13 (15.3%) of the negative ones. The greater frequency of altered DaT‐SPECT uptake among the RTQuIC‐positive cases is in line with the interpretation that these asymptomatic carriers are possibly those that will eventually evolve into manifest PD. The higher proportion of probable prodromal PD based on the MDS prodromal criteria33, 34 in those subjects with a positive RTQuIC reaction, also supports this hypothesis.
We found a positive RT‐QuIC test in two healthy controls. One of these individuals has developed an action tremor of the hands since the time of CSF acquisition and may be harboring a neurodegenerative disorder. The presence of phosphorylated a‐syn aggregates in the nervous system is not exceptional in living individuals without neurological diseases.44, 45, 46 These cases may not represent false‐positive cases but rather possibly be cases of incidental Lewy body disease. Postmortem studies in the brain show LTS in about 15–20% of elder population without parkinsonism or dementia during life.22, 47, 48
A major caveat of our study is the lack of neuropathological studies to establish a correlation between RT‐QuIC result and histological diagnosis. An additional limitation of our study is the small number of subjects studied, precluding generalization of the results. Still the good sensitivity and specificity found in the IPD group, practically identical to those encountered by others using similar techniques16, 19 supports the validity of our data. Further RT‐QuIC studies with greater number of LRRK2 mutation carriers, sufficient longitudinal follow‐up to assess phenoconversion in the asymptomatic carriers and, eventually, postmortem or in vivo49 studies to define the type of pathological lesions occurring in these subjects will be needed to validate our observation.
In conclusion, our results show that RT‐QuIC methodology detects a‐syn aggregation in CSF in a significant number of patients with LRRK2‐PD p.G2019S but less frequently than in patient with IPD. In our cohort of LRRK2‐NMC, almost 20% of subjects were positive for RT‐QuIC suggesting that a positive test in LRRK2‐NMC may possibly identify those at major risk to eventually develop parkinsonism. The high sensitivity and specificity of the results in IPD patients in this and previous studies support the notion that the presence of a‐syn aggregation in CSF detected by RT‐QuIC or similar a‐syn amplification methods, if appropriately validated, could contribute to the selection of candidates to receive disease modifying drugs, particularly treatments targeting a‐syn deposition.
Authors Contributions
E.Tolosa designed the study; A.Garrido, and A.Green contributed to the design of the study. G Fairfoul performed the experiments, A Green analyzed the RT‐QuIC data. A.Garrido, E. Tolosa, MJ. Marti and A. Green interpreted the results. A. Garrido and E. Tolosa and A. Green wrote the manuscript. All authors reviewed and commented on the manuscript.
Conflicts of Interest
Alicia Garrido has no competing interests.
Alison Green has no competing interests.
Graham Fairfoul has no competing interests.
Eduardo Tolosa has no competing interests.
Maria Jose Marti has not competing interests.
Acknowledgments
We thank the patients and their relatives for their participation in this study. We thank the Barcelona LRRK2 Study Group members for their contribution:
Yaroslau Compta, Francesc Valldeoriola, Esteban Muñoz, Manel Fernandez (Parkinson's Disease and Movement Disorders Unit, Institut Clínic de Neurociències, Hospital Clinic de Barcelona, Barcelona, Spain), Ramiro Álvarez, Dolores Vilas, Lourdes Ispierto (Neurology Service, Hospital Universitari Germans Trias i Pujol Badalona, Barcelona, Spain), Oriol De Fàbregues, Jorge Hernández‐Vara (Neurology Service, Hospital Universitari Vall D'Hebron, Barcelona, Spain), Víctor Puente (Neurology Service, Hospital Del Mar, Barcelona, Spain), Matilde Calopa, Serge Jauma, Jaume Campdelacreu (Neurology Service, Hospital Universitari de Bellvitge, Barcelona, Spain), Angels Bayés (Parkinson's Group, Clínica Teknon, Barcelona, Spain), Asunción Ávila, Nuria Caballol (Neurology Service, Hospital de Sant Joan d'Espí ‐ Moises Broggi, Sant Joan d'Espí, Barcelona, Spain), Miquel Aguilar (Neurology Service, Hospital Universitari Mutua de Terrasa, Barcelona, Spain), Pilar Casquero (Hospital Mateu Orfila, Maó, Menorca, Islas Baleares, Spain).
References
- 1. Trinh J, Zeldenrust FMJ, Huang J, et al. Genotype‐phenotype relations for the Parkinson's disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord 2018;33:1857–1870. 10.1002/mds.27527. [DOI] [PubMed] [Google Scholar]
- 2. Healy DG, Falchi M, O'Sullivan SS, et al. ; International LRRK2 Consortium . Phenotype, genotype, and worldwide genetic penetrance of LRRK2‐associated Parkinson's disease: a case‐control study. Lancet Neurol 2008;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hentati F, Trinh J, Thompson C, et al. LRRK2 parkinsonism in Tunisia and Norway: a comparative analysis of disease penetrance. Neurology 2014;83:568–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee AJ, Wang Y, Alcalay RN, et al. ; Michael J. Fox LRRK2 Cohort Consortium . Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non‐Ashkenazi Jewish ancestry. Mov Disord 2017;32:1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaig C, Ezquerra M, Marti MJ, et al. LRRK2 mutations in Spanish patients with Parkinson disease: frequency, clinical features, and incomplete penetrance. Arch Neurol 2006;63:377–382. [DOI] [PubMed] [Google Scholar]
- 6. Schneider SA, Alcalay RN. Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord 2017;32:1504–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson's disease. Int J Clin Exp Pathol 2008;1:217–231. [PMC free article] [PubMed] [Google Scholar]
- 8. Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson's disease. Mov Disord 2012;27:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalia LV, Lang AE, Hazrati LN, et al. Clinical correlations with Lewy body pathology in LRRK2‐related Parkinson disease. JAMA Neurol 2015;72:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajput A, Dickson DW, Robinson CA, et al. Parkinsonism, LRRK2 G2019S, and tau neuropathology. Neurology 2006;67:1506–1508. [DOI] [PubMed] [Google Scholar]
- 11. Giasson BI, Covy JP, Bonini NM, et al. Biochemical and pathological characterization of LRRK2. Ann Neurol 2006;59:315–322. [DOI] [PubMed] [Google Scholar]
- 12. Shi M, Bradner J, Hancock AM. Cerebrospinal fluid biomarkers for Parkinson's disease diagnosis and progression. Ann Neurol 2011;69:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mollenhauer M, Cullen V, Kahn I, et al. Direct quantification of CSF alpha‐synuclein by ELISA and first cross‐sectional study in patients with neurodegeneration. Exp Neurol 2008;213:315–325. [DOI] [PubMed] [Google Scholar]
- 14. Tokuda T, Qureshi MM, Ardah MT, et al. Detection of elevated levels of α‐synuclein oligomers in CSF from patients with Parkinson disease. Neurology 2010;75:1766–1772. [DOI] [PubMed] [Google Scholar]
- 15. Williams SM, Schult P, Sierks MR. Oligomeric a‐synuclein an b‐amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases. Eur J Neurosci 2016;43:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fairfoul G, McGuire LI, Pal S, et al. Alpha‐synuclein RT‐QuIC in the CSF of patients with alpha‐synucleinopathies. Ann Clin Transl Neurol 2016;3:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron‐to‐neuron transmission of alpha‐synuclein. Proc Natl Acad Sci U S A 2009;106:13005–13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luk KC, Kehm V, Carroll J, et al. Pathological α‐synuclein transmission initiates Parkinson‐like neurodegeneration in nontransgenic mice. Science 2012;338:949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahnawaz M, Tokuda T, Waragai M, et al. Development of a biochemical diagnosis of Parkinson disease by detection of α‐synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 2017;74:163–172. [DOI] [PubMed] [Google Scholar]
- 20. Dauvilliers Y, Schenck CH, Postuma RB, et al. REM sleep behaviour disorder. Nat Rev Dis Primers 2018;4:19. [DOI] [PubMed] [Google Scholar]
- 21. Simon‐Sanchez J, Marti‐Masso JF, Sanchez‐Mut JV, et al. Parkinson's disease due to the R1441G mutation in Dardarin: a founder effect in the Basques. Mov Disord 2006;21:1954–1959. [DOI] [PubMed] [Google Scholar]
- 22. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 24. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 25. Schwab JF, England AC. Projection technique for evaluating surgery in Parkinson's disease In: Gillingham F. J., Donaldson M. C., eds. Proceedings of the third symposium on Parkinson's disease. pp. 152–157. Edinburgh, UK: Churchill Livingstone, 1969. [Google Scholar]
- 26. Meara J, Mitchelmore E, Hubson P. Use of the GDS‐15 geriatric depression scale as screening instrument for depressive symptomatology in patients with Parkinson's disease and their carers in the community. Age Ageing 1999;28:35–38. [DOI] [PubMed] [Google Scholar]
- 27. Steer RA, Rissmiller DJ, Beck AT. Use of the Beck Depression Inventory‐II with depressed geriatric inpatients. Behav Res Ther 2000;30:311–318. [DOI] [PubMed] [Google Scholar]
- 28. Visser M, Marinus J, Stiggelbount AM, Van Hilten JJ. Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA‐AUT. Mov Disord 2004;19:1306–1312. [DOI] [PubMed] [Google Scholar]
- 29. Doty RL, Bromley SM, Stern MB. Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration 1995;4:93–97. [DOI] [PubMed] [Google Scholar]
- 30. Stiasny‐Kolster K, Mayer G, Schafer S, et al. The REM sleep behavior disorder screening questionnaire a new diagnostic instrument. Mov Disord 2007;22:2386–2393. [DOI] [PubMed] [Google Scholar]
- 31. Hagell P, Broman JE. Measurment properties and hierarchical item structure of the Epoworth Slepiness Scale in Parkinson's disease. J Sleep Res 2007;16:102–109. [DOI] [PubMed] [Google Scholar]
- 32. Benamer TS, Patterson J, Grosset DG, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]‐FP‐CIT SPECT imaging: the [123I]‐FP‐CIT study group. Mov Disord 2000;15:503–510. [PubMed] [Google Scholar]
- 33. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord 2015;30:1600–1611. [DOI] [PubMed] [Google Scholar]
- 34. Mirelman A, Saunders‐Pullman R, Alcalay R, et al. Application of the movement disorder society prodromal criteria in healthy G2019S‐LRRK2 carriers. Mov Disord 2018;33:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vilas D, Shaw LM, Taylor P, et al. Cerebrospinal fluid biomarkers and clinical features in leucine‐rich repeat kinase 2 (LRRK2) mutation carriers. Mov Disord 2016;31:906–914. [DOI] [PubMed] [Google Scholar]
- 36. Gaig C, Martí MJ, Ezquerra M, et al. G2019S LRRK2 mutation causing Parkinson's disease without Lewy bodies. J Neurol Neurosurg Psychiatry 2007;78:6286–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ling H, Kara E, Bandopadhyay R, et al. TDP‐43 pathology in a patient carrying G2019S LRRK2 mutation and a novel p.Q124E MAPT. Neurobiol Aging. 2013;34:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruffmann C, Giaccone G, Canesi M, et al. Atypical tauopathy in a patient with LRRK2‐G2019S mutation and tremor‐dominant Parkinsonism. Neuropathol Appl Neurobiol 2012;38:382–386. [DOI] [PubMed] [Google Scholar]
- 39. Vilas D, Sharp M, Gelpi E, et al. Clinical and neuropathological features of progressive supranuclear palsy in Leucine rich repeat kinase (LRRK2) G2019S mutation carriers. Mov Disord 2018;33:335–338. [DOI] [PubMed] [Google Scholar]
- 40. Mamais A, Raja M, Manzoni C, et al. Divergent α‐synuclein solubility and aggregation properties in G2019S LRRK2 Parkinson's disease brains with Lewy body pathology compared to idiopathic cases. Neurobiol Dis 2013;58:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 42. Hasegawa K, Stoessl AJ, Yokoyama T, et al. Familial parkinsonism: study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Parkinsonism Relat Disord 2009;15:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sierra M, Martínez‐Rodríguez I, Sánchez‐Juan P, et al. Prospective clinical and DaT‐SPECT imaging in premotor LRRK2 G2019S‐associated Parkinson disease. Neurology 2017;89:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minguez‐Castellanos A, Chamorro CE, Escamilla‐Sevilla F, et al. Do alpha‐synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology 2007;68:2012–2018. [DOI] [PubMed] [Google Scholar]
- 45. Navarro‐Otano J, Navarro‐Otano J, Gelpi E, et al. Alpha‐synuclein aggregates in epicardial fat tissue in living subjects without parkinsonism. Parkinsonism Relat Disord 2013;19:27–31. [DOI] [PubMed] [Google Scholar]
- 46. Sánchez‐Ferro Á, Rábano A, Catalán MJ, et al. In vivo gastric detection of α‐synuclein inclusions in Parkinson's disease. Mov Disord 2015;30:517–524. [DOI] [PubMed] [Google Scholar]
- 47. Wakisaka Y, Furuta A, Tanizaki Y, et al. Age‐associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol 2003;106:374–382. [DOI] [PubMed] [Google Scholar]
- 48. Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 2009;68:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vilas D, Iranzo A, Tolosa E, et al. Assessment of α‐synuclein in submandibular glands of patients with idiopathic rapid‐eye‐movement sleep behaviour disorder: a case‐control study. Lancet Neurol 2016;15:708–718. [DOI] [PubMed] [Google Scholar]


