Abstract
Chronic kidney disease (CKD) is a major cause of morbidity and premature mortality and represents a significant global public health issue. Underlying this burden are the many complications of CKD, including mineral and bone disorders, anemia, and accelerated cardiovascular disease. Hyperphosphatemia and elevated levels of fibroblast growth factor 23 (FGF23) have been identified as key independent risk factors for the adverse cardiovascular outcomes that frequently occur in patients with CKD. Auryxia® (ferric citrate; Keryx Biopharmaceuticals, Inc., Boston, MA, USA) is an iron-based compound with distinctive chemical characteristics and a mechanism of action that render it dually effective as a therapy in patients with CKD; it has been approved as a phosphate binder for the control of serum phosphate levels in adult CKD patients treated with dialysis and as an iron replacement product for the treatment of iron deficiency anemia in adult CKD patients not treated with dialysis. This review focuses on Auryxia, its mechanism of action, and the clinical attributes that differentiate it from other, non-pharmaceutical-grade, commercially available forms of ferric citrate and from other commonly used phosphate binder and iron supplement therapies for patients with CKD. Consistent with the chemistry and mechanism of action of Auryxia, multiple clinical studies have demonstrated its efficacy in both lowering serum phosphate levels and improving iron parameters in patients with CKD. Levels of FGF23 decrease significantly with Auryxia treatment, but the effects associated with the cardiovascular system remain to be evaluated in longer-term studies.
Key Points
| Patients with chronic kidney disease (CKD) frequently have high levels of phosphate and low levels of iron in the blood, both of which are associated with elevated risks of cardiovascular disease and death. |
| The drug Auryxia (ferric citrate) has a special formulation that enables it to both increase iron levels in patients with CKD who are not receiving dialysis and decrease phosphate levels in patients with CKD who are receiving dialysis. |
Introduction
Chronic kidney disease (CKD) represents a major global public health concern [1]. The United States Renal Data System estimated that in 2017, 30 million American adults had CKD [2]. CKD is associated with serious comorbidities, most importantly cardiovascular disease [1, 2]. Furthermore, the financial burden of CKD is high; in the USA, with total Medicare expenditures exceeding $64 billion in 2015 [2]. Magnifying this burden is the undertreatment of CKD [3], especially in the earlier stages of the disease, highlighting the need for better awareness of all therapeutic options with regard to their efficacy, safety, tolerability, and cost effectiveness.
Perturbations of Bone and Mineral Metabolism and Iron Parameters in Chronic Kidney Disease
Alterations of bone and mineral metabolism and anemia are common and occur early in the course of CKD and when left untreated carry an increased risk for adverse outcomes [4, 5]. Approximately 36% of hemodialysis patients in the USA have hyperphosphatemia (when defined as a phosphate concentration > 5.5 mg/dL) [6], although older surveys cite higher percentages [7–9]. Hyperphosphatemia is an independent risk factor for cardiovascular events, and has been shown to be associated with a higher mortality rate [10, 11]; however, it is important to note that the evidence supporting the association between phosphorus levels and mortality is derived from observational studies, which are potentially subject to confounding. A National Health and Nutrition Examination Survey estimated that 15.4% of patients with CKD in the USA are anemic, defined as having blood hemoglobin levels ≤ 12 g/dL in women and ≤ 13 g/dL in men, representing 4.8 million affected individuals [4]. As with hyperphosphatemia, patients with CKD who also have anemia are at increased risk of cardiovascular disease and death, among other comorbidities [4]. It is expected that, in theory, successful treatment of hyperphosphatemia and anemia in patients with CKD could help avoid adverse clinical outcomes; however, with the exception of renal replacement, no interventions have yet been proven to improve outcomes.
The pathophysiologic networks that regulate bone and mineral metabolism and iron distribution are complex. An increasing number of studies support the idea that fibroblast growth factor 23 (FGF23), a key phosphate-regulating hormone, is associated with adverse outcomes in CKD [12]. Along with parathyroid hormone (PTH), FGF23 is an important factor involved in the disordered bone and mineral metabolism that contributes to CKD morbidity and mortality [5, 12]. Normally, FGF23 regulates phosphate and vitamin D metabolism through a negative endocrine feedback loop it shares with PTH; however, production of 1,25-dihydroxyvitamin D3 is inhibited by FGF23 but stimulated by PTH, whereas both FGF23 and PTH share the phosphaturic effect [12–14]. In patients with early CKD, as renal function begins to decrease, compensatory increases in FGF23 and PTH may help maintain normal phosphate balance, mainly by stimulating greater urinary phosphate excretion [13, 15]. These increases in FGF23 and PTH in early CKD precede frank hyperphosphatemia, which is observed only with advanced renal disease after the compensatory mechanisms have been overwhelmed [13, 15]. In a cross-sectional study of patients representing a spectrum of CKD severity, increased FGF23 levels were significantly associated with deteriorating renal function [15]. For reasons that are not yet clear, FGF23 also may be related to the development of iron deficiency anemia; in a recent cohort study, elevated concentrations of FGF23 were associated with a higher incidence of anemia, particularly in patients with iron deficiency [16]. Importantly, increased serum FGF23 concentrations are associated with increased risk of cardiovascular events and death in CKD [12]. Several studies have highlighted the relationships of inflammation and iron deficiency anemia, both commonly present in CKD, with FGF23 levels that rise early in the course of the disease [17–19]. Thus, interventions that decrease levels of FGF23 may favorably affect several important outcomes in patients in CKD, although this has yet to be established in long-term clinical studies.
Treatment of Hyperphosphatemia and Iron Deficiency Anemia in Chronic Kidney Disease
With the high prevalence of hyperphosphatemia among hemodialysis patients in the USA, restoration of phosphate balance using phosphate binders has long been a therapeutic goal in CKD management [7–9]. In the 1970s to 1990s, aluminum-based and then calcium-based phosphate binders were often used for the control of hyperphosphatemia in patients with end-stage renal disease [7, 20]. However, associations with aluminum toxicity for aluminum-based binders and hypercalcemia and metastatic calcification for calcium-based binders motivated the development of calcium-free, aluminum-free phosphate binders [7, 20–22]. In fact, the Kidney Disease: Improving Global Outcomes (KDIGO) 2017 guidelines for CKD-mineral bone disorder recommend restricting the dose of calcium-based phosphate binders in adult patients with stage 3–5 CKD receiving phosphate-lowering treatment [23]. However, the Dialysis Clinical Outcomes Revisited trial, which compared mortality among hemodialysis patients treated with calcium-based phosphate binders and sevelamer hydrochloride, failed to demonstrate the superiority of the non-calcium-containing sevelamer hydrochloride with respect to death and hospitalization rates [24]. Ultimately, improvements in clinical outcomes in patients with hyperphosphatemia may not be achieved by improving phosphate balance alone.
Iron deficiency anemia in CKD has long been treated with iron supplementation using either oral or intravenous (IV) therapy [25]. The decision between oral and IV iron should involve an assessment of benefit and risk. Most oral iron formulations may be less expensive but are often associated with adverse gastrointestinal (GI) effects, whereas IV iron may be more effective than oral iron but carries the risks of adverse reactions during IV administration and potential for long-term iron overload [25–28]. IV administration may be the preferred route of administration of iron for hemodialysis patients partly because it is easily administered during hemodialysis; however, this is not necessarily true for patients that are not on dialysis (or are on peritoneal dialysis) [29].
Auryxia: an Iron-Based Treatment for Hyperphosphatemia and Iron Deficiency Anemia in Chronic Kidney Disease
Iron-based compounds, such as ferric citrate (Auryxia®; Keryx Biopharmaceuticals, Inc., Boston, MA, USA), represent a new category of phosphate binders [30]. Auryxia has the advantage of dual functionality (i.e. controlling serum phosphate levels and treating iron deficiency anemia) [31]. In the USA, Auryxia is indicated as a phosphate binder for the control of serum phosphate levels in adult patients with CKD treated with dialysis and as an iron replacement product for the treatment of iron deficiency anemia in adult patients with CKD not on dialysis [31]. Similar ferric citrate products are approved for use in other countries under different brand names. In the European Union, ferric citrate coordination complex (Fexeric® Keryx Biopharma UK Ltd, London, UK) is indicated for the control of hyperphosphatemia in adult patients with CKD [32]. In Japan, ferric citrate hydrate (Riona® Torii Pharmaceutical Co., Ltd., Tokyo, Japan) is indicated to treat hyperphosphatemia in patients with CKD [33, 34]. In Taiwan, ferric citrate (Nephoxil®, Panion & BF Biotech Inc., Taipei, Taiwan) is indicated for controlling hyperphosphatemia in adult patients with CKD undergoing hemodialysis [35]. This review focuses on Auryxia, its mechanism of action, and the clinical attributes that differentiate it from other, non-pharmaceutical-grade, commercially available forms of ferric citrate and from other commonly used phosphate binder and iron supplement therapies for patients with CKD.
Chemical Composition of the Active Pharmaceutical Ingredient (API) in Auryxia
The API of Auryxia is not a single compound but rather a solid mixture of ferric citrate coordination complexes (FCCCs) with the following chemical formula: iron (+ 3), x (anion of 1,2,3-propanetricarboxylic acid, 2-hydroxy-), y (H2O), where x ranges from 0.70 to 0.87 and y ranges from 1.9 to 3.3 [31, 36]. As opposed to iron salts, which readily dissociate into their component ions in water, the bonds that coordinate the central metal atom with the surrounding ligands allow the complex to retain its identity as a unit with properties different from those of its components. Furthermore, Auryxia has different properties from those of commercial-grade ferric citrate [36], which was the material investigated in early studies. Most important among these differences among ferric citrate preparations is that the API of Auryxia has a defined molar ratio of ferric iron (Fe3+) to citrate anions [37], whereas ferric citrate from commercial sources may have variable molar ratios of ferric iron, citric acid, and associated hydrates [36]. It is important to first establish what is known about ferric citrate in general before understanding more about the specific physicochemical profile of the pharmaceutical-grade ferric citrate that constitutes the API of Auryxia.
Structural Chemistry of Ferric Citrate
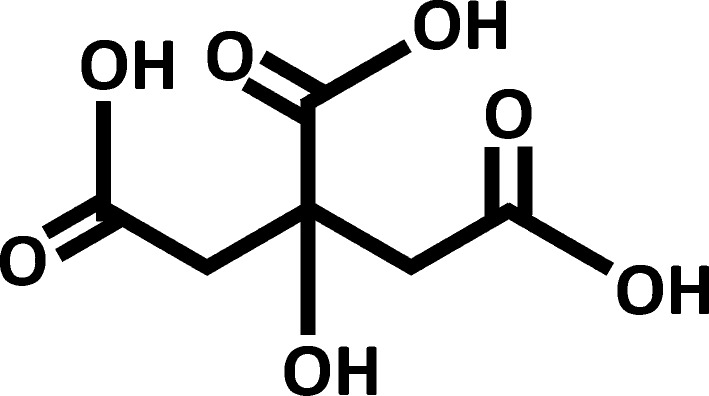
As already noted, “ferric citrate” is a common name for a large group of metallo-complexes comprising ferric ions (Fe3+) and citrate ligands with various degrees of protonation. These complexes are characterized by different iron nuclearities, ratios of iron to citrate, and ligand coordination modes. Naturally occurring ferric citrate complexes play an important role in iron solubilization, mobilization, and utilization in all forms of life [38]. Citric acid is an α-hydroxy tricarboxylic acid (Fig. 1), capable of binding ferric ions and forming a series of stable species in aqueous solution over a wide pH range [39]. This action prevents the hydrolysis of ferric ions that leads to the formation of insoluble ferric oxides and ferric hydroxides under physiological pH. It is known that iron levels in physiological (i.e. biological, living) systems are regulated by citric acid chelation of ferric ions or through redox reactions of ferric citrate [40, 41]. In medicine, it has long been known that citric acid enhances the bioavailability of iron [42], and several iron citrate preparations are commercially available for use as iron supplements in foods. Because ferric citrate can form a series of interrelated complexes, it is soluble over a broad range of pH in the stomach and intestine (where phosphate binding occurs) and in the duodenum (the primary site of oral iron absorption) [43].
Fig. 1.
Structure of citric acid
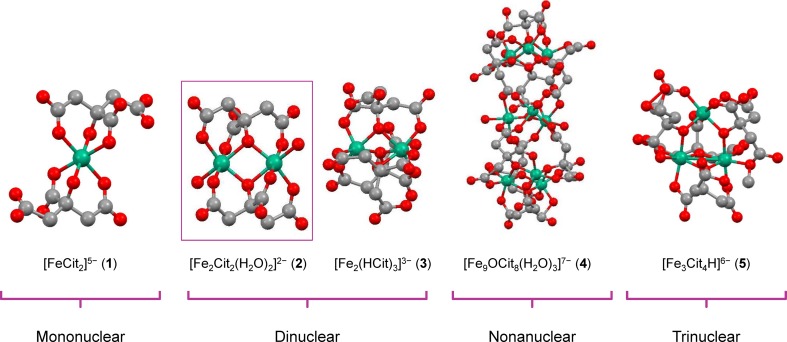
Although ferric citrate complexes have been investigated and used clinically as iron supplements [44], no structural data were available until 1994. High solubility in aqueous solution and the many diverse species of ferric citrate complexes had been the main obstacles to obtaining single crystals for structural elucidation by x-ray crystallography. By introducing a variety of cations into solutions prepared by mixing ferric salts and sodium citrate or citric acid in water, several crystalline compounds were obtained and subjected to structural studies. To date, the crystal structures of five ferric citrate complexes are known in the literature (Fig. 2); these consist of one mononuclear species, [FeCitrate2]5− (complex 1) [45]; two dinuclear species, [Fe2Citrate2(H2O)2]2− (complex 2) and [Fe2(HCitrate)3]3− (complex 3) [46]; one nonanuclear species, [Fe9OCitrate8(H2O)3]7− (complex 4) [47]; and one trinuclear species, [Fe3Citrate4H]6− (complex 5) [48]. Ferric citrate complex 2 appears to be particularly relevant to the physiologic interactions. The reports of Ferguson and coworkers from the group of Johann Deisenhofer in 2002, as well as Sauter and Braun in 2004, showed that the dinuclear complex 2 is recognized, absorbed, and transferred across membranes by the outer membrane transporter FecA in Escherichia coli, establishing the biological relevance of this species [49, 50]. Earlier work had shown the remarkable kinetic stability of complex 2, as demonstrated by the recrystallization of its pyridinium salt after the dissolution of the crude material in warm pure water; crystals usually grew slowly at room temperature over a period of approximately 3 to 6 days [46]. Such kinetic stability suggests that the complex may be stable in aqueous solution over long time frames. Later mass spectrometry experiments in aqueous solution likewise found a spectrum of complexes, among which again a dinuclear species was prominent [37].
Fig. 2.
Chemical structures of ferric citrate coordination complex anions determined by X-ray crystallography. Green-blue spheres represent iron atoms, red spheres represent oxygen atoms, and gray spheres represent carbon atoms. Cit citrate.
Based on data from Matzapetakis et al. [45]; Shweky et al. [46]; Bino et al. [47]; and Tenne et al. [45–48]
Ferric Citrate as API of Auryxia
The API of Auryxia has a defined molar ratio of ferric iron to citrate anions, predominantly in the molar ratio of 2:2, whereas ferric citrate from commercial sources may have variable molar ratios of ferric iron and citric acid [36]. The API in Auryxia is described in US patents 7,767,851; 8,338,642; 8,609,896 and 8,901,349 [51–54]; chemical analysis shows that this material contains a positively charged Fe(III) complex, presumably [Fe(H2O)6]3+, and a negatively charged complex, [Fe2Citrate2(H2O)2]2− [36]. The major component in the API corresponds to the dinuclear complex 2 (Fig. 2).
Unlike simple iron salts, the FCCCs in Auryxia help maintain ferric iron in solution at the varying conditions in different portions of the GI tract (i.e. at various pH levels) [36]. The relatively high solubility of the pharmaceutical-grade ferric citrate in Auryxia contrasts with some commercial iron salts, particularly at high pH; for example ferrous (Fe2+) sulfate, an iron supplement commonly used to treat iron deficiency anemia, is practically insoluble at high pH [36, 43, 44]. An additional distinguishing characteristic of the FCCCs in Auryxia is their large surface area, which is at least 16 times that of commercial-grade ferric citrate [51]. High surface area generally favors rapid disintegration and therefore dissolution [55]. In the case of Auryxia, the rate of dissolution of its API at pH 8 is 3.08 times the rate of commercial-grade ferric citrate; the solubility of the Auryxia FCCCs is vital to the absorption of their iron [36, 51].
Absorption of Iron from Auryxia
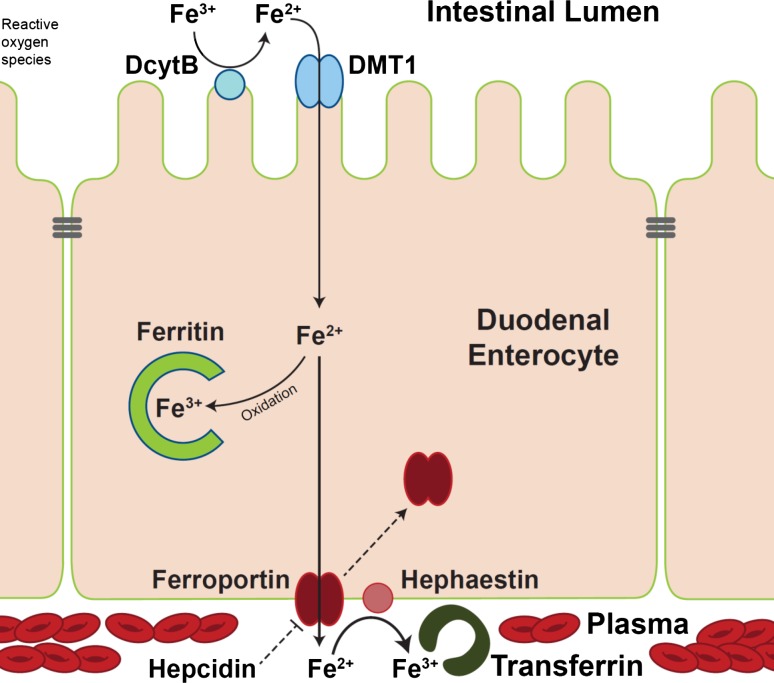
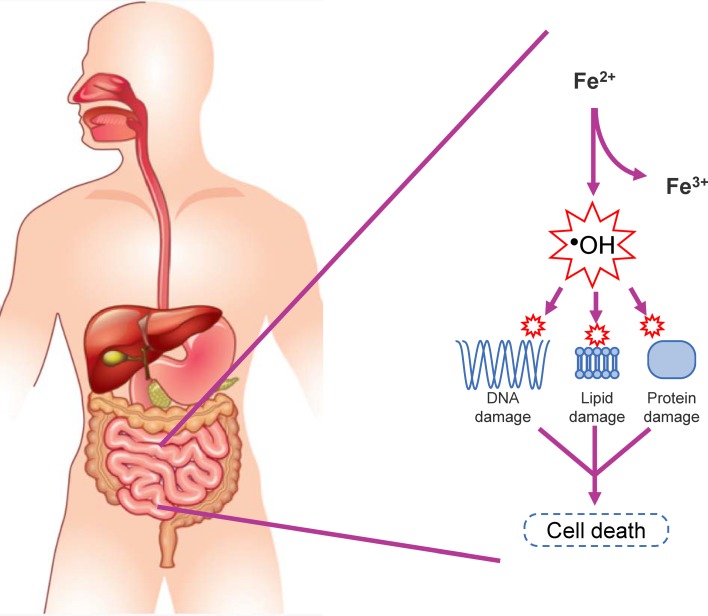
Once the ferric citrate in Auryxia is ingested, the exact chemistry and structure of the resulting mixed citrate and ferric phosphate compounds in the stomach and intestines is unknown. It has long been thought that the low pH of the stomach is important for delivering soluble iron into the intestinal tract [43], although the solubility of Auryxia over a broad range of pH might make that factor less critical. Auryxia is believed to use the conventionally described and highly regulated enterocytic pathway of iron absorption [56], in which ferric iron (such as in the API of Auryxia) is enzymatically reduced to the ferrous state, absorbed primarily in the duodenum, and finally transported into plasma and made available for erythropoiesis (Fig. 3) [57–59]. In contrast, when ferrous sulfate is used for iron supplementation, the reductive step in iron absorption may be bypassed, potentially leading to more rapid absorption, transferrin saturation and the release of substantial amounts of iron not bound to transferrin [60, 61]. Preliminary data from ongoing work suggest that the “conventionally” described pathway is the main route for iron absorption, but some contributions from other pathways (e.g. paracellular pathway [62], transcellular pathway [63], gut microbiota [64]) have not been excluded (T. Ganz, private oral communication, March 4, 2019 [manuscript in preparation]). Of further interest is that ferric iron, such as in Auryxia and unlike ferrous iron, is not easily oxidized [65]. Ferrous iron, during oxidation, can catalyze the formation of free radicals, causing GI mucosal cell damage and erosions of the GI mucosa that likely account for the reported increased incidence of GI adverse effects with ferrous iron compared with ferric iron products (Fig. 4) [65–71].
Fig. 3.
Overview of iron absorption pathway. DcytB duodenal cytochrome B, DMT1 divalent metal transporter 1
Fig. 4.
Iron misregulation and generation of reactive oxygen species
Phosphate Binding Capacity of Auryxia
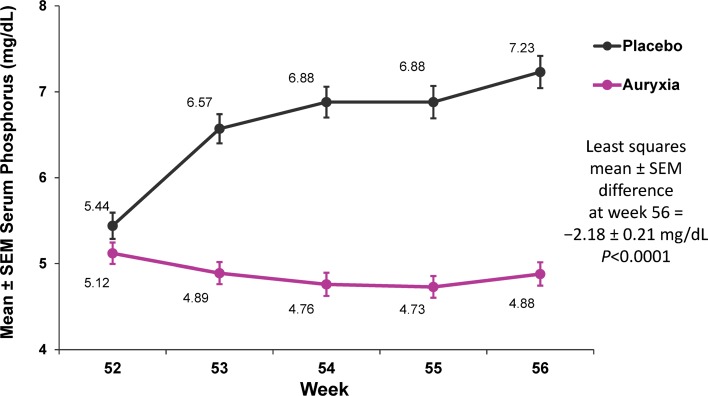
Although some details of the iron chemical species present in the GI tract after ingestion of Auryxia are unclear, the subsequent impact of the FCCCs on phosphate levels is well established. The ferric iron from the API of Auryxia binds dietary phosphorus in the GI tract to form insoluble ferric phosphate, which precipitates and is excreted, thus decreasing intestinal phosphorus absorption and lowering blood phosphate levels [31, 72]. Multiple clinical studies have demonstrated the efficacy of Auryxia in lowering serum phosphate levels across the spectrum of CKD [72–76]. For example, results from a Phase 3, randomized, controlled trial in patients with CKD treated with dialysis showed that Auryxia effectively reduced serum phosphate compared with placebo (analysis of covariance adjusted least squares mean treatment difference: − 2.18 mg/dL [95% CI: − 2.59, − 1.77]; p < 0.0001; Fig. 5) [72]. These findings were confirmed in a recent retrospective chart review that showed that Auryxia reduced and maintained serum phosphate levels over a 6-month period in adult dialysis patients with CKD [73]. Although the main focus of the studies was on control of hyperphosphatemia, improvements in iron parameters, such as increases in ferritin levels and transferrin saturation (TSAT), also were noted [72–74, 77]. Another consequence of the precipitation reaction between ferric iron (in Auryxia) and dietary phosphate is the release of citrate, which can be absorbed and converted to bicarbonate, in theory helping to correct metabolic acidosis, a common complication of CKD [78, 79].
Fig. 5.
Serum phosphate levels in patients with chronic kidney disease treated with dialysis. The displayed 4-week, open-label, placebo-controlled period followed a 52-week, open-label, active-controlled period.
Based on data from the study reported in Lewis et al. [72]
A non-calcium, iron-based phosphate binder, sucroferric oxyhydroxide (Velphoro®; Fresenius Medical Care North America, Waltham, MA, USA), was shown to effectively lower and maintain serum phosphate levels for over 1 year in patients receiving dialysis [80]. Treatment with sucroferric oxyhydroxide did not significantly affect iron-related parameters such as TSAT, serum iron, and blood hemoglobin concentrations, which remained unchanged over the long-term [80].
Iron Supplementation with Auryxia
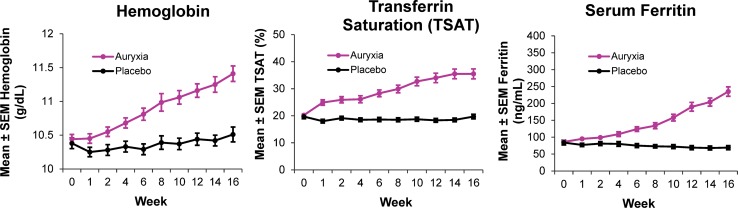
The efficacy of Auryxia for the treatment of iron deficiency anemia in patients with non-dialysis-dependent CKD (NDD-CKD) was tested in a Phase 3 placebo-controlled trial [56]. In that study, treatment with Auryxia significantly improved hemoglobin levels, TSAT, and serum ferritin levels versus placebo (p < 0.001; Fig. 6). Diarrhea was the most common adverse reaction leading to discontinuation of Auryxia (2.6% of patients) in the 16-week, placebo-controlled trial; 12 patients (10%) in the Auryxia treatment group discontinued Auryxia because of an adverse reaction, compared with 10 patients (9%) in the placebo control arm [31]. Tolerability in the GI tract with Auryxia is consistent with the expected low level of reactive oxygen species, anticipated based on chemical considerations and regulated absorption, relative to ferrous iron products. Furthermore, the chelate effect (such as provided by the coordination of ferric iron by citrate) has been observed to improve GI tolerability in a study of a different oral iron product [81]. Notably, although citrate (a component of Auryxia) is known to promote absorption of aluminum in the GI tract, no changes in mean serum aluminum levels were seen after treatment with Auryxia [56, 82, 83].
Fig. 6.
Iron parameters in patients with iron deficiency anemia and non–dialysis-dependent chronic kidney disease. *Between-group difference of 0.84 g/dL (95% CI 0.58, 1.10; p < 0.001); †between-group difference of 18.4% (95% CI 14.6, 22.2; p < 0.001); ‡between-group difference of 170.3 ng/mL (95% CI 144.9, 195.7; p < 0.001).
Adapted from Fishbane et al. [56]
Iron Overload
As mentioned previously, long-term administration of medicinal iron raises a concern about eventual iron overload, and the risk is higher with IV iron products than with oral products, because IV administration bypasses the physiologic regulation of iron absorption [25–28]. Indeed, with the exception of one patient who also received IV iron, iron overload has not been observed in patients after treatment with Auryxia in clinical studies [31]. Furthermore, in patients with CKD treated with dialysis, treatment with Auryxia led to few serious adverse events in organ systems usually affected by iron overload [72]. Ferric iron, such as in Auryxia, is delivered relatively slowly and consistently, likely allowing the iron-regulatory peptide hormone, hepcidin, to slowly upregulate, as reflected in maintenance of clinically appropriate levels of iron markers [44, 84].
The iron-storage protein, ferritin, and the iron-carrier protein, transferrin, are both critical for iron homeostasis and have traditionally served as markers of iron status; however, there are caveats for their use as indicators of iron overload [57, 85–88]. Neither serum ferritin nor TSAT alone is accurate as a standalone measure of iron overload; serum ferritin is severely affected by inflammation, and TSAT, which is proportional to serum iron, is affected by the timing of the sample relative to IV or oral iron administration [86–88]. However, waiting at least 48 hours to draw blood after iron administration [89] allows a more accurate picture of iron overload using TSAT; TSAT tends to stabilize within 2 weeks after beginning IV iron administration, so that high TSAT values at this point or later suggest iron overload or the inability to regulate iron; this timeline has not been studied extensively with oral iron [90–92]. In patients with iron deficiency anemia and NDD-CKD who were treated with Auryxia, 17.9% had transient elevations of TSAT ≥ 70%, yet none had iron overload [56]. Persistent increases in serum ferritin in the absence of clinically identifiable episodes of inflammation, but accompanied by increased TSAT, together may suggest iron overload. The only definitive outcome that indicates clinically significant iron overload is dysfunction of the end organs.
Auryxia and Riona share the same API, although the dosage forms and strengths differ (210 mg ferric iron per pill of Auryxia; approximately 62 mg ferric iron per pill of Riona) [31, 93]. The United States Food and Drug Administration (FDA) label for Auryxia carries a warning for iron overload recommending that iron parameters (e.g. serum ferritin and TSAT) should be assessed prior to initiating Auryxia and should be monitored while on therapy [31]. In a 56-week, Phase 3, randomized, controlled trial in patients with CKD treated with dialysis in which concomitant use of IV iron was permitted, 19% of patients treated with Auryxia had at least one measurement of serum ferritin > 1500 ng/mL, as compared with 10% of patients treated with the active controls (sevelamer carbonate and/or calcium acetate) [72]. Therefore, the FDA label includes a recommendation that patients receiving IV iron may require a reduction in dose or discontinuation of the IV iron therapy [31]. Because elevations in serum ferritin have been observed in previous studies of Auryxia and Riona [72, 94], a recent retrospective analysis of a Japanese Phase 3 trial in hemodialysis patients with hyperphosphatemia investigated the factors that may be associated with these elevations; the factor most strongly associated with elevations in serum ferritin, second only to the dose of Riona, was reduction in the dose of erythropoiesis-stimulating agent (ESA), presumably causing decreased utilization of iron for erythropoiesis [82, 84]. Importantly, no studies of Auryxia and Riona supported an association with clinically significant iron overload despite the observed elevations in serum ferritin levels [72, 82, 84, 94, 95].
Clinical Characteristics of Auryxia
Effects on Iron Levels When Used to Treat Hyperphosphatemia
The chemical attributes of Auryxia and its biological interactions are reflected in its clinical characteristics. For example, when used to treat hyperphosphatemia, it may also have beneficial effects on measures of iron status in disease settings where functional or true iron deficiency and hyperphosphatemia coexist. In patients with CKD treated with dialysis, studies have shown that treatment with Auryxia reduces serum phosphate and improves iron parameters [72, 95]. In a Phase 3, 56-week, placebo- and active-controlled trial in patients with dialysis-dependent CKD, Auryxia (dosed and titrated to maintain serum phosphate control) significantly improved iron parameters compared with active controls (sevelamer carbonate and/or calcium acetate) as early as Week 12 (ferritin: mean difference of 281.8 ± 42.9 ng/mL at week 52, p < 0.001; TSAT: mean difference of 9.55% ± 1.58%, p < 0.001) [96]. Additionally, two studies have suggested that Auryxia may reduce the need for IV iron when used as a phosphate binder in patients with CKD treated with dialysis [72, 96, 97]. Lower percentages of patients required IV iron in the Auryxia group compared with an active control group at all time points over the 52-week active control period; at the end of this period, 85.4% and 69.0% of patients did not receive IV iron at week 52 in the Auryxia and active control groups, respectively (p < 0.001) [96]. The cumulative dose of IV iron was less in the Auryxia group as compared with the active control group over the entire 52-week active control period (median [interquartile range] dose of 12.9 [1.0–28.9] mg/week with Auryxia vs 26.8 [13.4–47.6] mg/wk with active control; p < 0.001). Similarly, in the same study and over the same period, the cumulative dose of ESAs was lower in the Auryxia group compared with the active control group (median [interquartile range] dose of 5303 [2023–9695] units/week with Auryxia vs 6954 [2664–12,375] units/week with active control; p = 0.04) [96].
The effects of treatment with Auryxia could lead to clinical outcomes that are different from those with other therapies. Of note, treatment to a higher hemoglobin target has been associated with increased risk of vascular thrombosis, and higher ESA doses have been explored as a plausible mechanism for this increased risk [98]; therefore, a reduction of ESA dose may lower risk of cardiovascular events, thereby decreasing hospitalization. Infections are also an important driver of readmission after hospitalization in patients with CKD who are receiving hemodialysis [99]. In the aforementioned 56-week, Phase 3 trial in patients with end-stage renal disease on dialysis, treatment with Auryxia was associated with reductions in overall hospitalizations, cardiac-related hospitalizations, and infection-related hospitalizations as compared with active control [100]. Treatment with Auryxia also reduced health-care costs; the cost savings were attributed to a decrease in use of IV iron and ESA and to a reduction in the number of hemodialysis sessions that were missed as a result of hospitalizations [101, 102].
Thus, the ability of Auryxia to deliver iron, because of its chemical composition, is an added benefit when it is already being used to control phosphate levels and may improve clinical outcomes that are unrelated to hyperphosphatemia. The benefit of Auryxia as a therapy for the treatment of iron deficiency may be particularly important in light of recent research that has implicated IV iron therapy in non-alcoholic fatty liver disease (NAFLD) [103, 104]. In a longitudinal study of 7 patients undergoing IV iron therapy, hepatic proton density fat fraction and liver iron concentration levels increased significantly, suggesting that iron overload in these dialysis patients may have led to or exacerbated NAFLD [103].
Effects on Serum Phosphate Levels When Used to Treat Iron Deficiency Anemia
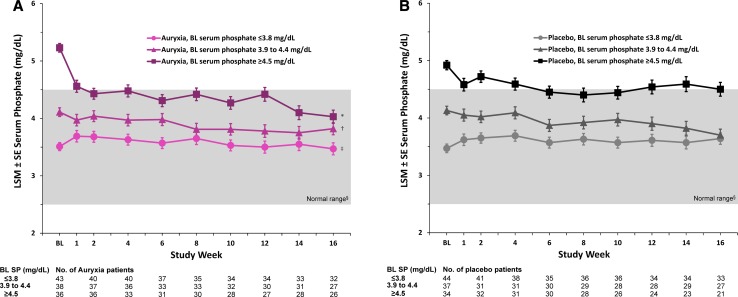
Additional studies have shown that treatment with Auryxia improves iron parameters in patients with CKD and iron deficiency anemia without negatively perturbing serum phosphate when kidney function is sufficient to maintain normal phosphate levels [56, 105]. A post hoc analysis of the Phase 3 trial in patients with iron deficiency anemia and NDD-CKD showed that, when given as an iron replacement product, Auryxia did not significantly reduce serum phosphate compared with placebo among patients with baseline serum phosphate concentrations within the population reference range of 2.5–4.5 mg/dL (Fig. 7) [105]. The effect of Auryxia on serum phosphorus depends on baseline phosphate levels, declining only when the initial levels are excessive; the greatest reductions in serum phosphate were seen in patients with the highest baseline serum phosphate concentrations [105]. Therefore, as a converse of the situation in which Auryxia is used primarily as a phosphate binder, treating patients with iron deficiency anemia using Auryxia may have the additional benefit of reducing high serum phosphate and FGF23 levels. The evident lack of hypophosphatemia is interesting; one possibility is that patients with residual renal function may avoid hypophosphatemia during Auryxia treatment by retaining more phosphate in the kidneys to compensate for losses by excretion in the gut. In fact, in a Phase 2 study, when patients with CKD stages 3–5 were given Auryxia as a phosphate binder, both mean serum phosphate and 24-hour urinary phosphate significantly decreased compared with control [75].
Fig. 7.
Changes in mean serum phosphate levels stratified by tertiles of baseline serum phosphate levels. a Patients treated with Auryxia. b Patients who received placebo. BL baseline, LSM least squares mean, SP serum phosphorus. Data are LSM (SE) serum phosphate estimates derived from a mixed-effects model for repeated measures analysis with fixed-effects terms for treatment (Auryxia vs placebo), baseline covariate, visit, treatment-by-visit interaction, treatment-by-covariate interaction, and treatment-by-visit-by-covariate interaction. *p = 0.006, †p = 0.438, and ‡p = 0.236 vs placebo. §The normal laboratory reference range of serum phosphate levels is indicated by the gray background.
Adapted from Block et al. [105]
Effects on FGF23 Levels
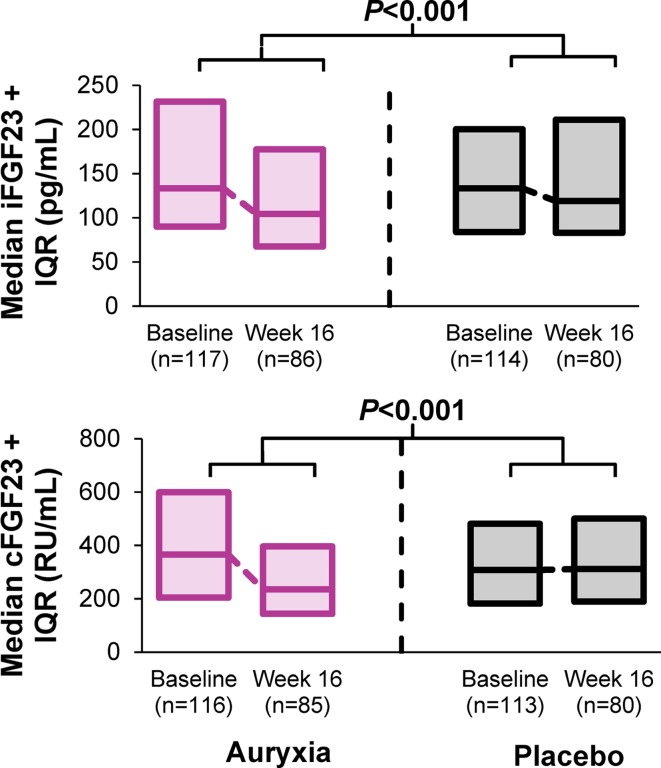
The dual function of Auryxia in reducing serum phosphate and treating iron deficiency simultaneously decreases circulating levels of FGF23, a key phosphate-regulating hormone whose plasma concentration increases as CKD progresses [12, 56, 105]. In a Phase 3 study in patients with NDD-CKD and iron deficiency anemia, levels of FGF23 were reduced significantly more between baseline and Week 16 with Auryxia treatment compared with placebo (Fig. 8) [56]. This was true both for the intact bioactive form of FGF23 (iFGF23) and for its carboxy-terminal cleavage product (cFGF23), whose biological activities are under investigation [106]. Similarly, an open-label Japanese study in patients on hemodialysis showed that treatment with Riona compared with lanthanum carbonate led to significantly lower levels of iFGF23, independent of phosphate levels (change from baseline in iFGF23: − 6160 vs − 1118 pg/mL, respectively; p = 0.026) [97]. Although this conjecture is still speculative, reduction of FGF23 levels could have a beneficial effect on the progression of CKD and cardiovascular disease. In mice treated with Auryxia, the concentrations of FGF23 decreased and CKD disease progression was slowed [107]. More definitive clinical trials will be needed to validate the hypothesis that ferric citrate slows CKD progression by lowering FGF23 [108].
Fig. 8.
Changes in FGF23 levels. cFGF23 carboxy-terminal cleavage product of fibroblast growth factor 23, iFGF23 intact fibroblast growth factor 23, IQR interquartile range.
Based on data from the study reported in Fishbane et al. [56]
Summary
Consistent with its chemistry and mechanism of action, Auryxia is effective both in reducing serum phosphate levels in patients treated with dialysis and in improving iron parameters in CKD patients not on dialysis. Overall, across both CKD patient populations, treatment with Auryxia was considered to have good safety and was similarly well tolerated compared with other study treatments. In dialysis patients, 21% discontinued Auryxia due to an adverse reaction versus 14% who discontinued active control (which patients had tolerated before enrollment); in patients with NDD-CKD, 10% discontinued Auryxia due to an adverse reaction versus 9% who discontinued placebo [31]. The balance of these characteristics distinguishes Auryxia from commercial-grade ferric citrate and differentiates it from other common therapies used to treat hyperphosphatemia and iron deficiency anemia in patients with CKD.
Acknowledgements
The authors acknowledge Keryx Biopharmaceuticals, Inc. (Boston, MA, USA; now a wholly owned subsidiary of Akebia Therapeutics, Inc., Cambridge, MA, USA) for supporting the preparation of this manuscript. Under the authors’ direction, Madhura Mehta, Ph.D., and Michael J. Theisen, Ph.D. (CHC Group, LLC, North Wales, PA, USA) provided writing and formatting assistance. Editorial assistance in the form of proofreading, copyediting, and fact checking was also provided by CHC Group. The authors exercised full editorial control and provided input on and approval of the content of the manuscript at each stage of development. All authors have been involved in research relating to Auryxia: TG conducts research studying the mechanism of iron absorption from Auryxia, AB is involved in research studying the chemical structure of Auryxia, and IBS studies the use of Auryxia in children.
Compliance with Ethical Standards
Conflict of interest
TG is a scientific cofounder and shareholder of Intrinsic LifeSciences and Silarus Pharma. He is a consultant for Ablynx, Ambys, Akebia Therapeutics, Gilead, Global Blood Therapeutics, Keryx Biopharmaceuticals, La Jolla Pharmaceutical Company, Sierra Oncology and Vifor. He has received Grant support from Akebia Therapeutics and Keryx Biopharmaceuticals. AB was a consultant to Keryx and received support for characterizing the structural composition of Auryxia. IBS is a consultant for Akebia Therapeutics, Keryx Biopharmaceuticals, Amgen, and Ultragenyx. He has received Grant support from Amgen and AbbVie.
Funding
Support was provided by Keryx Biopharmaceuticals, Inc., which is now a wholly owned subsidiary of Akebia Therapeutics, Inc.
References
- 1.Braun L, Sood V, Hogue S, et al. High burden and unmet patient needs in chronic kidney disease. Int J Nephrol Renovasc Dis. 2012;5:151–163. doi: 10.2147/IJNRD.S37766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System. 2017 USRDS annual data report: executive summary [online]. https://www.usrds.org/2017/view/Default.aspx. Accessed 7 June 2018.
- 3.Vest BM, York TR, Sand J, et al. Chronic kidney disease guideline implementation in primary care: a qualitative report from the TRANSLATE CKD study. J Am Board Fam Med. 2015;28(5):624–631. doi: 10.3122/jabfm.2015.05.150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salanova Villanueva L, Sanchez Gonzalez C, Sanchez Tomero JA, et al. Bone mineral disorder in chronic kidney disease: klotho and FGF23; cardiovascular implications. Nefrologia. 2016;36(4):368–375. doi: 10.1016/j.nefro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 6.US-DOPPS Practice Monitor. Serum phosphorus (most recent) national sample [online]. http://www.dopps.org/DPM. Accessed 18 Mar 2019.
- 7.Friedman EA. An introduction to phosphate binders for the treatment of hyperphosphatemia in patients with chronic kidney disease. Kidney Int Suppl. 2005;96:S2–S6. doi: 10.1111/j.1523-1755.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 9.Block GA. Prevalence and clinical consequences of elevated Ca × P product in hemodialysis patients. Clin Nephrol. 2000;54(4):318–324. [PubMed] [Google Scholar]
- 10.Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74(2):148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16(6):1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 12.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82(7):737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isakova T, Wahl P, Vargas G, et al. FGF23, PTH and phosphorus metabolism in the chronic renal insufficiency cohort. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17(5):1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 16.Nam KH, Kim H, An SY, et al. Circulating fibroblast growth factor-23 levels are associated with an increased risk of anemia development in patients with nondialysis chronic kidney disease. Sci Rep. 2018;8(1):7294. doi: 10.1038/s41598-018-25439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis C, David V. Inflammation regulates fibroblast growth factor 23 production. Curr Opin Nephrol Hypertens. 2016;25(4):325–332. doi: 10.1097/MNH.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7(7):1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–146. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malluche HH, Mawad H. Management of hyperphosphataemia of chronic kidney disease: lessons from the past and future directions. Nephrol Dial Transplant. 2002;17(7):1170–1175. doi: 10.1093/ndt/17.7.1170. [DOI] [PubMed] [Google Scholar]
- 21.Salusky IB, Goodman WG. Cardiovascular calcification in end-stage renal disease. Nephrol Dial Transpl. 2002;17(2):336–339. doi: 10.1093/ndt/17.2.336. [DOI] [PubMed] [Google Scholar]
- 22.Alfrey AC, LeGendre GR, Kaehny WD. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294(4):184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suki WN, Zabaneh R, Cangiano JL, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72(9):1130–1137. doi: 10.1038/sj.ki.5002466. [DOI] [PubMed] [Google Scholar]
- 25.Rottembourg J, Rostoker G. Use of iron therapy in chronic kidney disease. Arch Clin Nephrol. 2016;1(1):001–003. [Google Scholar]
- 26.Shepshelovich D, Rozen-Zvi B, Avni T, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68(5):677–690. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Bonovas S, Fiorino G, Allocca M, et al. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(2):e2308. doi: 10.1097/MD.0000000000002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Vecchio L, Longhi S, Locatelli F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J. 2016;9(2):260–267. doi: 10.1093/ckj/sfv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roger SD. Practical considerations for iron therapy in the management of anaemia in patients with chronic kidney disease. Clin Kidney J. 2017;10(suppl 1):i9–i15. doi: 10.1093/ckj/sfx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai AB, Jang SM, Wegrzyn N. Iron-based phosphate binders—a new element in management of hyperphosphatemia. Expert Opin Drug Metab Toxicol. 2016;12(1):115–127. doi: 10.1517/17425255.2016.1110573. [DOI] [PubMed] [Google Scholar]
- 31.Auryxia (Ferric Citrate) Full prescribing information. Boston: Keryx Biopharmaceuticals Inc.; 2017. [Google Scholar]
- 32.Fexeric (Ferric Citrate Coordination Complex) Summary of product characteristics. London: Keryx Biopharma UK Ltd.; 2015. [Google Scholar]
- 33.Torii Pharmaceuticals Co., Ltd. Mainstay products [online]. https://www.torii.co.jp/en/company/product.html. Accessed 7 June 2018.
- 34.Pharmaceuticals and Medical Devices Agency. New drugs approved in FY 2013 [online]. https://www.pmda.go.jp/files/000153463.pdf. Accessed 2 May 2018.
- 35.Panion & BF Biotech, Inc. NEPHOXIL capsule 500 mg [online]. https://www.pbf.com.tw/. Accessed 2 Aug 2018.
- 36.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Data on file. Boston: Keryx Biopharmaceuticals; 2018. [Google Scholar]
- 37.Silva AM, Kong X, Parkin MC, et al. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009;40:8616–8625. doi: 10.1039/b910970f. [DOI] [PubMed] [Google Scholar]
- 38.Milewska MJ. Citric-acid—its natural and synthetic derivatives. Z Chem. 1988;28(6):204–211. [Google Scholar]
- 39.Pierre JL, Gautier-Luneau I. Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance. Biometals. 2000;13(1):91–96. doi: 10.1023/a:1009225701332. [DOI] [PubMed] [Google Scholar]
- 40.Guerinot ML, Meidl EJ, Plessner O. Citrate as a siderophore in Bradyrhizobium japonicum. J Bacteriol. 1990;172(6):3298–3303. doi: 10.1128/jb.172.6.3298-3303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langman L, Young IG, Frost GE, et al. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govindaraj T, KrishnaRau L, Prakash J. In vitro bioavailability of iron and sensory qualities of iron-fortified wheat biscuits. Food Nutr Bull. 2007;28(3):299–306. doi: 10.1177/156482650702800306. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs A, Miles PM. Role of gastric secretion in iron absorption. Gut. 1969;10(3):226–229. doi: 10.1136/gut.10.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. Sci World J. 2012;2012:846824. doi: 10.1100/2012/846824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matzapetakis M, Raptopoulou CP, Tsohos A, et al. Synthesis, spectroscopic and structural characterization of the first mononuclear, water soluble iron–citrate complex, (NH4)5Fe(C6H4O7)2·2H2O. J Am Chem Soc. 1998;120(50):13266–13267. [Google Scholar]
- 46.Shweky I, Bino A, Goldberg DP, et al. Syntheses, structures, and magnetic properties of two dinuclear iron(III) citrate complexes. Inorg Chem. 1994;33(23):5161–5162. [Google Scholar]
- 47.Bino A, Shweky I, Cohen S, et al. A novel nonairon(III) citrate complex: a “ferric triple-decker”. Inorg Chem. 1998;37(20):5168–5172. [Google Scholar]
- 48.Tenne D, Bogoslavsky B, Bino A. Ferric ammonium citrate—what’s in it? Inorg Chem. 2015;2015(25):4159–4161. [Google Scholar]
- 49.Ferguson AD, Chakraborty R, Smith BS, et al. Structural basis of gating by the outer membrane transporter FecA. Science. 2002;295(5560):1715–1719. doi: 10.1126/science.1067313. [DOI] [PubMed] [Google Scholar]
- 50.Sauter A, Braun V. Defined inactive FecA derivatives mutated in functional domains of the outer membrane transport and signaling protein of Escherichia coli K-12. J Bacteriol. 2004;186(16):5303–5310. doi: 10.1128/JB.186.16.5303-5310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwok DWK, Stoynov NM, Inventors, Panion & BF Biotech, Inc., Assignee. Ferric organic compounds, uses thereof and methods of making same. US patent 7,767,851 B2, 3 Aug 2010.
- 52.Kwok DWK, Stoynov NM, Inventors; Panion & BF Biotech, Inc., Assignee. Ferric organic compounds, uses thereof and methods of making same. US patent 8,338,642 B2, 25 Dec 2012.
- 53.Kwok DWK, Stoynov NM, Inventors; Panion & BF Biotech, Inc., Assignee. Ferric organic compounds, uses thereof and methods of making same. US patent 8,609,896 B2, 17 Dec 2013.
- 54.Kwok DWK, Stoynov NM, Inventors; Panion & BF Biotech, Inc., Assignee. Ferric organic compounds, uses thereof and methods of making same. US patent 8,901,349 B2, 2 Dec 2014.
- 55.Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm. 2011;61(2):117–139. doi: 10.2478/v10007-011-0020-8. [DOI] [PubMed] [Google Scholar]
- 56.Fishbane S, Block GA, Loram L, et al. Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol. 2017;28(6):1851–1858. doi: 10.1681/ASN.2016101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffey R, Ganz T. Iron homeostasis: an anthropocentric perspective. J Biol Chem. 2017;292(31):12727–12734. doi: 10.1074/jbc.R117.781823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 59.Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307(4):G397–G409. doi: 10.1152/ajpgi.00348.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3(1):12–33. doi: 10.3390/pharmaceutics3010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dresow B, Petersen D, Fischer R, et al. Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals. 2008;21(3):273–276. doi: 10.1007/s10534-007-9116-5. [DOI] [PubMed] [Google Scholar]
- 62.Froment DP, Molitoris BA, Buddington B, et al. Site and mechanism of enhanced gastrointestinal absorption of aluminum by citrate. Kidney Int. 1989;36(6):978–984. doi: 10.1038/ki.1989.290. [DOI] [PubMed] [Google Scholar]
- 63.Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol. 2007;13(35):4716–4724. doi: 10.3748/wjg.v13.i35.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deschemin JC, Noordine ML, Remot A, et al. The microbiota shifts the iron sensing of intestinal cells. FASEB J. 2016;30(1):252–261. doi: 10.1096/fj.15-276840. [DOI] [PubMed] [Google Scholar]
- 65.Toblli JE, Cao G, Angerosa M. Ferrous sulfate, but not iron polymaltose complex, aggravates local and systemic inflammation and oxidative stress in dextran sodium sulfate-induced colitis in rats. Drug Des Devel Ther. 2015;9:2585–2597. doi: 10.2147/DDDT.S81863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhattacharyya A, Chattopadhyay R, Mitra S, et al. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94(2):329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383. doi: 10.1371/journal.pone.0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Troost FJ, Saris WH, Haenen GR, et al. New method to study oxidative damage and antioxidants in the human small bowel: effects of iron application. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G354–G359. doi: 10.1152/ajpgi.00422.2002. [DOI] [PubMed] [Google Scholar]
- 69.Dwyer DJ, Kohanski MA, Hayete B, et al. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaye P, Abdulla K, Wood J, et al. Iron-induced mucosal pathology of the upper gastrointestinal tract: a common finding in patients on oral iron therapy. Histopathology. 2008;53(3):311–317. doi: 10.1111/j.1365-2559.2008.03081.x. [DOI] [PubMed] [Google Scholar]
- 71.Haig A, Driman DK. Iron-induced mucosal injury to the upper gastrointestinal tract. Histopathology. 2006;48(7):808–812. doi: 10.1111/j.1365-2559.2006.02448.x. [DOI] [PubMed] [Google Scholar]
- 72.Lewis JB, Sika M, Koury MJ, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hain DJ, Marinaro M, Koeper DW, et al. Ferric citrate controls serum phosphorus in dialysis patients: retrospective data. Clin Nephrol. 2017;88(1):12–18. doi: 10.5414/CN109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dwyer JP, Sika M, Schulman G, et al. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis. 2013;61(5):759–766. doi: 10.1053/j.ajkd.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 75.Block GA, Fishbane S, Rodriguez M, et al. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3-5. Am J Kidney Dis. 2015;65(5):728–736. doi: 10.1053/j.ajkd.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Sinsakul M, Sika M, Koury M, et al. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121(1–2):c25–c29. doi: 10.1159/000341922. [DOI] [PubMed] [Google Scholar]
- 77.Kovesdy CP, Rowan CG, Foote B, et al. The effect of ferric citrate on IV iron, ESA utilization, and laboratory parameters in real-world dialysis patients. New Orleans: American Society of Nephrology; 2017. [Google Scholar]
- 78.Chen W, Abramowitz MK. Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 2014;15:55. doi: 10.1186/1471-2369-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hsu CH, Inventor. Methods for treating renal failure. US patent 5,753,706, 19 May 1998.
- 80.Floege J, Covic AC, Ketteler M, et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30(6):1037–1046. doi: 10.1093/ndt/gfv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duque X, Martinez H, Vilchis-Gil J, et al. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: a randomized controlled trial. Nutr J. 2014;13:71. doi: 10.1186/1475-2891-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yokoyama K, Akiba T, Fukagawa M, et al. Long-term safety and efficacy of a novel iron-containing phosphate binder, JTT-751, in patients receiving hemodialysis. J Ren Nutr. 2014;24(4):261–267. doi: 10.1053/j.jrn.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Van Buren PN, Lewis JB, Dwyer JP, et al. The phosphate binder ferric citrate and mineral metabolism and inflammatory markers in maintenance dialysis patients: results from prespecified analyses of a randomized clinical trial. Am J Kidney Dis. 2015;66(3):479–488. doi: 10.1053/j.ajkd.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokoyama K, Fukagawa M, Akiba T, et al. A prospective study examining the contribution to renal anemia treatment of ferric citrate hydrate, an iron-based oral phosphate binder, in hemodialysis patients with hyperphosphatemia: ASTRIO study (SA-PO818) New Orleans: American Society of Nephrology; 2017. [Google Scholar]
- 85.Kelly AU, McSorley ST, Patel P, et al. Interpreting iron studies. BMJ. 2017;357:j2513. doi: 10.1136/bmj.j2513. [DOI] [PubMed] [Google Scholar]
- 86.Lomer MC, Cook WB, Jan-Mohamed HJ, et al. Iron requirements based upon iron absorption tests are poorly predicted by haematological indices in patients with inactive inflammatory bowel disease. Br J Nutr. 2012;107(12):1806–1811. doi: 10.1017/S0007114511004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007;14(3):183–190. doi: 10.1097/MOH.0b013e3280d2b76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobune M, Miyanishi K, Takada K, et al. Establishment of a simple test for iron absorption from the gastrointestinal tract. Int J Hematol. 2011;93(6):715–719. doi: 10.1007/s12185-011-0878-8. [DOI] [PubMed] [Google Scholar]
- 89.Venofer (Iron Sucrose Injection) Full prescribing information. St Gallen: Vifor (International) Inc.; 2015. [Google Scholar]
- 90.Ferrari P, Kulkarni H, Dheda S, et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):77–83. doi: 10.2215/CJN.04190510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blunden RW, Lloyd JV, Rudzki Z, et al. Changes in serum ferritin levels after intravenous iron. Ann Clin Biochem. 1981;18(Pt 4):215–217. doi: 10.1177/000456328101800405. [DOI] [PubMed] [Google Scholar]
- 92.Van Wyck DB, Roppolo M, Martinez CO, et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68(6):2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 93.Pennoyer A, Bridgeman MB. Ferric citrate (Auryxia) for the treatment of hyperphosphatemia. Pharm Ther. 2015;40(5):329–339. [PMC free article] [PubMed] [Google Scholar]
- 94.Yokoyama K, Hirakata H, Akiba T, et al. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol. 2014;9(3):543–552. doi: 10.2215/CJN.05170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yokoyama K, Hirakata H, Akiba T, et al. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36(5):478–487. doi: 10.1159/000344008. [DOI] [PubMed] [Google Scholar]
- 96.Umanath K, Jalal DI, Greco BA, et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26(10):2578–2587. doi: 10.1681/ASN.2014080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maruyama N, Otsuki T, Yoshida Y, et al. Ferric citrate decreases fibroblast growth factor 23 and improves erythropoietin responsiveness in hemodialysis patients. Am J Nephrol. 2018;47(6):406–414. doi: 10.1159/000489964. [DOI] [PubMed] [Google Scholar]
- 98.Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2(6):1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 99.Lin E, Bhattacharya J, Chertow GM. Prior hospitalization burden and the relatedness of 30-day readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2019;30(2):323–335. doi: 10.1681/ASN.2018080858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodby R, Umanath K, Niecestro R, et al. Phosphorus binding with ferric citrate is associated with fewer hospitalizations and reduced hospitalization costs. Expert Rev Pharmacoecon Outcomes Res. 2015;15(3):545–550. doi: 10.1586/14737167.2015.995169. [DOI] [PubMed] [Google Scholar]
- 101.Rodby RA, Umanath K, Niecestro R, et al. Ferric citrate, an iron-based phosphate binder, reduces health care costs in patients on dialysis based on randomized clinical trial data. Drugs R D. 2015;15(3):271–279. doi: 10.1007/s40268-015-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brunelli SM, Sibbel SP, Van Wyck D, et al. Net budgetary impact of ferric citrate as a first-line phosphate binder for the treatment of hyperphosphatemia: a Markov microsimulation model. Drugs R D. 2017;17(1):159–166. doi: 10.1007/s40268-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rostoker G, Loridon C, Griuncelli M, et al. Liver iron load influences hepatic fat fraction in end-stage renal disease patients on dialysis: a proof of concept study. EBioMedicine. 2019;39:461–471. doi: 10.1016/j.ebiom.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chinnadurai R, Macdougall IC, Kalra PA. Treatment of anaemia in end-stage renal disease: a double-edged iron sword? EBioMedicine. 2019;40:31–32. doi: 10.1016/j.ebiom.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Block GA, Pergola PE, Fishbane S, et al. Effect of ferric citrate on serum phosphate and fibroblast growth factor 23 among patients with nondialysis-dependent chronic kidney disease: path analyses. Nephrol Dial Transpl. 2018 doi: 10.1093/ndt/gfy318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richter B, Faul C. FGF23 actions on target tissues—with and without klotho. Front Endocrinol (Lausanne). 2018;9:189. doi: 10.3389/fendo.2018.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Francis C, Courbon G, Neuburg S, et al. Ferric citrate administration reduces FGF23 production and improves renal function in a mouse model of CKD (FR-ORO70) New Orleans: American Society of Nephrology; 2017. [Google Scholar]
- 108.Block G, Block M, Smits G, et al. Randomized trial of the effects of ferric citrate in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2018;33(suppl 1):i637–i638. [Google Scholar]