Abstract
Background/purpose
The purpose of this study was to investigate whether poly-gamma-glutamic acid (γ-PGA), a naturally derived biomaterial, was suitable as an alternative antibacterial mouthwash in the absence of alcohol.
Materials and methods
Three bacterial strains, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, were used for testing the antibacterial activity of mouthwashes. In addition, cell viability, cytotoxicity, and genotoxicity experiments were conducted for testing the toxicity of mouthwashes.
Results
We demonstrated that 10000 ppm of γ-PGA without alcohol could efficiently inhibit 99% of bacterial growth. In addition, γ-PGA did not cause any cytotoxicity or genotoxicity.
Conclusion
10000 ppm of γ-PGA in an alcohol-free mouthwash is an alternative biomaterial for mouthwashes.
Keywords: Biomaterials, Mouthwash, Poly-gamma-glutamic acid (γ-PGA)
Introduction
Oral health is a foundation of a person's general good health and well-being. According to a World Health Organization fact sheet, 60–90% of school children and nearly 100% of adults have dental cavities worldwide. Hundreds of species of bacteria are present in the oral cavity, and some can cause various diseases such as dental caries, periodontal diseases, and even oral cancers.1 The majority of people have a habit of brushing their teeth in ways that only cleans ∼50% of a tooth's surface, thus a mouthwash may be used as an additional oral health care product. Table 1 summarizes the advantages and side effects of some materials that are used in commercially available mouthwashes. Although there is no perfect product, users can choose an appropriate mouthwash according to their needs.
Table 1.
The comparison of CHX, sodium fluoride, and HOCl in commercial mouthwashes.
| Antibacterial agent | Chlorhexidine (CHX) | Sodium fluoride | Hypochlorous Acid (HOCl) |
|---|---|---|---|
| Antibacterial mechanism | Damaging the cytoplasmic membrane to increase the inner permeability and to result in the membrane damage or loss of structural organization.22 | Releasing F− to create an over-acidification of the cytoplasm resulting in the disruption of bacteria.23 | Inhibiting DNA synthesis and causing partial inhibition of membrane protein synthesis.24 |
| Advantages | Bacteria will not develop to resist CHX.25 | Good routine home care oral hygiene in children due to anti-carcinogenic properties.4 | Dissolves in water, and does not cause damage to the environment.3 |
| Side effects or disadvantages | Staining on teeth and dental mucosa; alternates taste perception.2 | Risk of ingestion resulting in toxicity.4 | Easily degraded and required to be prepared freshly.3 |
It has been debated whether mouthwashes are helpful for oral care, because mouthwashes are made of chemicals that can cause side effects.2, 3, 4 Regular brushing and flossing is still recommended. However, mouthwashes are still beneficial especially for patients who are unable to brush their teeth. Radiation causes damage to the salivary glands, thus patients who undergo head and neck radiation are at high risk for the development of dental caries.5 Oral mucositis is another adverse event for some cancer patients who are treated with chemotherapy or radiotherapy.6 Due to the pain these patients encounter in the oral area, mouthwashes are a quick and efficient method of reducing oral diseases and maintaining oral health.7 However, most commercial mouthwashes usually contain alcohol to inhibit bacterial growth despite the irritation it causes to the users. Therefore, it is critical to use materials that can function as an antibacterial agent in the absence of alcohol.
Poly-gamma-glutamic acid (γ-PGA) is derived from Bacillus anthracis, and is known to be a biodegradable material. It is also difficult for proteases to catalyze γ-PGA, making it a potentially suitable antibacterial material.8 In this study, we investigated whether γ-PGA could be applied as a mouthwash in the absence of alcohol. We optimized the most suitable concentration of γ-PGA for inhibiting bacterial growth without causing cytotoxicity or genotoxicity.
Materials and methods
Preparation of γ-PGA-containing prospective mouthwash
Different concentrations of γ-PGA (Vedan Enterprise Corporation, Taichung, Taiwan) were dissolved in distilled deionized water. Green peppermint essential oil (Lixin Ltd., Taichung, Taiwan) was dissolved in glycerol (PanReac, Barcelona, Spain) and added into the prospective mouthwash. A trace of Brilliant Blue FCF (food grade dye, PanReac) was added to distinguish the mouthwash from water.
The composition of commercial mouthwashes
Three commercial mouthwash products were used in this study. CHX mouthwash (Day and Night mouthwash, Day and Night company, Taipei, Taiwan) contains 0.1–0.2% (weight/volume) CHX, menthol, and 4.6% of alcohol. Sodium fluoride mouthwash (Oral-B mouthwash, P&G, Taipei, Taiwan) includes 0.022% of sodium fluoride. HOCl mouthwash (Water god antibacterial mouthwash, Want–Want Ltd., Taipei, Taiwan) mainly contains 10–30 ppm of HOCl.
Culture of bacteria
Standard strains were used in this study: Escherichia coli (ATCC 23815), Staphylococcus aureus (ATCC 10832), and Pseudomonas aeruginosa (ATCC 10145). The bacteria were added into a centrifuge tube containing 10 ml of lysogeny broth in the laminar flow hood, and cultured at 150 rpm at 37 °C overnight. The next day, 1 ml of cultured bacteria was transferred into 9 ml of fresh broth and incubated at 37 °C. The optical density (OD) value of cultured bacteria was measured at 600 nm every hour to establish the growth curve of each bacterial strain.
The measurement of antibacterial activity
Monitoring bacterial growth by measuring the turbidity of bacterial cultures is a quick method, and it has been applied for testing antibacterial activity of materials.9,10 9 ml of mouthwash were mixed with 1 ml of bacterial suspension (OD value at 600 nm was 0.1, 106 CFU/ml) and the experiments were performed in triplicates. The mixtures were incubated at 37 °C for 8 h, and OD values at 600 nm were observed. The antibacterial activity (%) was calculated by the following formula:
[OD value in phosphate buffered saline (PBS)] – (OD value in prospective mouthwash or commercial mouthwash)/OD value in PBS.
Cell viability and cytotoxicity testing
The 3T3 cells were plated in a 96-well culture plate with a density of 3 × 104 cells per well and cultured overnight. For cell viability, 9 ml of mouthwash was mixed with 1 ml of medium and 200 μl of the mixture was added into each well and cells were incubated at 37 °C overnight. 15 μl of WST-8 reagent (water-soluble tetrazolium salts-8, Sigma, St Louis, MO, USA) was then added, and incubated at 37 °C for 90 min in the dark. After incubation, the OD value was measured at 450 nm by the ELISA reader (Tecan Austria GmbH, Sunrise Remote-F0393000). The viability was obtained by calculating the OD value of the mouthwash grown culture divided by the OD value of the control. For cytotoxicity, 50 μl of freshly prepared LDH reagent (lactate dehydrogenase, Clontech, Mountain View, CA, USA) was mixed with cells, and the OD value was measured at 490 nm by the ELISA reader after shaking at 100 rpm for 20 min. The cytotoxicity (%) was obtained according to the following formula:
[(OD value in mouthwash)-(OD value in control)]/[(OD value in total lysis)-(OD value in control)].
Genotoxicity test
5 х 105 of Chinese hamster ovary cells were plated and incubated at 37 °C for 4 h 15 ml of the mixture (mouthwash: medium = 1:9) was then added, and cells were incubated at 37 °C for 24 h. At 20 h, 200 μl of colchicine solution (Sigma) was added. At 24 h, cells were collected and fixed with 3 ml fixation solution (methanol: acetic acid = 3:1) at 4 °C for 15 min. Cells were placed on a 75% ethanol pre-treated glass, and dried at 55 °C in an oven. The glass was soaked in 5% Giemsa (Sigma) for 10 min, and the stained chromosomes were observed after rinsed and dried at 55 °C.
Results
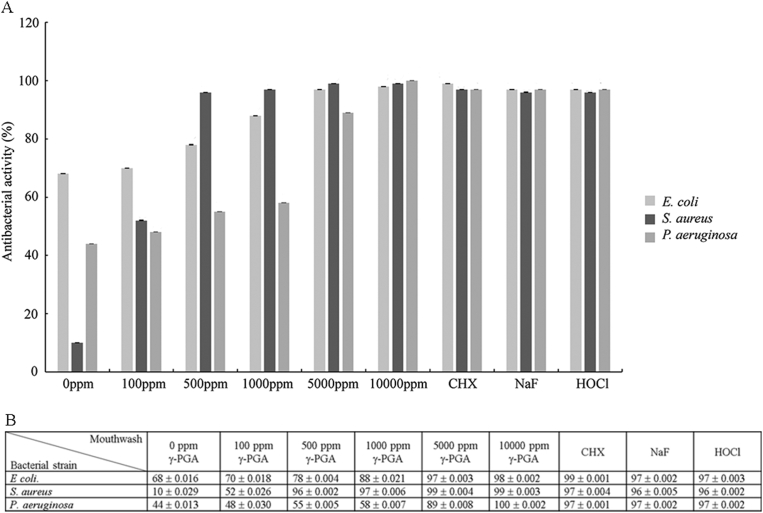
In order to evaluate the antibacterial effect of γ-PGA, different concentrations of γ-PGA were investigated (Fig. 1A). We discovered that the antibacterial activity in the absence of γ-PGA was 67.56%, 9.52%, and 67.56% for E. coli, S. aureus, and P. aeruginosa (Fig. 1B), respectively, suggesting that green peppermint essential oil may play a role in inhibiting bacterial growth. However, the antibacterial activity increased dramatically when γ-PGA was added and there was a dose-dependent effect. These results indicated that γ-PGA could efficiently inhibit the growth of the three tested bacterial strains. To investigate whether the antibacterial activity of γ-PGA was as effective as the commercial mouthwashes, we compared 10000 ppm γ-PGA mouthwash with mouthwashes that contained CHX, sodium fluoride, or HOCl (Fig. 1). All products showed higher than 95% antibacterial activity regardless of the strain of bacteria, indicating that the antibacterial activity of 10000 ppm of γ-PGA was as effective as the common known antibacterial materials.
Figure 1.
The inhibition of E. coli, S. aureus, and P. aeruginosa by different concentrations of γ-PGA compared with different commercial mouthwash products. (A) The x-axis shows the concentration of γ-PGA from 0 to 10000 ppm and mouthwashes containing chlorhexidine (CHX), sodium fluoride (NaF), and HOCl. The y-axis shows the antibacterial activity (%) of each concentration of γ-PGA or commercial mouthwash product. (B) The table lists the mean ± standard deviation for each testing condition.
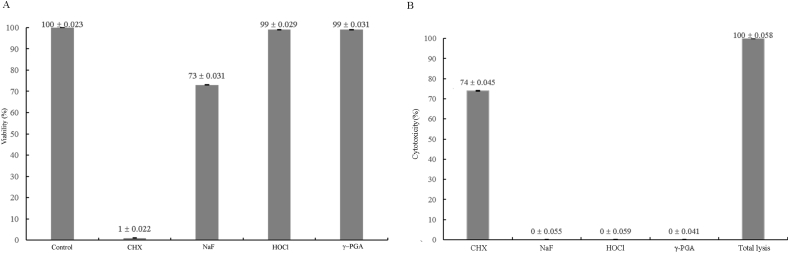
We next tested whether γ-PGA would affect cell survival or cause any toxicity in mouse embryonic fibroblasts (Fig. 2). The results showed that CHX mouthwash had the lowest viability (1.04%) and the viability of sodium fluoride mouthwash was 73.06% (Fig. 2A). Although CHX and sodium fluoride demonstrated efficient antibacterial activity, both materials did not promote cell viability. In contrast, the viability of HOCl— and γ-PGA-containing mouthwashes were higher than 98% indicating that both materials were sufficient for inhibiting bacterial growth and promoting cell viability. We then tested whether the high viability was due to low cytotoxicity. The results of the LDH assay demonstrated that indeed CHX caused high cytotoxicity (73.58%) while the cytotoxicity of sodium fluoride, HOCl, and γ-PGA was 0% (Fig. 2B). The results of the cell viability and cytotoxicity experiments indicated that γ-PGA did not cause any toxicity.
Figure 2.
γ-PGA shows high viability and low cytotoxicity. Mouthwashes containing sodium fluoride (NaF), HOCl or 10000 ppm γ-PGA show good cell viability (A) and low cytotoxicity (B) as tested by WST-8 (A) and LDH (B) assays, respectively.
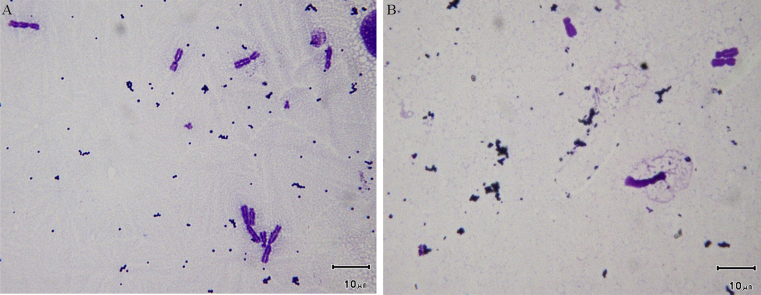
In addition, we investigated whether γ-PGA would cause any chromosomal abnormalities by observing the chromosomes in γ-PGA treated cells (Fig. 3). There were no abnormal chromosomes observed after cells were treated with 10000 ppm γ-PGA for 24 h (Fig. 3B) and there were no differences when compared to the negative control (Fig. 3A) indicating γ-PGA did not cause genotoxicity.
Figure 3.
No genotoxicity caused by γ-PGA. Chinese hamster ovary cells were treated either with culture medium (A) or 10000 ppm γ-PGA (B), and all chromosomes observed were normal. The scale bar demonstrates 10 μm.
Discussion
We investigated whether γ-PGA could be an antibacterial agent in the absence of alcohol for mouthwashes, and we tested its ability to inhibit the growth of three different kinds of bacteria. In addition, we tested whether γ-PGA would cause any cytotoxicity or genotoxicity in vitro. Our results showed that γ-PGA could indeed be an alternative antibacterial biomaterial for mouthwashes in the absence of alcohol.
We demonstrated that bacterial growth could be inhibited in the absence of γ-PGA. It was not surprising green peppermint oil could inhibit bacterial growth, since some herbal extracts have been shown to reduce dental plaque levels.11 However, the antibacterial activity was increased greatly in the presence of γ-PGA, especially when inhibiting the growth of S. aureus. Previous studies tested the antibacterial activity of γ-PGA when mixed with chitosan or magnetite nanoparticles, and the results showed that the mixtures could inhibit the growth of E. coli, S. aureus, or P. aeruginosa after culturing bacteria with the mixtures for 18–24 h.12 In this study, we tested the antibacterial activity of γ-PGA after culturing with bacteria for 18 h and found that 10000 ppm of γ-PGA had the same antibacterial effect as commercial mouthwashes. Further investigation needs to be done in order to determine whether γ-PGA can inhibit bacterial growth under this concentration for a shorter period of time.
CHX has been shown to cause cytotoxicity in canine embryonic fibroblasts and non-cytotoxic concentrations allowed the survival of bacteria.13 It has also been shown that CHX mouth rinses reduced the adhesion and proliferation of human gingival fibroblasts and keratinocytes.14 Although the safety of CHX-containing mouthwashes has been proven for short-term use or usage at lower concentrations,15,16 our results demonstrated that 10000 ppm of γ-PGA might be more suitable for mouthwashes due to its low cytotoxicity and genotoxicity.
When bacterial masses release metabolic products on the tooth surface, they are usually found on the margin of the gingival. The bacterial metabolic products then diffuse through the junctional epithelium and activate several host mechanisms including anti-inflammatory reactions.17 Anti-inflammatory cytokines, including interleukins (ILs), prostaglandins, tumor necrosis factor alpha (TNF-α), and matrix metalloproteinases, are produced from the gingival epithelium and enter into the connective tissue. The cytokines are thought to form a gradient of chemoattractant signals that guide the leukocytes to the location of the bacterial plaque for the consequent repair processes.18 It has been shown that γ-PGA can induce the production of IL-12p40 and IL-6 in mice when delivered as nanoparticles,19 and an increase of IL-10 has been observed when γ-PGA nanoparticles were delivered to human allergen-derived dendritic cells.20 Anti-inflammatory activities of γ-PGA have also been demonstrated when conjugated with l-phenylalanine ethylester in nanoparticles (γ-PGA-Phe-NPs), where the results showed that IL-1α, IL-1β, IL-6, and TNF-α were generated when γ-PGA-Phe-NPs were applied on rat middle ear mucosa.21 The mechanisms of how the γ-PGA-containing mouthwash inhibits the growth of bacteria and whether it has anti-inflammatory activity are still unknown, thus further investigation should be focused on these topics. However, the efficacy and safety of γ-PGA in mouthwashes shown here should be supportive of the fact that it is an alternative biomaterial for oral care.
Our studies have shown that 10000 ppm of γ-PGA can efficiently inhibit the growth of E. coli, S. aureus, and P. aeruginosa. Unlike some commercial mouthwash reagents such as CHX, γ-PGA was characterized to promote high cell viability with low cytotoxicity while being non-genotoxic. Our results demonstrated that γ-PGA is an alternative antibacterial biomaterial that can be potentially applied in mouthwashes in the absence of alcohol.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgement
This work and all the commercial products were supported by Purzer Pharmaceutical Co., Ltd (Taipei, Taiwan).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2019.01.002.
Contributor Information
Feng-Huei Lin, Email: double@ntu.edu.tw.
Hsu-Wei Fang, Email: hwfang@ntut.edu.tw.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chocolatewala N., Chaturvedi P., Desale R. The role of bacteria in oral cancer. Indian J Med Paediatr Oncol. 2010;31:126–131. doi: 10.4103/0971-5851.76195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanz M., Serrano J., Iniesta M., Santa Cruz I., Herrera D. Antiplaque and antigingivitis toothpastes. Monogr Oral Sci. 2013;23:27–44. doi: 10.1159/000350465. [DOI] [PubMed] [Google Scholar]
- 3.Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodontal Res. 2002;37:389–398. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani M.H., Asghari G., Hajiahmadi M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (Randomized double-blind controlled clinical trial) Dent Res J. 2011;8:S58–S63. [PMC free article] [PubMed] [Google Scholar]
- 5.Hong C.H., Napenas J.J., Hodgson B.D. A systematic review of dental disease in patients undergoing cancer therapy. Support Care Canc. 2010;18:1007–1021. doi: 10.1007/s00520-010-0873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarvizadeh M., Hemati S., Meidani M., Ashouri M., Roayaei M., Shahsanai A. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv Biomed Res. 2015;4:44. doi: 10.4103/2277-9175.151254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joyston-Bechal S., Hayes K., Davenport E.S., Hardie J.M. Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive programme using chlorhexidine and fluoride. Caries Res. 1992;26:384–390. doi: 10.1159/000261473. [DOI] [PubMed] [Google Scholar]
- 8.Shih I.L., Van Y.T. The production of poly-(gamma-glutamic acid) from microorganisms and its various applications. Bioresour Technol. 2001;79:207–225. doi: 10.1016/s0960-8524(01)00074-8. [DOI] [PubMed] [Google Scholar]
- 9.Wahab R., Khan F., Mishra Y.K., Musarrat J., Al-Khedhairy A.A. Antibacterial studies and statistical design set data of quasi zinc oxide nanostructures. RSC Adv. 2016;6:32328–32329. [Google Scholar]
- 10.Wahab R., Khan S.T., Dwivedi S., Ahamed M., Musarrat J., Al-Khedhairy A.A. Effective inhibition of bacterial respiration and growth by CuO microspheres composed of thin nanosheets. Colloids Surfaces B Biointerfaces. 2013;111:211–217. doi: 10.1016/j.colsurfb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Gupta D., Nayan S., Tippanawar H.K. Are herbal mouthwash efficacious over chlorhexidine on the dental plaque? Pharmacogn Res. 2015;7:277–281. doi: 10.4103/0974-8490.155874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J.M., Yang J.H., Huang H.T. Chitosan/polyanion surface modification of styrene-butadiene-styrene block copolymer membrane for wound dressing. Mater Sci Eng C Mater Biol Appl. 2014;34:140–148. doi: 10.1016/j.msec.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez I.R., Nusbaum K.E., Swaim S.F., Hale A.S., Henderson R.A., McGuire J.A. Chlorhexidine diacetate and povidone-iodine cytotoxicity to canine embryonic fibroblasts and Staphylococcus aureus. Vet Surg. 1988;17:182–185. doi: 10.1111/j.1532-950x.1988.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 14.Balloni S., Locci P., Lumare A., Marinucci L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol Vitro. 2016;34:88–96. doi: 10.1016/j.tiv.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Najafi M.H., Taheri M., Mokhtari M.R. Comparative study of 0.2% and 0.12% digluconate chlorhexidine mouth rinses on the level of dental staining and gingival indices. Dent Res J. 2012;9:305–308. [PMC free article] [PubMed] [Google Scholar]
- 16.Quirynen M., Avontroodt P., Peeters W., Pauwels M., Coucke W., van Steenberghe D. Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J Clin Periodontol. 2001;28:1127–1136. doi: 10.1034/j.1600-051x.2001.281207.x. [DOI] [PubMed] [Google Scholar]
- 17.Kornman K.S., Page R.C., Tonetti M.S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Cekici A., Kantarci A., Hasturk H., Van Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uto T., Wang X., Sato K. Targeting of antigen to dendritic cells with poly(gamma-glutamic acid) nanoparticles induces antigen-specific humoral and cellular immunity. J Immunol. 2007;178:2979–2986. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 20.Broos S., Lundberg K., Akagi T. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine. 2010;28:5075–5085. doi: 10.1016/j.vaccine.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson J.S., Broos S., Akagi T. Amphiphilic gamma-PGA nanoparticles administered on rat middle ear mucosa produce adjuvant-like immunostimulation in vivo. Acta Otolaryngol. 2014;134:1034–1041. doi: 10.3109/00016489.2014.918278. [DOI] [PubMed] [Google Scholar]
- 22.Jones C.G. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 23.Sutton S.V., Bender G.R., Marquis R.E. Fluoride inhibition of proton-translocating ATPases of oral bacteria. Infect Immun. 1987;55:2597–2603. doi: 10.1128/iai.55.11.2597-2603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna S.M., Davies K.J. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem J. 1988;254:685–692. doi: 10.1042/bj2540685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronroos L., Matto J., Saarela M. Chlorhexidine susceptibilities of mutans streptococcal serotypes and ribotypes. Antimicrob Agents Chemother. 1995;39:894–898. doi: 10.1128/aac.39.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.