Abstract
Background
Exposure to anesthetics during early life may impair cognitive functions. However, the underlying mechanisms remain largely unknown. We set out to determine effects of sevoflurane anesthesia on folate metabolism and myelination in young non-human primates, mice and children.
Methods
Young rhesus macaque and mice received 2.5 to 3% sevoflurane daily for three days. DNA and RNA sequencing and immunohistochemistry among others were used in the studies. We performed unbiased transcriptome profiling in prefrontal cortex of rhesus macaques and mice after the sevoflurane anesthesia. We constructed a brain blood barrier-crossing AAV-PHP.EB vector to harbor ERMN expression in rescue studies. We measured blood folate levels in children after anesthesia and surgery.
Findings
We found that thymidylate synthase (TYMS) gene was downregulated after the sevoflurane anesthesia in both rhesus macaque and mice. There was a reduction in blood folate levels in children after the anesthesia and surgery. Combined with transcriptome and genome-wide DNA methylation analysis, we identified that ERMN was the primary target of the disrupted folate metabolism. Myelination was compromised by the anesthesia in the young mice, which was rescued by systematic administration of folic acid or expression of ERMN in the brain through brain-specific delivery of the adeno-associated virus. Moreover, folic acid and expression of ERMN alleviated the cognitive impairment caused by the sevoflurane anesthesia in the mice.
Interpretation
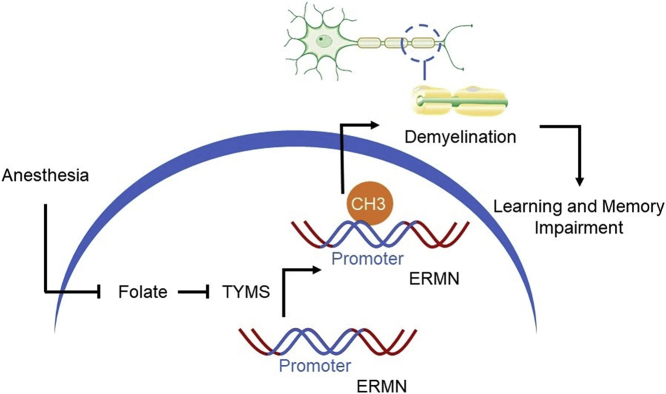
General anesthesia leads to disrupted folate metabolism and subsequently defects in myelination in the developmental brain, and ERMN is the important target affected by the anesthesia via epigenetic mechanisms.
Keywords: Sevoflurane, Folate, Methylation, ERMN, Myelination, Neurotoxicity
Graphical abstract
Research in context.
Evidence before this study
Millions of children in the United States have surgery under anesthesia every year. Clinical studies show that children who undergo multiple exposures to anesthesia and surgery (anesthesia/surgery) at an earlier age have increased the risk of developing cognitive impairment. Moreover, there have been many studies showing that anesthetics induce cognitive impairment, apoptosis, synaptic deficiency, neuroinflammation, Tau phosphorylation, and other structure and functional changes in the brains of young animals. However, the mechanism of the anesthesia neurotoxicity in the developmental brain remains mostly unknown.
Added value of study
Thymidylate synthase (TYMS) gene, a critical gene implicated in folate metabolism, was downregulated after the multiple exposures of the sevoflurane anesthesia in young rhesus macaques and mice, the blood folate levels decreased after surgeries under general anesthesia in children, ERMN, an essential gene involved in myelination, was the primary target of the disrupted folate metabolism. Finally, the sevoflurane anesthesia compromised myelination in the brain during the early postnatal stage in young mice, which was rescued by systematic administration of folic acid or expression of ERMN in the brain through brain-specific delivery of the adeno-associated virus.
Implications of all the available evidence
This study indicates that general anesthesia during the early postnatal period leads to disrupted folate metabolism and subsequently defects in myelination in the young brain, and the myelination-related gene ERMN is the crucial target gene affected by the general anesthesia via epigenetic mechanisms. Importantly, administration of folate acid can rescue the myelination defects as well as the cognitive impairments induced by the sevoflurane anesthesia.
Alt-text: Unlabelled Box
1. Introduction
The widespread and growing use of anesthesia in children makes its safety a significant health issue of interest. Each year, millions of children in the United States have surgery under anesthesia (anesthesia/surgery). Clinical investigations demonstrated that children who had anesthesia/surgery might experience an increased risk of developing cognitive impairment and changes in brain structures ([[1], [2], [3], [4]], reviewed in [5,6]). Specifically, children less than four years old who had received three or more, but not one, exposures to anesthesia/surgery could be at higher risk to develop cognitive impairments before the age of 15 [1,7]. However, a single and short duration exposure to anesthesia/surgery might not be associated with the increased risk of developing cognitive impairment in children [8,9]. A recent prospective study showed that children who had multiple exposures of anesthesia and surgery had impairments in processing speed and fine motor abilities, but not significant reductions in the intelligence quotient [10]. Similarly, animal studies have shown that multiple, but not single, exposures of anesthesia in the young mice can induce neuroinflammation [11], Tau phosphorylation [12], and synaptic dysfunction [13,14]. Collectively, multiple exposures of anesthesia and surgery are needed to cause the anesthesia neurotoxicity in developing brain.
Besides, it has been reported that general anesthetics induce cognitive impairment and neurotoxicity in young animals, including non-human primate [[15], [16], [17], [18]]. Animal studies showed that exposure to 1%–1.5% isoflurane for 5 h in infant rhesus macaques induced neurotoxicity at one year of age in the rhesus macaques [15]. Furthermore, the epigenetic change such as methylation was observed in multiple, but not single, exposures to anesthesia in animals [19]. Neural-related gene silencing caused by DNA methylation was suggested to contribute to the general anesthesia-induced neurotoxicity in rhesus macaque [20,21]. Therefore, in the current study, we combined with transcriptome and genome-wide DNA methylation analysis to investigate the epigenetic mechanism, including ERM-like protein (ERMN) gene and thymidylate synthase (TYMS) gene, of the anesthesia-induced neurotoxicity in the prefrontal cortex of infant rhesus macaques and mice.
Non-neuronal cells include glia, ependymal and epithelial cells, and pericytes. Glial cells are generally classified into microglia, astrocytes, oligodendrocytes [22]. Previous studies showed that anesthetic isoflurane was able to impair oligodendrocytes of the rhesus macaque [18,23,24]. Specifically, isoflurane anesthesia caused oligodendrocyte apoptosis in neonatal and fetal rhesus macaque brain. Besides, neonatal monkeys exposed to anesthetic isoflurane repetitively exhibited deficits in motor reflex and elevated anxiety levels at one month of age [15].
Sevoflurane, the most commonly used anesthetic in children, also led to visual recognition memory impairment and anxiety behaviors in infant rhesus macaque [16]. These findings suggest that repetitive or prolonged anesthesia exposures during early postnatal brain development stages caused cognitive impairment and anxiety-like behaviors in rodents or non-human primate. Moreover, these results indicated the need for further research into anesthesia neurotoxicity in the developing brain. Thus, employing sevoflurane, we set out to study the molecular mechanism underlying the anesthesia neurotoxicity in young rhesus macaques and mice and to explore the potential therapeutic interventions in the rodent and non-human primate models.
The objective of the studies was to determine the effects of sevoflurane anesthesia on folate metabolism in the brain tissues of young rhesus macaque and mice, and in the blood of children. The hypothesis to be tested in the present studies was that sevoflurane anesthesia in early postnatal time disrupted the folate metabolism, leading to defects in myelination and cognitive impairment, via epigenetic mechanisms, including ERMIN, a gene involved in myelination [25], and TYMS, a gene involved in folate-mediated one-carbon metabolism pathway [26,27].
2. Materials and methods
We carried out clinical studies in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). We performed all of the animal experiments complying with National Institutes of Health guide for the care and use of Laboratory animals.
2.1. Rhesus macaque and mice anesthesia
The animal studies were performed according to the guidelines and regulations of the Institute of Laboratory Animal Science, Peking Union Medical College and Chinese Academy of Medical Science (Beijing, China). Efforts were made to minimize the number of animals in the studies. The use of rhesus macaque in research at the Institute of Laboratory Animal Science was approved by the Institutional Animal Care and Use Committee (Protocol number #XC17001). The rhesus macaques were purchased from the Institute of Laboratory Animal Science, Peking Union Medical College and Chinese Academy of Medical Science (Beijing, China). There were three female rhesus macaques in the control group, and two female and one male rhesus macaques in the anesthesia group. Given the limited number of rhesus macaques in the studies, we did not determine the potential sex difference of the anesthesia neurotoxicity in the rhesus macaques. The rhesus macaques received 6%–8% anesthetic sevoflurane with 100% oxygen for the induction (2 to 4 min) of the general anesthesia, and then received 2.5%–3% sevoflurane and 100% oxygen with endotracheal intubation for 4 h for the maintenance of the general anesthesia. All the animals returned to their mothers in the cages after the anesthesia. The temperatures of the rhesus macaques were maintained by placing the rhesus macaques in a warm box (37 °C). The rhesus macaques received the sevoflurane anesthesia on postnatal day (P) 7 and then on 21 and 35 days. The heart rate and peripheral capillary oxygen saturation (SpO2) were monitored. The rhesus macaque in the control group received three times of the maternal separations with the same time of duration (4 h). The values of heart rate and SpO2 of the rhesus macaques under the sevoflurane anesthesia were not significantly deviated from the values observed in the awake rhesus macaques of the control group (Fig. S4). After a 30 min recovery from the anesthesia, the rhesus macaques were returned to their cage accompanied by their mother(s). We only used sevoflurane, but not other anesthetics, in the current studies. We killed rhesus macaques by decapitation under brief sevoflurane anesthesia (3% sevoflurane for 5 min) and harvested the prefrontal cortex of the rhesus macaques at the end of the third sevoflurane anesthesia on P35. C57BL/J6 mice (Shanghai SLAC Laboratory Animal, Zhangjiang Area, Shanghai, P. R. China) at P6 were used in the studies. The animal protocol was approved by the Institutional Animal Care and Use Committee at Shanghai Ninth People's Hospital, Shanghai, China. The mice were obtained from our breeding. The mice were housed in a temperate- and humidity-controlled environment (20–22 °C; 12-hour light: dark on a reversed light cycle) with free access to water and food. Given it was challenging to identify the sex in the young mice, we did not allocate the equal number of female and male mice in the anesthesia or control group and did not determine the sex-dependent effects of anesthesia neurotoxicity in the young mice in the present studies. The mice received the sevoflurane anesthesia as described in the previous studies [11,12]. Specifically, the mice received the anesthesia with 3% sevoflurane with 60% O2 at the flow rate of 1 L/min for 2 h daily with three days on P6, P7, and P8 in an anesthetizing chamber. The mice in the control group received 60% O2 at identical flow rates and in an identical anesthetizing chamber. The anesthetizing chamber was placed in a warm box to maintain the rectal temperature of the mice at 37 ± 0.5 °C. We used the anesthesia with 3% sevoflurane plus 60% oxygen because the previous studies [11,12,14,28] had demonstrated that such anesthesia-induced cognitive impairment in the young mice. There was no death of mice or rhesus macaque in the current studies. The mice were decapitated under brief anesthesia (3% sevoflurane for 3 min) for the harvest of the cortex and olfactory bulb at the end of the anesthesia on P8, P14 or P30. A different group of the mice was tested for spatial learning and memory function in Morris Water Maze from P30 to P34.
2.2. Morris water maze
Morris water maze (MWM) experiments were performed using the methods described in our previous studies. Specifically, the MWM test was performed in a circular tank (diameter 1.8 m) filled with opaque water. A platform (diametric distance, 10 cm) was submerged below the water surface in the center of the target quadrant. A black curtain surrounded the pool. The temperature of the water in the pool was kept at 20 °C. We tested the P30 mice in the MWM for five days (P30 to P34) with four trials daily. The water maze and the video recording device was used to track the swimming path of each mouse, and the data were analyzed by software (Coulbourn instruments, Holliston, MA). Both escape latency (the time for the mouse to reach the platform) in the MWM training from P30 to P34 and platform-crossing times (the counts the mouse moved across the original area of the removed platform) in MWM probe test on P34 were measured. Mouse body temperature was maintained by using a heating device to dry the mice before putting them back to the home cage.
2.3. Construction of RNA sequencing libraries and sequencing
Total RNA was extracted from the samples by Trizol reagent (Invitrogen) separately. The RNA quality was checked by Agilent 2200 and kept at −80 °C. The RNA with RIN (RNA integrity number) >7.0 is acceptable for cDNA library construction. A TruSeq Stranded Total RNA with Ribo-Zero Gold kit (Illumina, USA) was used to prepare the RNA-seq library. rRNA was removed, and strand-specific RNA-Seq libraries were prepared following the manufacturer's instructions. Briefly, ribosome depleted RNA was fragmented and then used for first- and second-strand complementary DNA (cDNA) synthesis with random hexamer primers. The dUTP mix was used for second-strand cDNA synthesis, which allows for the removal of the second strand. The cDNA fragments were end repaired, A-tailed and ligated with indexed adapters. The ligated cDNA products were purified and treated with uracil DNA glycosylase to remove the second-strand cDNA. Purified first-strand cDNA was subjected to PCR amplification, and the libraries were quality controlled with a Bioanalyzer 2200 (Agilent, Santa Clara, CA) and sequenced by HiSeq X (Illumina, San Diego, CA) on a 150 bp paired-end run.
2.4. RNA sequencing mapping
Before read mapping, clean reads were obtained from the raw reads by removing the adaptor sequences and low-quality reads. The clean reads were then aligned to the Rhesus monkey genome (GCF_000772875.2, NCBI) using the Hisat2 [29]. HTseq was used to get gene counts, and RPKM method was used to determine the gene expression [29]. We applied EB-Seq algorithm [30] to filter the differentially expressed genes (DEGs), after the meaningful analysis, P-value and FDR analysis [31] under the following criteria: i) Fold Change >1.5 or <2/3; ii), P value<0.05, FDR < 0.05.
2.5. WGBS DNA library construction and sequencing
Genomic DNA was extracted and purified using the Qiagen DNeasy Kit. Then concentrations and sample quality were determined by NanoDrop. DNA libraries were prepared by following the Accel-NGS Methyl-Seq DNA Library kit. Generally, the protocol consists of the following steps: Extracted DNA was fragmented into 350 bp by Bioruptor. The fragmented DNA was purified and converted by bisulfite. Then the bisulfite-converted DNA was denatured and ligated with an adaptor. After extension of the primer annealed to the first adapter, a dsDNA adapter was ligated to the other end of the copied DNA, and the products were performed indexing PCR to create the final DNA libraries. The libraries were quality controlled with a Bioanalyzer 2200 (Agilent, Santa Clara, CA) and sequenced by HiSeq X (Illumina, San Diego, CA) on a 150 bp paired-end run.
2.6. Whole-genome bisulfite sequencing data processing
Raw sequence reads were quality-trimmed using trim galore. Then the clean data were aligned to the Rhesus monkey genome (GCF_000772875.2, NCBI) using Bismark [32]. After the removal of PCR duplicates, the numbers of methylated and unmethylated cytosines were counted for each CpG site. Differences in methylation between groups were measured using a logistic regression model built in methylKit [33], allowing us to identify the differentially methylated region (DMRs, 1000 bp) with at least 25% difference in methylation levels and a q value <0.01. Finally, the related genes and the genome features were assigned to the DMRs based on the ChIP seeker package [34].
2.7. Go analysis
Gene ontology (GO) analysis was performed to facilitate elucidating the biological implications of the differentially expressed genes or differentially methylated genes in the experiment [35]. We downloaded the GO annotations from NCBI (http://www.ncbi.nlm.nih.gov/), UniProt (http://www.uniprot.org/) and the Gene Ontology (http://www.geneontology.org/). Firstly, based on the GO database (such as NCBI/UNIPROT/SWISSPROT/AMIGO) and the differential genes, GO annotations were performed from three layers: Biological Process, Molecular Function, and Cellular Component. Then, we obtained the GO Term ID (usually known as Gene Ontology ID) of differential genes. Subsequently, we obtained the following indicators: “x”- the number of differential genes in the specified GO Term; “K”- the total number of differential genes; “n”- the total number of genes in the specified GO Term; “N”- the total number of genes.
The P value of each GO Term was calculated by Fisher's exact test, and p < 0.05 was used as a criterion for significant enrichment in order to screen out significantly enriched GO Terms. At the same time, the P value was corrected by false discovery rate (FDR) through the Benjamini-Hochberg (BH) algorithm. The lower FDR showed a more probable result, which was the significant level of GO Term. Besides, under the same P value, the GO term with larger enrichment retained a more significant influence on the experimental results. The formula for enrichment was as follows: (ng/Ng)/(na/Na).
2.8. Signed gene co-expression network analysis (WGCNA)
Signed gene co-expression network analysis [36] was performed across all tissue samples from BrainSpan (http://www.brainspan.org/static/download.html) using the standard method with a power of 6 to cluster the spatiotemporal-expression patterns. We then constructed the co-expression networks (weight > 0.1) based on the ERMN and related gene.
3. Clinical investigation
3.1. Study population
The study protocol was approved by the Human Research Ethics Committee of Shanghai 9th People's Hospital and Shanghai Jiao Tong University in Shanghai, P. R. China [SH9H-2018-T53–2, the clinical trial number is NCT03746340]. The parents of participants signed the informed consent before the enrollment in the study. We followed STROBE (Strengthening the Reporting of Observational studies in Epidemiology) to conduct this study. The inclusion criteria were: (1) children under 3 years old who had surgery under general anesthesia for the first time; (2) children with no significant past medical history and participation in other clinical trials in last 3 months; (3) American Society of Anesthesiologists (ASA) Physical Status I and II. The exclusion criteria were (1) severe liver and kidney diseases; (2) hemodynamic instability (hemorrhage shock or blood pressure drop >30% from baseline blood pressure); (3) egg and milk allergy; (4) family history of malignant hyperthermia; (5) short or long duration of anesthesia and surgery (longer than 120 min or shorter than 30 min). Accordingly, 30 patients were eligible to participate in the studies, and 6 of them declined to participate; thus, 24 patients were enrolled in the studies. Four patients were excluded from the studies due to the hemolysis of blood samples, and consequently, 20 patients (6 to 36 months old, 65% male) were included for the final data analysis. The flow diagram was shown in Fig. S1.
3.2. Anesthesia and surgery
All participants had surgery under sevoflurane anesthesia with standard perioperative care, including maintaining hemodynamic stability during operation and postoperative pain management. Specifically, the participants received the anesthetic sevoflurane (6% to 8%), together with fentanyl (0.003 mg/kg) and rocuronium (0.75 mg/kg) for the induction of anesthesia and endotracheal intubation. The participants received 2.0–2.5% of sevoflurane for the maintenance of anesthesia. We collected blood samples and measured the levels of folate in the blood. We only included the participants who had single surgery under anesthesia with the duration <120 min because the majority of the patients in our hospital have one surgery with the duration of fewer than 120 min. Therefore, we could be able to recruit sufficient participants without difficulties in the present studies. The arterial blood (2 milliliter) from each participant was collected immediately after the start of the general anesthesia and then at the end of the surgery. The blood was allowed to stand in the anti-coagulation tube at room temperature for 30 min and was then centrifuged to separate plasma, red blood cells and the buffy coat at 4 degree Celsius (C). The plasma was used to measure the levels of folate in the pathology lab of the hospital. Specifically, a Chemiluminescent Microparticle Folate Binding Protein assay, with the ARCHITECT Folate Reagent Kit (Abbott Laboratories, Chicago, IL, USA), was used to determine serum folate level.
3.3. Reverse transcription PCR and real-time quantitative PCR
Total RNA of the prefrontal cortex of the mice that had the sevoflurane anesthesia was extracted by Trizol (Invitrogen) according to the manufacturer's instructions and our previous studies. RNA was reversely transcribed into cDNA using the Prime-Script RT reagent Kit (Takara). Target genes were determined by using a standard SYBR-Green method on the ABI7500 Real-Time PCR System (Applied Bio-systems, USA) and normalized to the expression of GAPDH. PCR experiments were repeated three times, each using separate sets of cultures. The relative expression level of the target gene was presented as 2−∆∆Ct using the relative quantification method. The primer sequences used in this study were as follows: macaque ERMN:PF: 5′- CAAGAAACAAGTGCTGATGAAATGAC-3′, RF:5′- CTGGTGACCTTTGTTCTTCCTGTC-3′; macaque TYMS: PF: 5′- TGGGGCAGAATACAGAGATATGG-3′, RF:5′- TGATGGTGTCAATCACTCTTTGC-3′; mouse ERMN: PF: 5′- CCAACAGCCTAGCAGTCAAAC-3′, RF:5′- GGGTTCAACCTTGTAGTATGCCT-3′; mouse TYMS: PF: 5′- GGAAGGGTGTTTTGGAGGAGT-3′, RF:5′- GCTGTCCAGAAAATCTCGGGA-3′.
3.4. Western blotting
The brain tissues of different groups of mice were lysed in cell lysis buffer. The lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free dry milk and incubated with primary antibodies against ERMN (1:500 dilution, Biobyt #orb183427, UK), Thymidylate Synthase (1:2000 dilution, Abcam #ab7398, USA), DYKDDDDK Tag (1:1000 dilution, Cell Signaling Technology #14793, USA) and β-actin (1:1000 dilution, Cell Signaling Technology #3700, USA) at 4 °C overnight. The bound antibodies were detected with horseradish peroxidase-conjugated secondary anti-rabbit IgG (1:2000 dilution, Cell Signaling Technology #7074, USA), anti-mouse IgG (1:2000 dilution, Cell Signaling Technology #7076, USA), anti-goat IgG (1:2000 dilution, Santa Cruz #sc-2354, USA) and visualized using the enhanced chemiluminescence (ECL, Thermo Fisher, USA). The relative levels of the target protein to β-actin were determined by densitometric analysis using the Image J software.
3.5. Immunohistochemistry
Mice were perfused with 4% polyformaldehyde (PFA) in PBS. The brains of the mice were post-fixed for 24 h in the same fixative solution and were dehydrated in 15% and 30% sucrose in PBS in turn for 24 h. Serials of 40-μm-thick coronal sections were cut on a cryostat, and slices containing olfactory bulbs and cortex were collected and subjected to immunohistochemistry (IHC) staining. Fluorescent images were acquired using a Zeiss 780 confocal microscope (Andor Spinning disk dragonfly, Oxford instruments, Belfast, Northern Ireland) with a 10× objective lens. Fluorescent signals were visualized by using a laser wavelength of 561 nm for Alexa Fluor555, 488 nm for Alexa Fluor 488, and 405 nm for DAPI, respectively. The thickness of Z-stacking sections was 10 μm, and a montage of 2048 × 2048-pixel images was made of an entire unilateral olfactory bulb and bilateral cortex. The fluorescent quantification process was performed by using ImageJ software. Briefly, the region of interest (ROIs) was manually drawn around the fluorescent signals, and the fluorescent intensity was measured by using the ImageJ plugin ROI manager. The intensity of background with no signal in each slice was used as the reference value for normalizing data in all images. Integrated optical density (IOD) from each mouse was measured according to the methods described in the previous study [37].
3.6. Intracardial injection
Adeno-associated virus (AAV) (pAAV-CAG-EGFP-2A-ERMN-3FLAG), which can cross the blood-brain barrier through the blood circulation system, was purchased from Obio Technology Corp. (Shanghai, China) to over-express the levels of ERMN. It was carried out by the micropipette (0.3 milliliter, BD #328838, USA) and injected into the left ventricle of each of the mice. In each mouse, the injection was made into the slightly left of the middle of the chest (10 μl volume). AAVs (5.97 × 1012 VG/milliliter) were obtained from Obio Technology (Shanghai) Corp.
3.7. Statistics
The data obtained from clinical studies were presented as means ± SEM. The data obtained from molecular and biochemistry experiment and escape latency of MWM was presented as means ± SD. The platform crossing time of MWM was presented as medians with interquartile range. Student's t-test was used for the comparison between the mean of two samples. Paired t-test was used for the comparison of the same index before and after the time point. The values of Integrated optical density (IOD) of immunohistochemistry staining in four groups were analyzed using one-way analysis of variance (ANOVA) and the Tukey post hoc test (α = 0.05). Two-way analysis of variance (ANOVA) with repeated measurement was used to evaluate the difference of escape latency of the mice in different groups in the MWM test. Post hoc analysis was used to compare the change in escape latency of each group of every day during the MWM test, and cut-off α was adjusted using the Bonferroni method. Mann–Whitney U test was used to compare the difference in the platform crossing times of the mice in the different groups. The protein levels of ERMN and TYMS were found to have a normal distribution and analyzed by two-way ANOVA without repeated measurement to evaluate the interaction of different groups. The studies employed two-tailed hypothesis, and statistically significant P values were <0.05. We used the software SPSS 21.0 (SPSS Inc., IBM Corporation, Chicago, IL, USA) and Prism 6 (GraphPad, USA) to evaluate all of the data in the studies.
3.8. Data deposition
The sequencing data of mRNA and DNA methylation in prefrontal cortex of the rhesus macaques has been uploaded to a publicly available database. [The raw data was uploaded to Gene Expression Omnibus (GEO) DataSets: GSE119625].
4. Results
4.1. Sevoflurane anesthesia reduced TYMS and ERMN expression and was associated with a reduction in folate levels
Previous works showed that anesthesia induced myelination damage of the central nervous system and caused behavior changes (e.g., anxiety) in infant rhesus macaque [18,23,24]. To address the molecular mechanisms, we performed multiple exposures of sevoflurane anesthesia in infant rhesus macaque on a postnatal day 7 (P7), P21 and P35, lasting for 4 h each time [16]. After the anesthesia, we harvested prefrontal cortex of the rhesus macaques and performed the RNA sequencing, as well as whole-genome methylation analysis to examine whether and how the sevoflurane anesthesia affected the gene expression and epigenetic status genome-wide. We harvested the brain tissues of the young rhesus macaque immediately after the third sevoflurane anesthesia because the harvested brain tissues immediately following the anesthesia would be better for the detection of the changes in mRNA levels and preparation of the RNA-seq analysis based on the outcomes obtained from the previous studies [16]. We found that the thymidylate synthase coding gene TYMS was downregulated after the sevoflurane anesthesia among the genes altered (Fig. 1a), validated by real-time PCR using the cortex harvested from the rhesus macaque (Fig. 1b, p =0.013, N = 3, Student's t-test). TYMS is an essential gene for the folate-dependent enzyme and is involved in folate-mediated one-carbon metabolism pathway which takes part in DNA methylation by providing methyl donor [26,27]. Previous reports showed that folate and other metabolites were altered in patients after anesthesia [38]. Folate is a crucial source of the one-carbon group used to methylate DNA. Low folate status may lead to aberrant DNA methylation and result in misregulation of the expression of genes [39]. Since folate metabolism plays a critical role in regulating epigenetic regulation including DNA methylation, we wonder whether genomic methylation levels may also be altered in young animals after the anesthesia.
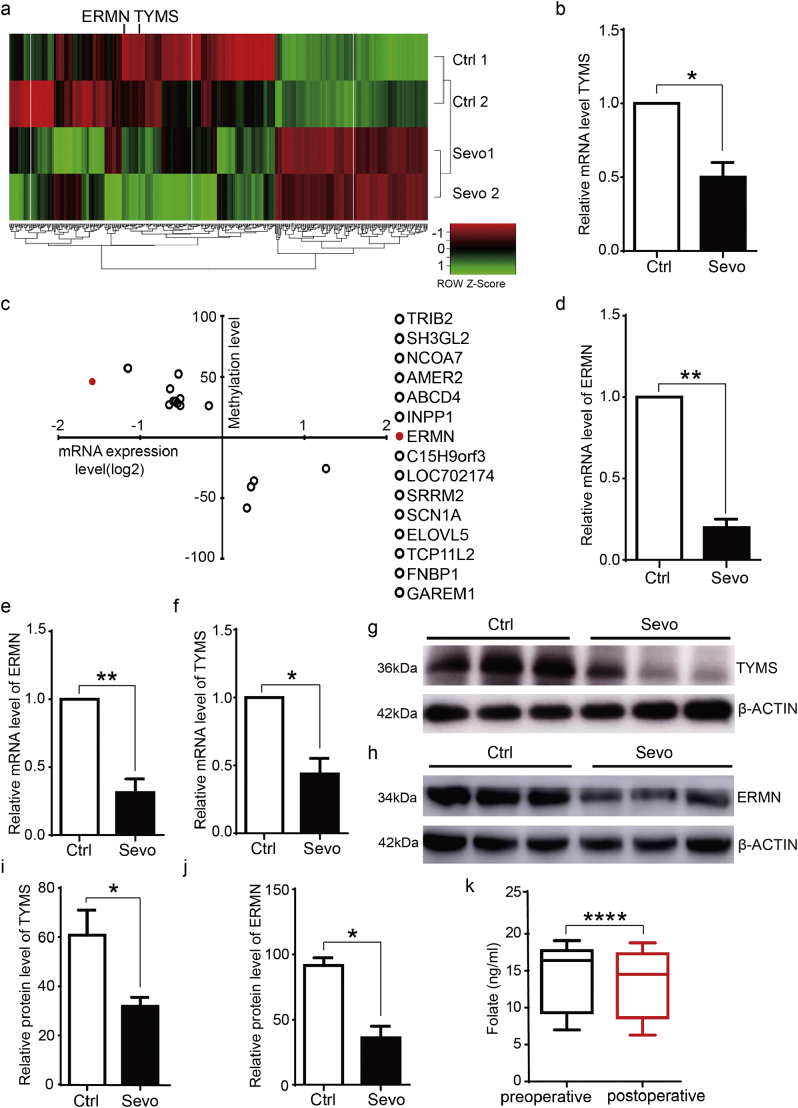
Fig. 1.
Sevoflurane reduced the expression of TYMS and ERMN in both macaque and mice prefrontal cortex and was associated with the reduction of folate levels in blood in children.
a. RNA sequencing assay indicated that the sevoflurane anesthesia induced the downregulation of TYMS and ERMN in the cortex of rhesus macaque (N =2).
b. The sevoflurane anesthesia decreased the mRNA level of TYMS detected by quantitative RT-PCR (N = 3). The data shown are the mean ± SD, p= 0.013, Student's t-test.
c. The whole genome methylation analysis showed that there was higher ERMN promoter methylation in the cortex of the rhesus macaque after the sevoflurane anesthesia as compared to that in the control condition (N = 2).
d. qRT-PCR assay confirmed the downregulation of ERMN mRNA levels in cortex tissues of rhesus macaque following the sevoflurane anesthesia as compared to those in the cortex tissues of rhesus macaque following the control condition (N = 3). The data shown are the mean ± SD, p = 0.0013, Student's t-test.
The down-regulation of ERMN (e) and TYMS (f) was detected by qPCR in the prefrontal cortex of P8 mice following the sevoflurane anesthesia as compared to the control condition. The data shown are the mean ± SD, p = 0.007 and p = 0.014, Student's t-test. Representative pictures showed that the sevoflurane anesthesia reduced the protein levels of TYMS (g) and ERMN (h), detected by Western blot analysis in the prefrontal cortex of P8 mice (N = 3).
The quantification of the Western blot in g and h showed that the sevoflurane anesthesia decreased the protein levels of TYMS (i, p = 0.022, N = 3, Student's t-test) and ERMN (j, p = 0.035, N = 3, Student's t-test).
k. There were postoperative reductions in the blood levels of folate in the participants who had surgery under sevoflurane anesthesia: (median: 14.50; interquartile range: 8.65, 17.33) versus (median: 16.40; interquartile range: 9.32, 17.75) (p < 0.0001, N = 20, Mann–Whitney U test).
The data shown are the mean ± SD, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. The Wilcoxon-Mann-Whitney test used for the analysis of the data that were not normally distributed for the comparison of two samples. A two-sided Student's t-test was used for the analysis of the data that were normally distributed for the comparison of two samples.
Control: ctrl.; sevoflurane: sevo.; ERM-like protein: ERMN; thymidylate synthase: TYMS.
Combining mRNA and methylation levels [the raw data was uploaded to Gene Expression Omnibus (GEO) Database: GSE119625], we identified several candidate genes with increasing methylation in promoter regions but decreased expression levels. These data suggest that DNA methylation, an important epigenetic pathway, played a critical role in regulating gene expression during the sevoflurane anesthesia (Fig. 1c). Among these candidate genes, we found that EAR-like protein (ERMN), a myelination-related gene, was significantly downregulated in rhesus macaque prefrontal cortex (Fig. 1c), confirmed by real-time PCR (Fig. 1d, p = 0.0013, N = 3, Student's t-test).
After confirming the downregulation of TYMS and ERMN in the prefrontal cortex of young rhesus macaques, we asked whether the protein levels of TYMS and ERMN were also regulated in young rodent models after the sevoflurane anesthesia. We treated P6 mice with 3% of sevoflurane two hours daily for three days (P6, P7, and P8) and then harvested the prefrontal cortex to examine the levels of mRNA and proteins. We found that the mRNA levels of TYMS and ERMN were consistently downregulated in the prefrontal cortex of the mice after the anesthesia [(Fig. 1e (p = 0.007, N = 3, Student's t-test), Fig. 1f (p = 0.014, N = 3, Student's t-test)]. Moreover, the sevoflurane anesthesia in mice also reduced the protein levels of TYMS and ERMN (Fig. 1g, h, i, and j) in the prefrontal cortex of the mice. This evidence indicated that the sevoflurane anesthesia decreased TYMS and ERMN levels in both rhesus macaques and mice, suggesting the evolutionally conserved mechanisms of the early developing brain in response to the sevoflurane anesthesia.
Since general anesthesia was able to disrupt the folate metabolism in rhesus macaques and mice, we examined whether anesthesia could also alter folate metabolism in humans. A total of 20 participants (6 to 36 months old, 65% male) were enrolled in the clinical study (Table S1, Fig. S1). All participants had elective surgeries under general anesthesia with sevoflurane for the duration <120 min (Table S2). We found that there were reduced folate levels in the blood of the children who had surgery under sevoflurane anesthesia (Fig. 1k, 14.50 ng/milliliter versus 16.40 ng/milliliter, p < 0.0001, N = 20, Mann–Whitney U test). These data suggest the potential impairment of folate metabolism after the sevoflurane anesthesia in young patients.
4.2. Sevoflurane anesthesia influenced the myelination-related process and signaling pathway in rhesus macaque prefrontal cortex
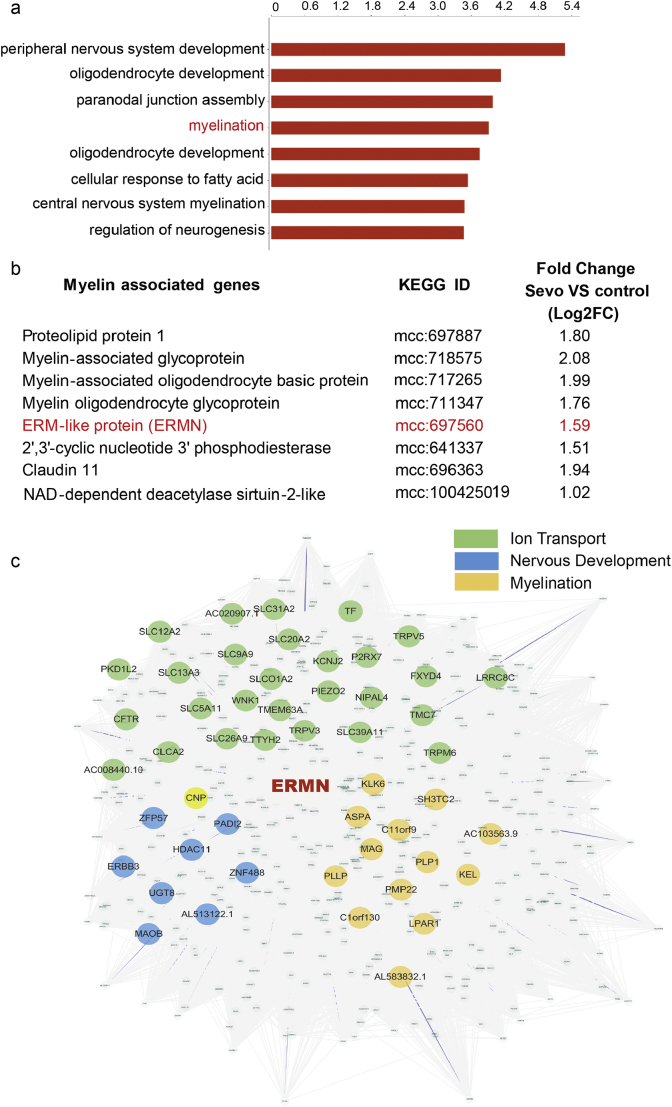
With Gene Ontology analysis of RNA-seq data in the prefrontal cortex of rhesus macaques, we found that the sevoflurane anesthesia might influence the myelination-related physiological process (Fig. 2a, Fig. S2). Numerous myelin-associated genes were significantly reduced by the sevoflurane anesthesia, including ERMN (Fig. 2b). Notably, the previous studies reported an association between childhood exposure to multiple anesthesia/surgery and the increased risk of learning disability and attention-deficit/hyperactivity disorder (ADHD) [1,7,40,41]. Myelin damage in white matter microstructure is associated with ADHD [42,43]. We, therefore, performed signed gene co-expression network analysis (WGCNA) [36] which crossed all tissue samples from BrainSpan (http://www.brainspan.org/static/download.html) using the standard method with a power of 6 to cluster the spatiotemporal-expression patterns. We then constructed the co-expression networks (weight > 0.1) based on the ERMN and related gene. WGCNA indicated that ERMN was related to the myelination-associated regulatory genes, the ion transport, and central nervous system development in humans (Fig. 2c). These data suggest that ERMN could be involved in the regulation of brain development and function.
Fig. 2.
Sevoflurane anesthesia affected the myelination-related physiological process and signaling pathway in macaque prefrontal cortex.
a. The sevoflurane anesthesia influenced the myelination-related physiological process in rhesus macaque prefrontal cortex as detected by Gene ontology (GO) analysis.
b. The sevoflurane anesthesia decreased the myelination-related gene expression in the rhesus macaque prefrontal cortex by Gene Ontology (GO) analysis.
c. Signed gene co-expression network analysis (WGCNA) demonstrated that the downregulation of ERMN was associated with the gene expression of myelination relation, ion transport, and nervous system development.
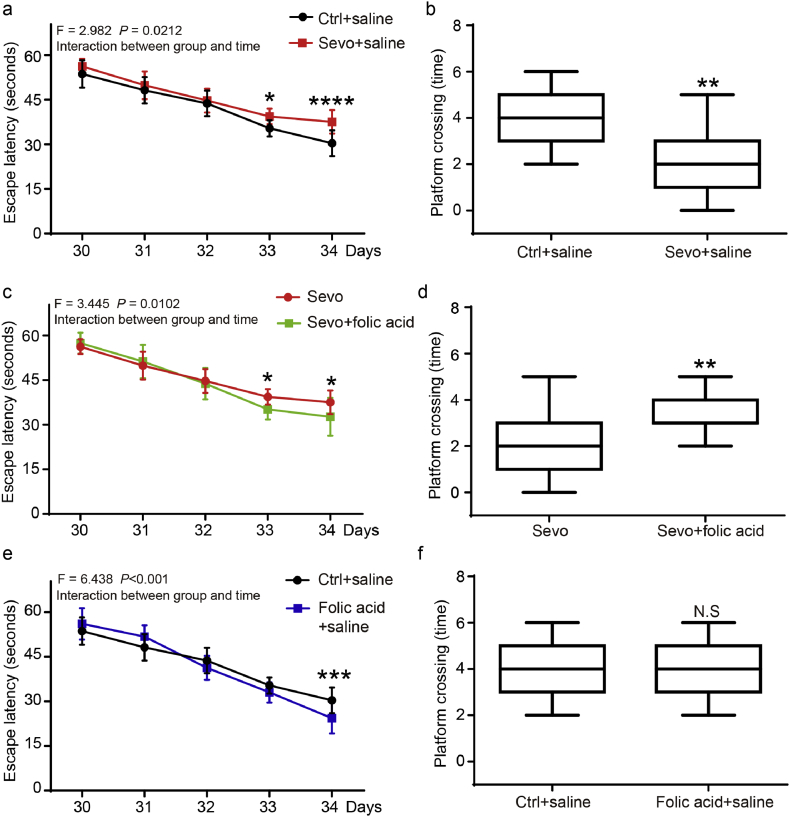
4.3. Folic acid mitigated the sevoflurane anesthesia-induced damage of the myelin sheath and cognitive impairment in young mice
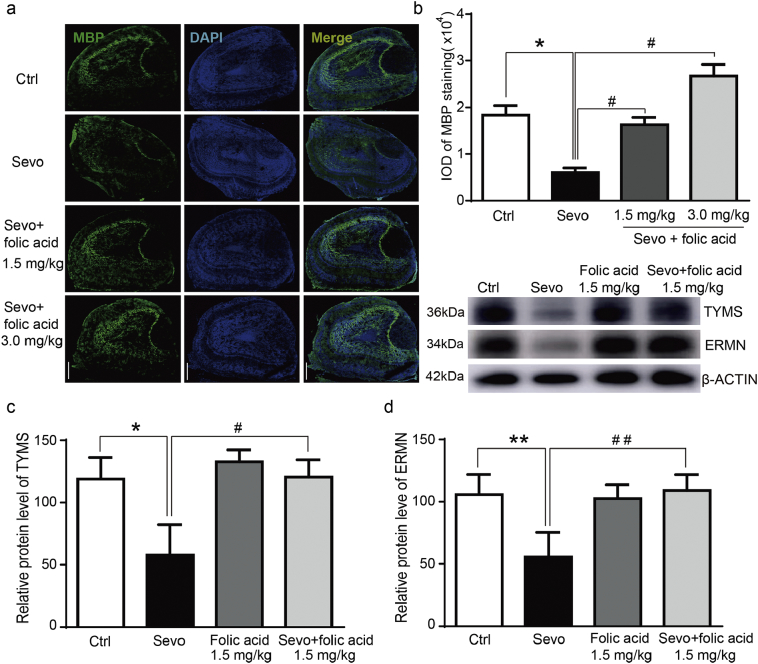
Folic acid is a clinically available compound and is widely used in various conditions. Therefore, we asked whether folic acid could mitigate the sevoflurane anesthesia-induced changes in mice. We injected folic acid intraperitoneally with two doses (1.5 mg/kg, 3.0 mg/kg) into the mice at 1 h before the sevoflurane anesthesia on P6, P7, and P8. We then performed Western blotting to detect the protein level of ERMN and TYMS at P8. We also used immunohistochemistry staining to detect the myelination markers myelin basic protein (MBP) at P14 and employed Morris Water Maze (MWM) to assess the cognitive function at P30 to P34. We found that the treatment with folic acid in two doses significantly rescued the deficits in myelination after the sevoflurane anesthesia by assessing myelination under various conditions via immunohistochemistry staining of MBP protein in olfactory bulbs [(Fig. 3a), (Fig. 3b, crtl + saline vs. sevo + saline: p = 0.012, N = 3, one-way ANOVA; sevo + saline vs. sevo + folic acid 1.5 mg/kg: p = 0.018; sevo + saline vs. sevo + folic acid 3.0 mg/kg: p = 0.019; N = 3, one-way ANOVA)]. We also found that folic acid (1.5 mg/kg) restored the decreased protein level of the ERMN and TYMS caused by the sevoflurane anesthesia in the prefrontal cortex of mice [(Fig. 3c), (Fig. 3d, sevo + saline vs. sevo + folic acid 1.5 mg/kg: p = 0.007, N = 3, two-way ANOVA), (Fig. 3e, sevo + saline vs. sevo + folic acid 3.0 mg/kg: p = 0.002; N = 3, two-way ANOVA)]. These data indicated that folic acid played an important role in regulating myelination process affected by the sevoflurane anesthesia during early life stages.
Fig. 3.
Folic acid mitigated the damage of the myelin sheath and cognitive impairment in mice caused by the sevoflurane anesthesia.
a. Immunofluorescence staining of myelin basic protein (MBP) indicated that folic acid (1.5 mg/kg and 3.0 mg/kg) mitigated the sevoflurane anesthesia-induced damage of the myelin sheath in the young mice olfactory bulb at P14. Scale bar indicates 100 μm.
b. The values of Integrated optical density (IOD) showed the quantification of MBP level of immunofluorescence staining (N = 3). Sevoflurane anesthesia reduced the MBP levels in the young mice olfactory bulb as compared to the control condition (p = 0.012, one-way ANOVA). Both 1.5 mg/kg and 3.0 mg/kg folic acid attenuated the sevoflurane-induced damage of the myelin sheath as demonstrated by the immunofluorescence staining of the myelin basic protein (MBP) level (p = 0.018 and p = 0.019, one-way ANOVA). The values of Integrated optical density (IOD) of MBP staining in four groups were analyzed by using one-way analysis of variance (ANOVA) and the Bonferroni post hoc test (α = 0.05). c. Western blotting indicated that folic acid mitigated the sevoflurane-induced decrease in the protein levels of TYMS and ERMN. Quantification of the Western blot in Fig. 3c showed the protein levels of TYMS (Fig. 3d) and (Fig. 3e) ERMN (N = 3). Folic acid attenuated the sevoflurane anesthesia-reduced changes in TYMS protein levels in the prefrontal cortex of the P8 mice (F = 11.28, p = 0.007, two-way ANOVA). Folic acid also attenuated the sevoflurane anesthesia-induced changes in the ERMN protein levels in the prefrontal cortex of the P8 mice (F = 17.1, p = 0.002, two-way ANOVA). The data were analyzed by using a Two-way ANOVA and a Bonferroni post hoc test (α = 0.05). Control: ctrl.; sevoflurane: sevo.; ERM-like protein: ERMN.; thymidylate synthase: TYMS.
Furthermore, we wonder whether folic acid could be able to alleviate the cognitive defects in young animals caused by the sevoflurane anesthesia. We measured spatial memory in Morris Water Maze from P30 to P34. Two-way ANOVA with repeated measurement showed the significant interaction of anesthesia (sevoflurane versus control) and time (P30 to P34), and the sevoflurane anesthesia induced cognitive impairment as evidenced by increases in the escape latency and decrease in the platform crossing time in previous target platform [(Fig. 4a, ctrl + saline vs. sevo + saline, p = 0.0212, two-way ANOVA), (Fig. 4b, ctrl + saline vs. sevo + saline, p = 0.006, Mann–Whitney U test), N = 15]. Folic acid (1.5 mg/kg) mitigated the cognitive impairment induced by the sevoflurane anesthesia [(Fig. 4c, sevo vs. sevo + folic acid, p = 0.0102, two-way ANOVA); (Fig. 4d, sevo vs. sevo + folic acid, p = 0.0049, Mann–Whitney U test) N = 15]. Folic acid improved learning and memory function by increasing the escape latency than the saline control group (Fig. 4e, ctrl + saline vs. folic acid + saline, p < 0.001, N = 15, two-way ANOVA). There was no significant difference in the platform crossing time between the mice in the folic acid group and the mice in the control group (Fig. 4f, ctrl + saline vs. folic acid + saline, p = 0.217, N = 15, Mann–Whitney U test). These data demonstrated the potential use of folic acid in the prevention or treatment of the cognitive impairment associated with anesthesia and surgery in children pending further investigation.
Fig. 4.
Folic acid treatment attenuated the cognitive impairment caused by sevoflurane anesthesia.
a. Two-way ANOVA with repeated-measurement analysis showed a significant interaction of anesthesia (ctrl + saline vs. sevo + saline) and time (days 30 to 34) on the escape latency of MWM (F = 2.982, p = 0.0212*, N = 15, two-way ANOVA). A post-hoc test (Bonferroni) showed that the mice in sevoflurane anesthesia group took a longer period (escape-latency) to locate the position of a platform in the MWM test as compared to the control mice at P33 and P34.
b. The mice that received the sevoflurane anesthesia also demonstrated fewer platform-crossing-times as compared to the mice that received the control condition (p = 0.006**, N = 15, Mann–Whitney-U test).
c. A two-way ANOVA with repeated-measurement analysis showed a significant interaction between anesthesia (sevo + folic acid vs. control + sevo) and time (days 30 to 34) on the escape latency of MWM (F = 3.445, p = 0.0102*, N = 15, two-way ANOVA). Post-hoc test (Bonferroni) showed that the mice in the sevo plus folic acid group were able to locate the position of a platform within a shorter time (escape latency) compared to the mice in the sevoflurane alone at P33 and P34.
d. The folic acid mitigated the sevoflurane anesthesia-induced decrease in the platform-crossing times (p = 0.0049**, Mann–Whitney-U test, N = 15).
e. A two-way ANOVA with repeated-measurement analysis showed a significant interaction between treatment (ctrl + saline vs. folic acid + saline) and time (days 30 to 34) on the escape latency of MWM (F = 6.438, p < 0.001***, N = 15, two-way ANOVA). Post-hoc test (Bonferroni) showed that the mice in the folic acid group were able to locate the position of a platform in a shorter time (escape latency) compared to the mice in the saline group at P34.
f. There is no significant difference between the mice in the saline group and the mice in the folic acid group in platform-crossing-times (p = 0.217, Mann–Whitney-U test, N = 15).
(Control: ctrl.; sevoflurane: sevo.; MWM: Morris Water Maze.)
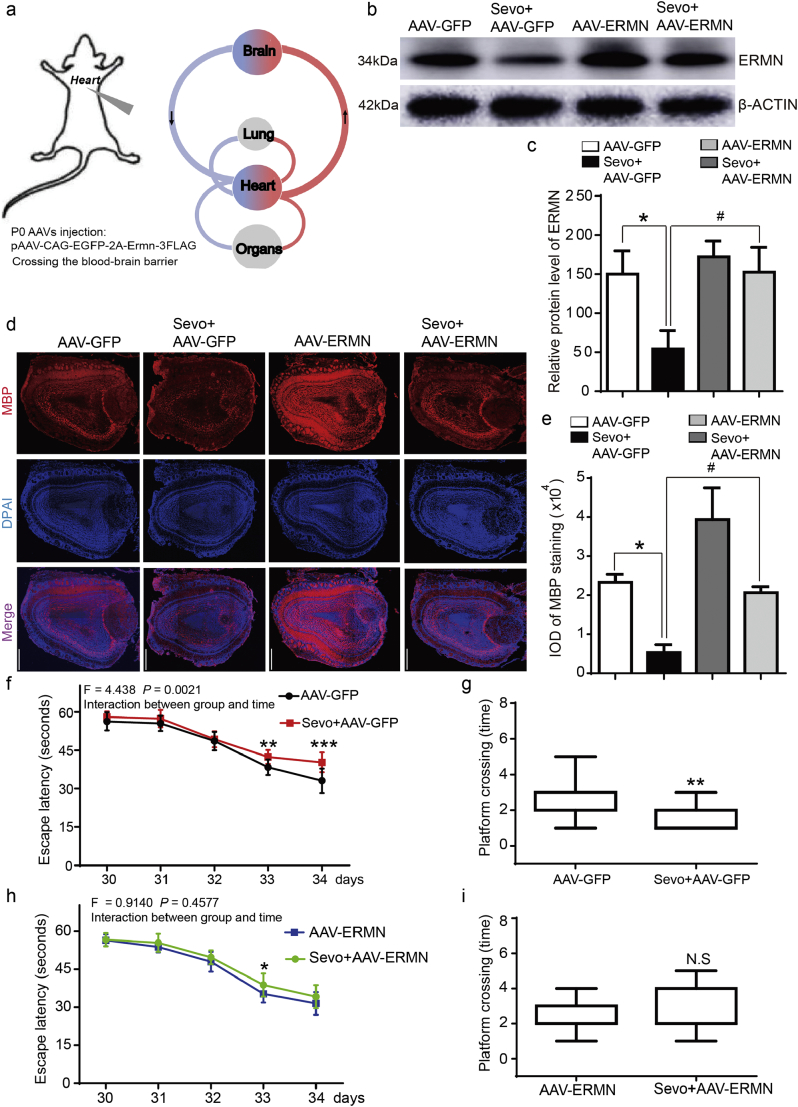
4.4. Restoration of ERMN signaling alleviated the damage of myelin sheath and cognitive impairment induced by the sevoflurane anesthesia
To examine whether re-introducing ERMN could be able to rescue the defects of myelination, we constructed a brain blood barrier (BBB)-crossing AAV-PHP.EB vector harboring GFP or ERMN expression cassette (Fig. 5a). After injection of AAV-PHP.EB vectors into the heart at P0, GFP or ERMN was able to cross the BBB and was expressed in the brain (Fig. S3). Western blots confirmed the over-expression of the ERMN in the brain tissues of the mice at P8 (Fig. 5b, Fig. 5c, sevo +AAV-GFP vs. sevo +AAV-ERMN, p = 0.0075, N = 3, two-way ANOVA).
Fig. 5.
Restoration of ERMN signaling alleviated the defects of the myelin sheath and cognitive impairment induced by sevoflurane anesthesia.
a. The diagram showed the process of ectopic expression of ERMN from the AAV-PHP.EB vectors virus crossing the blood-brain barrier.
b. Western blot detection of the ERMN overexpression in the brains of mice treated by sevoflurane anesthesia or control condition at P8.
c. Quantification of the western blot assay in Fig. 5b showed the ERMN overexpression attenuated the sevoflurane anesthesia-induced reduction in the ERMN protein levels in the young mice prefrontal cortex at P8 (F = 10.97, p = 0.0075##, two-way ANOVA).
d. The ERMN overexpression attenuated the sevoflurane anesthesia-induced damage of the myelin sheath in the young mice olfactory bulb at P14.
e. The values of Integrated optical density (IOD) showed the quantification of MBP level of immunohistochemistry staining (N = 3). The ERMN overexpression attenuated the sevoflurane anesthesia-induced damage of the myelin sheath as demonstrated by the MBP level (F = 25.02, p = 0.0009, two-way ANOVA).
f. After the injection of AAV-PHP.EB vectors into the heart at P0, AAV-GFP were allowed to cross the BBB and were primarily expressed in the brains of mice. In the mice treated with sevoflurane, Two-way ANOVA with repeated-measurement analysis showed a significant interaction of anesthesia (ctrl + AAV-GFP vs. sevo + AAV-GFP) and time (days 30 to 34) on the escape latency of MWM (F = 4.438, p = 0.0021**, N = 15, two-way ANOVA). A post-hoc test (Bonferroni) showed that the mice in the sevoflurane anesthesia group took a longer time (escape latency) to locate the position of a platform as compared to the control mice at P33 and P34.
g. The AAV-GFP mice that received the sevoflurane anesthesia also demonstrated fewer platform-crossing-times as compared to the mice that were in the control condition (p = 0.001**, Mann–Whitney-U test, N = 15).
h. After the injection of AAV-PHP.EB vectors into the heart at P0, AAV-ERMN were allowed to cross the BBB and were expressed in the brains. In the mice injected with AAV-ERMN, the sevoflurane anesthesia did not induce cognitive impairment in the mice tested in the MWM. No significant interaction (F = 0.914, p = 0.4577, N = 15, two-way ANOVA) between group (ctrl + AAV-ERMN vs. sevo + AAV-ERMN) and time (P30 to P34) was observed, and the sevoflurane anesthesia did not significantly increase the time (escape latency) for the mice to locate the platform in the MWM test as compared to the control condition,
i. The AAV-ERMN mice that received the sevoflurane anesthesia did not have decreased platform crossing times as compared to the mice that received the control condition (p = 0.9596, Mann–Whitney-U test, N = 15).
(Control: ctrl.; sevoflurane: sevo.; AAV-GFP: Adeno-associated virus- Green fluorescent protein; AAV-ERMN: Adeno-associated virus- ERM-like protein).
We then investigated whether the re-introduction of ERMN could be able to rescue the defects of myelination caused by the sevoflurane anesthesia in the brain tissues at P14. We found that myelin sheath around olfactory bulb was damaged by the sevoflurane anesthesia (measured by MBP staining), whereas the over-expression of ERMN mitigated the change (Fig. 5d, Fig. 5e, sevo +AAV-GFP vs. sevo +AAV-ERMN, p = 0.0009, N = 3, two-way ANOVA).
Finally, we found that the delivery of ERMN into the brain tissues of young mice mitigated the sevoflurane anesthesia-induced cognitive impairment, assessed in the Morris water maze [(Fig. 5f, ctrl + AAV-GFP vs. sevo + AAV-GFP, p = 0.0021, two-way ANOVA); (Fig. 5g, ctrl + AAV-GFP vs. sevo + AAV-GFP, p = 0.001, Mann–Whitney U test); (Fig. 5h, ctrl + AAV-ERMN vs. sevo + AAV-ERMN, p = 0.4577, two-way ANOVA); (Fig. 5i, ctrl + AAV-ERMN vs. sevo + AAV-ERMN, p = 0.9596, Mann–Whitney U test); (Fig. 5f, Fig. 5g, N = 15]. This evidence indicated that ERMN played a critical role in regulating myelination during early brain development and cognitive function in the young mice, which were compromised by the sevoflurane anesthesia.
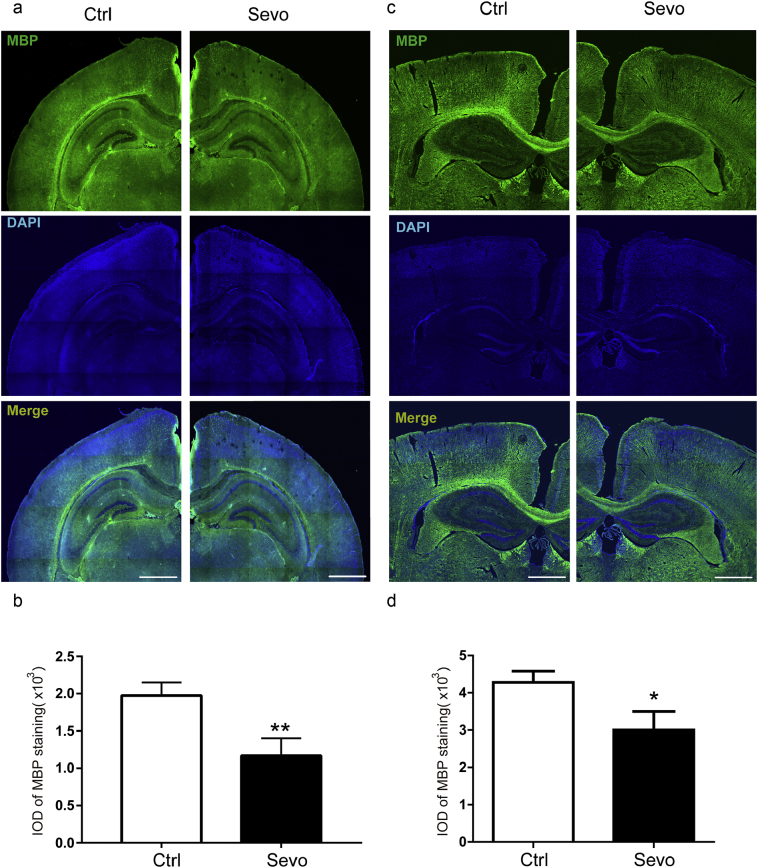
4.5. Sevoflurane induced myelin damage in the cortex of the brain of the young mice
We further investigated whether the sevoflurane anesthesia was able to induce myelin damage elsewhere in the regions of the brain more relevant to the cognitive function. The immunohistochemistry staining showed that the sevoflurane anesthesia reduced the MBP levels in the cortex of the mice at P14 (Fig. 6a and b, p = 0.0092, N = 3, Student's t-test) and P30 (Fig. 6c, Fig. 6d, p = 0.019, N = 3, Student's t-test).
Fig. 6.
Sevoflurane anesthesia damaged the myelin sheath in the cortex of young mice. a. Sevoflurane induced damage of the myelin sheath in the young mice cortex at P14 mice. b. The values of Integrated optical density (IOD) showed the quantification of myelin basic protein (MBP) level of immunohistochemistry staining (p = 0.0092, N = 3, Student's t-test). c. Sevoflurane anesthesia also induced damage of the myelin sheath in the young mice cortex at P30. d. Sevoflurane anesthesia induced damage of the myelin sheath as demonstrated by the MBP level (p = 0.019, N = 3, Student's t-test). Scale bar indicates 100 μm. (Control: ctrl.; sevoflurane: sevo.).
5. Discussion
In the current studies, we showed that surgery under sevoflurane anesthesia was associated with decreased levels of serum folate in young patients. Sevoflurane anesthesia decreased TYMS levels in the prefrontal cortex in both young rhesus macaque and mice. TYMS is an essential folate-dependent enzyme, and the decrease of TYMS usually causes DNA methylation imbalance [39]. DNA methylation, one of the epigenetic modifications, is essential for the development of the central nervous system and can be affected by sevoflurane [[19], [20], [21],44]. In the current study, we found the increased methylation in the promoter region of ERMN, detected by whole-genome DNA methylation analysis, and decreased mRNA levels of ERMN and TYMS, assessed by RNA-seq, after multiple exposures to the sevoflurane anesthesia in infant rhesus macaque. TYMS contributes to methylation by taking part in folate-mediated one-carbon metabolism (FOCM) [27]. FOCM is a crucial pathway essential for DNA methylation as a methyl donor [45]. Previous studies found that both folate reduction and decreased TYMS expression levels can affect DNA methylation. Taken together, these data suggest that the decrease of folate by sevoflurane may affect the global DNA methylation in the primate brain which further affects the expression of ERMN.
It is well known that ERMN is a cytoplasmic protein located in the outer tongue of the myelin sheath and the paranodal loops of oligodendrocytes [25,46]. Isoflurane has been shown to induce apoptosis of oligodendrocytes, especially in the primate brain [18,23,24]. Oligodendrocytes are a particular class of glial cells responsible for myelination in the central nervous system. The sevoflurane anesthesia also decreased the mPFC myelin fiber density in the cortex of mice, assessed by using immunohistochemistry for MBP in the current studies (Fig. 6). Overexpression of ERMN rescued the effect of the sevoflurane anesthesia on the reduction of myelination (Fig. 5).
A recent study reported an association between the multiple exposures to anesthesia and surgery and the increased risk of learning disability and attention-deficit/hyperactivity disorder (ADHD) in children [1,7,40,41]. Myelin damage in white matter microstructure is associated with ADHD [42,43]. Taken together, the data in current studies demonstrated that ERMN-mediated myelination might play an essential role in the anesthesia-induced neurotoxicity in the young brain.
Previous studies in rodents and rhesus macaque show that anesthetics can induce neuronal apoptosis which can lead to cognitive impairment [reviewed in6]. However, it is unknown whether anesthesia can also cause cognitive impairment via the non-apoptosis mechanism. The data in the current studies revealed that anesthesia with sevoflurane could change the folate metabolism and demyelination. Moreover, treatment with the ERMN overexpression in the brain mitigated the sevoflurane anesthesia-induced myelination impairment and cognitive impairment in the mice. In consideration of facts that the immature myelination is associated with motor deficits and childhood exposure to multiple anesthesias increases the risk of ADHD [7,40,41], these findings suggest that general anesthesia could impair the neuronal transportation and communication, leading to cognitive impairment and emotional behavior disorder, pending further investigation. Infant rhesus macaques exposed repeatedly to sevoflurane (three exposures of 4 h each time) and isoflurane (three exposures of 5 h each time) had increased anxiety-like behavior at six months and 12 months of age as compared with the unexposed controls [15,16]. The prefrontal cortex is a crucial region implicated in anxiety-like behavior. The findings in the current studies that sevoflurane anesthesia caused damage to the myelin sheath would promote more research regarding the role of myelin damage in the anesthesia neurotoxicity in the young brain.
The previous studies showed that fasting increases the serum folate levels potentially due to the enterohepatic recirculation of folate [47]. Specifically, the serum folate concentration increased from a mean of 14.8 ng/milliliter to 29.3 ng/milliliter following the 36 h fasting time and decreased to 22.1 ng/milliliter during the 6 h of refeeding time [47]. Therefore, the decrease in the blood levels of folic acid in children after anesthesia and surgery observed in the current studies might not be the result of fasting before the anesthesia and surgery. The changes in the levels of blood folic acid in children who had the anesthesia and surgery were mild (14.50 versus 16.40 ng/milliliter), which could be because the participants in the current studies only had a single round of anesthesia/surgery. These mild changes might likely not be associated with any behavioral changes in cognitive function since single exposure of anesthesia/surgery was not associated with cognitive impairment in children [8,9,41]. Thus, the results from the current human studies only demonstrated that we established a system in children to determine the effects of general anesthesia on the blood levels of folate. Moreover, these findings suggest that future studies, employing the established system, to determine the association between blood levels of folate and cognitive function in children are warranted
There were several limitations in the current studies. First, the animal models in rhesus macaques had potential concerns of hypoxia and hypotension following general anesthesia. However, the anesthesia we used in the present studies was the same one used in the previous studies [11,16,28], which did not show hypoxia and hypotension. We only used these established systems of anesthesia in rhesus macaque and mice to determine the role of folate metabolism in the anesthesia neurotoxicity in developing the brain. Second, we did not perform the immunohistochemistry staining of MBP in the brain tissues of rhesus macaque mainly due to the limited supply of the rhesus macaque. However, the immunohistochemistry staining of MBP in the brain tissues of mice was performed. The systematical evaluation of the effects of anesthesia on the levels of MBP and other biomarkers of white matter injury should be performed by immunohistochemistry staining, Western blot and other methods in rhesus macaque in the future.
In conclusion, blood folate levels were reduced after surgery under sevoflurane anesthesia in young children. The folate-dependent enzyme TYMS was downregulated in the prefrontal cortex of rhesus macaque and mice after multiple exposures to sevoflurane anesthesia, which might lead to the change of DNA methylation in the brain. The methylation in the promoter region of myelination development-related gene ERMN was increased, and the expression of mRNA levels of ERMN was decreased. ERMN-mediated myelination could contribute to the sevoflurane anesthesia-induced neurotoxicity and cognitive impairment in young mice. Folic acid and ERMN expression mitigated these detrimental effects induced by the sevoflurane anesthesia. These data revealed that the folate-ERMN-myelination cascade might serve as the underlying mechanism of the postoperative neurocognitive disorder in young animals and children. The disruption of myelination and communication between surviving neurons in the young brain could be a more important factor in postoperative neurocognitive disorder than the acute loss of neurons at the time of anesthesia and surgery.
Availability of data and material
The datasets analyzed and/or used in the present study are available from the corresponding author upon request.
Declaration of interests
Dr. Zhongcong Xie provides consulting service to Baxter (invited speaker), Hengrui, Novartis, Tongji University, Shanghai Jiao Tong University and Central South University.
Author contributions
Hong Jiang, Lei Zhang, Zilong Qiu: established research concept and experimental design; Lei Zhang, Zhenyu Xue, Qidong Liu, Siwei Xi, Yanyong Cheng, Jingjie Li, and Chong Xiao: obtained the data, performed data analysis and interpretation of data; Lei Zhang, Hong Jiang, and Zilong Qiu: wrote the manuscript. Hong Jiang, Lei Zhang and Zilong Qiu: obtained funding; Yuan Shen and Zhongcong Xie: provided consultation for the studies and preparation of the manuscript; Lei Zhang, Yunbo Liu: provided administrative, technical, and material support.
Funding
This work was supported by NSFC Grants (#81571028, # 81771132, #81870818, #31625013, #91732302, #81720108012), Shanghai Brain-Intelligence Project from STCSM (16JC1420501) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDBS01060200). The research was supported by the Open Large Infrastructure Research of Chinese Academy of Sciences. National Key R&D Program of China 2017YFA0105201, CAMS Innovation Fund for Medical Sciences CIFMS 2016-I2M-2-001, Program of Shanghai Academic Research Leader #19XD1404300. The research was also sponsored by the Shanghai Science and Technology Committee #17ZR1416400.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.04.048.
Contributor Information
Zhongcong Xie, Email: zxie@mgh.harvard.edu.
Zilong Qiu, Email: zqiu@ion.ac.cn.
Hong Jiang, Email: jiangh1173@sh9hospital.org.
Appendix A. Supplementary data
Supplements data - The information of patients, the methods and results of experiments in rhesus macaques.
References
- 1.Wilder R.T., Flick R.P., Sprung J. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flick R.P., Katusic S.K., Colligan R.C. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5) doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backeljauw B., Holland S.K., Altaye M., Loepke A.W. Cognition and brain structure following early childhood surgery with Anesthesia. Pediatrics. 2015;136(1):e1–e12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block R.I., Magnotta V.A., Bayman E.O., Choi J.Y., Thomas J.J., Kimble K.K. Are Anesthesia and surgery during infancy associated with decreased white matter integrity and volume during childhood? Anesthesiology. 2017;127(5):788–799. doi: 10.1097/ALN.0000000000001808. [DOI] [PubMed] [Google Scholar]
- 5.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl. 1):i61–i68. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vutskits L., Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705–717. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 7.Hu D., Flick R.P., Zaccariello M.J. Association between exposure of young children to procedures requiring general Anesthesia and learning and Behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127(2):227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A.J., Disma N., de Graaff J.C. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387(10015):239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L.S., Li G., Miller T.L. Association between a single general Anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315(21):2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner D.O., Zaccariello M.J., Katusic S.K. Neuropsychological and Behavioral outcomes after exposure of young children to procedures requiring general Anesthesia: the Mayo Anesthesia safety in kids (MASK) study. Anesthesiology. 2018;129(1):89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen X., Dong Y., Xu Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118(3):502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao G., Zhang J., Zhang L. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology. 2014;121(3):510–527. doi: 10.1097/ALN.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H., Liufu N., Dong Y. Sevoflurane acts on Ubiquitination-proteasome pathway to reduce postsynaptic density 95 protein levels in young mice. Anesthesiology. 2017;127(6):961–975. doi: 10.1097/ALN.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Dong Y., Zhou C., Zhang Y., Xie Z. Anesthetic sevoflurane reduces levels of hippocalcin and postsynaptic density protein 95. Mol Neurobiol. 2015;51(3):853–863. doi: 10.1007/s12035-014-8746-1. [DOI] [PubMed] [Google Scholar]
- 15.Coleman K., Robertson N.D., Dissen G.A. Isoflurane Anesthesia has long-term consequences on motor and Behavioral development in infant rhesus macaques. Anesthesiology. 2017;126(1):74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarado M.C., Murphy K.L., Baxter M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119(3):517–523. doi: 10.1093/bja/aew473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple Anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123(5):1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenning K.J., Noguchi K.K., Martin L.D. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ju L.S., Yang J.J., Morey T.E. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth. 2018;121(2):406–416. doi: 10.1016/j.bja.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ju L.S., Jia M., Sun J. Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal Sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res. 2016;29(2):243–255. doi: 10.1007/s12640-015-9585-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y., Wang Y., Yao R. Enhanced neuroinflammation mediated by DNA methylation of the glucocorticoid receptor triggers cognitive dysfunction after sevoflurane anesthesia in adult rats subjected to maternal separation during the neonatal period. J Neuroinflammation. 2017;14(1):6. doi: 10.1186/s12974-016-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argente-Arizon P., Freire-Regatillo A., Argente J., Chowen J.A. Role of non-neuronal cells in body weight and appetite control. Front Endocrinol (Lausanne) 2015;6:42. doi: 10.3389/fendo.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creeley C.E., Dikranian K.T., Dissen G.A., Back S.A., Olney J.W., Brambrink A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120(3):626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72(4):525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockschnieder D., Sabanay H., Riethmacher D., Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci. 2006;26(3):757–762. doi: 10.1523/JNEUROSCI.4317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotanda K., Hirota T., Matsumoto N., Ieiri I. MicroRNA-433 negatively regulates the expression of thymidylate synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa cells. BMC Cancer. 2013;13:369. doi: 10.1186/1471-2407-13-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw G.M., Yang W., Perloff S. Thymidylate synthase polymorphisms and risks of human orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2013;97(2):95–100. doi: 10.1002/bdra.23114. [DOI] [PubMed] [Google Scholar]
- 28.Xu G., Lu H., Dong Y. Coenzyme Q10 reduces sevoflurane-induced cognitive deficiency in young mice. Br J Anaesth. 2017;119(3):481–491. doi: 10.1093/bja/aex071. [DOI] [PubMed] [Google Scholar]
- 29.Anders S., Pyl P.T., Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leng N., Dawson J.A., Thomson J.A. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29(8):1035–1043. doi: 10.1093/bioinformatics/btt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 32.Krueger F., Andrews S.R. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27(11):1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akalin A., Kormaksson M., Li S. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012;13(10):R87. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu G., Wang L.G., He Q.Y. ChIPseeker: an R/bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31(14):2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- 35.Ashburner M., Ball C.A., Blake J.A. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinfo. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballaz S.J., Perez J., Waselus M., Akil H., Watson S.J. Interaction between cholecystokinin and the fibroblast growth factor system in the ventral tegmental area of selectively bred high- and low-responder rats. Neuroscience. 2013;255:68–75. doi: 10.1016/j.neuroscience.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myles P.S., Chan M.T., Leslie K., Peyton P., Paech M., Forbes A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth. 2008;100(6):780–786. doi: 10.1093/bja/aen085. [DOI] [PubMed] [Google Scholar]
- 39.Crider K.S., Yang T.P., Berry R.J., Bailey L.B. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Adv Nutr. 2012;3(1):21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprung J., Flick R.P., Katusic S.K. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87(2):120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warner D.O., Zaccariello M.J., Katusic S.K. Neuropsychological and Behavioral outcomes after exposure of young children to procedures requiring general Anesthesia: the Mayo Anesthesia safety in kids (MASK) study. Anesthesiology. 2018;129(1):89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameis S.H., Lerch J.P., Taylor M.J. A diffusion tensor imaging study in children with ADHD, autism Spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry. 2016;173(12):1213–1222. doi: 10.1176/appi.ajp.2016.15111435. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z.M., Bralten J., Cao Q.J. White matter microstructural alterations in children with ADHD: categorical and dimensional perspectives. Neuropsychopharmacology. 2017;42(2):572–580. doi: 10.1038/npp.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori K., Iijima N., Higo S. Epigenetic suppression of mouse Per2 expression in the suprachiasmatic nucleus by the inhalational anesthetic, sevoflurane. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leopardi P., Marcon F., Caiola S. Effects of folic acid deficiency and MTHFR C677T polymorphism on spontaneous and radiation-induced micronuclei in human lymphocytes. Mutagenesis. 2006;21(5):327–333. doi: 10.1093/mutage/gel031. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann M.L., Weigel T.K., Elkahloun A.G., Herkenham M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci Rep. 2017;7 doi: 10.1038/srep46548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahill E., McPartlin J., Gibney M.J. The effects of fasting and refeeding healthy volunteers on serum folate levels. Int J Vitam Nutr Res. 1998;68(2):142–145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplements data - The information of patients, the methods and results of experiments in rhesus macaques.
Data Availability Statement
The datasets analyzed and/or used in the present study are available from the corresponding author upon request.