Abstract
Rheumatic valve disease is an endemic problem that is responsible for substantial morbidity and mortality in many countries. Unlike the rheumatic mitral valve, aortic repair continues to be challenging. A thorough understanding of the underlying mechanisms; structural and functional, is essential for repair. We here describe various methods of repair and outline our favoured techniques.
Keywords: Rheumatic aortic, aortic repair, rheumatic repair, aortic valve repair
Introduction
Rheumatic valve infection is prevalent in developing countries. Affecting 10:1,000–15:1,000 in endemic countries, it is responsible for the death of more than 300,000 patients/year. It is the most common cause for heart valve surgery (1,2) (Figure 1). Although predominantly affecting the mitral valve, rheumatic aortic valve disease is present in up to 30% of cases causing a more serious impact on left ventricular function, quality of life and overall prognosis (1). Nine percent of patients have isolated aortic stenosis, 14% isolated incompetence and 6% have mixed lesions. Rheumatic post-inflammatory lesions leave the aortic valve with thickened, fibrotic, shrunken cups usually with fusion at the commissures and sometimes calcification.
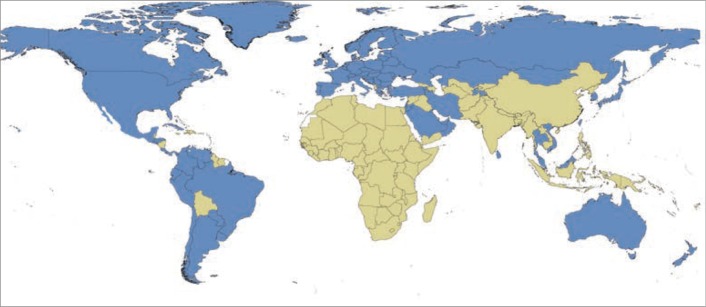
Figure 1.
World map showing endemic countries with rheumatic heart disease (based on estimated childhood mortality due to rheumatic heart disease greater than 0.15 deaths per 100,000 population among children aged 5 to 9 years) (Endemic in green and non-endemic in blue) (From Watkins et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med. 2017;377(8):713-722) (2).
Repair of the rheumatic mitral valve is particularly important for the sake of improved ventricular remodeling, contractility and overall survival in addition to avoiding anticoagulation in a young cohort with a tendency to poor compliance. A somewhat difficult undertaking, specialized centers have shown very good results especially in rheumatic mitral and tricuspid valve incompetence (3). However, the rheumatic aortic valve continuous to defy attempts at reliable repair due to its peculiar anatomical and histopathological characteristics (4) (Figures 2,3). Recent attempts at achieving this goal continue (5). We here outline the different methods being applied.
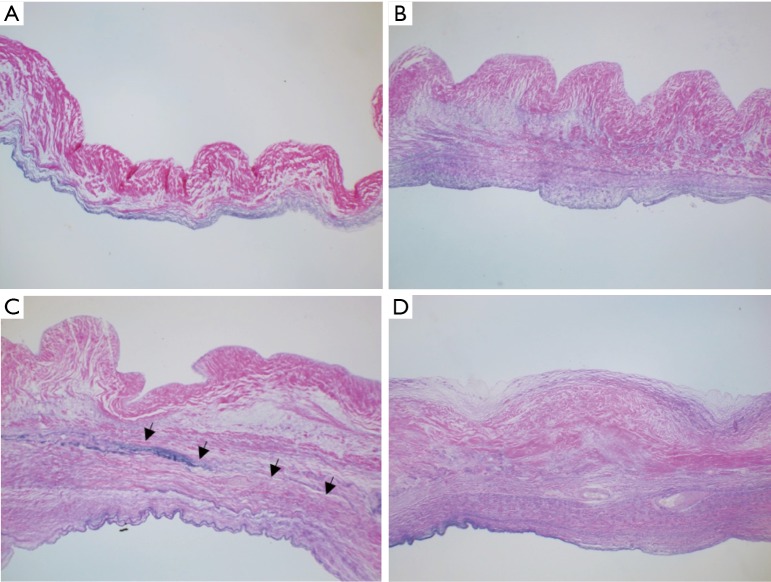
Figure 2.
Haematoxylin-eosin staining showing different stages of thickening and vascularization of the cusps (HE, ×40). (A) Normal aortic valve; (B) early stage of rheumatic valve affection with mild thickening of the ventricular aspect of the valve not interfering with mobility; (C) later stage; fibrosis confined to the ventricular surface of the leaflet with further thickening; (D) advanced (mature) stage with further fibrosis and vascularization as well as clear line of demarcation between the leaflet and fibrous tissue and mild degree of fibrosis on the aortic surface of the cusp. Arrows indicate clear line of demarcation between the leaflet and fibrous tissue.
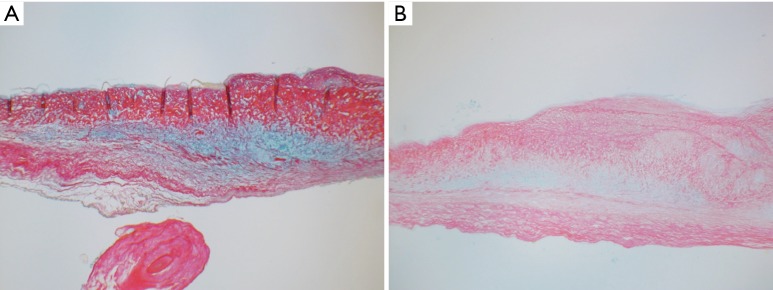
Figure 3.
Alcian blue and sirius red staining of aortic valve cusps (Alcian Blue and Sirius Red, ×40). (A) Healthy aortic valve showing normal amount of glycan layer (GAGs) necessary for improved mobility; (B) rheumatic aortic valve, glycan layer is diminished but still present.
Surgical treatment of rheumatic aortic valve disease
Valve replacement provides good early results but long-term outcomes are limited by the cumulative risk of valve-related complications (6). Mechanical aortic valve replacement remains, however, the classical treatment for severe rheumatic aortic valve infection, especially for young patients. This remains far from optimal due to increased risk of thrombosis and embolism since this disease mainly affects patients of low socio-economic profile with limited access to follow-up and control of anticoagulation. Additionally, stented bioprosthetic replacements for rheumatic aortic valve disease have a tendency for patient-prosthesis mismatch, as patients commonly have a small, fibrotic annulus, particularly females. Additionally, from our personal experience bioprostheses, including stentless prostheses tend to degenerate very quickly in young patients. It therefore follows that available valve substitutes are not optimal for use in this relatively young population (7). Hence, valve-conserving restorative operations are preferred if they can perform reliably.
Timing of surgery for rheumatic lesions is still largely unanswered. The currently accepted guidelines for non-rheumatic aortic valve disease are being used to guide timing (6). Recent evidence supports rather early intervention before the setting of irreversible myocardial damage. This is particularly the case for aortic regurgitation (AR) as it poses both a diastolic as well as a systolic volume overload, causing earlier irreversible damage as compared with aortic stenosis (8).
How to repair the rheumatic aortic valve
Effective repair of the rheumatic aortic valve requires thorough understanding of the pathophysiology of rheumatic valve disease, the histological disease process and the dynamics of flow across the valve and within the aortic root in health and disease. Changes in size, shape and dynamism of the aortic valve and root can be characterized by modern imaging techniques and need to be addressed during operative repair (9-11).
Many techniques have been proposed for repair of rheumatic aortic valve disease (5,12-15) (Table 1). Some rheumatic aortic valves have obvious commissural fusion that can be dealt with by sharp dissection which, if carried out alone, residual gradient would result from stiff aortic leaflets.
Table 1. Different techniques for rheumatic aortic valve repair.
| For post inflammatory rheumatic affection/thickening: |
| Peeling (decortication) |
| Shaving (thinning) |
| For associated rheumatic aortic stenosis: |
| Commissurotomy |
| Decalcification |
| For pure rheumatic aortic incompetence: |
| Free edge plication |
| Subcommissural suture plication |
| Augmentation |
| Of the body |
| Of the free edge |
| Single cusp replacement |
| Of all cusps (Neocuspidization-Ozaki Technique) |
The leaflets are mobilized by a process of peeling (or decortication) of the cusps, with focal decalcification where necessary. Starting from a tissue plane on the ventricular surface of the leaflet beneath the aortic annulus, peeling can sometimes significantly improve cusp mobility; increasing its pliability, surface area and extent of coaptation. This decortication technique is possible because the rheumatic disease process spares the elastica and part of the fibrosa. We believe it yields better results than simple shaving of the leaflet body or edge, which makes the leaflet thinner but does not improve its mobility (12) (Figures 2,3).
Cusp extension/augmentation can be done by patching a ribbon of pericardium (or other tissue) to the free edge of the cusp. This has not shown good results in our experience and that of other groups especially if it is done in one or two cusps only (Figure 4). This technique has been superseded by total cusp replacement, which has shown very good mid-term results, especially by the Ozaki group (13). This technique, however, is to be considered a stentless aortic valve replacement rather than repair.
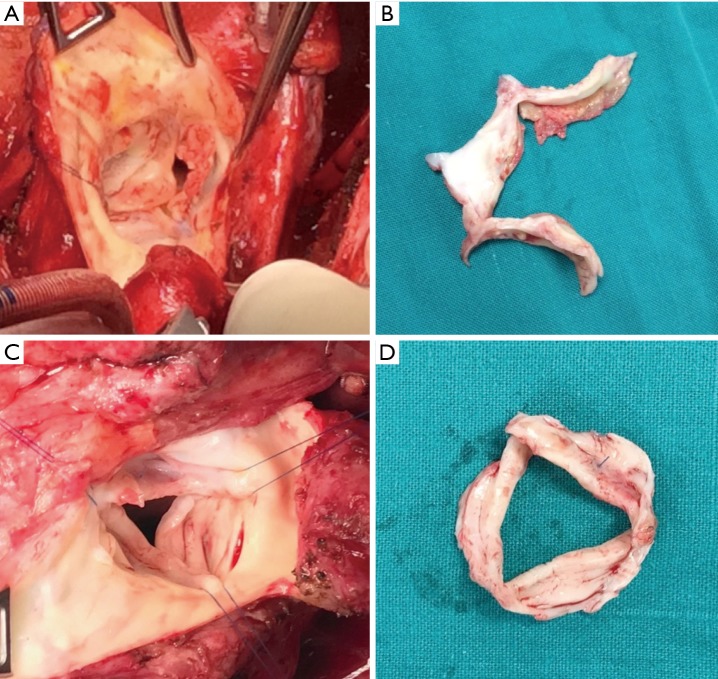
Figure 4.
Early and midterm images after rheumatic aortic repair by cusp extension of the free edge. (A,B) Redo operation 1 year after free edge extension of the non-coronary cusp with extra cellular matrix. (A) Intraoperative image; (B) the excised aortic valve. The ribbon shaped patch has degenerated completely while native cusps remain short and thickened. (C,D) Redo operation 3 years after free edge extension of the non-coronary cusp with autologous pericardium. (C) Intraoperative image; (D) the excised aortic valve. The pericardium is thickened, fibrotic and shrunken.
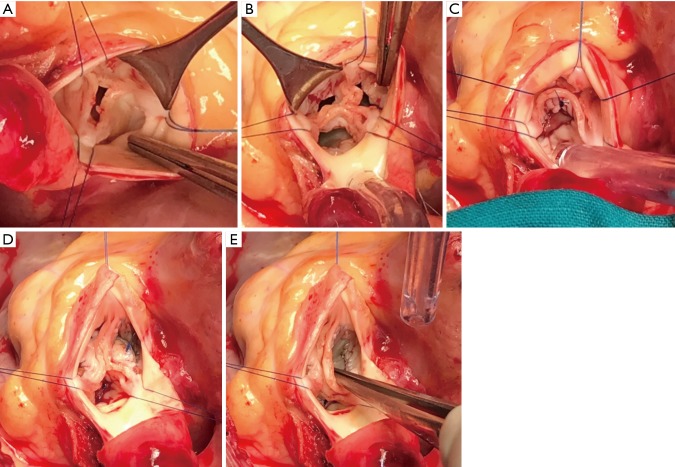
Patching the aortic cusps seems to degenerate quickly when performed at the free edge. However, from the experience in patching holes in the belly of the cusps, following healed endocarditis, away from the free edge had superior longevity. We therefore elect to perform aortic cusp augmentation by incising the base of the cusps, freeing the coapting edge of the aortic leaflet and patching the “belly” of the aortic leaflet (Figure 5). This has shown improved results in our hands.
Figure 5.
Repair of rheumatic aortic valve regurgitation. (A) The rheumatic aortic valve; (B) incision along the annulus with release of the free edge of all three cusps; (C) pericardial patch of the left coronary cusp; (D) pericardial patch of the non-coronary cusp; (E) the final result showing improved coaptation of the free edge with good leaflet substance.
No perfect material exists to replace or augment the diseased aortic cusps. Fresh pericardium is soft and potentially living tissue that we have favored whilst performing cusp extension. Treated pericardium offers better handling but has accelerated degeneration. PTFE has been tried by other groups with less than optimal results. Extracellular matrix, in our experience, degenerated very rapidly in the aortic position especially when used to extend the free aortic leaflet edge (5,14,15). In the past, we have used dura mater with apparently good results, but they are no longer available. Tissue engineering of patches or entire valves is an emerging area of surgical research (16). While not currently in use, such technologies will hopefully be available in the near future potentially improving outcomes at a lower cost. Tissue-engineered products could also be stent mounted and delivered percutaneously making them even more attractive in limited-resource settings.
Which rheumatic aortic valves to repair
Repair of mild to moderate aortic lesions during concomitant mitral valve surgery gives acceptable results. This is because the valve is not yet burnt out, there is still some leaflet tissue to use for repair and mild residual or recurrent degrees of aortic incompetence, in this setting, is well-tolerated. Another good cohort is younger children where the valve is more amenable to repair and the cumulative risk of replacement is more substantial.
Need for specialized centers (10)
Especially with valve repair, ensuring quality is an important consideration for surgical programs in limited-resource settings. Post-operative outcomes have been recorded in a robust manner for congenital heart disease surgery for children in these settings (17). The same practices could be easily extended to patients requiring rheumatic valve repair, since patient demographics and healthcare providers overlap significantly.
Joint effort of teams with interest in reconstructive valve surgery is bound to lead to better and more reproducible results. More efforts are needed on ensuring quality of post-surgical care, including anticoagulation, for those living in remote or deprived areas (6,18).
Conclusions
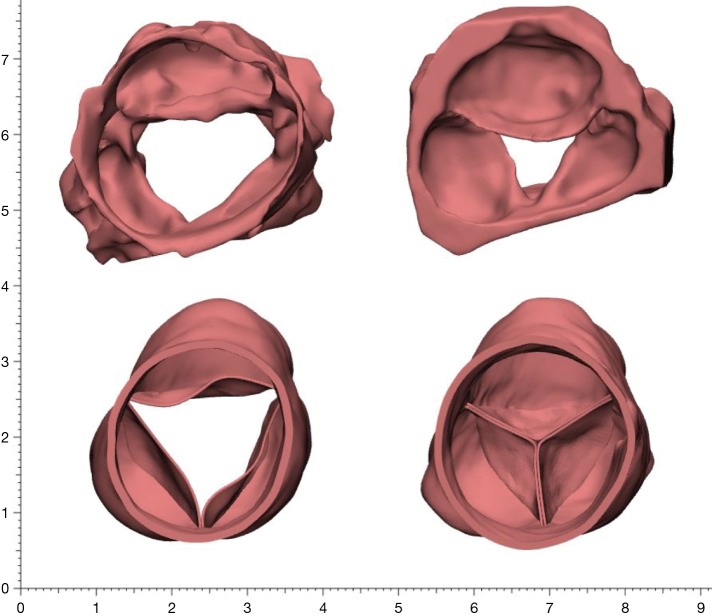
Rheumatic aortic valve disease is a problem without a perfect solution. Surgical techniques are still evolving and the efficacy of current practices needs to be validated by studying larger numbers of patients with long-term follow-up focusing specifically on ventricular function and, importantly, quality of life which is often significantly impaired (18). A tailored approached should be followed based on patient age, body habitus, compliance to medications and size and shape of the aortic annulus and root. We believe that the Ross procedure still offers the best option for this young cohort for its improved survival, positive impact on left ventricular function, exercise tolerance and quality of life in addition to its clear advantage of avoiding anticoagulation it is limited however by the availability of homografts (19,20) (Figure 6).
Figure 6.
Difference in mobility and pliability between a rheumatic aortic root and a pulmonary autograft aortic root. (Top) 3D Segmentation using CT angiography of the aortic root in a patient with advanced rheumatic aortic valve disease not suitable for repair; (Bottom) same patient following pulmonary autograft replacement of the aortic valve and root (Ross) with regurgitant area 0 mm2 and excellent coaptation.
More effort should be invested in optimizing cusp replacement and extension by further understanding of aortic complex dynamics and investment in a tissue-engineered cusp tissue.
Acknowledgments
Heba Aguib, Mohamed Nagi (Aswan Heart Centre, Magdi Yacoub Foundation): for providing segmentation and 3D reconstruction of the aortic valve and root; Najma Latif, Padmini Sarathchandra (Harefield Heart Science Centre, Imperial College London): for providing the histological sections.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yacoub M, Mayosi B, ElGuindy A, et al. Eliminating acute rheumatic fever and rheumatic heart disease. Lancet 2017;390:212-3. 10.1016/S0140-6736(17)31608-2 [DOI] [PubMed] [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med 2017;377:713-22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 3.Chotivatanapong T, Lerdsomboon P, Sungkahapong V. Complex surgical repair of rheumatic mitral stenosis. Ann Cardiothorac Surg 2015;4:480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair: from structure to function: Part I. Circulation 2004;109:942-50. 10.1161/01.CIR.0000115633.19829.5E [DOI] [PubMed] [Google Scholar]
- 5.d’Udekem Y, Sharma V. Repair Options in Rheumatic Aortic Valve Disease in Young Patients. World J Pediatr Congenit Heart Surg 2013;4:392-6. 10.1177/2150135113496440 [DOI] [PubMed] [Google Scholar]
- 6.Choudhary SK, Talwar S, Airan B. Choice of prosthetic heart valve in a developing country. Heart Asia 2016;8:65-72. 10.1136/heartasia-2015-010650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antunes MJ. Challenges in rheumatic valvular disease: Surgical strategies for mitral valve preservation. Glob Cardiol Sci Pract 2015;2015:9. 10.5339/gcsp.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carabello BA. Assessment of the patient with valvular heart disease: An integrative approach. Aswan Hear Cent Sci Pract Ser 2011;2011:15 10.5339/ahcsps.2011.15 [DOI] [Google Scholar]
- 9.Yacoub MH, Kilner PJ, Birks EJ, et al. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg 1999;68:S37-43. 10.1016/S0003-4975(99)00745-6 [DOI] [PubMed] [Google Scholar]
- 10.Watkins DA, Beaton AZ, Carapetis JR, et al. Rheumatic Heart Disease Worldwide. J Am Coll Cardiol 2018;72:1397-416. 10.1016/j.jacc.2018.06.063 [DOI] [PubMed] [Google Scholar]
- 11.Timek TA. Love the root, not the flowers everyone sees. J Thorac Cardiovasc Surg 2018;156:937-8. 10.1016/j.jtcvs.2018.04.068 [DOI] [PubMed] [Google Scholar]
- 12.Talwar S, Saikrishna C, Saxena A, et al. Aortic Valve Repair for Rheumatic Aortic Valve Disease. Ann Thorac Surg 2005;79:1921-5. 10.1016/j.athoracsur.2004.11.042 [DOI] [PubMed] [Google Scholar]
- 13.Ozaki S, Kawase I, Yamashita H, et al. Midterm outcomes after aortic valve neocuspidization with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg 2018;155:2379-87. 10.1016/j.jtcvs.2018.01.087 [DOI] [PubMed] [Google Scholar]
- 14.Nosál’ M, Poruban R, Valentik P, et al. Initial experience with polytetrafluoroethylene leaflet extensions for aortic valve repair. Eur J Cardiothorac Surg 2012;41:1255-7. 10.1093/ejcts/ezr214 [DOI] [PubMed] [Google Scholar]
- 15.Przybylski R, Pawlak S, Śliwka J, et al. Aortic cusp extension valvuloplasty: repair with an extracellular patch. Kardiochir Torakochirurgia Pol 2015;12:314-7. 10.5114/kitp.2015.56780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yacoub MH. In Search of Living Valve Substitutes. J Am Coll Cardiol 2015;66:889-91. 10.1016/j.jacc.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 17.Hickey PA, Connor JA, Cherian KM, et al. International quality improvement initiatives. Cardiol Young 2017;27:S61-8. 10.1017/S1047951117002633 [DOI] [PubMed] [Google Scholar]
- 18.Watkins D, Zuhlke L, Engel M, et al. Seven key actions to eradicate rheumatic heart disease in Africa: the Addis Ababa communiqué. Cardiovasc J Afr 2016;27:184-7. 10.5830/CVJA-2015-090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet 2010;376:524-31. 10.1016/S0140-6736(10)60828-8 [DOI] [PubMed] [Google Scholar]
- 20.Sampath Kumar A, Talwar S, Saxena A, et al. Ross procedure in rheumatic aortic valve disease. Eur J Cardiothorac Surg 2006;29:156-61. 10.1016/j.ejcts.2005.11.021 [DOI] [PubMed] [Google Scholar]