Abstract
Stevia leaves contain various components, such as flavonoids, labdanes, chlorophylls, sterols, triterpenoids, mono-disaccharides, organic acids and inorganic salts. Stevia is known to accumulate diterpenoid steviol glycosides, which are approximately 300 times sweeter than regular sugar. Stevioside and rebaudioside A are the main diterpenic glycosides in stevia. Steviol glycosides are the secondary metabolites responsible for the sweetness of stevia. The main objectives of the present study were to determine the concentrations of diterpenic glycosides (stevioside and rebaudioside A) in three stevia varieties (Stevia rebaudiana) via the HPLC-UV technique and to amplify the UGT76G1 gene by PCR using gene-specific primers. The expression levels of the UGT76G1 gene were determined in the three stevia varieties. The PCR products were sequenced and analyzed, and the nucleotide sequences of the UGT76G1 gene were submitted to GenBank and assigned to the following three varieties: Egy1 (MH087463), China1 (MH087464) and Sponti (MH087465). Cluster analysis was used to separate the three varieties into two major clusters based on their phylogenetic relationship. In addition, chemical analysis was carried out to evaluate stevioside and rebaudioside A. The present study concluded that Egy1 and Sponti are closely related varieties as they fall in the same cluster, while China1 forms a separate cluster. Bioprospecting studies could be useful for selection of superior ecotypes of Stevia rebaudiana.
Subject terms: Genomic analysis, Genomics
Introduction
Stevia plants are an important source of commercial steviol glycosides (SGs)1. SGs are used as alternative natural sweeteners and have applications in the control of diseases caused by modern lifestyles, such as obesity, diabetes, hypertension and cardiac blockage2–4. Up to thirty percent of diterpenoid steviol glycosides accumulate in dry stevia leaves5. Steviol glycosides are sweeter than sugar and noncalorific sweeteners that are used worldwide. Eight different steviol glycosides are produced in stevia plants. Stevioside constitutes a majority of sweeteners (60~70%) with high potential medicinal value6–8. Rebaudioside A is of particular interest, due to the desirable flavor profile of this compound and is considered to be antidiabetic, noncariogenic and mutagenic9. Diterpene glycosides are currently used in different varieties of food products (pickled vegetables, dried sea food, beverages, candies, chewing gum, yogurt, etc.). Different techniques are used to determine the glycoside content in plants (e.g., gas chromatography, HPLC, LC-MS, infrared spectroscopy). HPLC is a reliable method that has been used to determine the composition of Stevia rebaudiana10–13. Plant UDP-glycosyltransferases (UGTs) are a unique group of enzymes that transfer sugar residue from an activated donor to an acceptor molecule14–19. UDP-glycosyltransferases were mostly unidentified until recently, and detailed functional characterization of these enzymes is only just beginning. Complete genome sequencing uncovered 112 full-length candidate UGTs in Arabidopsis, and these results led to the characterization of many new activities20–24. In Stevia, UDP-glycosyltransferases are involved in the production of steviol glycosides, compounds that are unique in the plant world due to their intense sweetness and high concentration in leaves25,26. In stevia, kaurene is transformed to steviol, the backbone of the sweet glycosides; steviol is also transformed to many glycosides by uridine-diphosphate-dependent glycosyltransferases (UGTs)27–29. The C-19 carboxylate and C-13 alcohol oxygenated functional groups of steviol provide attachment points for the sugar side chains that determine the identity of the glycosides. The addition of the C-13-glucose to steviol is catalyzed by UGT85C2, first yielding steviolmonoside and then steviolbioside; the addition of the C-19-glucose is catalyzed by UGT74G1, yielding stevioside30,31; and finally, glucosylation of the C-3′ of the glucose at the C-13 position is catalyzed by UGT76G1, yielding rebaudioside A27,32. The UGT76G1 gene is responsible for the conversion of stevioside to rebaudioside A and improves the organoleptic properties of steviol glycosides33; therefore, the present study was focused on UGT76G1. To differentiate between three stevia varieties, biochemical analysis of the three varieties was carried out, to determine the concentrations of both stevioside and rebaudioside A in stevia leaves using HPLC. Moreover, the gene expression levels of UGT76G1 in the three stevia varieties. Furthermore, the UGT76G1 gene was characterized via gene sequencing to determine the genetic similarity of the three stevia varieties, and the obtained sequences were submitted to GenBank to identify accession numbers.
Material and Methods
Sample collection
Mature leaves (3 months old) of three stevia varieties (Egy1, China1 and Sponti of S. rebaudiana) were collected from the middle parts of plants (between internodes 10 and 15) from the Sugar Crops Research Institute, Agricultural Research Center (SCRI-ARC), Ministry of Agriculture, Egypt. Cooperating with Botany and Microbiology Department, College of Science, King Saud University, Saudi Arabia.
Sweet diterpene glycoside extraction
The collected leaves were oven-dried (E. Schulz & Co. Inh. Franz. Skorezewsh KOMEG Technology, China) at 50 °C and then ground to a fine powder according to34 with some modifications as follows: 5 g of dried leaf powder was extracted with 50 ml of hot methanol using a Soxhlet apparatus for 2 hrs. The extract was filtered using Whatman no. 1 filter paper, and the residue was re-extracted twice with methanol at room temperature. The filtrate was further concentrated in rotary flash evaporator (Type 349, James Jobling and Co. Ltd., England) at 60 °C to 10 ml, and then, 50 ml of distilled water was added to the concentrated extract. A phase separation step was performed to remove plant pigments as follows: 25 ml of diethyl ether was added to the extract in a 500-ml separatory funnel (Sigma Aldrich), and the aqueous phase was collected and extracted with butanol. Finally, the butanol upper layer was collected and refrigerated overnight at 4 °C to allow the purified glycosides to form crystals. Then, the crystals were separated by filtration and analyzed using HPLC34. Each stevia sample was extracted twice.
Preparation of the stevioside standard
Stevioside standard preparation was carried out according to the method described by Nishiyama et al.35 with minor modifications as follows: dried leaves of S. rebaudiana Bertoni (10 g) collected from Sugar Crops Research Institute (SCRI), Agricultural Research Center (ARC), Ministry of Agriculture, Egypt, were extracted by soaking leaves in 1.0 liter of hot distilled water (85 °C) for 30 minutes. The resulting liquid fractions were filtered using a Buchner filtration system, and the leaves were then washed with an additional volume of hot water (50 ml). The aqueous solution was concentrated to 50 ml in a freeze-drier (Edwards model EF03, England). The extract was defatted by ethyl acetate followed by extraction with isobutyl alcohol (150 ml). The aqueous phase was discarded, and the organic phase was evaporated until dryness by using a rotary evaporator (Type 349, James Jobling and Co. Ltd., England) at 70 °C. The dry pellets were dissolved in hot methanol (100 ml) and allowed to crystallize overnight. The crystals were separated by filtration and dissolved in boiling methanol (50 ml) to obtain a concentrated solution. The solution was clarified with active charcoal (B.D.H. Laboratory Chemicals Division, Poole, England) and left to recrystallize. The procedure was repeated three times until the formation of colorless crystals. The pure solution of the stevioside standard was subjected to HPLC analysis.
Analysis of SGs by HPLC
High-performance liquid chromatography (HPLC) technology can be used to directly measure the levels of steviol glycosides (rebaudioside A and stevioside) in Stevia rebaudiana Bertoni36. The levels of stevia sweetener compounds were estimated at the Central Laboratory, Faculty of Science, Alexandria University. Leaf extracts were separated and identified by HPLC according to37 as follows: the stevioside solution was filtered through a Millipore membrane (13 mm diameter, 0.5 μm pore size) and analyzed using HPLC with a stevioside standard as an internal standard (10 mg/ml). Different extracts of stevia leaves were injected into an HPLC instrument (Shimadzu, Tokyo, Japan; model SPD-6AV) equipped with an LC-GA UV-vis detector and an Alex C-R 4 A recorder. The separation was carried out on a Zorbax NH2 column (25 cm × 0.4 mm I.D.; Dupont, Wilmington, DE, USA) with acetonitrile (HPLC grade, Fisons Co., England) as the mobile phase (acetonitrile: water (80: 20 v/v), adjusted to pH 5 with H3PO4). The flow rate was 2 ml/min; the UV detection wavelength was 210 nm; the recorder chart speed was 20 nm/min; and the analysis was performed at ambient temperature (25 °C). Two samples per variety were analyzed, and the quantities of stevioside and rebaudioside A were calculated from the area under each peak.
PCR amplification of the stevia UGT76G1 gene
Fresh leaf samples of three stevia varieties (three months old), namely, China1, Egy1 and Sponti, were used to extract total genomic DNA using the DNA Mini-Prep Kit (BIO BASIC, Canada). The stevia UGT76G1 gene was amplified by using gene-specific primers UGT76G1 FP (5′ AACGTCAGTCAAACCCAATG3′) and UGT76G1 RP (5′ CTCACATAACCAACAACCATCC3′) according to33. The PCR was performed in a 25-µl reaction mixture containing 1 µl of 100 ng/µl DNA, 1 µl of each primer at a concentration of 0.01 nmol/µl, 12.5 µl of master mix (2.5 µl of 10 × PCR buffer, 1.5 µl of 25 mM MgCl2, 2.0 µl of 2.5 mM dNTP, and 0.125 µl of Taq DNA polymerase) (BIOLINE, UK) and 9.5 µl of water (H2O). The PCR program was as follows: denaturation for 5 min at 95 °C; 35 cycles of 40 s at 94 °C, 40 s at 55 °C and 2 min at 72 °C; and 72 °C for 10 min. Then, 2% agarose gel electrophoresis with ethidium bromide was used to separate the PCR product. The image was recorded using a gel documentation system (Alpha Image, USA). Clearly separated DNA bands of PCR products (approximately 1500 bp) were cut from the gel and purified using the GF-1 AmbiClean Kit (PCR & Gel, Vivantis, WE CARE, Malaysia). Then, the purified PCR products were submitted for sequencing at Macrogene (Korea). The sequences were analyzed and compared by NCBI BLAST (http://blast.ncbi.nlm.nih.gov); the sequences were aligned to generate a phylogenetic tree by using Molecular Evolutionary Genetics Analysis (MEGA5) software. The sequences were submitted to GenBank (gb-admin@nbci.nlm.nih.gov), and each sequence was assigned a GenBank accession number.
Gene expression analysis of UGT76G1 (qRT-PCR)
RNA extraction and cDNA synthesis
Total RNA was isolated from stevia leaves using the BS82314-50 Preps EZ-10 Spin Column Plant RNA Mini-Prep Kit (BIO BASIC, Canada). For cDNA synthesis, the first strand of cDNA was synthesized using M-MuLV reverse transcriptase (New England Bio Labs Inc.). The samples were incubated at 42 °C for 1 hr and then 72 °C for 10 minutes. cDNA samples were stored at −20 °C. Every 20 µl of the reverse transcription mixture contained 1 µl of template RNA, 2 µl of oligo (dT) primer, 2 µl of 10 × M-MuLV buffer, 1 µl of M-MuLV RT (200 U/µl), 1 µl of 10 mM dNTP mix, and nuclease-free water to a total volume of 20 µl.
Quantitative real-time PCR analysis (qRT-PCR)
Primer design for the UGT76G1 gene is recorded in Table (1). The stevia actin gene was used as an internal control for data normalization. For relative quantification of gene expression, qRT-PCR was conducted in an Eppendorf Master Cycler ep realplex using the following PCR cycling conditions: 2 min at 95 °C, followed by 40 cycles of 5 s at 95 °C, 10 s at 60 °C and 5 s at 72 °C; then, melting curve analysis was performed according to33. Expression of the UGT76G1 gene was determined by quantitative qRT-PCR on a Thermo Scientific PikoReal 96 real-time PCR system (www.thermoscientific.com/pikoreal) with the SYBR Green SensiFASTTM SYBR® No-ROX Kit (BIOLINE). Relative quantification by real-time PCR was performed in a 10 µl volume containing 1 μl of cDNA, 5 µl of 2 × SensiFAST SYPBR® No-ROX mix, 0.5 µl of each primer, and 3 µl of H2O. For quantitative real-time PCR data analysis, relative expression of UGT76G1 was calculated based on the threshold cycle using the 2−ΔΔCq method38. The expression levels of target genes were normalized using the stevia actin gene as an internal control, and the relative transcript levels were calculated as follows according to39.
Table 1.
Primer set designed for qRT-PCR used in the current study.
| Gene | Primer sequence | Reference |
|---|---|---|
| Stevia actin | F-5′ CCCGCCATGTATGTCGCCATTCAA 3′ | Madhav et al. (2012) |
| R-5′ TCAGTGAGGTCACGACCAGCAAGA 3′ | ||
| UGT76G1 | F-5′ AACGTCAGTCAAACCCAATG 3′ | Yang et al.33 |
| R-5′ CTCACATAACCAACAACCATCC 3′ |
Statistical analysis
Data analysis was performed by using the Excel software program; gene expression was examined for three biological replicates of each variety. A t-test at p < 0.05 was applied to determine significant differences in gene expression between the three varieties, and standard deviations were calculated for the means of the biological replicates.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Results
Chemical analysis of stevia sweeteners
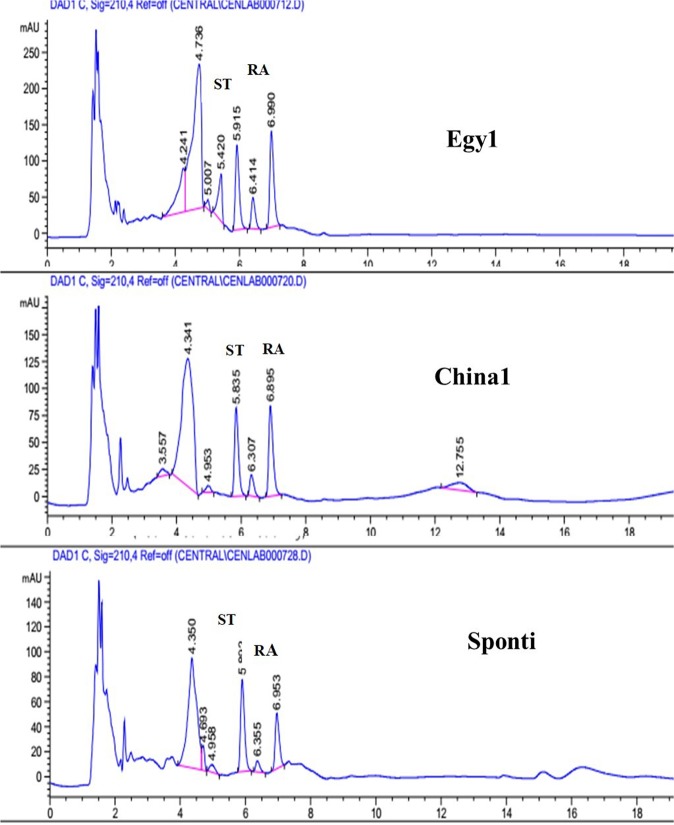
HPLC was used to determine the levels of stevioside and rebaudioside A. The results shown in Table (2) and Fig. (1A–C) indicate that the highest stevioside content was observed in the Sponti variety (21.46%), followed by China1 (0.18%) and finally Egy1 (12.27%). The range from lowest to highest value was 12.27 to 21.46% (with a 9.19% increase observed for the Sponti variety, Table 2). The highest levels of rebaudioside A were observed in China1 and Egy1 (15.54% and 14.48%, respectively), while a value of 13.02% was observed for the Sponti variety (Table 2).
Table 2.
Percentage of the stevioside and rebaudioside A sweeteners in stevia leaves (values are the means of two readings for each variety).
| Egy1 | China1 | Sponti | |||
|---|---|---|---|---|---|
| Stevioside % | Rebaudioside A % | Stevioside % | Rebaudioside A % | Stevioside % | Rebaudioside A % |
| 12.27 | 14.48 | 14.18 | 15.54 | 21.46 | 13.02 |
Figure 1.
HPLC of stevia varieties Egy1 (A), China1 (B)and Sponti (C).
PCR amplification of UGT76G1
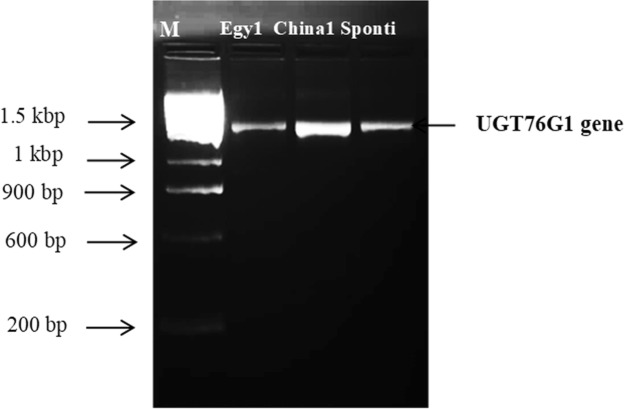
The UGT76G1 gene was amplified from the stevia varieties by PCR using the designed primers (UGT76G1 F and R). The PCR assay showed an amplification product of the expected size (1.51 kb), as shown in Fig. (2). These data are consistent with33, in which a product with same molecular weight was detected.
Figure 2.
PCR products of the UGT76G1 gene amplified from Egy1, China1 and Sponti.
UGT76G1 gene sequencing and phylogenetic analysis
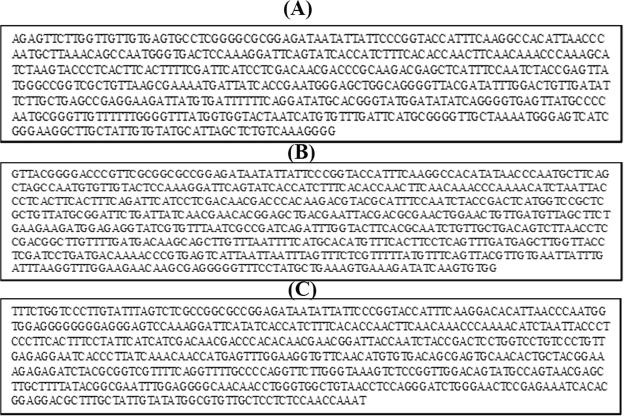
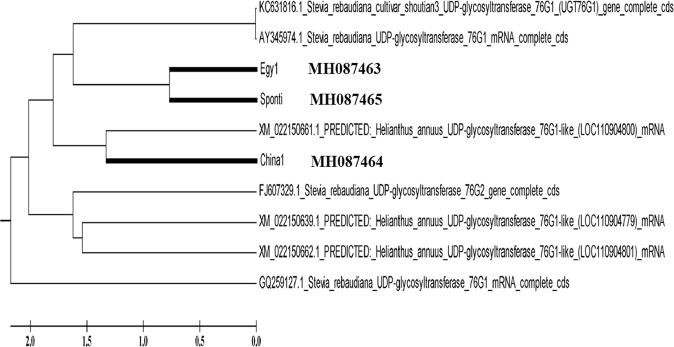
The polymerase chain reaction amplification products of the UGT76G1 gene were sequenced and analyzed to determine nucleotide similarity among the three stevia varieties, as shown in Fig. (3). Data were submitted to GenBank for identification of the accession numbers for each sequence (Table 3, Figs 4 and 5). The partial sequence of the UGT76G1 gene was aligned and compared to the sequences in GenBank. A dendrogram was generated using MEGA5 software to examine the phylogenetic relationship of UGT76G1 among the three stevia varieties, namely, Egy1, China1 and Sponti. The observed similarity might be the result of the existence of a common ancestor for Egy1 and Sponti, and this ancestor might differ from the ancestor of China1 (Fig. 6). The generated genetic similarity dendrogram for the three stevia varieties classified the populations into two major groups: Group I (Egy1 and Sponti) and Group II (China1). The results showed that Egy1 and Sponti belong to the same cluster (Figs 6 and 7).
Figure 3.
Nucleotide sequencing of the UGT76G1 gene from stevia plants of different varieties: (A) Egy1 (519 bp); (B) China1 (634); (C) Sponti (549).
Table 3.
Accession numbers of the UGT76G1 gene in GenBank.
Figure 4.
Multiple DNA sequence alignments of UGT76G1 genes with the genes from three different varieties of stevia. Completely conserved residues across all the aligned sequences are marked with an asterisk (*) below. Absent nucleotides are indicated by dashes (−).
Figure 5.
Multiple DNA sequence alignments of the UGT76G1 genes with the genes from three different varieties of stevia available GenBank. Completely conserved residues across all the aligned sequences are marked with an asterisk (*) below. Absent nucleotides are indicated by dashes (−).
Figure 6.
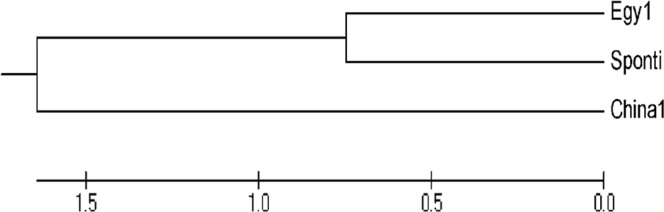
Dendrogram illustrating the phylogenetic relationship of different varieties based on DNA nucleotide sequencing of the UGT76G1 gene.
Figure 7.
Dendrogram illustrating the phylogenetic relationship of different varieties based on DNA nucleotide sequencing of the UGT76G1 gene and comparison with the same gene listed in GenBank.
Gene expression analysis of UGT76G1
The data presented in Table (4) and Fig. (8) indicate that the gene expression of UGT76G1 varied significantly between the three stevia varieties under investigation. Based on the obtained results, China1 showed the highest gene expression level, exhibiting a ΔΔCq value of 0.178, followed by Egy1, which exhibited a relatively low expression level (ΔΔCq value of 0.119). The lowest gene expression level was reported for the Sponti variety, which showed a ΔΔCq value of 0.074, and these values were significantly different for the three varieties (p = 0.05). The relative gene expression of UGT76G1 was significantly high between China1 and Egy1 (value of 0.002), followed by China1 and Sponti and Egy1 and Sponti (0.0001 and 0.0007, respectively). These results support the finding obtained using HPLC analysis that showed a significant increase in rebaudioside A concentrations in China1 compared to Sponti.
Table 4.
Relative gene expression of the stevia UGT76G1 gene in three varieties using RT-quantitative PCR.
| Variety | Average of 2− ∆∆Cq | Gene expression in stevia varieties relative to that in China1 |
|---|---|---|
| China1 | 0.178 ± 0.012 | 1.00 |
| Egy1 | 0.119 ± 0.007 | 0.628 |
| Sponti | 0.074 ± 0.002 | 0.376 |
Figure 8.
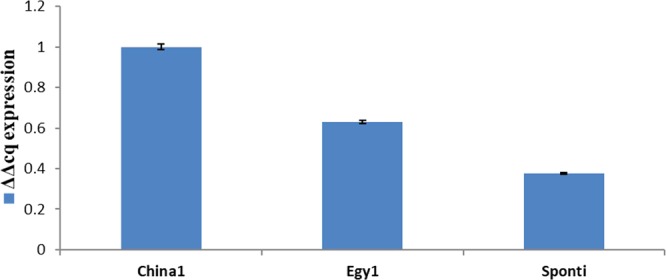
Quantitative estimation of UGT76G1 gene expression in stevia leaves.
Discussion
The stevioside content observed in this study was higher than that reported by Parris et al.39, in which the concentration ranged from 2.8–5.49%25 and 6.98–12.16%. For rebaudioside A content, China1 exhibited the highest value (15.54%), while Sponti exhibited the lowest value (13.02%). A low value for rebaudioside content was also reported by40. The variation in stevioside content and rebaudioside A content was further speculated to be associated with increasing altitude, resulting in decreasing temperature and in turn in accumulation of stevioside. Recently, stevia has received much attention. Rebaudioside A represents 30–40% of total glycosides41, but in the current study, the rebaudioside A levels of the stevia varieties China1, Egy1 and Sponti were 15.54%, 14.48% and 13.02% of the total glycosides, respectively. Therefore, these varieties are considered good materials for the study of the synthesis of steviol glycosides. The results of the present study are consistent with those of previous studies that determined stevioside, rebaudioside A and steviol levels via different methods42. HPLC and NIR spectroscopy models have been used to directly measure the steviol glycoside content in S. rebaudiana Bertoni to decrease the cost and complexity of operation36. The UGT76G1 gene was amplified from the stevia varieties by PCR using the designed primers (UGT76G1 F and R). The PCR assay showed an amplification product of the expected size (1.51 kb), which is in accordance with Li et al.24. The phylogenetic analysis indicated that the three sequences were grouped with UGT76G1 in the same cluster, indicating a close relatedness between UGTSr and UGT76G1. The phylogenetic analysis showed a close relationship between UGTSr and UGT76G143. The UGT76G1 obtained from the Egy1 variety (accession number MH087463) was closely related to S. rebaudiana KC631816.1 and AY345974.1 (UGT76G1 genes), with a nucleotide sequence similarity of 88%. Additionally, the nucleotide sequence from China1 (accession number MH087464) showed high nucleotide sequence similarity (98%) with Stevia rebaudiana KC631816.1, AY345974.1 and FJ607329.1 (UGT76G2 genes). In contrast, the nucleotide sequence obtained from Sponti (accession number MH087465) was closely related to KC631816.1 and AY345974.1 with a similarity of 87%. Moreover, China1 showed high similarity with XM_022150661.1 (Helianthus annuus UGT76G1), while Egy1, China1 and Sponti showed low similarity with FJ607329.1 (Stevia rebaudiana UGT76G2), XM_022150639.1 (Helianthus annuus UGT76G1), XM_022150662.1 (Helianthus annuus UGT76G1), and GQ259127.1 (Stevia rebaudiana UGT76G1). In a previous study, a mutation in the UGT76G1 gene was found to cause a reduction in rebaudioside A accumulation to 0.2% compared to normal plants, which usually exhibited 30–40% accumulation of total glycosides33. It was concluded that these three UGTs were not expressed at higher levels than any of the other UGTs. The expression of these genes was in the following order: UGT85C2 > UGT76G1 > UGT74G1. In the present study, we used qRT-PCR, a highly sensitive and specific method, to accurately measure the transcript levels of the three varieties44. Increased transcript levels of the UGT76G1 enzymes increased the level of the final product of the biosynthetic pathway, rebaudioside A45,46. Rebaudioside A accumulation is one of the most important traits contributing to the economic value of stevia crops47.
Conclusion
The present study investigated the relationship among three Stevia varieties using HPLC and molecular techniques. Sequence analysis was used to determine the nucleotide similarity of the stevia varieties China1, Egy1, and Sponti. The three varieties were clustered into two major groups and showed close similarity to UGT76G1. Phylogenetic relationships among different sequences of S. rebaudiana UGT76G1 were studied. A dendrogram of genetic similarities among the three varieties was constructed. The results indicated that the three varieties were clustered into two major groups: Group I (Egy1 and Sponti) and Group II (China1). This similarity might be the result of the existence of a common ancestor for Egy1 and Sponti, and this ancestor might be different from the ancestor of China1. The results obtained for the gene expression of UGT76G1 indicated that China1 showed the highest gene expression levels compared to the other two varieties, and the gene expression level of UGT76G1 was significantly higher in China1 than in the two varieties.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group (no. RG 1435-011). The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Author Contributions
Nader R. Abdelsalam wrote the main text of the paper, contributed to discussion of the results and prepared the tables and figures. William A. Botros performed DNA extraction, PCR and sequencing data analysis. Ahmed E. Khaled designed the experimental work. Mohamed A. Ghonema provided the plant materials and performed the HPLC analysis. Shimaa G. Hussein performed DNA extraction, PCR and sequencing data analysis. Hayssam M. Ali reviewed and edited the paper and prepared the tables and figures. Mohamed S. Elshikh reviewed the main text of the paper and designed the tables and figures.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gopal S, et al. Molecular dissection of transcriptional reprogramming of steviol glycosides synthesis in leaf tissue during developmental phase transitions in Stevia rebaudiana Bert. Scientific Reports. 2017;7(1):11835. doi: 10.1038/s41598-017-12025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh MH, et al. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: a two-year, randomized, placebo-controlled study. Clinical Therapeutics. 2003;25(11):2797–2808. doi: 10.1016/S0149-2918(03)80334-X. [DOI] [PubMed] [Google Scholar]
- 3.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Stevia: Steps to developing a new sweetener. Metabolism: Clinical and Experimental. 2004;53(1):73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Samant SS, Wilkes K, Odek Z, Seo HS. Tea-induced calmness: Sugar-sweetened tea calms consumers exposed to acute stressor. Scientific reports. 2016;16(6):36537. doi: 10.1038/srep36537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geuns JMC. Molecules of Interest Stevioside. Phytochemistry. 2003;64:913–921. doi: 10.1016/S0031-9422(03)00426-6. [DOI] [PubMed] [Google Scholar]
- 6.Jeppesen PB, et al. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism. 2003;52(3):372–378. doi: 10.1053/meta.2003.50058. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, et al. Stevioside does not cause increased basal insulin secretion orb-cell desensitization as does the sulphonylurea, glibenclamide: studies in vitro. Life Sci. 2006;78:1748–1753. doi: 10.1016/j.lfs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Ferri LA, et al. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytother Res. 2006;20:732–736. doi: 10.1002/ptr.1944. [DOI] [PubMed] [Google Scholar]
- 9.Yadav SK, Guleria P. Steviol glycosides from stevia: Biosynthesis pathway review and their application in foods and medicine. Crit. Rev. Food Sci. Nutr. 2012;52:988–998. doi: 10.1080/10408398.2010.519447. [DOI] [PubMed] [Google Scholar]
- 10.Kovylyaeva, G., et al. Glycosides from Stevia rebaudiana. Chem. Nat. Compounds43, 81–85, 0009-3130/07/4301-0081 (2007).
- 11.Aranda-González Irma, Moguel-Ordoñez Yolanda, Betancur-Ancona David. Determination of Rebaudioside A and Stevioside in Leaves of <i>S. rebaudiana</i> Bertoni Grown in México by a Validated HPLC Method. American Journal of Analytical Chemistry. 2015;06(11):878–885. doi: 10.4236/ajac.2015.611083. [DOI] [Google Scholar]
- 12.Cacciola Francesco, Delmonte Pierluigi, Jaworska Karolina, Dugo Paola, Mondello Luigi, Rader Jeanne I. Employing ultra high pressure liquid chromatography as the second dimension in a comprehensive two-dimensional system for analysis of Stevia rebaudiana extracts. Journal of Chromatography A. 2011;1218(15):2012–2018. doi: 10.1016/j.chroma.2010.08.081. [DOI] [PubMed] [Google Scholar]
- 13.Martono Y, Trihandaru S, Rondonuwu FS. Determination of Stevioside and Rebaudioside A in Stevia rebaudiana Bertoni Leaves Using near Infrared Spectroscopy and Multivariate Data Analysis. Indonesian Journal of Chemistry. 2018;18(4):664–671. doi: 10.22146/ijc.25580. [DOI] [Google Scholar]
- 14.Hughes J, Hughes MA. Multiple secondary plant product UDP-glucose glucosyltransferases genes expressed in cassava (Manihot esculenta Crantz) cotyledons. DNA Seq. 1994;5:41–49. doi: 10.3109/10425179409039703. [DOI] [PubMed] [Google Scholar]
- 15.Paquette, S., Moller, B. L. & Bak, S. On the origin of family 1 plant glycosyltransferases. Phytochemistry, 62, 399–413, 0031-9422/03/$ (2003). [DOI] [PubMed]
- 16.Behroozi P, Baghizadeh A, Saei A, Kharazmi S. Quantitative analysis of uridine diphosphate glycosyltransferase UGT85C2, UGT74G1 and UGT76G1 genes expression in Stevia rebaudiana under different irrigations. Russian journal of plant physiology. 2017;64(1):67–72. doi: 10.1134/S1021443717010034. [DOI] [Google Scholar]
- 17.Nguyen Thi Thanh Hanh, Seo Changseop, Kwak So-Hyung, Kim Jeesoo, Kang Hee-Kyoung, Kim Seong-Bo, Kim Doman. Enzymes in Food Biotechnology. 2019. Enzymatic Production of Steviol Glucosides Using β-Glucosidase and Their Applications; pp. 405–418. [Google Scholar]
- 18.Ghaheri Matin, Kahrizi Danial, Bahrami Gholamreza, Mohammadi-Motlagh Hamid-Reza. Study of gene expression and steviol glycosides accumulation in Stevia rebaudiana Bertoni under various mannitol concentrations. Molecular Biology Reports. 2018;46(1):7–16. doi: 10.1007/s11033-018-4250-4. [DOI] [PubMed] [Google Scholar]
- 19.Petit E, Jacques A, Daydé J, Vallejo V, Berger M. UGT76G1 polymorphism in Stevia rebaudiana: New variants for steviol glycosides conjugation. Plant Physiology and Biochemistry. 2019;135:563–569. doi: 10.1016/j.plaphy.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Jones P, Messner B, Nakajima J-I, Scha¨ ffner AR, Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavanol glycoside biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 2003;45:43910–43918. doi: 10.1074/jbc.M303523200. [DOI] [PubMed] [Google Scholar]
- 21.Lim E-K, Higgins GS, Li Y, Bowles DJ. Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem. J. 2003;373:987–992. doi: 10.1042/bj20021453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messner B, Thulke O, Scha¨ ffner AR. Arabidopsis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta. 2003;217:138–146. doi: 10.1007/s00425-002-0969-0. [DOI] [PubMed] [Google Scholar]
- 23.Jackson RG, et al. Identification and biochemical characterization of an Arabidopsis indole- 3-acetic acid glucosyltransferase. J. Biol. Chem. 2001;276:4350–4356. doi: 10.1074/jbc.M006185200. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Baldauf S, Lim E-K, Bowles DJ. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J. Biol. Chem. 2001;276:4338–4343. doi: 10.1074/jbc.M007447200. [DOI] [PubMed] [Google Scholar]
- 25.Brandle JE, Rosa N. Heritability for yield, leaf: stem ratio and stevioside content estimated from a landrace cultivar of Stevia rebaudiana. Can. J. Plant Sci. 1992;72:1263–1266. doi: 10.4141/cjps10086. [DOI] [Google Scholar]
- 26.Starratt AN, Kirby CW, Pocs R, Brandle JE. Rebaudioside F, a diterpene glycoside from Stevia rebaudiana. Phytochemistry. 2002;59:367–370. doi: 10.1016/S0031-9422(01)00416-2. [DOI] [PubMed] [Google Scholar]
- 27.Richman AS, Gijzen M, Starratt AN, Yang Z, Brandle JE. Diterpene synthesis in Stevia rebaudiana: Recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J. 1999;19:411–421. doi: 10.1046/j.1365-313X.1999.00531.x. [DOI] [PubMed] [Google Scholar]
- 28.Richman A, et al. The major sweet glucosides of Stevia rebaudiana. Plant J. 2005;41:56–67. doi: 10.1111/j.1365-313X.2004.02275.x. [DOI] [PubMed] [Google Scholar]
- 29.Humphrey TV, Richman AS, Menassa R, Brandle JE. Spatial organization of four enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis. Plant Molecular. Biol. 2006;61:47–62. doi: 10.1007/s11103-005-5966-9. [DOI] [PubMed] [Google Scholar]
- 30.Shibata, H., Sonoke, S., Ochiai, H., Nishihashi, H. & Yamada, M. Glucosylation of steviol and steviol-glucosides in extracts from Stevia rebaudiana Bertoni. Plant Physiol, 95, 152–15 6, 0032-0889/91/95/01 52/05 (1991). [DOI] [PMC free article] [PubMed]
- 31.Shibata H., Sawa Y., Oka T., Sonoke S., Kim K.K., Yoshioka M. Steviol and Steviol-Glycoside: Glucosyltransferase Activities in Stevia rebaudiana Bertoni - Purification and Partial Characterization. Archives of Biochemistry and Biophysics. 1995;321(2):390–396. doi: 10.1006/abbi.1995.1409. [DOI] [PubMed] [Google Scholar]
- 32.Brandle JE, Telmer PG. Steviol glycoside biosynthesis. Phytochemistry. 2007;68:1855–1863. doi: 10.1016/j.phytochem.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Yang, Y. H. et al. Base substitution mutations in uridinediphosphate-dependent glycosyltransferae 76G1 gene of Stevia rebaudiana causes the low levels of rebaudioside A Mutations inUGT76G1, A key gene of steviol glycosides synthesis, Plant Physiology and Biochemistry 80, 220–225, 10.1016/j.plaphy.2014, 04.005 (2014). [DOI] [PubMed]
- 34.Harmanjit K. Chromatographic determination of stevioside in leaf parts of in vitro and in vivo regenerated plants of Stevia rebaudiana. International Journal of Natural Products Research. 2011;1(4):2249–2253. [Google Scholar]
- 35.Nishiyama, P., Alvarey, M. & Vieira, L. Quantitative analysis of stevioside in the leaves of Stevia rebaudiana by Near Infrared reflectance spectroscopy. 3. Sci. Food Agric. 59, 277–281, 0022-5142/92/$05.00 (1992).
- 36.Yu C, Xu K, Shi Y. The spectrum model established for measuring the contents of Rebaudioside A and Stevioside quickly in the leaves of Stevia rebaudiana Bertoni. Energy Procedia. 2011;5:855–861. doi: 10.1016/j.egypro.2011.03.151. [DOI] [Google Scholar]
- 37.Makapugay, H. C., Nanayakkara, N. P. D. & Kinghorn, A. D. Improved high-performance liquid chromatographic separation of the Stevia rebaudiana sweet diterpene glycosides using linear gradient elution, J. Chromatogr, 283, 390–395, 002 l-9673/84 (1984).
- 38.Livak, K. & Schmittgen, T. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method, Methods25, 402–408, 1046-2023/01 (2001). [DOI] [PubMed]
- 39.Parris CA, Shock CC, Qian M. Dry leaf and steviol glycoside productivity of Stevia rebaudiana in the Western United States. HortScience. 2016;51(10):1220–1227. doi: 10.21273/HORTSCI11149-16. [DOI] [Google Scholar]
- 40.Pereira C, Storck L, Lopes SJ, Martin TN, Bisognin DA. Dry biomass and glycosides yield from Stevia rebaudiana leaves under different harvesting times. Bioscience. Journal. 2016;32(6):1462–1471. doi: 10.14393/BJ-v32n6a2016-31574. [DOI] [Google Scholar]
- 41.Yadav AK, Singh S, Dhyani D, Ahuja PS. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)] Can. J. Plant Sci. 2011;91:1–27. doi: 10.14393/BJ-v32n6a2016-31574. [DOI] [Google Scholar]
- 42.Gardana C, Scaglianti M, Simonetti P. Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-highperformance liquid chromatography–mass spectrometry. Journal of Chromatography A. 2010;1217:1463–1470. doi: 10.1016/j.chroma.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Madhav H, Salini B, Mohankumar C. Functional and structural variation of uridine diphosphate glycosyltransferase (UGT) gene of Stevia rebaudianae UGTSr involved in the synthesis of rebaudioside A. Plant Physiol. Biochem. 2013;63:245–53. doi: 10.1016/j.plaphy.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed A, Ceunen S, Geuns J, Ende W, Ley M. UDP-dependent glycosyltransferases involved in the biosynthesis of steviol glycosides. Plant Physiology. 2011;168:1136–1141. doi: 10.1016/j.jplph.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Behroozi P, Baghizadeh A, Saei A, Kharazm S. Quantitative Analysis of Uridine Diphosphate Glycosyltransferase UGT85C2, UGT74G1 and UGT76G1 Genes Expression in Stevia rebaudiana under Different Irrigations. Russian Journal of Plant Physiology. 2017;64(1):67–72. doi: 10.1134/S1021443717010034. [DOI] [Google Scholar]
- 46.Zhang SS, et al. Mutations in the uridine diphosphate glucosyltransferase 76G1 gene result in different contents of the major steviol glycosides in Stevia rebaudiana. Phytochemistry. 2019;162:141–147. doi: 10.1016/j.phytochem.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Francisco F, Pereira GP, Machado MP, Kanis LA, Deschamps C. Characterization of Stevia rebaudiana Bertoni accessions cultived in southern Brazil. J. Agric. Sci. 2018;10:353. doi: 10.5539/jas.v10n3p353. [DOI] [Google Scholar]