Abstract
Two new cationic surfactants, n-hexadecyl-3-methylpyridinium bromide and n-heptadecyl-3-methylpyridinium bromide have been synthesized and characterized in solid state by FT-IR, and in solution by 1H- and 13C-NMR spectroscopy. The values of critical micelle concentration (CMC) were determined by UV-visible spectroscopy and conductometry. Interaction of synthesized surfactants with two anionic drugs, i.e., diclofenac sodium {[2-(2, 6-Dichloroanilino) phenyl] acetic acid} and ketoprofen [(RS)-2-(3-benzoylphenyl) propionic acid] was studied by UV-visible spectroscopy. Binding constant (K), Gibb's free energy (ΔG) and number of drug molecules (n) per micelle were also calculated. These synthesized surfactants were proved to be efficient in increasing the solubility and bioavailability of drug molecules. In order to check the carrier efficiency of synthesized surfactants against bioactive coordinate, on complexes, interaction of recently reported bioactive zinc complexes was tested with synthesized cationic surfactants by conductometric measurements. Mole fractions (Xcmc) and Gibbs free energy (ΔGcmc) values were also calculated. Both surfactants were further screened for anti-fungal and anti-bacterial activities.
Keywords: Analytical chemistry, Inorganic chemistry, Pharmaceutical chemistry, Cationic surfactants, CMC, Drug-/complex-surfactant interactions
1. Introduction
Surface active agents are amphipathic compounds possessing polar and non-polar moieties as head and tail respectively. Depending on the charge present on head moieties they can be classified as; anionic, cationic, amphoteric and non-ionic surfactants [1]. One of the exciting features of surface active agents is self-aggregation which results in the formation of micelles which is considered to be an alternative mechanism to interfacial adsorption. Micellization is accompanied with reduction in free energy of system, increment in entropy which has a spontaneous mechanism [1]. The critical micelle concentration (CMC) is defined as the minimum concentration at which surfactant molecules starts to aggregate that can be spotted as an inflection point when any of the physicochemical property is plotted against concentration of surfactant molecules [2, 3]. The CMC value can be influenced by many factors including structure, chemical nature of hydrophobic and hydrophilic moieties, temperature, presence of electrolyte, solvent, nature of counter ions, pH etc [2].
In bio-medical field the limited therapeutic efficiency of drugs, which may be in metal complexes, is generally related to their low solubility in aqueous media. Surfactants have potential to act as drug delivery systems in which micelles not only solubilize but also increase bioavailability of drug molecules. They can stay in body for a longer time and protect drug molecules from adverse effects of biological surroundings [4]. The further use of micelle in drug delivery systems is also to reduce degradation rate of drug molecules. Micelles interact with drug molecules via either through their outer surfaces or by incorporation of drug molecules in their core [5]. Ionic surfactants being an important group of surfactants have also established their importance due to their interactions with different inorganic and organic bio-active, DNA, and proteins. The path of interaction is governed by the interaction of hydrophobic or hydrophilic sites of drugs and that of surfactants [5].
Surfactants have multitude of industrial applications and are used in cosmetics, corrosion inhibition, biocides, fabric softeners, emulsifier, detergents etc. Moreover they are effective bactericides and fungicides [6, 7, 8, 9, 10, 11, 12, 13].
In the present work we have reported the synthesis of two N-(n-alkyl)-3-methylpyridinium based cationic surfactants that are characterized in solid state by FTIR spectroscopy whereas in solution by 1H- and 13C-NMR. The critical micelle concentration (CMC) has been measured by UV-visible spectroscopy and conductometry. Interaction of synthesized surfactants with two anionic drugs has been studied by UV-visible spectroscopy. Moreover, in order to prolong the bioavailability and hence increasing the bio-active potential of zinc metal complexes, their interaction with synthesized surfactants has also been investigated. The anti-microbial activities of surfactants have also been studied.
2. Experimental
2.1. Materials and methods
3-methylpyridine and 1-bromoalknane was procured from Alfa-Aesar (USA). Ethanol, methanol, chloroform, toluene were obtained from Merck Chemicals (Germany). All these chemicals were of analytical grade and used without further purification. Toluene was used after drying for further synthesis. Melting points were recorded by Sanyo electro thermal melting point apparatus. 1H and 13C-NMR were recorded in CDCl3 as solvent on Bruker AC spectrometer at 300.13 MHz for 1H and at 75.47 MHz for 13C. FT-IR spectra were recorded on thermo Nicolet-6700 spectrophotometer in the frequency range of 4000–400 cm−1. To determine CMC of the surfactant molecules and to study drugs interaction with surfactants, Shimadzu double beam Spectrophotometer 1800 was used. Inolab 720 precision conductivity meter was used to determine electrical conductivity of surfactant molecules at room temperature and was also used to study their interaction with metal complexes.
2.2. General procedure for the synthesis of surfactants
Equimolar quantities (10 mM) of 3-methylpyridine and 1-bromoalkane were dissolved in 35 mL of toluene in 250 mL round bottom flask. The reaction mixture was refluxed for 12 h with continuous stirring. Diethyl ether (20–25 mL) was added to cold solution and stirred. Separating funnel was then used to separate the solvents and the desired product. The product was washed with diethyl ether. The general reaction is:
The structures and numbering for interpretation of 1H- and 13C-NMR are given in scheme 1.
Scheme 1.
Numbering of carbon and proton atoms of surfactants (I & II) for interpretation of 1H- and 13C-NMR.
1H and 13C-NMR data of surfactants (I & II) are given as:
N-(n-hexadecyl)-3-methylpyridinium bromide (I)
1H-NMR (CDCl3, δ-ppm, 300 MHz): 9.36 (1H, H1, s), 8.28 (1H, H3, d), 8.05 (1H, H4, dd), 9.23 (1H, H5, d), 2.63 (3H, H6, s), 4.90 (2H, H7, t), 1.20–2.31 (28H, H8−21, m), 0.87 (3H, H22). 13C-NMR (CDCl3, δ-ppm, 75.5 MHz): 145.6 (C1), 139.5 (C2), 142.2 (C3), 127.8 (C4), 144.4 (C5), 26.1(C6), 61.8 (C7), 18.7–34.0 (C8–C22). FT-IR (υ cm−1): 1636 (C=N), 1149 (C–N), 1506 (C=C), 2919 (Aromatic –CH), 2852 (-CH3).
N-(n-heptadecyl)-3-methylpyridinium bromide (II)
1H-NMR (CDCl3, δ-ppm, 300 MHz): 9.31 (1H, H1, s), 8.28 (1H, H3, d), 8.04 (1H, H4, dd), 9.22 (1H, H5, d), 2.66 (3H, H6,s), 4.94 (2H,H7, t), 1.26–2.03 (30H, H8−22,m), 0.88 (3H, H23, t). 13C-NMR (CDCl3, δ-ppm, 75.5 MHz): 145.6 (C1), 139.6 (C2), 142.2 (C3), 127.8 (C4), 144.5 (C5), 26.1 (C6), 62.0 (C7), 18.8–34.1 (C8–C23). FT-IR (υ cm−1): 1644 (C=N), 1151 (C–N), 1506 (C=C), 2917 (Aromatic –CH), 2848 (-CH3).
2.3. Drug-surfactant interaction study
Two anionic drug solutions were prepared and divided into two parts. One part was used as reference solution and other was used to prepare different drug-surfactant solutions having concentration range from pre-micellar to post-micellar. To run auto zero, both cells were filled with deionized water. Then surfactant solutions of different concentrations having constant drug concentration were examined to study the influence of varying surfactant concentrations on the absorption spectra of drug. In order to avoid errors same stock solutions were used [14]. All the reported values are the average of at least triplicate independent measurements with relative standard deviation of ±3.5%.
2.4. Metal complex-surfactant interaction study
The conductivity measurements of complex-surfactant solutions were carried out to study the effect of complex-surfactant interaction on CMC value at 25 ± 0.1 0C. Conductivity measurements were performed by keeping the complex concentration constant and varying the concentration of surfactant solutions. All the reported values are the average of at least triplicate independent measurements with relative standard deviation of ±3.5%.
2.5. Antimicrobial activities
For antimicrobial studies synthesized surfacntants were tested against three fungal strains; Aspergillus flavous, Aspergillus fumigatus, Aspergillus niger, and four bacterial species, Bacillus subtilis (+), Staphylococcus aureus (+), Klebeseilla pneumonae (-), Escherichia coli (-). Disc diffusion method was used for antimicrobial study. At 45 °C broth culture of test sample was homogeneously mixed with 75 mL of nutrient agar medium, then decant into 14 cm antiseptic petri plate. The agar media was solidified. Then sterilized metallic borer was used to make 8 mm wells in media. Afterwards, respective wells were filled with 100 μL of DMSO solution of test sample at the amount of 1 mg mL−1. DMSO acted as negative (-) control while antibacterial and antifungal drugs Azithromycine (1 mg mL−1), Ciprofloxacine (1 mg mL−1) and Terbenafine (1 mg mL−1) were served as positive (+) control. At 37 °C, duplicate plated of each fungal strain and triplicate plates of each bacterial strain were incubated for 24 h.
3. Results and discussions
3.1. Characterization
The two surfacntants i.e., N-(n-hexadecyl)-3-methylpyridinium bromide (I) and N-(n-heptadecyl)-3-methylpyridinium bromide (II) were synthesized by simple addition reactions. The important physical properties of the compounds are reported in Table 1.
Table 1.
Physical properties of surfacntants (I and II).
| Surfacntant | MolecularFormula | Molecular Weight | Physical State | Melting Point (°C) | Color | CMC (m mol dm−3) | Solubility |
|---|---|---|---|---|---|---|---|
| I | C22H40NBr | 398 | Solid | 51–52 | White | 0.243 | Water, Methanol, Ethanol, Acetone, DMSO, Chloroform |
| II | C23H42NBr | 411.9 | Solid | 53–54 | White | 0.239 | As above |
The structural confirmation of synthesized surfactants was done by FT-IR, 1H- and 13C-NMR spectroscopy. In FT-IR spectra of both the surfactants, the characteristic peaks are at 1636-1644 cm−1 (C=N), 1149-1151 cm−1 (C–N), 1506 cm−1 (C=C), and 2917-2917 cm−1 (Aromatic –CH). In 1H-NMR the signal for H7 shifted from 3.39 ppm to 4.90 ppm (I) and 4.94 ppm (II) while in 13C-NMR the signal for C-7 shifted from 33.71 ppm (in alkyl bromides) to 61.8 ppm (I) and 62 ppm (II), respectively.
3.2. CMC determination
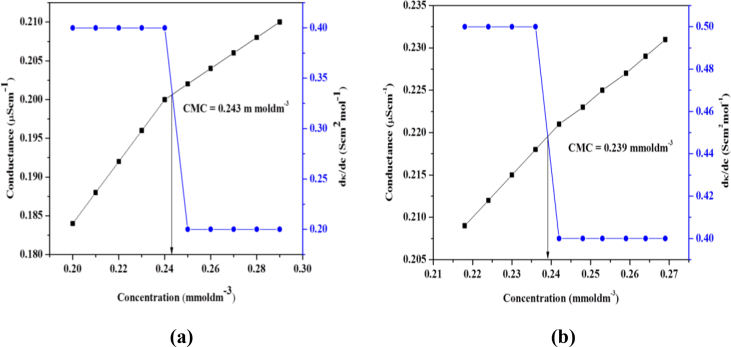
Conductivity measurements and UV-visible spectroscopy was used to investigate the CMC values of both surfactants. Firstly, standard solutions of surfactants were prepared in ethanol solvent and then conductivity measurements of varied surfactant concentrations were performed at 298 K. The data of conductivity obtained was plotted against concentration and CMC value was recorded. For CMC calculations, conductivity plots against varying surfactant concentrations are shown in Fig. 1. The CMC corresponds to the inflection point in the specific conductivity-concentration plot. In order to eliminate vagueness in results, differential conductance was plotted against concentration of surfactant and a reverse sigmoid shaped curve is formed which gave more precise CMC value [15, 16]. In pre micellar region, there is significant increase in conductance due to presence of free anions and cations but not in post micellar region probably due to micelle formation. CMC values for surfactants (I) and (II) were calculated as 0.243 and 0.239 mmol dm-3, respectively.
Fig. 1.
Plots of conductivity and differential conductivity vs concentrations of a) surfactant (I) and b) surfactant (II).
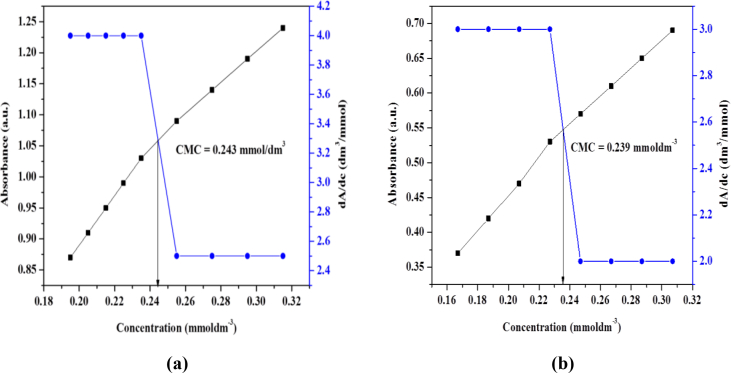
CMC values obtained from conductivity measurements were cross-checked by using UV-Visible spectroscopy. Firstly λmax was determined by plotting graph between absorbance and wavelength. Then absorbance values of surfactants (I) and (II) were recorded at λmax. The plotted graphs between absorbance and surfactant concentration are shown in Fig. 2. CMC values determined from both techniques are in close agreement.
Fig. 2.
Plots of absorbance and differential absorbance vs concentrations of a) surfactant (I) and b) surfactant (II).
It has been observed that by increasing chain length (even by one carbon), the value of CMC decreases [17].
3.3. Drug interaction studies
The drugs used for interaction studies are diclofenac sodium [{2-(2, 6-Dichloroanilino) phenyl} acetic acid] and ketoprofen [{(RS)-2-(3-benzoylphenyl) propionic acid}]. Both drugs are members of NSAIDs which are used as anti-pyretic and analgesics [18, 19]. These drugs have been interacted with surfactants to increase their bioavailability and controlled release rate in body fluids. Two types of interactions are possible, one involves the interaction with head of surfactants and in other drug molecules incorporated into hydrophobic core of micelles [20]. UV-Visible spectroscopy was used in order to study surfactant drug interactions.
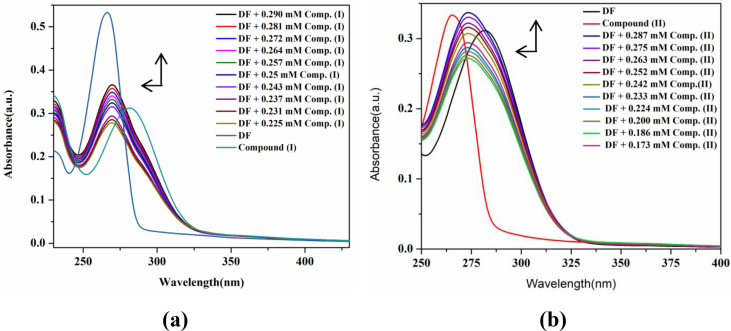
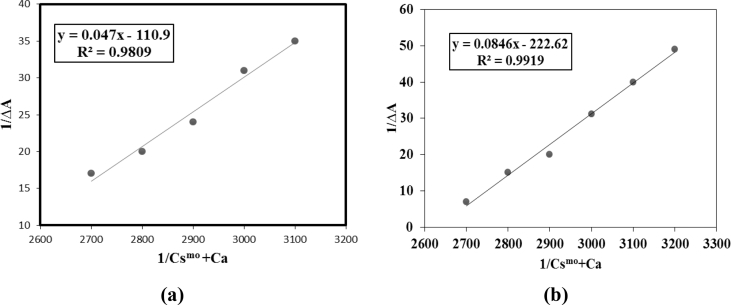
The λmax for diclofenac sodium was observed at 282 nm (Fig. 3). As drug was mixed with pre-micellar concentration of desired surfactants (I and II), a considerable blue shift of 8 nm was observed in its λmax. By approaching the post-micellar concentration, the rise in absorption of drug molecules was witnessed showing the increased bioavailability of drug molecules. By increasing surfactant concentration, absorption of drug increases [21] and this surfactant-drug interaction follows a straight line pattern as shown in Fig. 4. The value of Gibb's free energy (ΔG) and binding constant (K) were calculated from the Eqs. (1) and (2) [22]. Negative sign of Gibb's free energy (ΔG) shows that the reaction was spontaneous between drug and surfactant.
| (1) |
| (2) |
Fig. 3.
Absorption spectra of diclofenac sodium with a) surfactant (I) and b) surfactant (II).
Fig. 4.
Plots of 1/ΔA vs 1/Csmo + Ca for a) compound (I) and b) compound (II).
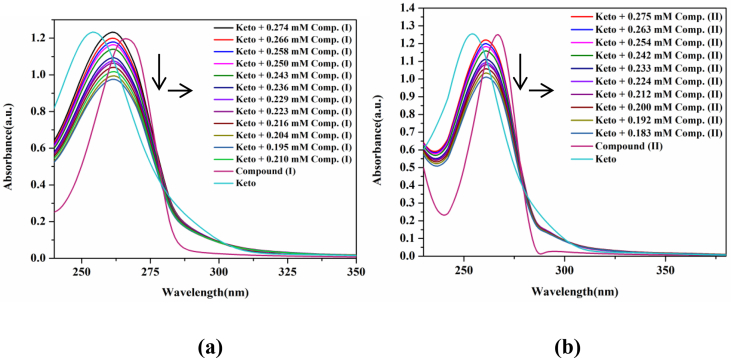
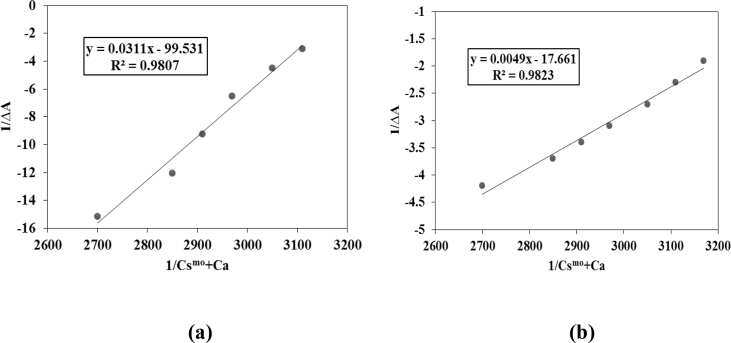
Ketoprofen gives λmax at 254 nm. When surfactants (I) and (II) was homogenized with KP, a bathochromic shift of 6 nm was observed in the λmax. Moving from pre micellar to post micellar concentration, a decrease in drug absorption was spotted as an indication that the drug is being incorporated into micellar core, thus the outside harmful environment cannot poison it and it can be safely transferred to its target (see Figs. 5 and 6).
Fig. 5.
Absorption spectra of KP with a) compound (I) and b) compound (II).
Fig. 6.
Plots of 1/ΔA vs 1/Csmo + Ca for a) compound (I) and b) compound (II).
In order to calculate the number of drug of drug molecules (n) per micelle following expressions were used [14].
| (3) |
In Eq. (3), ‘n’ gives number of drug molecules attached to micelles, ‘M’ represents micelle concentration and ‘Cm’ is concentration of drug solubilized in micelles.
| (4) |
‘Cs’ is concentration of surfactants. ‘N’ gives aggregation number. ‘CMC’ is critical micelle concentration of surfactants.
| (5) |
Where, ‘Ao’ and ‘A’ are absorbance of drug without and with surfactant concentration, respectively. ‘Eo’ and ‘Em’ gives corresponding absorptivities calculated from Beer lambert law. Table 2 gives the binding constant (K), Gibb's free energy (ΔG) and number of drug molecules (n) attached per micelle.
Table 2.
Calculated parameters for drug interaction studies.
| Compounds no. | Ao | A | Eo x 105 | Em X 105 | Cm x 10−5 mol/dm3 | Mx 10−5 mol/dm3 | N | K dm3/mol | -ΔG kJ/mol |
|---|---|---|---|---|---|---|---|---|---|
| I + DF | 0.317 | 0.332 | 3.17 | 3.32 | 0.100 | 0.025 | 4 | 2359 | 19.2 |
| II + DF | 0.317 | 0.333 | 3.17 | 3.33 | 0.100 | 0.035 | 3 | 2631 | 19.5 |
| I + KP | 1.267 | 1.252 | 18.1 | 17.8 | 0.070 | 0.14 | 0.5 | 3200 | 20.0 |
| II + KP | 1.267 | 1.003 | 18.1 | 14.7 | 0.077 | 0.17 | 0.4 | 3604 | 20.3 |
3.4. Complex interaction studies
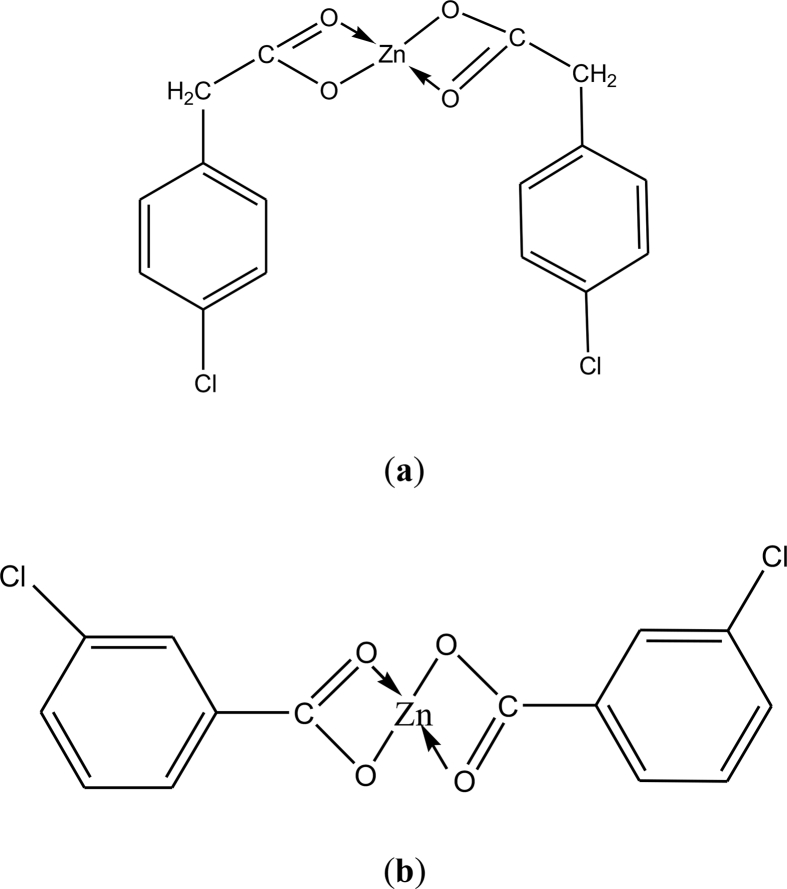
Zinc (II) complexes have wide range of biological applications. In order to increase controlled release rate and bioavailability of metal complexes based drugs in body fluids, interactions of synthesized surfactants were studied with our two recently reported synthesized zinc (II) complexes [23] i.e., (a) = [Zn (L1)2] and (b) [Zn (L2)2], through conductivity measurements. Here L1 = 3-chlorobenzoic acid and L2 = 2-(4-chlorophenyl) acetic acid. Solutions of 1 mM of each complex were prepared in ethanol and their specific conductance was measured in the presence of compound (I) and (II) to study the complex–surfactant interaction. The concentration of the complex was kept constant while that of the surfactants was varied from 0.05 mmol dm-3 to 0.75 mmol dm-3, in order to monitor the change in CMC of each surfactant upon the formation of Zn complex-surfactant adduct (see Fig. 7).
Fig. 7.
Zn (II) Complexes a) [Zn (L1)2] b) [Zn (L2)2].
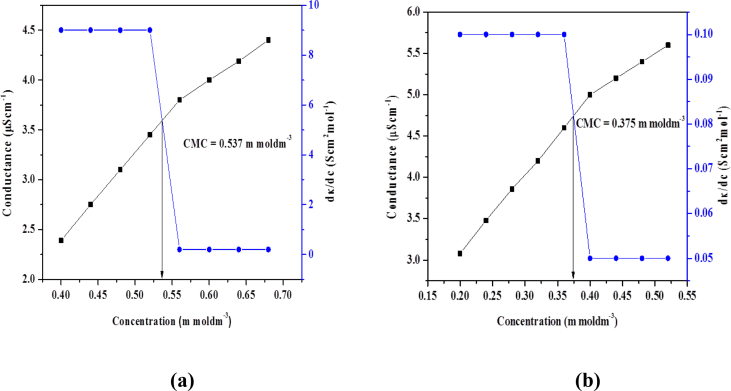
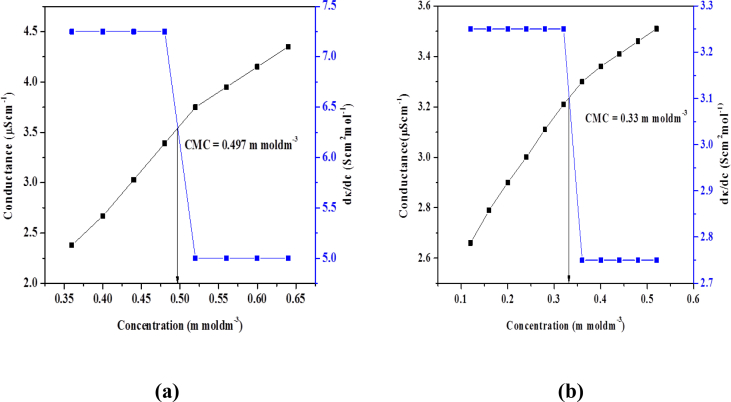
On interaction with complexes, the conductivity of surfactants increases hence their value of CMC. The increase in conductance was observed because of presence of free positive and negative ions of surfactants. The concentration of complex was kept constant. By increasing the concentration of compound (I) the value of CMC increases showing the strong interaction between surfactant and complex. In pre micellar region, there is significant increase in conductance due to presence of free anions and cations and surfactant molecules existed as monomers. In post micellar region, there is less increase in conductance due to micelle formation. However, the observed conductance is due to presence of bromide ions. Due to strong bonding between complex and cations of surfactants the CMC value increased. The role of complex is to reduce repulsion between nitrogen atoms of cationic head by decreasing its charge density. The complex decreases the micellization and increases the entropy [24]. If the complex is administered orally with surfactant of higher CMC, then on dilution the micelles will rupture and complex (drug) will be released to perform its activity but in case of surfactant of low CMC, the complex will reside in micelle and result in reduced bioavailability. This reduction in activity of complex reveals stronger interaction with micelles and this phenomenon is particularly very useful for prolonged delivery of pharmaceutical agents in biological systems. So CMC regulates release rate of drug in biological system [25] (see Figs. 8 and 9).
Fig. 8.
[Zn (L1)2] interactions with a) compound I and b) compound II.
Fig. 9.
[Zn (L2)2] interactions with a) compound I and b) compound II.
To calculate the ΔGm values of complex surfactant interactions, following expressions was used [25, 26].
| (6) |
Where β = degree of counter ion binding and can be calculated as:
| (7) |
α = degree of dissociation and calculated from the ratio of slopes before and after the CMC value:
| (8) |
S1 and S2 are slopes before and after the CMC value
| (9) |
Above expression is for the calculation of CMC in terms of mole fraction.
Following table gives the ΔGm values of interaction of surfactants with different zinc complexes (see Table 3).
Table 3.
Calculated parameter for complex interaction studies.
| Compounds no. | Complexes | CMC [mM] | -ΔGm (kJ/mol) |
|---|---|---|---|
| I | Zn(L1)2 | 0.537 | 36.46 |
| II | Zn(L1)2 | 0.375 | 40.51 |
| I | Zn(L2)2 | 0.497 | 39.87 |
| II | Zn(L2)2 | 0.33 | 42.41 |
Negative sign of ΔGm shows that complex surfactant interactions are spontaneous. Surfactant-complex interaction plays a significant role in augmentation of complex solubility.
3.5. Anti-microbial activity
Ant-bacterial and anti-fungal activities of all compounds are shown in Tables 4 and 5.
Table 4.
Anti-bacterial activity of compounds (I & II).
| Compounds no. |
Bacillus subtilis |
Staphylococcus aureus |
Klebeseilla pneumonae |
Escherichia coli |
|---|---|---|---|---|
| (+) | (+) | (-) | (-) | |
| Zone of inhibition (mm) | ||||
| I | 15 | 21 | 12 | 17 |
| II | 17 | 19 | 9 | 14 |
| Azithromycine | 28 | 24 | 21 | 22 |
| Ciprofloxacine | 22 | 24 | 26 | 29 |
Table 5.
Anti-fungal activity of compounds (I & II).
| Compounds no. | Aspergillus fumigatus | Aspergillus niger | Aspergillus flavous |
|---|---|---|---|
| % inhibition | |||
| I | 43 | 57 | 55 |
| II | 57 | 59 | 49 |
| Terbenafine | 100 | 100 | 100 |
Compound I showed significant activity with Staphylococcus aureus and Escherichia coli. Compound II showed effective anti-bacterial activity against Bacillus subtilis and Staphylococcus aureus. All compounds are significant bactericides.
Compound I displayed efficient anti-fungal activity against Aspergillus niger and Aspergillus flavous while compound II exhibited substantial activity against Aspergillus niger and Aspergillus fumigatus. Hence both compounds are good fungicides.
4. Conclusion
Cationic surfactants, N-(n-hexadecyl)-3-methylpyridinium bromide (I) and N-(n-heptadecyl)-3-methylpyridinium bromide (II) have been synthesized and characterized by FT-IR, 1H and 13C-NMR spectroscopy. Critical micelle concentration (CMC) values are determined by UV-Visible spectroscopy and results are further verified by conductometry. CMC values determined for compound I and II are 0.243 and 0.239 mM, respectively. Interaction of cationic surfactants with two anionic drugs i.e., diclofenac sodium and ketoprofen is studied by UV-Visible spectroscopy. The Binding constant (K), Gibb's free energy (ΔG) and drug molecules attached per micelle (n) are also reported. The synthesized cationic surfactants are found effective in increasing the solubility and bioavailability of drugs. By varying alkyl chain length, we can vary CMC so we can control retention time of drugs in body. In order to check the effective interaction of synthesized surfactants towards bio-active coordination complexes, two bio-active zinc complexes are interacted and studied with surfactants by conductometric technique. Metal complex-surfactant interaction results in increase in the CMC value of surfactants. The negative value of ΔG in case of interactions of surfactants with drugs and complexes show spontaneous type of interactions. Synthesized compounds are proved to be potentially anti-bacterial and anti-fungal agents.
Declarations
Author contribution statement
Kalsoom Akhter, Kaleem Ullah: Performed the experiments; Wrote the paper.
Rabia Talat, Faizan Ullah: Performed the experiments.
Ali Haider, Saqib Ali: Conceived and designed the experiments.
Nasir Khalid: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Saqib Ali was supported by the Pakistan Academy of Sciences under grant No. PAS-76.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Ali Haider, Email: ahaider@qau.edu.pk.
Saqib Ali, Email: drsa54@hotmail.com.
References
- 1.Azum N., Rub M.A., Asiri A.M. Interaction of antipsychotic drug with novel surfactants: micellization and binding studies Chinese. J. Chem. Eng. 2018;26:566–573. [Google Scholar]
- 2.Rub M.A., Azum N., Asiri A.M. Binary mixtures of sodium salt of ibuprofen and selected bile salts: interface, micellar, thermodynamic, and spectroscopic Study. J. Chem. Eng. Data. 2017;62:3216–3228. [Google Scholar]
- 3.Kumar D., Rub M.A. Studies of interaction between ninhydrin and Gly-Leu dipeptide: influence of cationic surfactants (m-s-m type Gemini) J. Mol. Liq. 2018;269:1–7. [Google Scholar]
- 4.Farías T., De Menorval L.C., Zajac J., Rivera A. Solubilization of drugs by cationic surfactants micelles: conductivity and 1H NMR experiments. Colloids Surf., A. 2009;345(1):51–57. [Google Scholar]
- 5.Fayyaz S., Ali S., Khalid N., Shah A., Ullah F. One pot synthesis and properties of cationic surfactants. J. Surfactants Deterg. 2016;19(4):841–848. [Google Scholar]
- 6.Gonçalves L.M., Kobayakawa T.G., Zanette D., Chaimovich H., Cuccovia I.M. Effects of micelles and vesicles on the oximolysis of p-nitrophenyl diphenyl phosphate: a model system for surfactant-based skin-defensive formulations against organophosphates. J. Pharm. Sci. 2009;98(3):1040–1052. doi: 10.1002/jps.21506. [DOI] [PubMed] [Google Scholar]
- 7.Shaban S.M., Aiad I., Moustafa A.H., Aljoboury O.H. Some alginates polymeric cationic surfactants; surface study and their evaluation as biocide and corrosion inhibitors. J. Mol. Liq. 2019;273:164–176. [Google Scholar]
- 8.Shaban S.M., Saied A., Tawfik S.M., Abd-Elaal A., Aiad I. Corrosion inhibition and biocidal effect of some cationic surfactants based on Schiff base. J. Ind. Eng. Chem. 2013;19(6):2004–2009. [Google Scholar]
- 9.Mishra S. Ester quats: the novel class of cationic fabric softeners. J. Oleo Sci. 2007;56(6):269–276. doi: 10.5650/jos.56.269. [DOI] [PubMed] [Google Scholar]
- 10.Maurya Neetish Kumar, Mandal Ajay. Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based EOR application in oil reservoirs. Chem. Eng. Res. Des. 2018;132:370–384. [Google Scholar]
- 11.Puchta Cationic surfactants in laundry detergents and laundry aftertreatment aids. J. Am. Oil Chem. Soc. 1984;61(2):367–376. [Google Scholar]
- 12.Talat R., Fayyaz S., Ali S., Khalid N., Haider A., Shah A., Ullah F. Designing of new cationic surfactant based micellar systems as drug carriers: an investigation into the drug cell membrane interactions. J. Dispersion Sci. Technol. 2019;40 [Google Scholar]
- 13.Paluch E., Piecuch A., Obłąk E., Lamch Ł., Wilk K.A. Antifungal activity of newly synthesized chemo degradable dicephalic-type cationic surfactants. Colloids Surfaces B Biointerfaces. 2018;164(1):34–41. doi: 10.1016/j.colsurfb.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.M., Shah S.S.A. UV-visible study of partitioning of pyrene in an anionic surfactant sodium dodecyl sulfate. J. Dispersion Sci. Technol. 2008;29(10):1401–1407. [Google Scholar]
- 15.Azum N., Naqvi A.Z., Akram M. Properties of mixed aqueous micellar solutions formed by cationic alkanediyl-α, ω-bis (tetradecyldimethylammonium bromide) and alkyltrimethylammonium bromides: fluorescence and conductivity studies. J. Chem. Eng. Data. 2009;54(5):1518–1523. [Google Scholar]
- 16.Mata J., Varade D., Bahadur P. Aggregation behavior of quaternary salt based cationic surfactants. Thermochim. Acta. 2005;428(1):147–155. [Google Scholar]
- 17.Engin Özdil S., Akbaş H., Boz M. Synthesis and physicochemical properties of double-chain cationic surfactants. J. Chem. Eng. Data. 2015;61(1):142–150. [Google Scholar]
- 18.Brogden R., Heel R.C., Pakes G.E., Speight T.M., Avery G.S. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20(1):24–48. doi: 10.2165/00003495-198020010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kantor T.G. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 1986;6(3):93–102. doi: 10.1002/j.1875-9114.1986.tb03459.x. [DOI] [PubMed] [Google Scholar]
- 20.Rangel-Yagui C.O., Pessoa A., Jr., Tavares L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005;8(2):147–163. [PubMed] [Google Scholar]
- 21.Zhang L., Dong Y., Zhang X., Guo X. Micellization of lactosylammonium surfactants with different counter ions and their interaction with DNA. J. Chem. Eng. Data. 2016;61(9):2969–2978. [Google Scholar]
- 22.Mehta S.K., Bhasin K.K., Kumar A., Dham S. Micellar behavior of dodecyldimethylethyl ammonium bromide and dodecyltrimethylammonium chloride in aqueous media in the presence of diclofenac sodium. Colloids Surf., A. 2006;278(1):17–25. [Google Scholar]
- 23.Ullah K., Sirajuddin M., Zubair M., Haider A., Ali S., Ullah F., Dutkiewicz G., Kubicki M., Rizzoli C. Designing of homo and heteroleptic zinc (II) carboxylates: synthesis, spectroscopic characterizations, DNA binding study, CTAB interaction and in vitro antimicrobial evaluations. JICS. 2019;16(6):1–15. [Google Scholar]
- 24.Shah S.S., Khan M.S., Ullah H., Awan M.A. Solubilization of amphiphilic hemicyanine dyes by a cationic surfactant, cetyltrimethylammonium bromide. J. Colloid Interface Sci. 1997;186(2):382–386. doi: 10.1006/jcis.1996.4649. [DOI] [PubMed] [Google Scholar]
- 25.Shah F.A., Khan A.M., Sabir S., Ali S. CTAB–tributylstannic [3-(3′, 4′-dichlorophenylamido) propanoate] interaction: a tool for predicting organotin (IV) complex–cell membrane interaction parameters. Colloid Polym. Sci. 2016;294(1):87–94. [Google Scholar]
- 26.Chakraborty T., Chakraborty I., Ghosh S. Sodium carboxymethylcellulose− CTAB interaction: a detailed thermodynamic study of polymer− surfactant interaction with opposite charges. Langmuir. 2006;22(24):9905–9913. doi: 10.1021/la0621214. [DOI] [PubMed] [Google Scholar]