Introduction
Diabetes mellitus (DM) is a group of metabolic diseases,1 and several studies have demonstrated a relationship between DM and periodontitis.2, 3, 4, 5 Diabetic complications include an increase in oxidative stress, inflammation, and advanced glycation end product formation. Poorly controlled DM is strongly associated with clinical attachment loss and edentulism in people with DM,6 and periodontal therapy influences glycemic control in type 2 DM (T2DM).7 A meta-analysis showed that scaling and root planning (SRP) improved the levels of glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG), although statistically nonsignificant.3 Other systematic reviews and meta-analysis have reported that nonsurgical periodontal therapy (NSPT) significantly reduces mean HbA1c levels and also FPG levels.4,5
As periodontitis is caused due to dental plaque accumulation, maintaining oral hygiene is essential for preventing inflammation from periodontitis. Poor oral hygiene increases the risk of periodontitis by 2- to 5-fold,8 and plaque removal and control has been shown to be fundamentally important in preventing periodontal disease occurrence and progression.9 However, only a few studies have reported that oral hygiene instructions (OHIs) improve glycemic control in patients with T2DM.10, 11, 12
Furthermore, patients with DM recognize the characteristic oral malodor as “a disagreeable odor that emanates from the mouth.”13,14 Oral malodor is socially awkward for others to point out. As diabetes causes abnormal liver metabolism, abnormal ketone metabolism in the body results in acetone metabolism.15 We hypothesized that HbA1c level and oral malodor may decrease by regulating a patient's oral hygiene instructed by a dental hygienist. The aim of our study was to investigate the effects of OHIs on glycemic control and oral malodor in patients with T2DM.
Materials and methods
This study was reviewed and approved by the Ethical Committee of Tokyo Medical and Dental University (Faculty of medical No. 2189, Faculty of dentistry No. 1232, and Biomaterial Research Institute No. 2015-06) and conformed to the Declaration of Helsinki. This trial was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000018894).
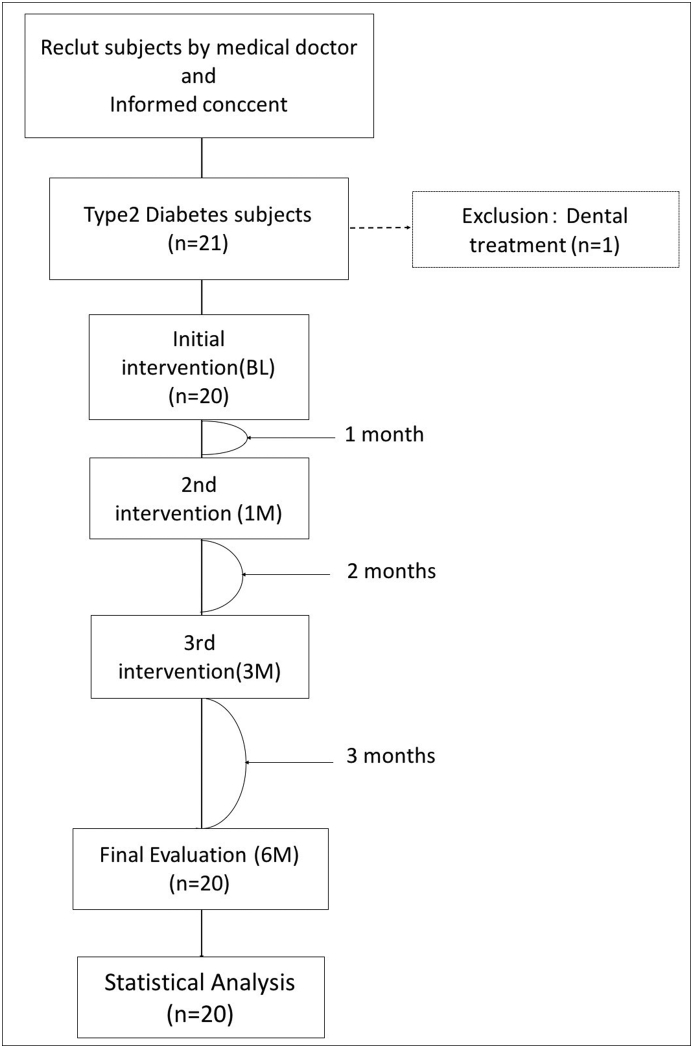
Patients were recruited from the Department of Diabetes, Endocrinology and Metabolism, Medical Hospital, Tokyo Medical and Dental University. Informed consent was obtained from all participants included in the study. The study included (1) patients diagnosed with T2DM and controlled by a medical doctor and (2) those with the ability to independently brush their teeth. Exclusion criteria were patients who (1) were edentulous and (2) did not have periodontitis, and (3) sought dental care for acute inflammation. Subgingival debridement was postponed until the end of the study. Fig. 1 shows a flow diagram depicting the study design. Initial intervention was performed at baseline (BL) and continued after 1, 3, and 6 months (1M, 3M, and 6M, respectively) after BL. The examinations were conducted four times, and OHIs were given three times. The study was conducted from September 2016 to March 2017.
Figure 1.
In total, 21 patients participated in the study, but one patient was excluded owing to the need for dental treatment at initial intervention. Finally, 20 patients received all interventions.
Diabetic condition
Blood tests were conducted to evaluate FPG and HbA1c levels. Diabetes duration, diabetic complaints, medications, and questionnaire data on smoking habits were also collected.
Periodontal examination
Dental examination was carried out by one dentist (KS) at BL. One dental hygienist (KT) measured probing pocket depth (PPD), bleeding on probing (BOP), and O'Leary plaque control record (PCR)16 at all investigation times. PPD was measured on a 6-point scale per tooth, and BOP was counted over 30 s following BOP.
Inflammatory cytokine expression in gingival crevicular fluid
Inflammatory cytokine expression in the gingival crevicular fluid (GCF) was measured using an enzyme-linked immunosorbent assay (ELISA), “Gingival crevicular fluid biomarker test” (Health Science Research Institute West Japan CO., LTD, Kyoto, Japan), according to the manufacturer's instructions.17 Briefly, GCF was collected from the upper and lower buccal side using a collection brush. The brush containing the GCF sample was immersed in a 0.05 M Tris hydrochloride buffer solution (pH 7.5) containing 0.1% bovine serum albumin (Serological Proteins, Inc., Kankakee, IL, USA) and 0.1% sodium azide (Nacalai Tesque CO., LTD, Kyoto, Japan). Cytokine measurements using ELISA were performed by Health Science Research Institute West Japan.
Oral malodor measurement
One trained odor judge (KT) scored breath odor levels (0–5) using organoleptic scoring (OLS).13 Volatile sulfur compounds (VSCs) are the major compounds that cause oral malodor.18 These compounds were measured using a gas sensor breath meter (BTESSTRON, REX. CO., LTD, Hyogo, Japan), with values ranging from 0 to 3000 ppb (normal = 0–250 ppb, mild = 251–600 ppb, medium = 601–1500 ppb, and severe = 1501–3000 ppb). For measuring breath acetone concentration, patients held a deep inhalation for 15 s and then exhaled into a 3-l sample bag; the collected breath air samples were measured using bio-sniffer.19
Oral health instructions
Patients received OHIs from one dental hygienist (KT) at each intervention. After interviewing the patient's lifestyle and daily brushing habits, the dental hygienist instructed each patient to use a plaque substitute solution (Dental Disclosure Solution Fluid Light, Satoh Dental Material Co., LTD, Tokyo, Japan) and noted the O'leary PCR.16 The instruction contents included toothbrush (DENT. EX Slimhead II 33M, Lion Dental Products Co., Ltd., Tokyo, Japan) techniques and interdental cleaning using dental floss, interdental brushing, or both. The toothbrush technique instructed the horizontal scrubbing method to avoid biasing the patient's dexterity. Then, the dental hygienist removed the supragingival plaque using a toothbrush and interdental flossing.
Statistical analysis
IBM SPSS statistics 22.0 was used for comparing oral and blood statuses at 1M, 3M and 6M with those as BL using one-way repeated measures ANOVA. Following the analysis of normal distribution of all data using Shapiro–Wilk test, normally-distributed data were expressed in mean ± standard deviation (SD), and non-normally distributed data were expressed in median with Interquartile range (25%–75%). The number of teeth, PPD% of 4–5 mm, PPD% of ≥6 mm, VSCs, and BOP and lactoferrin (Lf), antitrypsin (AT), and aspartate aminotransferase (AST) levels were analyzed using Wilcoxon test. PCR, breath acetone concentration, FPG level, and HbA1c level were analyzed using a paired t-test. OLS and VSCs were rank-analyzed using chi-square test. The correlation of interexaminer reliability was analyzed using intraclass correction coefficients. The correlation of intraexaminer between the dentist and the dental hygienist was not analyzed because the examinations in their charge were different. All statistical analyses were set at 0.05 significance level, and subsequent post hoc tests were Bonferroni-corrected (p < 0.0167).
Results
Summary of health characteristics (Table 1)
Table 1.
Characteristics of subjects at BL.
| Mean ± SD | Range | |
|---|---|---|
| Male/Female (n) | 9/11 | |
| age (years) | 63.9 ± 9.5 | 46 to 78 |
| BMI | 26.1 ± 4.5 | 16.2 to 33.8 |
| Diabetic duration (years) | 5.7 ± 7.5 | 1 to 31 |
| smorking/non smorking (n) | 2/18 | |
| Diabetic complication (n) | ||
| none | 7 | |
| neuropathy | 3 | |
| retinopathy | 5 | |
| nephrooathy | 9 | |
| gangrene of foot | 1 | |
| FPG (mg/dl) | 119.3 ± 26.7 | 88 to 209 |
| HbAlc (%) | 7.1 ± 0.7 | 5.9 to 9.1 |
| WBC (×103/μl) | 6.8 ± 1.7 | 3.8 to 9.9 |
| RBC (×103/μl) | 469.9 ± 63.9 | 361.0 to 569.0 |
| T-Chol (mg/dl) | 179.9 ± 19.1 | 143.0 to 214.0 |
| HDL-C (mg/dl) | 62.9 ± 17.4 | 41.0 to 102.0 |
| LDL-C (mg/dl) | 99.5 ± 16.9 | 73.0 to 125.0 |
| TG (mg/dl) | 134.8 ± 75.3 | 52.0 to 302.0 |
| CRE | 69.0 ± 14.9 | 39.3 to 98.6 |
A total of 21 patients were recruited for this study, and 20 patients (9 males and 11 females) completed the study. The mean age of the patients was 63.9 ± 9.5 years, BMI was 26.1 ± 4.5 kg/m2, and diabetes duration was 5.7 ± 7.5 years. Two patients were smokers, and 18 were nonsmokers. Regarding diabetic complications, three patients had diabetic neuropathy, five had retinopathy, nine had nephropathy, and one had gangrene of the foot. Seven patients had no diabetic complications. There was no significant correlation between the subsequent outcomes and these complications.
Blood test results at BL
Blood tests at BL revealed the following results: mean FPG level, 119.3 ± 26.7 mg/dl; HbA1c level, 7.1% ± 0.7%; white blood cell count, 6.8 ± 1.7 × 103/μl; red blood cell count, 469.9 ± 63.9 × 103/μl; total cholesterol level, 179.9 ± 19.1 mg/dl; HDL cholesterol level, 62.9 ± 17.4 mg/dl; LDL cholesterol level, 99.5 ± 16.9 mg/dl; and triglyceride (TG) level, 134.8 ± 75.3 mg/dl.
Periodontal examination (Table 2)
Table 2.
The results of number of teeth, periodontal examination, plaque control, gingival crevicular fluid.
| Base line |
1 month |
3 month |
6 month |
|||||
|---|---|---|---|---|---|---|---|---|
| Median | Interquartile range [25%, 75%] | Median | Interquartile range [25%, 75%] | Median | Interquartile range [25%, 75%] | Median | Interquartile range [25%, 75%] | |
| Number of teeth (n) % of sites with Probing Pocket Depth (mm) |
25 | [19, 26] | 25 | [19, 26] | 25 | [19, 26] | 25 | [19, 26] |
| 4–5 | 20.1 | [11.2, 30.8] | 23.4 | [7.5, 27.8] | 15.4 | [4.0, 23.1] | 5.0* | [0.9, 15.0] |
| 6 | 0.0 | [0.0, 0.0] | 0.0 | [0.0, 0.0] | 0.0 | [0.0, 0.0] | 0.0 | [0.0, 0.0] |
| % of sites with BOP | 7.7 | [3.8, 19.9] | 5.5 | [3.6, 11.5] | 7.7 | [1.1, 13.3] | 6.2 | [0.0, 11.9] |
| O'leary praque control record (%) (mean ± SD, range) Gingival crevicular fluid (GCF) |
48.2 ± 15.5 | 20.6 to 81.8 | 34.5 ± 11.2† | 11.1 to 53.5 | 34.9 ± 13.5† | 13.2 to 66.7 | 31.5 ± 15.6† | 6.0 to 62.0 |
| Lactoferrin ( g/ml) | 0.31 | [0.17, 0.57] | 0.27 | [0.13, 0.41] | 0.11* | [0.07, 0.32] | 0.1* | [0.05, 0.18] |
| α 1 antitrypsin ( g/ml) | 0.17 | [0.10, 0.26] | 0.15 | [0.09, 0.23] | 0.09 | [0.05, 0.21] | 0.07* | [0.03, 0.12] |
| Aspartate aminotransferase (IU/l) | 2.0 | [0.0, 4.0] | 1.0 | [0.0, 1.0] | 1.0* | [0.0, 2.0] | 0.0* | [0.0, 1.0] |
*: Comparison of baseline and 1,3 or 6th month data by Wilcoxson test. (p < 0.0167).
†: Comparison of baseline and 1,3 or 6th month data by Paired t-test. (p < 0.0167).
The correlation of interexaminer reliability was 0.91 for the dentist and 0.89 for the dental hygienist (p < 0.0001, respectively). The median (interquartile range) number of teeth was 25 (19–26), which remained unchanged during the intervention term. PPD% of 4–5 mm was 20.1% (11.2%–30.8%) at BL, 23.4% (7.5%–27.8%) at 1M, 15.4% (4.0%–23.1%) at 3M, and 5.0% (0.9%–15.0%) at 6 M. PPD% decreased significantly from BL to 6M (Wilcoxon test, p < 0.0167). PPD% of ≥6 mm was 0.0% (0.0%–0.0%) at BL, 0.0% (0.0%–0.0%) at 1M, 0.0% (0.0%–0.0%) at 3M, and 0.0% (0.0%–0.0%) at 6M. These interventions were not statistically significant. The percentage sites of BOP were 7.7% (3.8%–19.9%) at BL, 5.5% (3.6%–11.5%) at 1M, 7.7% (1.1%–13.3%) at 3M, and 6.2% (0.0%–11.9%) at 6M; there was no statistically significant improvement for each intervention at each time point compared with BL. The mean PCR values were 48.2% ± 15.5% at BL, 34.5% ± 11.2% at 1M, 34.9% ± 13.5% at 3M, and 31.5% ± 15.6% at 6M. The PCR significantly improved at 1M, 3M, and 6M intervention from BL (paired t-test, p < 0.0167).
Expression of inflammatory cytokines in the GCF (Fig. 2)
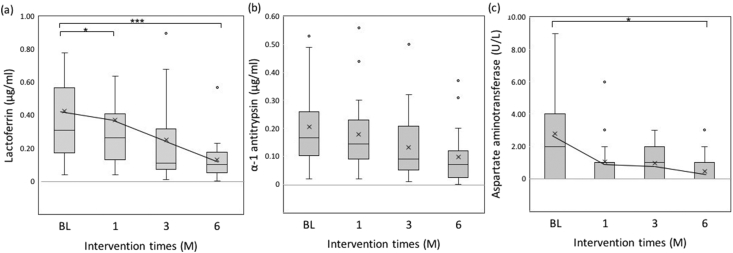
Figure 2.
The box represents the median 25 percentile values, and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. (a) Lactoferrin level was significantly decreased from BL to 1M (n = 17, Wilcoxon test, p = 0.015) and from BL to 6M (Wilcoxon test, p < 0.001). (b) Alpha-1 antitrypsin level was significantly decreased from BL to 6M (n = 17, Wilcoxon test, p = 0.004). (c) Aspartate aminotransferase level was significantly decreased from BL to 3M (n = 17, Wilcoxon test, p = 0.013) and from BL to 6M (Wilcoxon test, p = 0.006).
The median Lf levels were 0.31 (0.17–0.57) μg/ml at BL, 0.27 (0.13–0.41) μg/ml at 1M, 0.11 (0.07–0.32) μg/ml at 3M, and 0.10 (0.05–0.18) μg/ml at 6M, and it significantly decreased at 3M and 6M from BL (Wilcoxon test, p = 0.015, p < 0.001). The median AT levels were 0.17 (0.10–0.26) μg/ml at BL, 0.15 (0.09–0.23) μg/ml at 1M, 0.09 (0.05–0.21) μg/ml at 3M, and 0.07 (0.03–0.12) μg/ml at 6M. AT levels also decreased from BL to 6M (Wilcoxon test p = 0.004). The median AST levels were 2.0 (0.0–4.0) U/l at BL, 1.0 (0.0–1.0) U/l at 1M, 1.0 (0.0–2.0) U/l at 3M, and 0.0 (0.0–1.0) U/l at 6M. AST levels were significantly decreased from BL to 3M and 6M (Wilcoxon test, p = 0.013, p = 0.006).
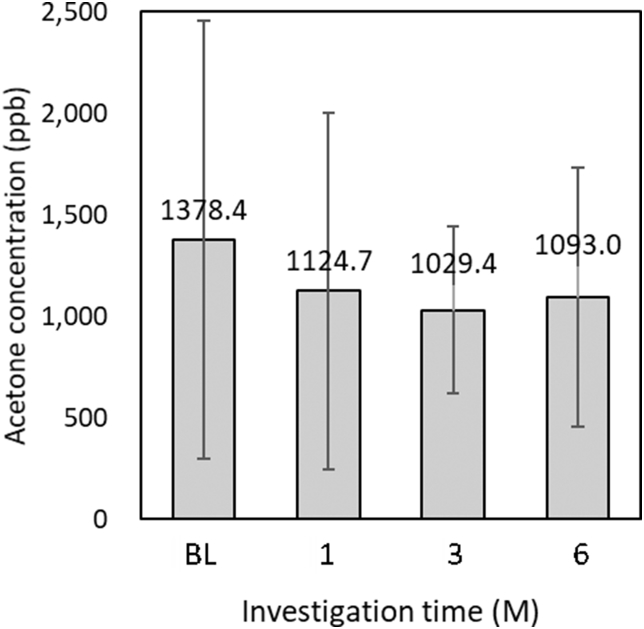
Oral malodor measurement (Figure 3, Figure 4, Figure 5)
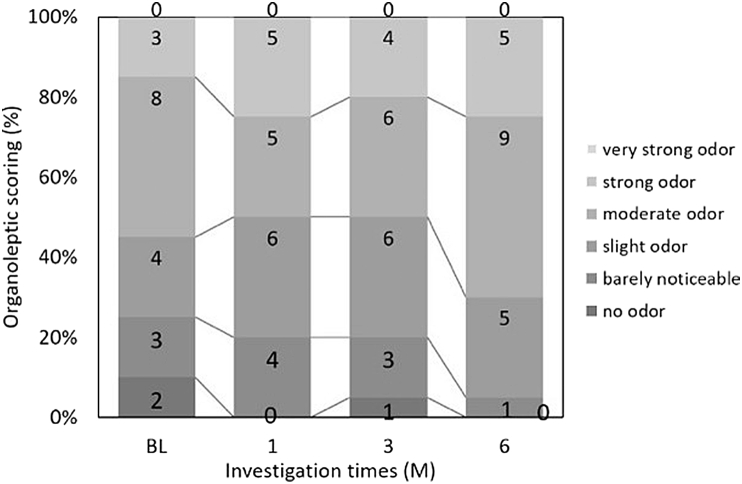
Figure 3.
A trained observer assessed the change in organoleptic score that measured the patient's oral malodor: not significant (n = 20, chi-square test).
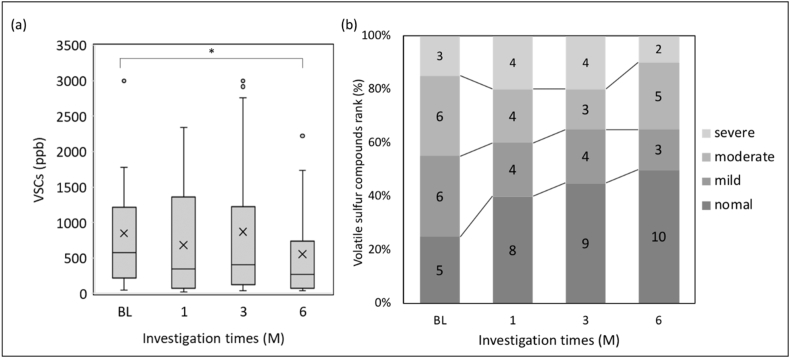
Figure 4.
(a) VSCs significantly decreased from BL to 6M (n = 20, Wilcoxon test p = 0.015). The box represents the median 25 percentile values, and the vertical bar indicates the minimum and maximum values. The “x” mark indicates the mean value. (b) Rank of VSCs. normal = 0–250 ppb, mild = 251–600 ppb, moderate = 601–1500 ppb, severe = 1501–3000 ppb: not significant (n = 20, chi-square test).
Figure 5.
Reduction in breath acetone concentration is shown: not significant (n = 17, paired t-test).
Fig. 3 shows the results of OLS, wherein the differences between each intervention from BL were not statistically significant. As shown in Fig. 4a, the median VSCs were 574.0 (217.0–1217.3) ppb at BL, 346.0 (69.3–1358.3) ppb at 1M, 408.0 (127.5–1226.5) ppb at 3M, and 272.5 (71.8–741.5) ppb at 6M. There was a significant decrease between VSCs at BL and those at 6M (Wilcoxon test, p = 0.015). Fig. 4b shows the VSC ranking. These interventions did not differ significantly from those at BL. As shown in Fig. 5, the mean breath acetone concentrations were 1378.4 ± 1078.0 ppb at BL, 1124.7 ± 877.3 ppb at 1M, 1029.4 ± 410.9 ppb at 3M, and 1093.0 ± 638.9 ppb at 6M. A decreasing tendency appeared, but without statistical significance.
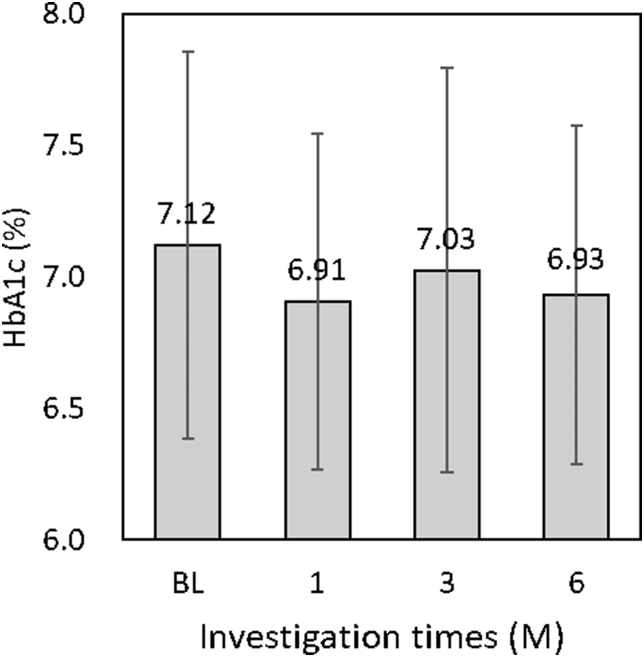
Glycemic control (Fig. 6)
Figure 6.
HbA1c level was decreased from BL (7.12%) to 6M (6.93%): not significant (n = 20, paired t-test).
The mean FPG levels were 118.8 ± 27.2 mg/dl at BL, 130.7 ± 28.3 mg/dl at 1M, 133.1 ± 45.0 mg/dl at 3M, and 127.6 ± 39.0 mg/dl at 6M, and these values were not statistically significantly reduced across time points. The mean HbA1c levels were 7.12% ± 0.74% at BL, 6.91% ± 0.64% at 1M, 7.03% ± 0.77% at 3M, and 6.93% ± 0.64% at 6M. There was a 0.2% decrease in the HbA1c level from BL to 6M, but it was not statistically significant.
Discussion
This study demonstrated the potential of oral hygiene control habits improve glycemic control and oral malodor in patients with T2DM.
The relationship between inflammation and glycemic control is well known.20, 21, 22, 23 In the present study, supragingival plaque removal was performed by a dental hygienist. PCR% was significantly reduced at 1M, 3M, and 6M compared to that at BL, suggesting that patients with T2DM followed the oral hygiene control habits. The mean HbA1c level decreased by 0.2% from BL to 6M, although the difference was not statistically significant. NSPT and OHIs were significantly reduced plaque index in patients with T2DM and chronic.10 Although the OHIs-only control group showed a reduction in their HbA1c level from 7.6% ± 1.5% at BL to 7.1% ± 1.2% at 3M, it was not statistically significant.10 Saengtipbovorn et al. reported that the intervention group in their study received not only OHIs but also monthly motivating interviews. The HbA1c level of the group significantly reduced from 7.39% ± 1.19% at BL to 7.10% ± 1.04% at the 3M follow-up.12 An improvement in HbA1c levels similar to that reported in the above mentioned study was observed in the present study. These results suggest that OHIs have the potential to influence glycemic control. Periodontal treatment should be administered to patients with periodontitis. OHIs are the easiest method to reduce oral inflammation. OHIs must be recommended to not only patients with T2DM with periodontitis but also all patients with T2DM to reduce inflammation.
O'Leary et al. had reported that periodontal tissue inflammation could be prevented by maintaining the PCR below 10%,16 which was not observed in the present study. There is a possibility that the more improved oral hygiene may lead to more effects on the glycemic control.
The levels of Lf, AT, and AST were selected in this study to evaluate inflammation. Lf is a granular protein of a neutrophil that has antibacterial activity against periodontitis bacteria.24 AT is a trypsin inhibitor that indicates leakage from capillaries and bleeding during inflammation.25 AST is an enzyme present in cells and is a marker of periodontal tissue destruction. In the present study, the expression of all the inflammatory cytokines in the GCF was significantly decreased after the implementation of OHIs. These results suggest that a patient's oral hygiene control habits improve not only adhesion of dental plaque but also periodontal tissue inflammation.
Reportedly, patients with T2DM with periodontitis and with poor glycemic control have high levels of inflammatory cytokines.26 Munenaga et al. reported that patients with T2DM and periodontitis-induced mild inflammation having a high-sensitivity C-reactive protein (hsCRP) level of >500 ng/ml showed a significant reduction in their HbA1c level after the administration of NSPT combined with antibiotics.27 An increased frequency of daily brushing tended to be closely related to TG and hsCRP levels.28 These reports indicate that improvement of periodontal status possibly reduces systemic inflammation and also that oral hygiene status is associated with systemic inflammation.
Oral malodor is primarily caused by caries, periodontal disease, residual tongue coating, and dental plaque remains.29 The present study aimed to improve oral malodor via OHIs and did not remove tongue coating. The amount of VSCs and the methyl mercaptan/hydrogen sulfide ratio were significantly increased in patients with periodontal disease.18 There were no significant improvements in OLS during the intervention period, but the amount of VSCs was significantly reduced. Professional tooth cleaning and SRP in combination with OHIs reduced VSCs.30 This result appears to suppress the accumulation of plaque as a cause of oral malodor reduction by OHIs. Self-perceived oral malodor has a negative influence on a patient's social interactions, and it improves with correct management.31 OHIs reduce oral malodor and may have the potential to affect social interaction by improving periodontal tissue inflammation. In this study, OLS was deteriorated 6M after treatment although PCR and VSCs were improved. The reason is presumed that the factors not-included in the analysis of this investigation might affected on the malodor such as tough coating or amount of residual plaque. The breath acetone concentrations in healthy individuals and patients with T2DM were reported to be 510 and 2350 ppb, respectively.19 In the present study, the mean breath acetone concentration in patients with T2DM was 1378.4 ppb, which was lower than that reported in the previous study. Patients with poor glycemic control have high breath acetone concentrations and strong oral malodor. In this study, the breath acetone concentration did not decrease, consistent with no change in glycemic control, because the patients could have undergone treatment with continuous diabetic care from their medical doctors. The mean HbA1c level appeared to be well controlled in the present study. Patients with poor glycemic control may be able to show more reduction in their oral malodor via OHIs.
A limitation of the present study is the relatively limited number of patients. The influence of OHIs could be considered compared with a control group without OHIs, but this could not be done due to ethical considerations. It was possible to investigate the impact of OHIs by designing a cross-sectional long-term study, and the patients had been receiving medication. Although the glycemic status of the patients changed from BL to the final assessment time, this study could not completely exclude the impact of medication on glycemic control. Further evidence is warranted to determine the effect of OHIs on systemic inflammation in patients with T2DM with periodontitis.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
None.
References
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graziani F., Gennai S., Solini A., Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J Clin Periodontol. 2018;45:167–187. doi: 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- 3.Sgolastra F., Severino M., Pietropaoli D., Gatto R., Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 2013;84:958–973. doi: 10.1902/jop.2012.120377. [DOI] [PubMed] [Google Scholar]
- 4.Teshome A., Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2017;17 doi: 10.1186/s12903-016-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson T.C., Weldon J.C., Worthington H.V. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;11 doi: 10.1002/14651858.CD004714.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowall B., Holtfreter B., Volzke H. Pre-diabetes and well-controlled diabetes are not associated with periodontal disease: the SHIP Trend Study. J Clin Periodontol. 2015;42:422–430. doi: 10.1111/jcpe.12391. [DOI] [PubMed] [Google Scholar]
- 7.Gay I., Tran D., Cavender A. The effect of periodontal therapy on glycaemic control in a Hispanic population with type 2 diabetes: a randomized controlled trial. J Clin Periodontol. 2014;41:673–680. doi: 10.1111/jcpe.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lertpimonchai A., Rattanasiri S., Vallibhakara S., Attia J., Thakkinstian A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J. 2017;67:332–343. doi: 10.1111/idj.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapple I.L., Van der Weijden F., Doerfer C. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42(Suppl. 16):S71–S76. doi: 10.1111/jcpe.12366. [DOI] [PubMed] [Google Scholar]
- 10.Raman R., Taiyeb-Ali T., Chan S., Chinna K., Vaithilingam R. Effect of nonsurgical periodontal therapy verses oral hygiene instructions on Type 2 diabetes subjects with chronic periodontitis: a randomised clinical trial. BMC Oral Health. 2014;14:79. doi: 10.1186/1472-6831-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.K., Choi S.H., Won K.C. The effect of intensive oral hygiene care on gingivitis and periodontal destruction in type 2 diabetic patients. Yonsei Med J. 2009;50:529–536. doi: 10.3349/ymj.2009.50.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saengtipbovorn S., Taneepanichskul S. Effectiveness of lifestyle change plus dental care (LCDC) program on improving glycemic and periodontal status in the elderly with type 2 diabetes. BMC Oral Health. 2014;14:72. doi: 10.1186/1472-6831-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg M., Kulkarni G.V., Bosy A., McCulloch C.A. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436–1440. doi: 10.1177/00220345910700110801. [DOI] [PubMed] [Google Scholar]
- 14.Tonzetich J., Ng S.K. Reduction of malodor by oral cleansing procedures. Oral Surg Oral Med Oral Pathol. 1976;42:172–181. doi: 10.1016/0030-4220(76)90121-3. [DOI] [PubMed] [Google Scholar]
- 15.Ruzsanyi V., Peter Kalapos M. Breath acetone as a potential marker in clinical practice. J Breath Res. 2017;11 doi: 10.1088/1752-7163/aa66d3. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary T.J., Drake R.B., Naylor J.E. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 17.Hanioka T., Matsuse R., Shigemoto Y., Ojima M., Shizukuishi S. Relationship between periodontal disease status and combination of biochemical assays of gingival crevicular fluid. J Periodontal Res. 2005;40:331–338. doi: 10.1111/j.1600-0765.2005.00807.x. [DOI] [PubMed] [Google Scholar]
- 18.Yaegaki K., Sanada K. Volatile Sulfur-compounds in mouth air from clinically healthy-subjects and patients with periodontal-disease. J Periodontal Res. 1992;27:233–238. doi: 10.1111/j.1600-0765.1992.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 19.Chien P.J., Suzuki T., Tsujii M. Biochemical gas sensors (Biosniffers) using forward and reverse reactions of secondary alcohol dehydrogenase for breath isopropanol and acetone as potential volatile biomarkers of diabetes mellitus. Anal Chem. 2017;89:12261–12268. doi: 10.1021/acs.analchem.7b03191. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista E.E., Franca C.M., Veni P. Antimicrobial photodynamic therapy combined with periodontal treatment for metabolic control in patients with type 2 diabetes mellitus: study protocol for a randomized controlled trial. Trials. 2015;16:229. doi: 10.1186/s13063-015-0757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 22.Mealey B.L., Oates T.W. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 23.Gurav A.N. Periodontal therapy -- an adjuvant for glycemic control. Diabetes Metab Syndr. 2012;6:218–223. doi: 10.1016/j.dsx.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Yadav N., Lamba A.K., Thakur A., Faraz F., Tandon S., Pahwa P. Effect of periodontal therapy on lactoferrin levels in gingival crevicular fluid. Aust Dent J. 2014;59:314–320. doi: 10.1111/adj.12203. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi S., Yamada H., Fukui M., Ito H.O., Sata M. Correlation between arteriosclerosis and periodontal condition assessed by lactoferrin and alpha1-antitrypsin levels in gingival crevicular fluid. Int Heart J. 2015;56:639–643. doi: 10.1536/ihj.15-218. [DOI] [PubMed] [Google Scholar]
- 26.Javed F., Al-Askar M., Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: a literature review. J Periodontol. 2012;83:156–161. doi: 10.1902/jop.2011.110207. [DOI] [PubMed] [Google Scholar]
- 27.Munenaga Y., Yamashina T., Tanaka J., Nishimura F. Improvement of glycated hemoglobin in Japanese subjects with type 2 diabetes by resolution of periodontal inflammation using adjunct topical antibiotics: results from the Hiroshima Study. Diabetes Res Clin Pract. 2013;100:53–60. doi: 10.1016/j.diabres.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi Y., Niu K., Guan L. Oral health behavior and metabolic syndrome and its components in adults. J Dent Res. 2012;91:479–484. doi: 10.1177/0022034512440707. [DOI] [PubMed] [Google Scholar]
- 29.Yaegaki K., Coil J.M., Kamemizu T., Miyazaki H. Tongue brushing and mouth rinsing as basic treatment measures for halitosis. Int Dent J. 2002;52:192–196. doi: 10.1002/j.1875-595x.2002.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 30.Deutscher H., Derman S., Barbe A.G., Seemann R., Noack M.J. The effect of professional tooth cleaning or non-surgical periodontal therapy on oral halitosis in patients with periodontal diseases. A systematic review. Int J Dent Hyg. 2018;16:36–47. doi: 10.1111/idh.12306. [DOI] [PubMed] [Google Scholar]
- 31.de Jongh A., van Wijk A.J., Horstman M., de Baat C. Self-perceived halitosis influences social interactions. BMC Oral Health. 2016;16:31. doi: 10.1186/s12903-016-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]