Abstract
Cryptococcal infection is a major cause of opportunistic infection in HIV/AID‐infected peoples. We determined cryptococcal antigenemia and cryptococcal meningitis among antiretroviral therapy (ART) initiated and ART‐naive HIV‐infected peoples. A cross‐sectional study was conducted at selected health facilities in Mekelle, Ethiopia. Blood was collected to determine CD4 and plasma cryptococcal antigen (CrAg). CSF CrAg and CSF culture and urease tests were also done. Socio‐demographic and clinical data were collected using a structured questionnaire and clinical chart review. From the enrolled study participants, 267 study participants had complete data, of which, 137 (51%) were females. From the study participants, 140 (52%) and 127 (48%) were ART experienced and ART naïve, respectively. The prevalence of cryptococcal antigenemia was 9 (3.4%). All the study participants, except one (CD4 = 120 cells/mm3), had CD4 count less than 100 cells/mm3. From CrAg‐positive peoples, 6 (4.7%) were ART naïve. Five CrAg‐positive peoples had cryptococcal meningitis. Being male, rural residence, and being hospitalized were associated with cryptococcal antigenemia. Cryptococcal infection poses a substantial risk of HIV‐positive peoples. This study provides relevant data for CrAg screening interventions in patients with low CD4 cell counts.
Keywords: ART‐experienced, ART‐naive, cryptococcal antigenemia, HIV infected, Mekelle
1. INTRODUCTION
Cryptococcal infection is a major cause of opportunistic infection in HIV/AIDS‐infected peoples. Cryptococcosis is one of the few infectious diseases that can be detected in asymptomatic peoples (Centers for disease control and Prevention (CDC), 2014; Meya, Rajasingham, Nalintya, Tenforde, & Jarvis, 2015). Asymptomatic cryptococcosis patients are positive for serum/plasma cryptococcal antigen (CrAg). The subclinical infectious state is known to precede clinically apparent disease by weeks to months, which eventually will lead to meningitis and mortality in HIV‐infected people (Centers for disease control and Prevention (CDC), 2014; Meya et al., 2015; Rajasingham, Meya, & Boulware, 2012).
The prevalence of cryptococcosis is high in Africa, especially in sub‐Saharan Africa, where it is found up to 28% of HIV‐infected patients with clinical signs of meningitis (Ngouana et al., 2015). In Africa, cryptococcal‐related infections place a high burden on healthcare resources. It causes a severe and often fatal meningoencephalitis in people living with HIV/AIDS, accounting for 42% to 71% of neuromeningeal cryptococcosis‐related deaths in sub‐Saharan Africa (Assogoba et al., 2015).
Testing for cryptococcal antigenemia is effective at reducing morbidity and mortality because patients who receive early antifungal treatment have better outcomes than those who receive delayed treatment (Kaplan et al., 2015; World Health Organization, 2011).
CrAg screening programs have begun in a number of countries with high burden of disease (Kaplan et al., 2015). However, in Ethiopia, a few ART clinics have started CrAg screening procedures. Generating evidence through research will support the existing prevention and control programs (Beyene, Woldeamanuel, Asrat, Ayana, & Boulware, 2013; Reepalu et al., 2015). In Ethiopia, the mortality rate due to cryptococcal meningitis is high (Berhe, Melkamu, & Amare, 2012). High prevalence of cryptococcal antigenemia has also been reported in ART sites in Addis Ababa and Adama (Alemu et al., 2013; Beyene et al., 2013, 2017 ). In contrast to these studies, one study has reported a low prevalence of cryptococcal infection in Adama (Reepalu et al., 2015). The existing studies in Ethiopia have shown a varying prevalence of cryptococcal antigenemia. Nevertheless, further assessment in different geographical regions is needed to address associated factor differences. The previous studies were also unable to assess the magnitude of cryptococcal meningitis. Therefore, this study is aimed at determining the cryptococcal antigenemia and associated factors among ART naïve and ART‐experienced HIV‐infected peoples.
2. METHODS
2.1. Study setting, population, and design
A cross‐sectional study was conducted at Mekelle, Ethiopia, from September 2016 to January 2017. Mekelle is situated 783 km north of the capital of Ethiopia, Addis Ababa. In the city, there are governmental and private health facilities providing HIV diagnostic and management clinics. This study was done among peoples who get ART‐related healthcare service (both outpatients and inpatients during the study period) from Mekelle Hospital, Ayder Comprehensive Specialized Hospital, and Mekelle Health Center. All HIV‐positive individuals aged 18 years and above with CD4 count less than or equal to 250cells/mm3 were eligible for this study. Peoples who had been diagnosed with a cryptococcal infection in the previous 2 years, those who were taking antifungal treatments, and severely ill patients that did not give consent were excluded from the study. A total of 280 study participants were included, considering 84% power to detect a 10% difference, with equal group sizes and two‐sided p = 0.05 and prevalence (P) from Adama (Sawadogo et al., 2016).
2.2. Data collection
A structured questionnaire was prepared to collect socio‐demographic data (age, gender, and residence) and clinical data (a headache, fever, nausea, cough, vomiting, visual changes and neck stiffness). History record cards were also reviewed for length of ART stay.
2.3. Sample collection, handling, and transport
Blood samples (3 ml) were collected by vein puncture in EDTA test tubes from each participant by an experienced laboratory professional. Specimens were transported at room temperature to Ayder Comprehensive Specialized Hospital ART laboratory for analysis. For study participants who had a positive plasma CrAg test, and who showed signs and symptoms of cryptococcal meningitis, 2 ml of cerebrospinal fluid (CSF) was collected following a lumbar puncture (LP) using a 21 gauge LP needle with a sterile test tube. LP was done by an experienced physician at the site of patient admission. CSF was immediately transported to Ayder Comprehensive Specialized Hospital Microbiology laboratory for analysis.
2.4. Ethics approval and consent to participate
The study protocol was evaluated and approved by the Research Ethics Review Committee of the College of Health Sciences, Mekelle University, and ethical clearance was obtained. Official support letters were obtained from Mekelle University and Tigray Regional Health Bureau. Moreover, prior to conducting the study, the purpose and objective of the study were described to the participants and a written informed consent was obtained. Laboratory examinations with a direct benefit in the health of the study participants were informed to physicians. The consent involves permission to disseminate the findings of the study through the scientific workshop and publish in reputable journals.
2.5. Laboratory testing
All laboratory tests for plasma CrAg test, CD4 testing, CSF CrAg test, and culture test were done at the time of data collection.
2.6. CD4 count
CD4 count was determined using FACSCount Immunecytometry analyzer (BD Biosciences, San Jose, USA).
2.7. Rapid CrAg lateral flow assay (LFA) test
Plasma and CSF samples were used to detect cryptococcal antigenemia and cryptococcal meningitis, respectively. Cryptococcal Antigen Lateral Flow test (ImmunoMycologics, Inc., Oklahoma, USA) was performed, and results were observed within 10 min and interpreted as per the manufacturer's instruction.
2.8. Culture for definitive diagnosis of cryptococcal meningitis
CSF was centrifuged at about 250 g for 10 min. The sediment was inoculated to Sabouraud Dextrose agar on two sterile petri dishes and was incubated at room temperature and at 37°C for up to 14 days. A moist white‐cream colored mucoid colonies appeared 3–4 days of incubation. For positive cultures, urease test was carried out on urea agar in a sterile test tube. A colony from the culture was inoculated and incubated at 37°C for 3–5 hr.
2.9. Data quality assurance
Reagents were checked for proper functioning using provided control reagents. CSF was collected aseptically to prevent contamination using 70% alcohol and 2% tincture of iodine. Sterility of media was checked by incubating random samples of prepared culture media at room temperature and at 37°C for 14 days.
2.10. Statistical analysis
The data were entered into the EPI Info version 7 (CDC, USA) every day. The data were imported from EPI Info and analyzed using Statistical Package for Social Sciences (SPSS) software version 22.0 (IBM, USA). Descriptive statistics were computed, and data were presented using figures and tables. Association between different variables with outcome was analyzed using Fisher's exact test. P‐values less than 0.05 were considered as statistically significant.
3. RESULTS
3.1. Socio‐demographic and clinical characteristics of study participants
Out of 280 respondents, a complete response (with socio‐demographic, clinical, and laboratory data) was obtained for only 267 study participants. Of these 267 study participants, about half 51% (n = 137) were females and 222 (83.1%) were urban residents. The mean (±SD) age was 38 (±10) years. Majority (86.5%) were on outpatient management, and 140 (52.4%) were on ART. At least one or more sign and symptoms were reported from all the study participants. Therefore, from all study participants, 33% (n = 87) had headache, 31% (n = 83) had cough, 28.8% (n = 77) had fever, 27.3% (n = 73) had nausea, 24.3% (n = 65) had vomiting, 20.6% (n = 55) had visual changes, and 11.6% (n = 31) had neck stiffness irrespective of CrAg status (Table 1 , Figure 1 ).
Table 1.
Cryptococcal antigenemia among ART‐naïve and ART‐initiated peoples (n = 267)
| ART naïve (n = 127) | On ART (n = 140) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | CrAg+:n(%) | CrAg−: n(%) | Total: n(%) | CrAg+:n(%) | CrAg−: n(%) | Total:n(%) | ||
| Gender | ||||||||
| Female | 1 (1.5) | 67 (98.5) | 68 (53.5) | 0 (0.00) | 69 (100.0) | 69 (49.3) | ||
| Male | 5 (8.5) | 54 (91.5) | 59 (46.5) | 3 (4.2) | 68 (95.8) | 71 (50.7) | ||
| Age (Years) | ||||||||
| 18–27 | 1 (4.8) | 20 (95.2) | 21 (16.5) | 0 (0.0) | 11 (100.0) | 11 (7.6) | ||
| 28–37 | 3 (5.5) | 52 (94.5) | 55 (43.3) | 1 (2.3) | 43 (97.7) | 44 (31.4) | ||
| 38–47 | 2 (4.9) | 39 (95.1) | 41 (32.3) | 2 (4.1) | 47 (95.9) | 49 (35) | ||
| 48–57 | 0 (0.0) | 7 (100.0) | 7 (5.5) | 0 (0.0) | 22 (100.0) | 22 (15.7) | ||
| 58–67 | 0 (0.0) | 3 (100.0) | 3 (2.4) | 0 (0.0) | 12 (100.0) | 12 (8.6) | ||
| 68–75 | — | — | — | 0 (0.0) | 2 (100.0) | 2 (1.4) | ||
| Residence | ||||||||
| Urban | 3 (2.8) | 103 (97.2) | 106 (83.5) | 1 (0.9) | 115 (99.1) | 116 (82.6) | ||
| Rural | 3 (14.3) | 18 (85.7) | 21 (16.5) | 2 (8.3) | 22 (91.7) | 24 (17.1) | ||
| Patients | ||||||||
| Out patients | 1 (1.0) | 102 (99.0) | 103 (81.1) | 0 (0.0) | 128 (100.0) | 128 (91.4) | ||
| Inpatients | 5 (20.8) | 19 (79.2) | 24 (18.9) | 3 (25.0) | 9 (75.0) | 12 (8.6) | ||
| CD4 count (cells/mm3) | ||||||||
| ≤50 | 4 (12.9) | 27 (87.1) | 31 (50.8) | 2 (6.7) | 28 (93.3) | 30 (42.5) | ||
| 51–100 | 1 (4.0) | 24 (96.0) | 25 (53.2) | 1 (4.5) | 21 (95.5) | 22 (46.8) | ||
| 101–150 | 1 (4.0) | 24 (96.0) | 25 (50.0) | 0 (0.0) | 25 (100.0) | 25 (50.0) | ||
| >150 | 0 (0.0) | 46 (100.0) | 46 (42.2) | 0 (0.0) | 63 (100.0) | 63 (57.8) | ||
| WHO stage | ||||||||
| Stage I | 0 (0.0) | 31 (100.0) | 31 (24.4) | 0 (0.0) | 102 (100.0) | 102 (72.9) | ||
| Stage II | 0 (0.0) | 31 (100.0) | 31 (24.4) | 0 (0.0) | 12 (100.0) | 12 (8.6) | ||
| Stage III | 0 (0.0) | 40 (100.0) | 40 (31.5) | 1 (6.7) | 14 (93.3) | 15 (10.7) | ||
| Stage IV | 6 (24.0) | 19 (76.8) | 25 (19.7) | 2 (18.2) | 9 (81.8) | 11 (7.9) | ||
| Sign and symptoms | ||||||||
| Headache | 4 (8.5) | 43 (91.5) | 47 (54.0) | 2 (6.7) | 28 (93.3) | 30 (46.0) | ||
| Cough | 4 (6.7) | 56 (93.3) | 60 (72.3) | 3 (13.0) | 20 (87.0) | 23 (27.7) | ||
| Fever | 2 (3.5) | 55 (96.5) | 57 (74.0) | 2 (10.0) | 18 (90.0) | 20 (26.0) | ||
| Nausea | 5 (13.2) | 33 (86.8) | 38 (52.1) | 2 (5.1) | 33 (94.9) | 35 (47.9) | ||
| Vomiting | 6 (15.4) | 33 (84.6) | 39 (60.0) | 1 (3.8) | 25 (96.2) | 26 (40.0) | ||
| Visual change | 3 (7.5) | 37 (92.5) | 40 (72.7) | 1 (6.7) | 14 (93.3) | 15 (27.3) | ||
| Neck stiffness | 3 (13.6) | 19 (86.4) | 22 (70.9) | 0 (0) | 9 (100) | 9 (29.1) | ||
CrAg+: positive for cryptococcal antigenemia; CrAg−: negative for cryptococcal antigenemia.
Figure 1.
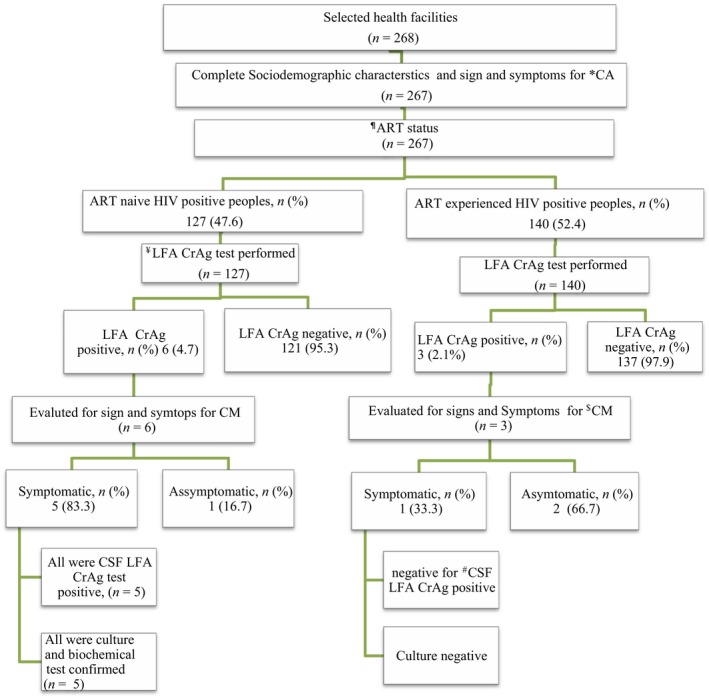
Study participant recruitment flowchart: ¶ART: antiretroviral therapy; *CA: cryptococcal antigenemia; $CM: cryptococcal meningitis; #CSF: cerebrospinal fluid; ¥LFA CrAg: lateral flow assay cryptococcal antigen
3.2. Prevalence of cryptococcal antigenemia
The prevalence of cryptococcal antigenemia was 9 (3.4%). All the study participants, except one (CD4 = 120 cells/mm3), had CD4 count less than 100 cells/mm3. The cryptococcal antigenemia‐positive peoples had one or more clinical sign and symptoms, of which, 6 (6.9%) had a headache, 4 (5.2%) had a fever, and 3 (9.7%) had neck stiffness. Six of the CrAg positives were ART naive, and 3 were ART experienced. From the total study participants with a CD4 count less than or equal to 50 cells/mm3, 6 (9.8%) were with cryptococcal antigenemia. Considering their ART status, among the ART‐naïve groups from those with a CD4 count of less than or equal to 50 cells/mm3, 4 (12.9) were positive for Cryptococcus. However, only one study participant with a CD4 count of 120 cells/mm3 was positive to Cryptococcus. Eight of the nine CrAg positive were on WHO stage IV of HIV/AIDS (Table 1).
3.3. Cryptococcal meningitis among cryptococcal antigenemia‐positive peoples
Cryptococcal antigen‐positive peoples were examined for signs and symptoms of meningitis. From 9 peoples with antigenemia, 5 from ART naïve and 1 from ART‐experienced groups had one or more signs and symptoms indicative for cryptococcal meningitis. Lumbar puncture was performed on those 6 study participant, of which, only 5 were positive for CSF LFA CrAg test. All CrAg positives were confirmed by culture and biochemical test (Figure 1 ).
3.4. Factors associated with cryptococcal infection
Among risk factors, being male (p = 0.017), living in rural areas (p = 0.008), and being hospitalized (p = 0.001) were statistically associated with cryptococcal antigenemia (Table 2).
Table 2.
Associated risk factors with cryptococcal antigenemia (n = 267)
| Variables | CrAg+ (n = 9): n (%) | CrAg− (n = 258): n (%) | p‐value* | |
|---|---|---|---|---|
| Gender | ||||
| Female | 1 (0.7) | 136 (99.8) | 1 | |
| Male | 8 (6.2) | 122 (93.8) | 0.017* | |
| Age (Years) | ||||
| 18–27 | 1 (3.1) | 31 (96.9) | ||
| 28–37 | 4 (4.0) | 95 (96.0) | ||
| 38–47 | 4 (4.4) | 86 (95.6) | NA | |
| 48–57 | 0 (0.0) | 29 (100.0) | ||
| 58–67 | 0 (0.0) | 15 (100.0) | ||
| 68–75 | 0 (0.0) | 2 (100.0) | ||
| Residence | ||||
| Urban | 4 (1.8) | 218 (98.2) | 1 | |
| Rural | 5 (11.1) | 40 (88.9) | 0.008* | |
| Patients | ||||
| Out patients | 1 (0.4) | 230 (99.6) | 1 | |
| Inpatient | 8 (22.2) | 28 (77.8) | 0.001* | |
| CD4 count(cells/µl) | ||||
| ≥100 | 1 (0.6) | 159 (99.4) | 1 | |
| <100 | 8 (7.5) | 99 (92.5) | 0.003* | |
| ART status | ||||
| ART naive | 6 (4.7) | 121 (95.3) | 1 | |
| On ART | 3 (2.1) | 137 (97.9) | 0.317 | |
CrAg+: positive for cryptococcal antigenemia; CrAg−: negative for cryptococcal antigenemia.
Fisher's exact test.
4. DISCUSSION
HIV‐infected peoples are at risk for different opportunistic infections, including fungal infections. Late presentations to care of HIV‐infected peoples will put them at risk for the development of the cryptococcal disease. In resource constraining settings, continuous surveillance of the cryptococcal infection in HIV‐infected peoples and associated factors is important to support national program related.
The prevalence of cryptococcal antigenemia in the current study is relatively higher than the prevalence reported from Adama (1.6%) (Reepalu et al., 2015) and much lower than the findings from Addis Ababa (8.4%) (Alemu et al., 2013) and Adama (10.2%) (Beyene et al., 2013). The difference in the prevalence rate might be due to improvements in a time of ART initiation for HIV‐infected peoples, which might reduce the incidence and high mortality associated with cryptococcal meningitis and other opportunistic infections (Alemu et al., 2013). However, in contrast to these, in a recently conducted study on HIV‐initiated peoples with a CD4 count of less than 150 cells/mm3 showed a prevalence of 6.2% (Beyene et al., 2017). The difference could be due to a large number of study participants with lower CD4 count are included (Beyene et al., 2017).
We compared our findings with other studies conducted outside Ethiopia. The findings from this study were similar to recent prevalence reports from Namibia (3.3%) (Sawadogo et al., 2016) and Tanzania (3.7%) (Letang et al., 2015) and slightly lower than the prevalence reported in South Africa (4.3%) (Longley et al., 2016). However, lower than the prevalence reported in two studies from Nigeria (12.7% and 8.9%) (Oladele et al., 2016; Osazuwa, Dirisu, Okuonghae, & Ugbebor, 2012) and two more from Tanzania (7.1% and 5.1%) (Magambo et al., 2014; Wajanga et al., 2011). We also compared our finding with studies in other developing countries like Indonesia (7.1%) (Ganiem et al., 2014), Vietnam (4%) (Smith et al., 2013), and Thailand (9.2%) (Pongsai, Atamasirikul, & Sungkanuparph, 2010). The reasons for the wide difference in the prevalence reports even within the same countries might be due to the difference in sample size, participant selection, study design, and seasonal variations on which the studies were conducted (Randhawa et al., 2011).
Considering cryptococcal meningitis (CM), in this study, high CM rates among ART‐naïve study participants were found. This is similar to other studies in Sub‐Saharan Africa. For example, in South Africa, CM was found 4/10 antigenemia‐positive cases (Longley et al., 2016), in Cameroon 41/146 of patients who had clinical signs of meningitis (Ngouana et al., 2015), and in Tanzania 15/17 cryptococcal antigenemia‐positive hospitalized patients (Wajanga et al., 2011). But we are unable to discuss further the findings from a statistical point of view due to the few numbers of study participants with CM.
However, in this study males, residing in rural areas, CD4 < 100 cells/mm3, and being hospitalized were statistically associated with cryptococcal antigenemia. Although cryptococcal infections occur in both sexes, a statistically significant difference in gender was also observed in other studies; this is likely due to poor health‐seeking behavior of men to present later to care (Alemu et al., 2013; Assogoba et al., 2015; Ngouana et al., 2015) and the interaction Cryptococcus with testosterone, which results in increased capsular polysaccharide release and Cryptococcus‐mediated macrophage death (McClelland et al., 2013). In addition to this, rural residents were at risk for cryptococcal infection. This might be supported by genotyping studies that dealt with the ubiquitous nature of Cryptococcus species providing strong evidence for additional origins of exposure like different species of plants, soil, and house dust (Chen et al., 2015; Cogliati, 2013). From the sign and symptoms, nausea and vomiting were observed in most of the participants tested positive in contrary to other studies (Hashimoto e Souza et al., 2013; Jarvis, Meintjes, Williams, Rebe, & Harrison, 2010; Makadzange & McHugh, 2014). In addition to this, those that were hospitalized for different reasons were at a significant risk of cryptococcal infections (Kaplan et al., 2015). In this study, higher prevalence of CrAg was observed in peoples with advanced immune‐suppression, which is supported by other similar studies, and WHO recommendations (Alemu et al., 2013; Ganiem et al., 2014; Osazuwa et al., 2012; Pongsai et al., 2010; Randhawa et al., 2011; Sawadogo et al., 2016; Smith et al., 2013; World Health Organization, 2011). Moreover, though a significant association was not found on their ART status, higher prevalence of Cryptococcus was observed in those that were ART naïve. Preventing new HIV infections, early HIV diagnosis, implementing early linkage to care, and ensuring timely initiation of ART with strict adherence would prevent incident cryptococcosis including Cryptococcal antigen screening and preemptive therapy with fluconazole (Asfaw et al., 2015; Smitson et al., 2014). Therefore, resource‐constrained settings, including Ethiopia, should work for improving their national program for the early ART initiation and promoting a cost‐effective preventive strategy to reduce the incidence and high mortality associated with cryptococcal meningitis in peoples with low CD4 count (Jarvis et al., 2013).
The limitations of this study are as follows: we were unable to see the independent effect of variables on cryptococcal antigenemia. It was also difficult to perform statistical analysis for Cryptococcal meningitis due to small sample size.
5. CONCLUSION
This study revealed that cryptococcal infection poses a substantial risk of HIV/AIDS‐infected peoples despite the widespread use of HAART. High prevalence of cryptococcal infection in ART‐naïve peoples was observed. Moreover, being male, living in rural areas, low CD4 count, and being hospitalized were statistically associated with cryptococcal infection. This provides relevant data for CrAg screening interventions in patients with low CD4 cell counts.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTION
HK participated in the conception of the title and study design, data collection, data analysis, data entry, and writing the manuscript. NS participated in the study during design and analysis. KH participated in data cleaning. AM was involved in the design and study monitoring. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank Clinton Health Access Initiative for donating LFA CrAg test kits and to Ayder Comprehensive Specialized Hospital for allowing us to do the research in the laboratory and for the logistic support. Our great appreciation also goes to healthcare workers of the study sites and to all study participants.
Hailu K, Niguse S, Hagos K, Abdulkader M. Cryptococcal antigenemia and associated risk factors among ART‐naïve and ART‐experienced HIV‐infected peoples at selected health institutions of Mekelle, Northern Ethiopia. MicrobiologyOpen. 2019;8:e746 10.1002/mbo3.746
DATA ACCESSIBILITY
The data will be available on request from the corresponding authors following permission from Research Ethics Review Committee of the College of Health Sciences, Mekelle University.
REFERENCES
- Alemu, A. S. , Kempker, R. R. , Tenna, A. , Smitson, C. , Berhe, N. , Fekade, D. , … Aseffa, A. (2013). High prevalence of cryptococcal antigenemia among HIV‐infected patients receiving antiretroviral therapy in Ethiopia. PLoS One, 8(3), e58377 10.1371/journal.pone.0058377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaw, A. , Ali, D. , Eticha, T. , Alemayehu, A. , Alemayehu, M. , & Kindeya, F. (2015). CD4 cell count trends after commencement of antiretroviral therapy among HIV‐infected patients in Tigray, Northern Ethiopia: A retrospective cross‐sectional study. PLoS One, 10(3), e0122583 10.1371/journal.pone.0122583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assogoba, K. , Belo, M. , Wateba, M. I. , Gnonlonfoun, D. D. , Ossou Inguiet, P. M. , Tsanga, B. B. , … Grunitzky, E. K. (2015). Neuromeningeal cryptococcosis in sub‐Saharan Africa: Killer disease with sparse data. Journal of Neurosciences in Rural Practice, 6, 221–224. 10.4103/0976-3147.153231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe, T. , Melkamu, Y. , & Amare, A. (2012). The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: A retrospective study. AIDS Research and Therapy, 9, 11 10.1186/1742-6405-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene, T. , Woldeamanuel, Y. , Asrat, D. , Ayana, G. , & Boulware, D. R. (2013). Comparison of cryptococcal antigenemia between antiretroviral‐naive and antiretroviral experienced HIV positive patients at two hospitals in Ethiopia, PLoS One, 8(10), e75585 10.1371/journal.pone.0075585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene, T. , Zewde, A. G. , Balcha, A. , Hirpo, B. , Yitbarik, T. , Gebissa, T. , … Boulware, D. R. (2017). Inadequacy of high‐dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)–positive human immunodeficiency virus‐infected persons in an ethiopian CrAg screening program. Clinical Infectious Diseases, 65(12), 2126–2129. 10.1093/cid/cix613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for disease control and Prevention (CDC) (2014). Prevalence and correlates of cryptococcal antigen positivity among the AIDS patients‐United States, 1986–2012. MMWR. Morbidity and Mortality Weekly Report, 63(27), 585–587. [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Litvintseva, A. P. , Frazzitta, A. E. , Haverkamp, M. R. , Wang, L. , & Fang, C. (2015). Comparative analyses of clinical and environmental populations of Cryptococcus neoformans in Botswana. Molecular Ecology, 24, 3559–3571. 10.1111/mec.13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati, M. (2013). Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An atlas of the molecular types. Scientifica., 2013, 1–23. 10.1155/2013/675213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganiem, A. R. , Indrati, A. R. , Wisaksana, R. , Meijerink, H. , Ven, A. V. , Alisjahbana, B. , & van Crevel, R. (2014). Asymptomatic cryptococcal antigenemia is associated with mortality among HIV‐positive patients in Indonesia. Journal of the International AIDS Society, 17, 18821 10.7448/IAS.17.1.18821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto e Souza, L. K. , Costa, C. R. , Fernandes Ode, F. , Abrão, F. Y. , Silva, T. C. , Treméa, C. M. , & Silva Mdo, R. (2013). Clinical and microbiological features of cryptococcal meningitis. Revista Da Sociedade Brasileira De Medicina Tropical, 46(3), 343–347. 10.1590/0037-8682-0061-2012 [DOI] [PubMed] [Google Scholar]
- Jarvis, J. N. , Harrison, T. S. , Lawn, S. D. , Meintjes, G. , Wood, R. , & Cleary, S. (2013). Cost‐effectiveness of cryptococcal antigen screening as a strategy to prevent HIV associated cryptococcal meningitis in South Africa. PLoS One, 8(7), e69288 10.1371/journal.pone.0069288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis, J. N. , Meintjes, G. , Williams, Z. , Rebe, K. , & Harrison, T. S. (2010). Symptomatic relapse of HIV‐associated cryptococcal meningitis in South Africa: The role of inadequate secondary prophylaxis. South African Medical Journal, 100, 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, J. E. , Vallabhaneni, S. , Smith, R. M. , Chideya‐Chihota, S. , Chehab, J. , & Park, B. (2015). Cryptococcal Antigen Screening and Early Antifungal Treatment to Prevent Cryptococcal Meningitis. Rev. JAIDS Journal of Acquired Immune Deficiency Syndromes, 68, S331–S339. 10.1097/QAI.0000000000000484 [DOI] [PubMed] [Google Scholar]
- Letang, E. , Müller, M. C. , Ntamatungiro, A. J. , Kimera, N. , Faini, D. , Furrer, H. , … Glass, T. R. (2015). Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: A preventable cause of early mortality. Open Forum Infectious Diseases, 2(2), ofv046 10.1093/ofid/ofv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley, N. , Jarvis, J. N. , Meintjes, G. , Boulle, A. , Cross, A. , Kelly, N. , et al. (2016). Cryptococcal antigen screening in patients initiating ART in South Africa: A prospective cohort Study. Clinical Infectious Diseases, 62(5), 581–587. 10.1093/cid/civ936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magambo, K. A. , Kalluvya, S. E. , Kapoor, S. W. , Seni, J. , Chofle, A. A. , Fitzgerald, D. W. , & Downs, J. A. (2014). Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV‐positive outpatients beginning antiretroviral therapy in Mwanza. Tanzania. J Int AIDS Soci., 17, 19040 10.7448/IAS.17.1.19040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadzange, A. T. , & McHugh, G. (2014). New approaches to the diagnosis and treatment of cryptococcal meningitis. Seminars in Neurology, 34(1), 47–60. 10.1055/s-0034-1372342 [DOI] [PubMed] [Google Scholar]
- McClelland, E. E. , Hobbs, L. M. , Rivera, J. , Casadevall, A. , Potts, W. K. , Smith, J. M. , & Ory, J. J. (2013). The role of host gender in the pathogenesis of Cryptococcus neoformans infections. PLoS One, 8(5), e63632 10.1371/journal.pone.0063632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meya, D. , Rajasingham, R. , Nalintya, E. , Tenforde, M. , & Jarvis, J. N. (2015). Preventing cryptococcosis‐ shifting the paradigm in the era of highly active antiretroviral therapy. Current Tropical Medicine Reports, 2(2), 81–89. 10.1007/s40475-015-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngouana, T. K. , Dongtsa, J. , Kouanfack, C. , Tonfack, C. , Fomena, S. , Mallie, M. , … Bertout, S. (2015). Cryptococcal meningitis in Yaounde (Cameroon) HIV infected patients: Diagnosis, frequency, and Cryptococcusneoformans isolate susceptibility study to fluconazole. Journal of Medical Mycology, 25(1), 11–16. 10.1016/j.mycmed.2014.10.016 [DOI] [PubMed] [Google Scholar]
- Oladele, R. O. , Akanmu, A. S. , Nwosu, A. O. , Ogunsola, F. T. , Richardson, M. D. , & Denning, D. W. (2016). Cryptococcal antigenemia in Nigerian patients with advanced human immunodeficiency virus: Influence of antiretroviral therapy adherence. Open Forum Infectious Diseases, 3(2), ofw055 10.1093/ofid/ofw055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osazuwa, F. , Dirisu, J. O. , Okuonghae, P. E. , & Ugbebor, O. (2012). screening for cryptococcal antigenemia in anti‐retroviral naïve AIDS patients in Benin City, Nigeria. Oman Medical Journal, 27(3), 228–231. 10.5001/omj.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongsai, P. , Atamasirikul, K. , & Sungkanuparph, S. (2010). The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV‐Infected patients with different ranges of CD4 cell counts. Journal of Infection, 60(6), 474–477. 10.1016/j.jinf.2010.03.015 [DOI] [PubMed] [Google Scholar]
- Rajasingham, R. , Meya, D. B. , & Boulware, D. R. (2012). Integrating cryptococcal antigen screening and preemptive treatment into routine HIV care. JAIDS Journal of Acquired Immune Deficiency Syndromes, 59(5), 85–91. 10.1097/QAI.0b013e31824c837e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa, H. S. , Kowshik, T. , Chowdhary, A. , Prakash, A. , Khan, Z. U. , & Xu, J. (2011). Seasonal variations in the prevalence of Cryptococcus neoformans var. grubii and Cryptococcus gattii in decayed wood inside‐trunk hollows of diverse tree species in north‐western India: A retrospective study. Medical Mycology, 49(3), 320–323. [DOI] [PubMed] [Google Scholar]
- Reepalu, A. , Balcha, T. T. , Yitbarek, T. , Jarso, G. , Sturegard, E. , & Bjorkman, P. (2015). Screening for cryptococcal antigenemia using the lateral flow assay in antiretroviral therapy‐naïve HIV‐positive adults at an Ethiopian hospital clinic. BMC Research Notes, 8, 702 10.1186/s13104-015-1707-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo, S. , Makumbi, B. , Purfield, A. , Ndjavera, C. , Mutandi, G. , Maher, A. , … Lowrance, D. W. (2016). Estimated prevalence of Cryptococcus antigenemia (CrAg) among HIV‐infected adults with advanced immunosuppression in namibia justifies routine screening and preemptive treatment. PLoS One, 11(10), e0161830 10.1371/journal.pone.0161830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, R. M. , Nguyen, T. A. , Ha, H. T. T. , Thang, P. H. , Thuy, C. , Lien, T. X. , & Harris, J. R. (2013). Prevalence of cryptococcal antigenemia and cost‐effectiveness of a cryptococcal antigen screening program ‐Vietnam. PLoS One, 8(4), e62213 10.1371/journal.pone.0062213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitson, C. C. , Tenna, A. , Tsegaye, M. , Alemu, A. S. , Fekade, D. , Aseffa, A. , … Kempker, R. R. (2014). No association of cryptococcal antigenemia with poor outcomes among antiretroviral therapy‐experienced hiv‐infected patients in Addis Ababa, Ethiopia. PLoS One, 9(1), e85698 10.1371/journal.pone.0085698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajanga, B. M. , Kalluvya, S. , Downs, J. A. , Johnson, W. D. , Fitzgerald, D. W. , & Peck, R. N. (2011). Universal screening of Tanzanian HIV‐infected adult inpatients with the serum cryptococcal antigen to improve diagnosis and reduce mortality: An operational study. Journal of the International AIDS Society, 14, 48 10.1186/1758-2652-14-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2011). Rapid Advice Diagnosis, Prevention and Management of Cryptococcal Disease in HIV‐infected Adults, Adolescents and Children. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available on request from the corresponding authors following permission from Research Ethics Review Committee of the College of Health Sciences, Mekelle University.


