Abstract
The Asian citrus psyllid, Diaphorina citri Kuwayama, is the most serious pest of citrus worldwide. It acts as a vector for a group of phloem‐limited bacteria (Candidatus Liberibacter spp.) that causes Huanglongbing (HLB) disease. Thus, D. citri management is an important strategy against HLB, and biological control is currently considered as the most effective method because of the unsustainable and negative side effects of chemical control. Here, we isolated a new strain of entomopathogenic fungus, Cordyceps javanica (GZQ‐1), from one cadaver of D. citri adult based on its morphological and phylogenetic data. Five conidial concentrations of the C. javanica pathogen (1 × 103, 1 × 104, 1 × 105, 1 × 106, and 1 × 107 conidia/ml) were assessed against six life stages of D. citri (1st‐5th instar nymphs and adults). Results showed that C. javanica GZQ‐1 was highly pathogenic to D. citri nymphs (69.49%–90.87% mortality) and adults (69.98% mortality). The LC50 and LT50 values of C. javanica against 1st‐2nd instar (younger), 3rd‐4th instar (middle aged), 5th instar (older), and adults were 1.20 × 105, 1.10 × 106, 4.47 × 106, 8.12 × 106 conidia/ml and 4.25, 4.51, 5.17, 5.49 days, respectively. Moreover, glasshouse experiments indicated that this C. javanica GZQ‐1 caused higher infection rates of D. citri adults compared to two other fungal strains we previously isolated in the laboratory, Cordyceps fumosorosea (IF010) and Metarhizium anisopliae (CNGD7).
Keywords: Asian citrus psyllid, biological control, Cordyceps javanica, entomopathogenic fungi, isolation
1. INTRODUCTION
The Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), is one of the most economically important pests of citrus worldwide. It serves as a vector of phloem‐limited bacteria (Candidatus Liberibacter spp.), which can cause Huanglongbing (HLB; citrus greening disease), a devastating disease of citrus (Bové, 2006; Lin, 1956). Thus, population management of D. citri is a basic and most important strategy in blocking the spread of HLB disease. Intensive chemical control of D. citri is the primary strategy currently advocated for HLB management. However, this strategy is pernicious and unsustainable with several negative side effects such as chemical residues, resistance, and pest resurgence being noted (Tansey, Vanaclocha, Monzo, Jones, & Stansly, 2017).
In view of the undesirable side effects of pesticides, biological control has become an advantageous development direction for the prevention and control of agricultural pests. Within this area, entomopathogenic fungi play a particularly important role. In addition to efficacy and cost, there are several advantages of using entomopathogenic fungi: they have broad‐spectrum insecticidal activity, diversified species range, complex metabolic types, and offer appropriate safety levels for humans and other non‐target organisms (Lacey, Frutos, Kaya, & Vail, 2001). They are also easy to mass‐produce and development of host resistance against them is unlikely to occur. They also infect their insect hosts in a unique way entering the hemocoel cavity through the external cuticle, where they absorb nutrients, produce immunosuppressive toxins (Vilcinskas & Götz, 1999), damage the host cells, and interact with the gut microbiota to promote host death. They finally kill the host, and the given fungus grows out the cuticle of the dead insect releasing conidiophores to infect other host individuals (Wang & Wang, 2017; Wei et al., 2017; Xiong et al., 2013). All this promotes their good application potential within invertebrate pest control strategies.
Different natural enemies of D. citri including parasitoids, predators, and entomopathogenic fungi have been reported and applied in practice to manage this pest. Among the parasitoids, Tamarixia radiate (Waterston) and Diaphorencyrtus aligarhensis (Shafee, Alam and Argarwal) have been utilized to control D. citri population in Mauritius, Reunion Island, and the United States (Chen & Stansly, 2014; Chen, Triana, & Stansly, 2017). The predatory natural enemies of D. citri investigated includes ladybirds, predatory mites, lacewings, spiders, ants, and preying mantis (Juan‐Blasco, Qureshi, Urbaneja, & Stansly, 2012; Michaud, Olsen, & Olsen, 2004; Navarrete, Mcauslane, Deyrup, & Peña, 2013).
Entomopathogenic fungi account for more than 60% of insect pathogenic microorganisms. The utilization of entomopathogenic fungi to control D. citri offers a great development opportunity on account of their significant epidemic potential and the convenience of production. Lezama‐Gutiérrez et al. (2012) determined the virulence of three entomopathogenic fungi against D. citri in the field and found 42%, 50%, and 22% mortality of D. citri adults when they infected with Beauveria bassiana, Metarhizium anisopliae, and Cordyceps fumosorosea (Wize) Kepler, B. Shrestha & Spatafora, comb. nov. (MycoBank MB820980), formerly Isaria fumosorosea, respectively. Ibarra‐Cortes et al. (2018) ascertained the fatality rate of C. fumosorosea, B. bassiana, and M. anisopliae to be 28%, 55%, and 43%, respectively, to D. citri adults, and 47%, 69%, and 100%, respectively, to the nymphs when applying 1 × 107 conidia/ml suspensions of the individual fungi under laboratory conditions.
In the present study, a new strain of entomopathogenic fungus, Cordyceps javanica (Friederichs & Bally), formerly Isaria javanica (Friederichs & Bally; Kepler et al., 2017), isolated from an adult cadaver of D. citri was identified through morphological as well as phylogenetic data. The pathogenicity of this newly isolated strain to D. citri was analyzed through bioassays against different developmental stages.
2. MATERIALS AND METHODS
2.1. Psyllid cadaver collection and fungus isolation
A new fresh entomopathogenic fungal strain was isolated from an adult D. citri cadaver collected from a glasshouse at South China Agricultural University (SCAU) in Guangzhou, China. The carcass was infiltrated in water containing 5% Tween‐80 and shaken vigorously to re‐suspend the fungal spores or the mycelium fragments present on the cuticle surface. Then 10 μl of this suspension was plated on standard PDA medium. After 1–2 days of incubation at 27°C, individual germinations (or mycelium regeneration) were transferred to a new plate. Thereafter, this isolate was submitted to several rounds of purification in order to follow morphological stability after the successive transfers. This purified strain was named as “GZQ‐1,” and deposited in Guangdong Microbial Culture Collection Center (GDMCC) with the deposition number GDMCC 60437.
Before morphological observation, this GZQ‐1 isolate was firstly cultivated on SDAY/4 medium (SDAY/4:10 g/L dextrose, 2.5 g/L peptone, 2.5 g/L yeast extract, and 15 g/L agar) in Petri dishes placed within a biochemical incubator (26 ± 1°C, 60% RH, L:D = 14:10). Fungal conidia were then harvested from plates after 5 days, suspended in 0.05% Tween 80 (v/v) and shaken on a vortex mixer for 10 min. The conidial suspension was then filtered, counted and adjusted to the target concentration for pathogenicity tests. The infected nymphs and adults of D. citri were observed and recorded under a binocular microscope (ZEISS, Discovery.V20, Germany), and preliminary morphology was identified based on phenotypic properties and pathogenic fungi morphology (Jaber, Mercier, Knio, Brun, & Kambris, 2016).
2.2. Morphological observation
For the morphometric evaluation of the GZQ‐1 isolate, its micro‐cultures were first grown on SDAY/4 and incubated at 27°C for 10 days. Slides were then prepared with lactophenol/blue cotton (10:1) and examined with phase contrast optics under an optical microscope Olympus BX51 (Microscopy GmbH, Gottingen, Germany). Images of the conidia were photographed digitally with an Axio Cam HRc camera (Carl Zeiss) using the Axion Vision SE64 Release 4.9.1 software.
To measure the growth rate and conidia yield of GZQ‐1, the fungi were cultured on SDAY/4 at 27°C for 10 days, and then the conidia were scraped from the plates and suspended in 10 ml of sterile water. Following this, the suspension was filtered through Miracloth held in a funnel and quantified using a hemocytometer. The growth rate of GZQ‐1 hypha was measured based on their morphology on SDAY/4 medium plate on day 10 of culturing. Both the examinations were repeated three times.
2.3. DNA extraction and ITS sequencing of the GZQ‐1 isolate
Total DNA of the GZQ‐1 isolate was isolated from each sample of the test strains using a fungal DNA kit following the standard manufacturer's instructions (Fungal DNA Kit; Sangon Biotech, Shanghai, China). Purified DNA specimens were amplified with universal primers, ITS4: 5’‐TCCTCCGCTTATTGATATGC‐3’, and ITS5: 5’‐GGAAGTAAAAGTCGTAACAAGG‐3’. Each PCR was carried out in 25 μl volumes, containing 12.5 μl 2 × HiFiTaq PCR StarMix buffer (GenStar, China), 1 μl of each primer, 1 μl of total DNA (53.1 ng/μl), and 9.5 μl of ddH2O. The reaction was then performed using the following reaction cycles: initial denaturation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s, then a final extension phase at 72°C for 10 min. PCR products were visualized by 1.0% agarose gel electrophoresis, stained with Gold View in 0.5 × TBE buffer (Sangon, Shanghai, China) and photographed under UV light. Following this the target PCR products were sent to The Beijing Genomics Institute (BGI; Shenzhen, China) for complete bidirectional sequencing with PCR primers.
The resulting sequences were checked and aligned using Lasergene v7.1 (DNASTAR, Inc., Madison, WI). Then the similarity of sequences compared with homologous sequences deposited in GenBank (Table 1) was calculated using “BLAST” tools on the National Center for Biotechnology Information (NCBI) website, and a resulting phylogenetic tree constructed using MEGA 6 software (Felsenstein, 1985; Saitou & Nei, 1987). C. javanica strain CBS 134.22 and C. fumosorosea strain CBS 107.10 were used as the Ex‐type strains. Metarhizium anisopliae strains ZJ and YD2‐1‐8 were used as the out group in the phylogeny analysis.
Table 1.
The reference entomopathogenic fungi from GenBank used in phylogenetic analysis. (“*” is the GenBank accession number of GZQ‐1; “N/A” unlabeled in NCBI)
| Species | GenBank number | Strain no. | Host | Location |
|---|---|---|---|---|
| Cordyceps javanica | MG742216* | GZQ‐1 | Diaphorina citri | Guangzhou, China |
| Cordyceps javanica | AY624186 | Ex‐type CBS 134.22 | Hypothenemus hampei | Java |
| Cordyceps javanica | MG837718 | ACP | Diaphorina citri | Fuzhou, China |
| Cordyceps javanica | KM234218 | CHE‐CNRCB 307/7 | Bemisia tabaci | Armeria, Mexico |
| Cordyceps javanica | KM234213 | CHE‐CNRCB 303/2 | Bemisia tabaci | Armeria, Mexico |
| Cordyceps javanica | KM234212 | CHE‐CNRCB 303 | Bemisia tabaci | Armeria, Mexico |
| Cordyceps javanica | KT225592 | CHE‐CNRCB 310 | Spodoptera littoralis | France |
| Cordyceps fumosorosea | AY624182 | CBS 244.31 | Butter | Ireland |
| Cordyceps fumosorosea | AY624183 | CBS 375.70 | Food | Japan |
| Cordyceps fumosorosea | AY624184 | Ex‐type CBS 107.10 | N/A | France |
| Beauveria bassiana | KX093961 | YT‐11 | Bombyx mori | Shandong, China |
| Beauveria bassiana | KF772868 | GA‐1 | Micromelalopha troglodyta | Hubei, China |
| Beauveria bassiana | KF772861 | YD‐1 | Dendroctonus punctatus | Hubei, China |
| Beauveria brongniartii | HQ380853 | ART376 | Melolontha melolontha | Innertkirchen, Switzerland |
| Beauveria brongniartii | JX110381 | SASR HHB32B | Galleria mellonella | South Africa |
| Beauveria brongniartii | JF947191 | ARSEF8153 | Agrilus planipennis | Ontario, Canada |
| Metarhizium anisopliae | JN377427 | ZJ | Rhynchophorus ferrugineus | Hainan,China |
| Metarhizium anisopliae | KX380792 | YD2‐1‐8 | Soil | Nanchang, China |
Phylogenetic hypotheses were analyzed independently for ITS with Maximum Likelihood (ML) using MEGA 6 software based on the Tamura‐Nei model for ITS, and a discrete gamma distribution was applied for each analysis with 1,000 bootstrap replicates (ML BS). The resulting trees are visualized in Figure 4.
2.4. Plants and insects in testing
Murraya paniculata (L) Jacks plants were used in this study. New seedling plants were cultured in 30‐cm‐diameter plastic pots containing a soil‐sand mixture (10% sand, 5% clay, and 85% peat) in a glasshouse at ambient temperature and photoperiod.
The colony of D. citri was first collected from M. paniculata plants at SCAU and then continuously reared on M. paniculata seedlings in a glasshouse onsite at SCAU at 26–28°C and 60%–65% relative humidity under a 14:10 hr (L:D) photoperiod. New plants with fresh shoots were provided for the D. citri culture once a month. For bioassay analysis, six life stages of D. citri were used: 1st–5th nymphal instars and mature adults. Nymphs were collected into Petri dishes using a fine camel hairbrush, and following morphological examination under a stereomicroscope, were classified into their individual nymphal stages based on morphological features.
2.5. Pathogenicity testing of the GZQ‐1 isolate
2.5.1. Laboratory bioassays
Experiments were performed in an artificial climate incubator (26 ± 1°C, 90% RH and photoperiod 14:10 hr (L:D)). Conidial suspensions of GZQ‐1 were diluted with sterile distilled water to five concentrations (1 × 103, 1 × 104, 1 × 105, 1 × 106, and 1 × 107 conidia/ml). The LC50 values and LT50 values of this GZQ‐1 isolate on D. citri younger nymphs (1st–2nd instar), middle and older nymphs (3rd–5th instar), and adults (3 days after emergence) were measured. Double sterile water was used as a negative control. Different instars of D. citri were sprayed with each concentration of conidial suspension as well as the ddH2O control (till the leaf surface was covered with beads of solution). Approximately 30–40 individuals of different instar D. citri nymphs or adults were examined in each bioassay, with each assay being replicated three times.
2.5.2. Glasshouse bioassays
The glasshouse bioassay of GZQ‐1 isolate to D. citri adults (3 days following emergence) were carried out in the autumn of 2017 at SCAU (average temperature 24–30°C, 50%–60% RH). C. fumosorosea (IF010) and M. anisopliae (CNGD7), which we have isolated in SCAU (Dai et al., 2017; Freed, Jin, & Ren, 2011) were also tested as positive controls. The conidial suspensions of these fungi (including GZQ‐1) were diluted with sterile distilled water to 1 × 107 conidia/ml. Approximately 30–40 insects were sprayed with the individual conidial suspensions or ddH2O as control in each bioassay, with each assay replicated three times.
The infected D. citri by the three entomopathogenic fungi were observed daily, and the dead individuals were removed and underwent further incubation to promote fungal growth. The average mortalities of D. citri caused by the tested fungi were calculated. The mathematical calculation of lethal concentrations LC50 and confidence limits were carried out by the method of probit analysis (Zhang et al., 2018).
3. RESULTS
3.1. Morphological identification of GZQ‐1 isolate
The morphological features of GZQ‐1 isolate are illustrated in Figure 1. The fungal colonies had a concentric ring pattern. Mycelium texture was velvet like. The center of the colony was pale pink while spores on the edge of the colony were white in color with radial growth (Figure 1a). The mature colony was brownish gray (Figure 1b). The conidiophores were straight with a long ovoid conidial shape linked into a chain, and the phialides were characterized by a wide globose basal portion with a long distal neck (Figure 1c,d). The morphological identification and infection observation revealed that GZQ‐1 isolate was Cordyceps javanica.
Figure 1.
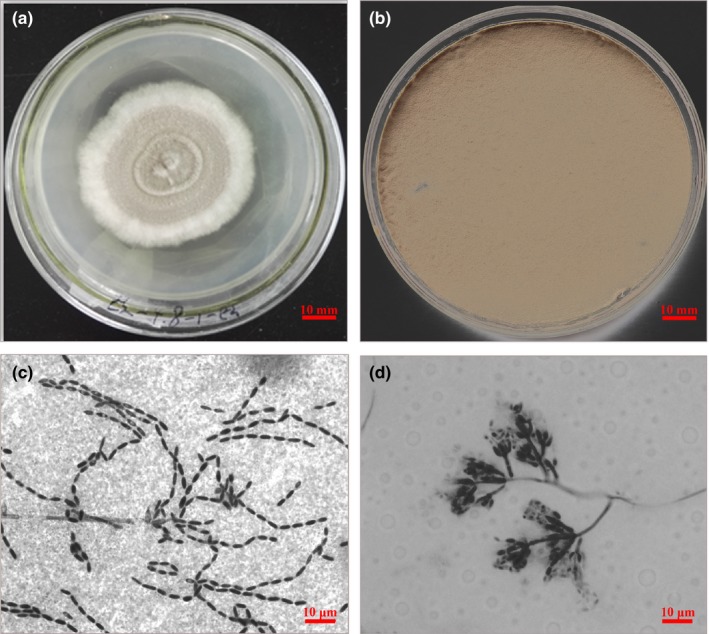
The morphology of Cordyceps javanica GZQ‐1 isolate on SDAY/4. (a) The upper side of C. javanica colony on SDAY/4 on 4th day; (b) The upper side of C. javanica mature colony on SDAY/4 on 14th day; (c) C. javanica GZQ‐1: chains of conidia. (d) C. javanica GZQ‐1: Phialides with developing conidia
When D. citri nymphs and adults were infected by the GZQ‐1 isolate in the glasshouse, the insects were observed to move slowly and suffer twitching of legs and antennae after 48 hr infection. Infected adult D. citri scratched actively, clung tightly to branches and leaves until they were completely covered with hyphae and finally died. Microscopic observation showed that fungal hyphae emerging from the tarsi and intersegmental regions of the legs of infected D. citri after 48–72 hr had grown from the metamere and intersegmental membranes. Then covering the whole of the D. citri including the wings, it caused death after seven days or longer (Figures 2 and 3).
Figure 2.
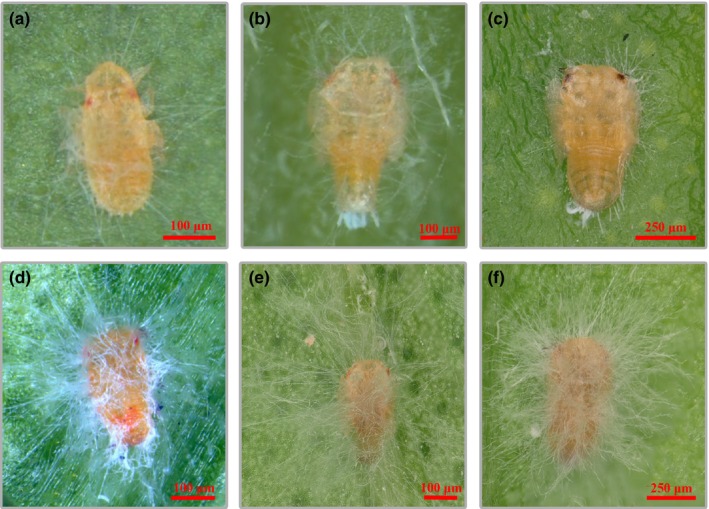
The infection phenotype of Diaphorina citri nymphs treated with Cordyceps javanica (1 × 107 conidia/ml). Panels a, b, and c were the 1st, 2nd, and 3rd nymphs of D. citri on 2nd day after infection while panels d, e, and f were the 1st, 2nd, and 3rd nymphs on the 7th day after infection
Figure 3.

The infection phenotype of Diaphorina citri older nymphs and adults treated with Cordyceps javanica (1 × 107 conidia/ml). Panels a, b, and c were the 4th and5th nymphs and mature adults of D. citri on 2nd day after infection while panels d, e, and f were the 4th and 5th nymphs and mature adults on the 7th day after infection
3.2. Sequencing of the ITS genes and phylogenetic analysis
Polymerase chain reaction amplification and DNA sequencing results indicated that the rDNA‐ITS gene of GZQ‐1 isolate was 616 bp (data not shown), following this the DNA sequence was submitted to GenBank; where it gain the GenBank accession number of MG742216. BLAST results in GenBank revealed that the ITS gene of GZQ‐1 was 100% homologous to the C. javanica strain (GenBank accession number MG837718). Moreover, phylogenetic analysis showed that GZQ‐1 isolate was closely clustered in the C. javanica clade (Figure 4), which supported our morphological identification that the GZQ‐1 isolate is a C. javanica strain.
Figure 4.
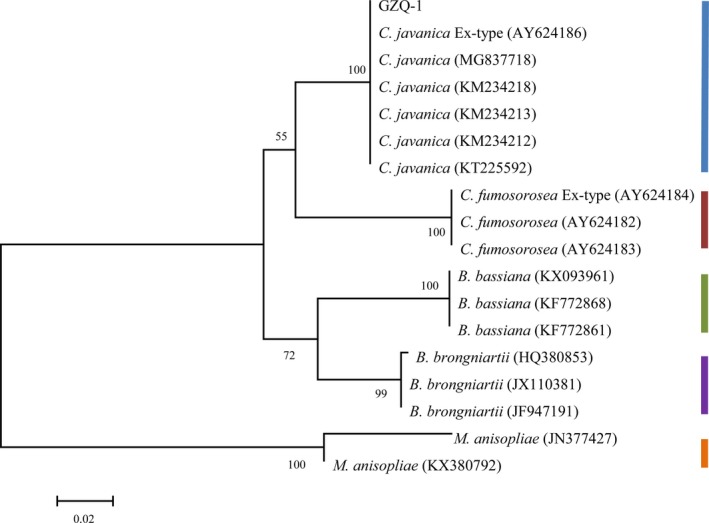
Majority rule consensus phylogram from the Bayesian analysis based on the sequences of the internal transcribed spacer (ITS) region for 18 isolates (GZQ‐1 isolate, Cordyceps javanica, Cordyceps fumosorosea, Beauveria brongniartii, Beauveria bassiana. Metarhizium anisopliae was used as the outgroup). Support values are shown for ML BS. The bars in different color show the different fungal species but different strains clustered into one branch in the phylogenetic tree
3.3. Pathogenicity testing of the GZQ‐1 isolate
3.3.1. Laboratory bioassays
After 10 days of incubation, the diameter of C. javanica colony reached 42.3 ± 1.86 mm and sporulation was 2.26 × 108 spores/g on SDAY/4 medium. The bioassay results showed that GZQ‐1 isolate has high pathogenicity to both the nymphs and adults of D. citri, with corrected mortality rates of 91.7% ± 2.36%, 88.3% ± 4.33%, 73.3% ± 3.32%, and 72.2% ± 2.94% to the 1st–2nd instar, 3rd‐4th instar, 5th instar nymphs, and adults of ACP following the 7th day of infection, respectively (Figure 5); there were significant differences among the mortality rates of 1st–2nd instar nymphs, 3rd–4th instar nymphs, 5th instar nymphs, and adult D. citri (p < 0.05).
Figure 5.
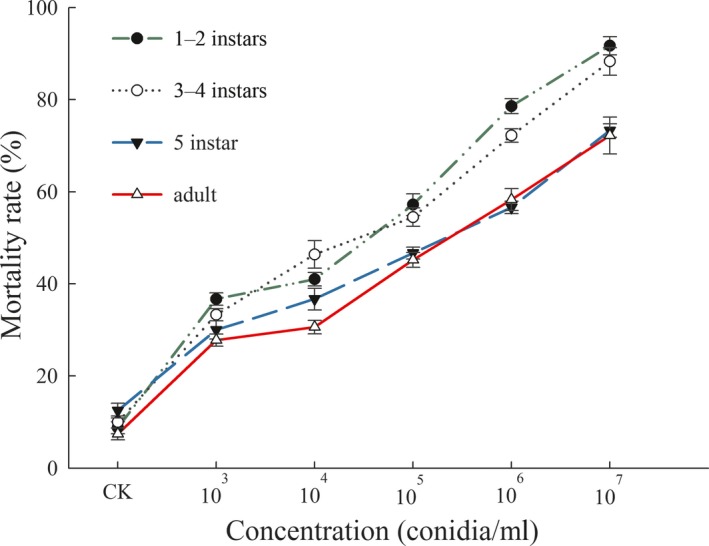
The mortality of Diaphorina citri different developmental stages treated with various concentrations of Cordyceps javanica (1 × 103, ×104, ×105, ×106, and ×107 conidia/ml). Data are mean ± SEM of three tests
Bioassay results showed that the LC50 values for C. javanica GZQ‐1 isolate acting on the different developmental stages of D. citri after 7 days were 5.19 × 105, 1.27 × 106, 4.47 × 106, and 8.12 × 106 conidia/ml, respectively (Table 2). While the LT50 values of GZQ‐1 isolate on different developmental stages of D. citri were 4.25, 4.51, 5.17, and 5.49 days, respectively (Table 3). Lethal concentration and death time increased with an increase in developmental stage of D. citri when sprayed with the same concentration of C. javanica GZQ‐1. This demonstrated that our C. javanica GZQ‐1 isolate was most effective against younger instar nymphs of D. citri.
Table 2.
Pathogenicity regression equations for LC50 values of Cordyceps javanica against different developmental stages of Diaphorina citri after 7 days of infection (1 × 107 conidia/ml)
| Insect stages | Regression virulence model | LC50% and 95% CI (conidia/ml) | R 2 |
|---|---|---|---|
| 1st−2nd instar nymph | Y = 0.129X − 0.085 | 5.19 (−13.2 ~ 19.55) × 105 | 0.634 |
| 3rd−4th instar nymph | Y = 0.132X − 0.164 | 1.27 (−1.02 ~ 3.07) × 106 | 0.724 |
| 5th instar nymph | Y = 0.145X − 0.44 | 4.47 (1.98 ~ 9.35) × 106 | 0.947 |
| Adult | Y = 0.436X − 2.07 | 8.12 (1.81 ~ 2.62) × 106 | 0.961 |
Table 3.
Pathogenicity regression equations for LT50 values of Cordyceps javanica against different developmental stages of Diaphorina citri after 7 days of infection (1 × 107 conidia/ml)
| Insect stages | Regression virulence model | LT50% and 95% CI (d) | R 2 |
|---|---|---|---|
| 1st−2nd instar nymph | Y = 0.549X − 2.335 | 4.25 (3.87 ~ 4.64) | 0.950 |
| 3rd−4th instar nymph | Y = 0.466X − 2.101 | 4.51 (4.08 ~ 4.96) | 0.976 |
| 5th instar nymph | Y = 0.449X − 2.322 | 5.17 (4.73 ~ 5.70) | 0.981 |
| Adult | Y = 0.486X − 2.668 | 5.49 (5.09 ~ 5.96) | 0.943 |
3.3.2. Glasshouse bioassays
The semi‐field pathogenicity of GZQ‐1 isolate to D. citri was compared with two other excellent entomopathogenic fungi, which have been screened under laboratory conditions: C. fumosorosea and M. anisopliae at 1 × 107 conidia/ml. Results showed that, when infected with C. javanica GZQ‐1 isolate, the survival rates of D. citri adults was 82.6% on the 3rd day, reducing to 35.7% on the 9th day and finally 18.3% on the 11th day. When infected with C. fumosorosea and M. anisopliae, their survival rates were 90.0% and 100% on the 3rd day, reducing to 60.0% and 66.7% on the 9th day and finally 22.3% and 20.0% on the 11th day, respectively. In contrast, only 7.7% mortality was recorded in the non‐infected controls on the 9th day (Figure 6). Results revealed that the infection and pathogenicity of C. javanica isolate to D. citri in semi‐field conditions was faster or higher than the two other fungal strains we previously isolated in the laboratory (C. fumosorosea (IF010) and M. anisopliae (CNGD7)) after 7 days of infection in the current study.
Figure 6.
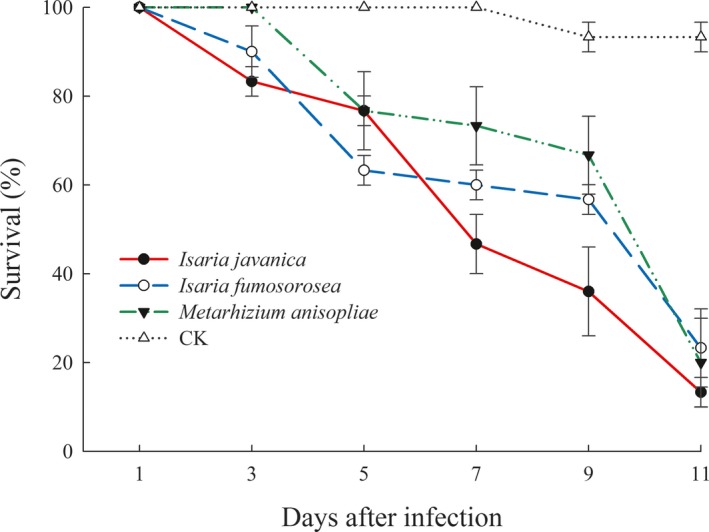
The survival rates of Diaphorina citri adults when infected with Cordyceps javanica GZQ‐1 isolate as well as Cordyceps fumosorosea and Metarhizium anisopliae (all are 1 × 107 conidia/ml) under glasshouse conditions. Data are mean ± SEM of three tests
4. DISCUSSION
Numerous insect pathogenic fungi including Paecilomyces varioti, C. fumosorosea, C. javanica, Beauveria. bassiana, M. anisopliae, Hirsutella citriformis, Acrostalagmus aphidium, Fusarium culmorum, Lecanicillium lecanii, Cladosporium oxysporum, Capnodium citri, and Stemphylium sp. have been reported to control different insect pests, but only a few studies have reported the pathogenicity of C. javanica against D. citri (Faria & Wraight, 2007; Gandarilla‐Pacheco, Galán‐Wong, López‐Arroyo, Rodríguez‐Guerra, & Quintero‐zapata, 2013; Medina‐Córdova, Rosales‐Mendoza, Hernández‐Montiel, & Angulo, 2018; Meyer, Hoy, & Boucias, 2008; Vega et al., 2008). Nguyen et al. (2017) isolated two highly virulent strains of C. javanica against diamondback moth Plutella xylostella with respective LT50 values of 2.52 and 2.55 days.
Based on the complete morphological and molecular characteristics described for Isaria, Cabanillas, Leon, Humber, Murray, and Jones (2013) correctly identified C. javanica from the erroneous differentiated I. poprawskii, which was collected from a cadaver of the sweet potato whitefly, Bemisia tabaci biotype B in the Lower Rio Grande Valley of Texas, USA. Following this, Mellin‐Rosas, Sanchez‐Gonzalez, Cruz‐Avalos, Montesinos‐Matias, and Arredondo‐Bernal (2016) reported that their C. javanica CHE‐CNRCB 303 isolate can cause 95% mortality of both D. citri nymphs and adults. In the current study, the C. javanica GZQ‐1 isolate is very similar to the C. javanica fungi descriptions published in regards to morphological features and phenotype of colony (Gallou et al., 2016); its conidial characteristic is also consistent to the findings of Gallou et al. (2016). Moreover, the growth speed and sporulation quantity of our C. javanica GZQ‐1 colony was faster than that reported by Yin, Qiong‐Bo, Guo‐Hua, and Mei‐Ying (2010) and Shimazu and Takatsuka (2010).
In terms of the pathogenicity bioassays, the LC50 and LT50 values of C. javanica GZQ‐1 isolate showed good biological control potential for D. citri management. Pinto, Filho, Almeida, and Wenzel (2012) reported the pathogenicity of B. bassiana (IBCB 66) to D. citri nymphs: the LC50 and LC90 values on the 10th day of application were 0.4 × 106 and 6.7 × 106 conidia/ml, respectively. Hoy, Singh, and Rogers (2010) determined the pathogenicity of C. fumosorosea (AsCP) to D. citri adults, here the LT50 value was 4.625 days at spore concentrations of 1 × 107 conidia/ml and the LC50 value was 6.8 × 107 conidia/ml after 8 days of fungal application. Our results indicated that our C. javanica GZQ‐1 isolate was similar too or even better than these high quality strains concerning their pathogenicity to D. citri.
Under semi‐field conditions, Dai et al. (2017) observed 62% and 65% mortality of D. citri when treated with 1 × 107 conidia/ml of B. bassiana (Bb‐E) and C. fumosorosea (IF‐B) after 7 days of infection, respectively. Lezama‐Gutiérrez et al. (2012) determined that the fatality rate of C. fumosorosea, B. bassiana, M. anisopliae to D. citri adults were 22%, 42%, and 50%, respectively, in the field. Compared to these studies and our current findings, the different pathogenicity rates of these fungi may be due to strain distinctions, different biotic, abiotic conditions, including host species, humidity, temperature, and soil type in the various studies (Bugeme, Maniania, Knapp, & Boga, 2008; Erler & Ates, 2015; Inyang, Mccartney, & Oyejola, 2000; Kim, Oh, Yoon, & Sung, 2017; Sharififard, Mossadegh, & Vazirianzadeh, 2012).
In conclusion, the efficacious strategy against Huanglongbing disease by suppressing D. citri populations has been required to affiliate a multi‐tactic integrated pest management (IPM) programme (Meyer et al., 2008; Weintraub & Beanland, 2006). In the current study, we identified a high pathogenic C. javanica GZQ‐1 isolate based on its morphology and phylogeny and estimated its potential use in Asian citrus psyllid biological control. Our study is expected to enrich the available resource library of entomopathogenic fungi and provide an alternative preparation for inclusion within D. citri IPM strategies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
DO and BLQ designed the study. DO, LHZ, and CFG performed the experiments and analyzed the data. XSC and SA participated in data analysis and photography. DO, BLQ, and SA wrote the paper. BLQ supported the grants.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
We thank Dr. Andrew G S Cuthbertson (Defra, UK) for his critical comments on an early draft of the manuscript. The work was supported by the National Key Research and Development Programme of China (2017YFD0200400), the Guangdong Science and Technology Innovation Leading Talent Program (2016TX03N273), the International Cooperation Project of Guangzhou Science and Technology Innovation Committee (201604030029, 201807010019); and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014‐19) to BLQ. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Ou D, Zhang L‐H, Guo C‐F, Chen X‐S, Ali S, Qiu B‐L. Identification of a new Cordyceps javanica fungus isolate and its toxicity evaluation against Asian citrus psyllid. MicrobiologyOpen. 2019;8:e760 10.1002/mbo3.760
DATA ACCESSIBILITY
All DNA sequences are submitted to GenBank with Accession number MG742216. All data generated or analyzed during this study are included in this published article.
REFERENCES
- Bové, J. M. (2006). Huanglongbing: A destructive, newly‐emerging, century‐old disease of citrus. Journal of Plant Pathology, 88, 7–37. [Google Scholar]
- Bugeme, D. M. , Maniania, N. K. , Knapp, M. , & Boga, H. I. (2008). Effect of temperature on virulence of Beauveria bassiana and Metarhizium anisopliae isolates to Tetranychus evansi . Experimental and Applied Acarology, 46, 275–285. 10.1007/s10493-008-9179-1 [DOI] [PubMed] [Google Scholar]
- Cabanillas, H. E. , de Leon, J. H. , Humber, R. A. , Murray, K. D. , & Jones, W. A. (2013). Isaria poprawskii sp nov (Hypocreales: Cordycipitaceae), a new entomopathogenic fungus from Texas affecting sweet potato whitefly. Mycoscience, 54, 158–169. 10.1016/j.myc.2012.09.009 [DOI] [Google Scholar]
- Chen, X. , & Stansly, P. A. (2014). Biology of Tamarixia radiata (Hymenoptera: Eulophidae), parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea): A mini review. Florida Entomologist, 97, 1404–1413. [Google Scholar]
- Chen, X. , Triana, M. , & Stansly, P. A. (2017). Optimizing production of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the citrus greening disease vector Diaphorina citri (Hemiptera: Psylloidea). Biological Control, 105, 13–18. 10.1016/j.biocontrol.2016.10.010 [DOI] [Google Scholar]
- Dai, X. Y. , Li, Y. Y. , Shen, Z. L. , Xu, W. M. , Wu, J. H. , Ren, S. X. , & Qiu, B. L. (2017). The biocontrol effects of Beauveria bassiana and Isaria fumosorosea on Asian citrus psyllid. Journal of South China Agricultural University, 38, 63–68. [Google Scholar]
- Erler, F. , & Ates, A. O. (2015). Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle. Journal of Insect Science, 15, 44 10.1093/jisesa/iev029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria, M. R. D. , & Wraight, S. P. (2007). Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43, 237–256. 10.1016/j.biocontrol.2007.08.001 [DOI] [Google Scholar]
- Felsenstein,, (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Freed, S. , Jin, F. L. , & Ren, S. X. (2011). Determination of genetic variability among the isolates of Metarhizium anisopliae var. anisopliae from different geographical origins. World Journal of Microbiology & Biotechnology, 27, 359–370. [Google Scholar]
- Gallou, A. , Serna‐Dominguez, M. G. , Berlanga‐Padilla, A. M. , Ayala‐Zermeno, M. A. , Mellin‐Rosas, M. A. , Montesinos‐Matias, R. , & Arredondo‐Bernal, H. C. (2016). Species clarification of Isaria isolates used as biocontrol agents against Diaphorina citri (Hemiptera: Liviidae) in Mexico. Fungal Biology, 120, 414–423. 10.1016/j.funbio.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Gandarilla‐Pacheco, F. L. , Galán‐Wong, L. J. , López‐Arroyo, J. I. , Rodríguez‐Guerra, R. , & Quintero‐zapata, I. (2013). Optimization of pathogenicity tests for selection of native isolates of entomopathogenic fungi isolated from citrus growing areas of México on Adults of Diaphorina citri Kuwayama (Hemiptera: Liviidae). Florida Entomologist, 96, 187–195. [Google Scholar]
- Hoy, M. A. , Singh, R. , & Rogers, M. E. (2010). Evaluations of a novel isolate of Isaria fumosorosea for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomologist, 93, 24–32. [Google Scholar]
- Ibarra‐Cortes, K. H. , Guzman‐Franco, A. W. , Gonzalez‐Hernandez, H. , Ortega‐Arenas, L. D. , Villanueva‐jimenez, J. A. , & Robles‐bermudez, A. (2018). Susceptibility of Diaphorina citri (Hemiptera: Liviidae) and its parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) to entomopathogenic fungi under laboratory conditions. Neotropical Entomology, 47, 131–138. 10.1007/s13744-017-0539-6 [DOI] [PubMed] [Google Scholar]
- Inyang, E. N. , Mccartney, H. A. , & Oyejola, B. (2000). Effect of formulation, application and rain on the persistence of the entomogenous fungus Metarhizium anisopliae on oilseed rape. Mycological Research, 104, 653–661. 10.1017/S0953756200002641 [DOI] [Google Scholar]
- Jaber, S. , Mercier, A. , Knio, K. , Brun, S. , & Kambris, Z. (2016). Isolation of fungi from dead arthropods and identification of a new mosquito natural pathogen. Parasites & Vectors, 9, 491 10.1186/s13071-016-1763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan‐blasco, M. , Qureshi, J. A. , Urbaneja, A. , & Stansly, P. A. (2012). Predatory Mite, Amblyseius swirskii (Acari: Phytoseiidae), for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomologist, 95, 543–551. [Google Scholar]
- Kepler, R. M. , Luangsaard, J. J. , Hyweljones, N. L. , Quandt, C. A. , Sung, G. H. , Rehner, S. A. , et al. (2017). A phylogenetically‐based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus, 8, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Oh, J. , Yoon, D. H. , & Sung, G. H. (2017). Suppression of a methionine synthase by calmodulin under environmental stress in the entomopathogenic fungus Beauveria bassiana . Environmental Microbiology Reports, 9, 612–617. [DOI] [PubMed] [Google Scholar]
- Lacey, L. A. , Frutos, R. , Kaya, H. K. , & Vail, P. (2001). Insect pathogens as biological control agents: Do they have a future? Biological Control, 21, 230–248. 10.1006/bcon.2001.0938 [DOI] [Google Scholar]
- Lezama‐Gutiérrez, R. , Molina‐Ochoa, J. , Chávez‐Flores, O. , Ángel‐Sahagún, C. A. , Skoda, S. R. , Reyes‐Martínez, G. , … Foster, J. E. (2012). Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae) in Persian lime under field conditions. International Journal of Tropical Insect Science, 32, 39–44. 10.1017/S1742758412000069 [DOI] [Google Scholar]
- Lin, K. (1956). Etiological studies of yellow shoot of citrus. Acta Phytopathologica Sinica, 2, 13–42. [Google Scholar]
- Medina‐Córdova, N. , Rosales‐Mendoza, S. , Hernández‐Montiel, L. G. , & Angulo, C. (2018). The potential use of Debaryomyces hansenii for the biological control of pathogenic fungi in food. Biological Control, 121, 216–222. 10.1016/j.biocontrol.2018.03.002 [DOI] [Google Scholar]
- Mellin‐Rosas, M. A. , Sanchez‐Gonzalez, J. A. , Cruz‐Avalos, A. M. , Montesinos‐Matias, R. , & Arredondo‐Bernal, H. C. (2016). Pathogenicity of entomopathogenic fungal strains on Diaphorina citri kuwayama under laboratory conditions. Southwestern Entomologist, 41, 791–799. [Google Scholar]
- Meyer, J. M. , Hoy, M. A. , & Boucias, D. G. (2008). Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. Journal of Invertebrate Pathology, 99, 96–102. 10.1016/j.jip.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Michaud, J. P. , Olsen, L. E. , & Olsen, L. E. (2004). Suitability of Asian citrus psyllid, Diaphorina citri, as prey for ladybeetles. BioControl, 49, 417–431. 10.1023/B:BICO.0000034605.53030.db [DOI] [Google Scholar]
- Navarrete, B. , Mcauslane, H. , Deyrup, M. , & Peña, J. E. (2013). Ants (Hymenoptera: Formicidae) Associated with Diaphorina citri (Hemiptera: Liviidae) and their role in its biological control. Florida Entomologist, 96, 590–597. [Google Scholar]
- Nguyen, H. C. , Tran, T. V. A. , Nguyen, Q. L. , Nguyen, N. N. , Nguyen, M. K. , Nguyen, N. T. T. , … Lin, K. H. (2017). Newly isolated Paecilomyces lilacinus and Paecilomyces javanicus as novel biocontrol agents for Plutella xylostella and Spodoptera litura . Notulae Botanicae Horti Agrobotanici Cluj‐Napoca, 45, 280 10.15835/nbha45110726 [DOI] [Google Scholar]
- Pinto, A. P. F. , Filho, A. B. , Almeida, J. E. M. D. , & Wenzel, E. I. M. (2012). Beauveria bassiana pathogenicity to Diaphorina citri and compatibility of the fungus with phytosanitary products. Pesquisa Agropecuária Brasileira, 47, 1673–1680. [Google Scholar]
- Saitou, N. , & Nei, M. (1987). The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Sharififard, M. , Mossadegh, M. S. , & Vazirianzadeh, B. (2012). Effects of temperature and humidity on the pathogenicity of the entomopathogenic fungi in control of the house fly, Musca domestica L. (Diptera: Muscidae) under laboratory conditions. Journal of Entomology, 9, 282–288. 10.3923/je.2012.282.288 [DOI] [Google Scholar]
- Shimazu, M. , & Takatsuka, J. (2010). Isaria javanica (anamorphic Cordycipitaceae) isolated from gypsy moth larvae, Lymantria dispar (Lepidoptera: Lymantriidae), in Japan. Applied Entomology and Zoology, 45, 497–504. [Google Scholar]
- Tansey, J. A. , Vanaclocha, P. , Monzo, C. , Jones, M. , & Stansly, P. A. (2017). Costs and benefits of insecticide and foliar nutrient applications to huanglongbing‐infected citrus trees. Pest Management Science, 73, 904–916. 10.1002/ps.4362 [DOI] [PubMed] [Google Scholar]
- Vega, F. E. , Posada, F. , Catherine Aime, M. , Pava‐Ripoll, M. , Infante, F. , & Rehner, S. A. (2008). Entomopathogenic fungal endophytes. Biological Control, 46, 72–82. 10.1016/j.biocontrol.2008.01.008 [DOI] [Google Scholar]
- Vilcinskas, A. , & Götz, P. (1999). Parasitic fungi and their interactions with the insect immune system. Advances in Parasitology, 43, 267–313. [Google Scholar]
- Wang, C. , & Wang, S. (2017). Insect pathogenic fungi: Genomics, molecular interactions, and genetic improvements. Annual Review of Entomology, 62, 73–90. 10.1146/annurev-ento-031616-035509 [DOI] [PubMed] [Google Scholar]
- Wei, G. , Lai, Y. , Wwang, G. , Chen, H. , Li, F. , & Wang, S. (2017). Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proceedings of the National Academy of Sciences of the United States of America, 114, 5994–5999. 10.1073/pnas.1703546114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, P. G. , & Beanland, L. (2006). Insect vectors of phytoplasmas. Annual Review of Entomology, 51, 91–111. 10.1146/annurev.ento.51.110104.151039 [DOI] [PubMed] [Google Scholar]
- Xiong, Q. , Xie, Y. , Zhu, Y. , Xue, J. , Li, J. , & Fan, R. (2013). Morphological and ultrastructural characterization of Carposina sasakii larvae (Lepidoptera: Carposinidae) infected by Beauveria bassiana (Ascomycota: Hypocreales: Clavicipitaceae). Micron, 44, 303–311. 10.1016/j.micron.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Yin, F. , Qiong-Bo, H. , Guo-Hua, Z. , & Mei-Ying, H. (2010). Effects of destruxins on entom opathogenic fungus Isaria javanicus and the joint toxicity of their mixtures against the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Acta Entomologica Sinica, 53, 61–67. [Google Scholar]
- Zhang, C. , Shao, Z. F. , Han, Y. Y. , Wang, X. M. , Wang, Z. Q. , Musa, P. D. , … Ali, S. (2018). Effects of Aschersonia aleyrodis on the life table and demographic parameters of Bemisia tabaci . Journal of Integrative Agriculture, 17, 389–396. 10.1016/S2095-3119(17)61773-8 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All DNA sequences are submitted to GenBank with Accession number MG742216. All data generated or analyzed during this study are included in this published article.


