Abstract
Introduction
The initial examination of cerebrospinal fluid (CSF) for proteins, glucose, and leukocytes, is still the gold standard investigation in some neurological conditions like meningitis. Aims and objective of the present study were to determine the accuracy of urinary reagent strip for semi-quantitative analysis of protein, glucose, leucocytes and erythrocytes in CSF along with its role in emergency for rapid diagnosis of neurological conditions.
Materials and methods
360 samples of CSF were received in emergency laboratory of a tertiary care hospital in a period of 6 months. All CSF samples were subjected to two types of tests-the definitive test and the index test. CSF microscopy for leucocyte and erythrocyte as well as biochemistry tests for protein and glucose on automated biochemistry analyser were considered as definitive test. The index test for protein, glucose, leucocyte and erythrocyte for the same sample was conducted by Combur-10 urinary reagent strip.
Result
The strip test showed a sensitivity of 99% and a specificity of 54% for proteins. With respect to glucose, the strip was highly sensitive (98%) as well as highly specific (92%).It showed a high sensitivity and specificity for leukocytes ≥ 10 cells/cumm i.e.100% and 96% respectively. For CSF erythrocytes sensitivity and specificity was 100%
Conclusion
Urinary reagent strip can be used routinely for rapid analysis of CSF. If implemented, this technique will be useful especially in emergency settings as well as in areas with limited resources.
Keywords: Cerebrospinal fluid analysis, Accuracy, Urinary reagent strips, Semi-quantitative, Emergency setting
Urinary reagent strip test: An alternative method for rapid analysis of CSF in neurological disorders in emergency settings lacking trained manpower and expensive equipments.
1. Introduction
Cerebrospinal fluid (CSF) examination is often performed in emergency to obtain diagnostic information of various life threatening conditions such as meningitis, subarachnoid haemorrhage, demyelinating diseases or carcinomas [1,2].Initial evaluation of CSF includes protein, glucose and microscopic (total and differential cell count) analysis which requires well-equipped laboratory and trained staff. Sometimes, these facilities are not available in the small set-ups with limited resources. So, the CSF samples from these areas are referred to higher laboratory for analysis which results in delay in diagnosis and starting of the initial treatment. In such cases, urinary reagent strip method can be used to assess CSF. These urinary reagent strips provide semi-quantitative estimation of CSF chemistry and cellularity. These are also simple to use, rapid, low cost and do not require any expertise [2]. Few studies had been conducted previously on CSF for detection of meningitis by this method [[2], [3], [4], [5]].
The present study was conducted to determine the accuracy of urinary reagent strip for semi-quantitative analysis of protein, glucose, leucocytes and erythrocytes in CSF along with its role in emergency for rapid diagnosis of meningitis and other neurological conditions. No study had included CSF erythrocytes or analysed neurological conditions other than meningitis by urinary reagent strip till now.
2. Materials & method
This single blinded prospective study was conducted in the Emergency Laboratory of a neuropsychiatric tertiary care hospital for a period of 6 months from November 2015 to May 2016. All consecutive 360 CSF samples were included in the study. Samples with quantity less than 2 ml were excluded from the study. After performing gross examination of CSF for appearance and color (normal is colorless and clear), both definitive test and the index test were carried out as described below.
2.1. Definitive test
Total cell count for leucocytes and erythrocytes were done manually from undiluted CSF sample on modified neubauer chamber immediately after receiving the sample. For differential count of leucocytes, CSF was diluted with Turk's fluid (1:1) and then manually performed by trained pathologist. After that, CSF was centrifuged at speed of 3000 g for 10 min. Smears were made from the sediment and stained with a Romanowsky stain. If CSF contains many cells, then a smear was made directly from the sample instead of centrifugation and then stained. This stained smear was examined for differential leucocyte count. Out of 360 cases, 75 cases were hemorrhagic.For hemorrhagic samples; we had serially diluted CSF by normal saline for effective dilution of the erythrocytes, so that it becomes effortless to count CSF leucocyte and red blood cell (RBC). We had calculated corrected leucocyte count and protein levels in hemorrhagic samples by following formula [3].
| Corrected CSF leucocyte count = Leucocyte count (blood) x RBC count (CSF) RBC count (blood) |
Other reference standard tests like CSF protein and glucose were performed in the biochemistry analyser by pyrogallol red method & glucose oxidase-peroxidase (GOD-POD) method respectively. In hemorrhagic CSF samples, corrected CSF protein was calculated by formula given below.
| Corrected CSF Protein = CSF protein-(Serum protein x 1000 x (1-Hematocrit/100) xCSF RBC(Blood RBC x 106) |
2.2. Index test
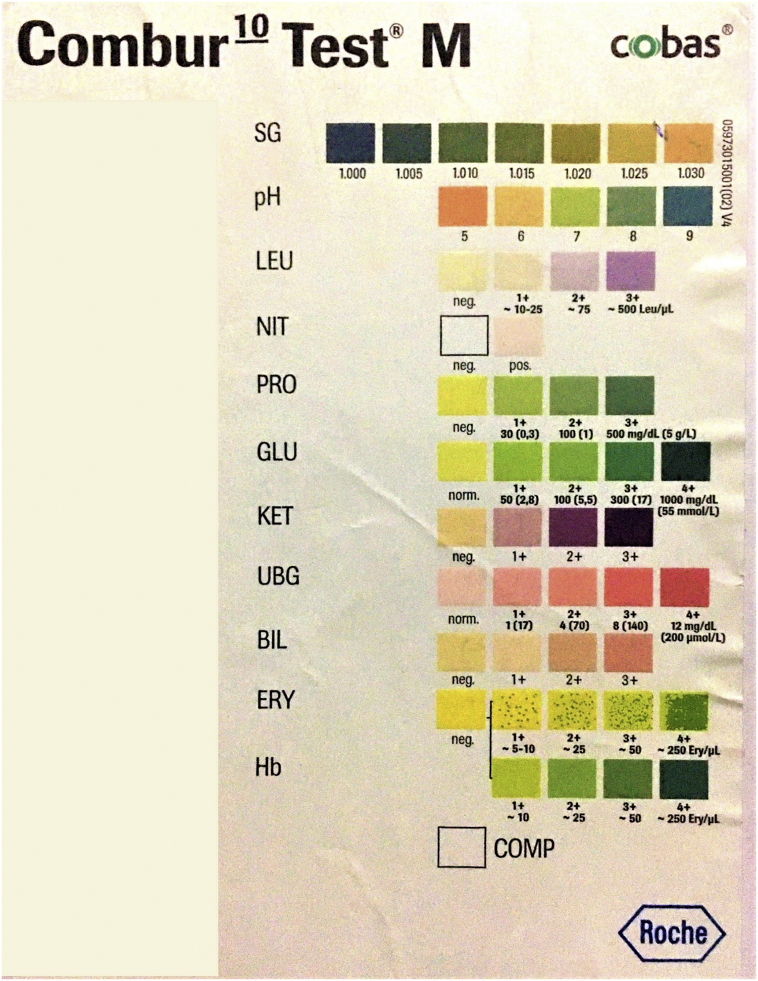
The combur-10 (Roche Diagnostics)urinary reagent strip was used as index test to detect CSF leucocytes by leucocyte esterase estimation, glucose by glucose oxidase-peroxidase method, protein levels by pyrogallol red method and erythrocytes by peroxidase method. The technicians, who performed the index test, were blinded to the results of definitive tests. Undiluted CSF was mixed with the micropipette and 2–3 drops of CSF was then added to patches of leucocytes, proteins, sugar and erythrocytes and reaction was noted after 60–120 s. Then, reaction colors of the test area were compared with the color chart on the label as shown in Fig. 1. Reference standard for all the parameters as shown on the label of strip are shown in Table 1.
Fig. 1.
Showing color chart scale as shown on the label of combur-10urinary reagent strip.
Table 1.
Reference standard for all the parameters as shown in strip label of combur-10urinary reagent strip.
| Parameter | Normal value in CSF | Principle of strip test | No color | 1+ | 2+ | 3+ | 4+ |
|---|---|---|---|---|---|---|---|
| CSF Leucocyte (cells/mm3) | <5 | Leucocyte esterase estimation | <10 | 10–75 | 75–499 | >500 | – |
| CSF Protein (mg/dl) | 15–45 | Protein error of pH indicator | <29 | 30–99 | 100–499 | >500 | – |
| CSF Glucose (mg/dl) | 40–70 | glucose oxidase-peroxidase method | <50 | 50–99 | 100–299 | 300–999 | 1000 |
| CSF Eythrocyte (cells/cumm) | Absent | Peroxidase method | <5 | 5–10 | 25 | 50 | 250 |
2.3. Statistical analysis
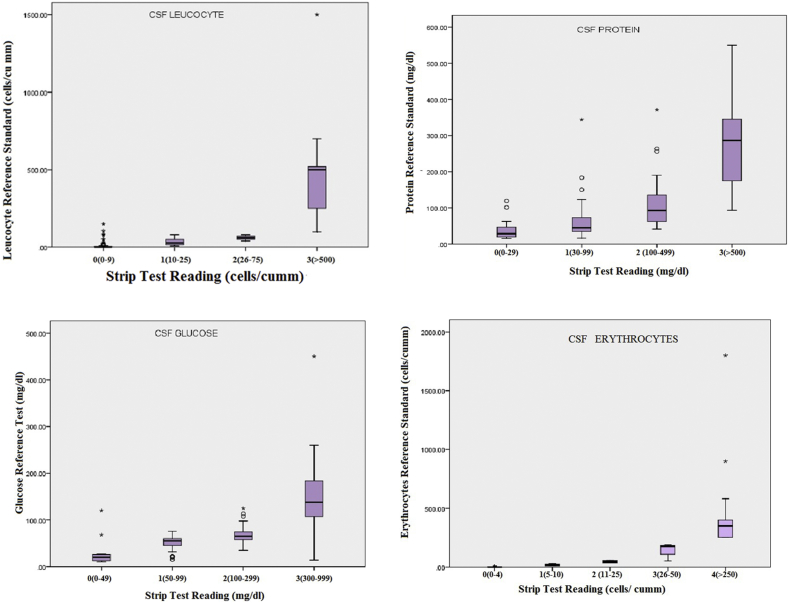
Statistical analysis was performed to derive the specificity, sensitivity, positive predictive value, negative predictive value, positive likelihood ratio (LR+)and negative likelihood ratio (LR−) and precision was estimated by calculating 95% confidence intervals (CI). Box- and- whisker graph is shown in Fig. 2.
Fig. 2.
Box & whisker plot showing the distribution of reference standard values for each strip color, (a) leucocytes, (b) proteins, (c) glucose and (d) erythrocyte. Horizontal line, box, whisker and dots indicate median (50th centile), 25th and 75th centile, ranges and outliers respectively.
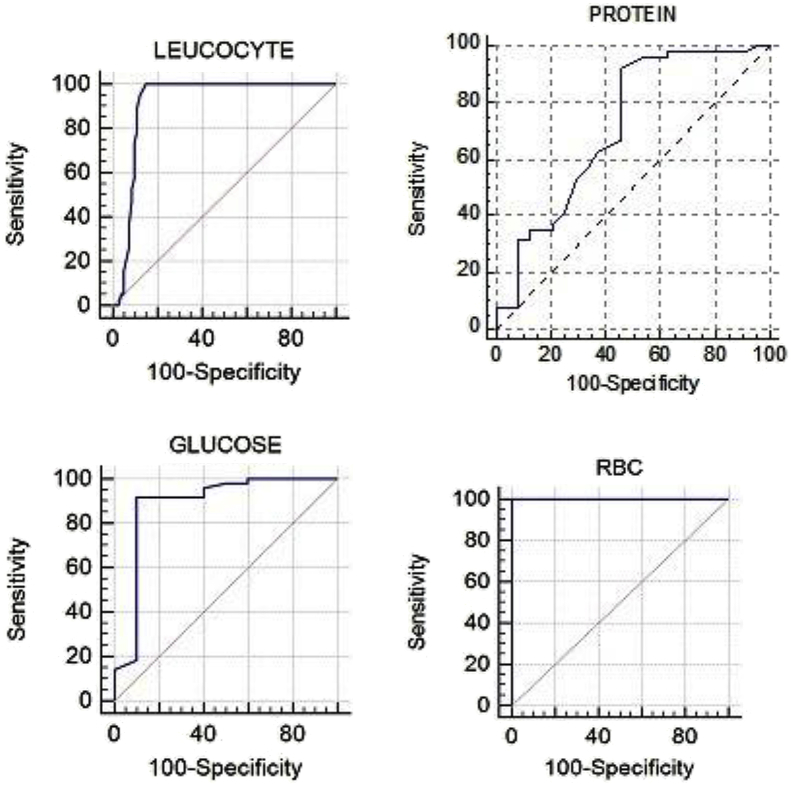
We also constructed receiver operating curves (ROC) to evaluate overall performance of index tests and estimated area under the curve (AUC) along with standard error (SE) as shown in Table 2 and Fig. 3. Data were analysed and graphed using SPSS 16 software, microsoft excel and trial version of software Medcalc.
Table 2.
Diagnostic accuracy of urinary reagent strip test when compared with reference standard values of parameters.
| CSF LEUCOCYTE (95% CI) | CSF PROTEIN (95% CI) | CSF GLUCOSE (95% CI) | CSF ERYTHROCYTE (95% CI) | |
|---|---|---|---|---|
| True Positive | 117 | 285 | 315 | 99 |
| False Positive | 09 | 33 | 03 | 00 |
| False Negative | 00 | 03 | 06 | 00 |
| True Negative | 234 | 39 | 36 | 261 |
| Sensitivity | 100% (90.9%–100%) | 98.9% (94.3%–99.9%) | 98.1% (93.4%–99.8%) | 100% (89.4%–100%) |
| Specificity | 96.3% (89.6%–99.2%) | 54.2% (32.8%–74.5%) | 92.3% (63.9%–99.8%) | 100% (95.9%–100%) |
| Positive Likelihood Ratio | 27 (8.9–81.9) | 2.2 (1.4–3.34) | 12.8 (1.9–83.8) | 00 |
| Negative Likelihood Ratio | 00 | 0.02 (0.00–0.14) | 0.02 (0.01–0.08) | 00 |
| Disease prevalence | 32.5% (24.2%–41.6%) | 80% (71.72%–86.75%) | 89.2% (82.2%–94.1%) | 27.5% (19.8 %–36.4%) |
| Positive Predictive Value (PPV) | 92.9% (80.5%–98.5%) | 89.6% (82.19%–94.70%) | 99.1% (94.9%–99.9%) | 100% (89.4%–100%) |
| Negative Predictive Value (NPV) | 100% (95.38%–100.00%) | 92.9% (66.13%–99.82%) | 85.7% (57.2%–98.2%) | 100% (95.9%–100%) |
| Area under the ROC curve (AUC) | 0.92 | 0.714 | 0.887 | 1.000 |
| Standard Error | 0.028 | 0.0705 | 0.0828 | 0.000 |
Fig. 3.
Receiver operator curves (ROC curve)for performance of reagent strips along with area under the curve (AUC), (a) leucocytes, (b) proteins, and (c) glucose and (d) erythrocytes.
3. Result
This study included 360 samples of patients with age ranged from 11 to 78 years, median being 25 years. Equal numbers of male and female cases were found i.e. male 174 cases and female 186 cases (ratio was 1:1).
Based on clinical, radiological and CSF findings, final diagnoses of neurological conditions were made as shown in Table 3.
Table 3.
Interpretation of CSF samples.
| Appearance | Glucose (mg/dl) | Protein (mg/dl) | Leucocyte (cells/mm3) | Type of Leucocyte | |
|---|---|---|---|---|---|
| Normal | Clear | 40–70 | 15–45 | 0–8 | CSF lymphonuclearcytosis |
| Bacterial meningitis | Turbid | <40 | 100–500 | ≥500 | CSF polymorphonuclearcytosis |
| Tuberculosis meningitis | Slightly opaque, cobweb formation | <40 | 50–300 | 100–600 | CSF lymphonuclearcytosis or Mixed |
| Aseptic meningitis | Clear | Normal | Normal to Mild increased | 5–300 | CSF lymphonuclearcytosis |
| Guillain–Barré syndrome | Clear | Normal | 100–500 | 0–8 | CSF lymphonuclearcytosis |
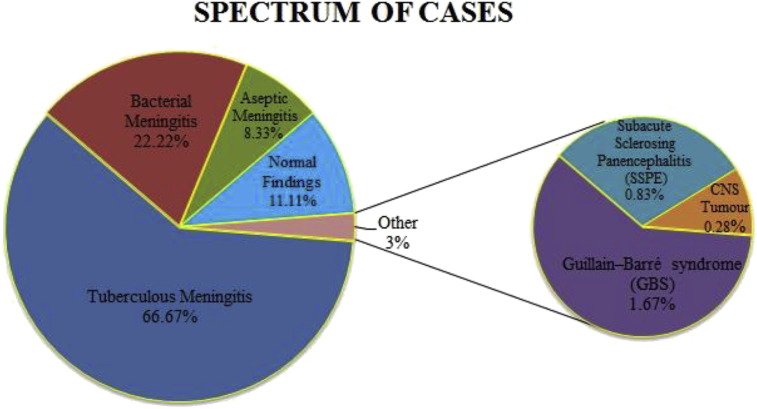
Most of the cases in our study were of meningitis [tuberculous: 240 cases (66%), bacterial: 80 cases (22%), aseptic: 30 cases(8%)]. There were 06 cases (1%) of Guillain–Barré syndrome (GBS), 03 cases (0.8%) of sub acute sclerosing panencephalitis (SSPE)and 01case(0.2%) of CNS tumor. 40 cases (11%) had normal findings as shown in Fig. 4.
Fig. 4.
Spectrum of neurological disorders studied.
A set point of paremeters on index test (combur-10 (Roche Diagnostics) urinary reagent strip) was a range of concentration representing a semi-quantitative value. Both definitive and strip tests were performed on each sample and the results were compared. Minimum value, maximum value, first quartile, median, third quartile, Inter quartile range and number of outliers were determined as shown in Table 4 and a box and whisker plot for combur-10 urinary reagent strip versus reference standards for leucocytes, proteins, glucose and erythrocyte were also plotted (Fig. 2).Reference standard values of all the parameters i.e. leucocyte, protein, glucose and erythrocytes were higher at their highest concentration i.e. >500 cells/cumm, >500 mg/dl, 300–999 mg/dl and >250 cells/cumm respectively.
Table 4.
Comparison between definitive test and strip tests.
| CSF Leucocyte | No: | Minimum | 1st quartile | Median | 3rd quartile | Maximum | Interquartile Range | No: of Outliers |
|---|---|---|---|---|---|---|---|---|
| 0 (0–9/cumm) | 270 | 0 | 0 | 0 | 5 | 150 | 05 | 17 |
| 1 (10–25/cumm) | 42 | 8 | 15 | 28 | 53 | 80 | 38 | 00 |
| 2 (25–75/cumm) | 21 | 40 | 50 | 60 | 75 | 80 | 25 | 00 |
| 3 (≥500/cumm) | 27 | 100 | 208 | 500 | 610 | 1500 | 403 | 01 |
| CSF Protein | ||||||||
| 0 (0–29 mg/dl) | 72 | 15 | 19 | 28 | 47 | 119 | 29 | 02 |
| 1 (30–99 mg/dL) | 153 | 16 | 34 | 45 | 73 | 344 | 39 | 03 |
| 2 (100–499 mg/dL) | 96 | 41 | 60 | 93 | 138 | 371 | 78 | 03 |
| 3 (>500 mg/dL) | 39 | 93 | 160 | 286 | 347 | 550 | 187 | 00 |
| CSF Glucose | ||||||||
| 0 (0–49 mg/dL) | 33 | 10 | 12 | 20 | 27 | 120 | 15 | 02 |
| 1 (50–99 mg/dL) | 147 | 15 | 45 | 55 | 61 | 76 | 16 | 02 |
| 2 (100–299 mg/dL) | 153 | 35 | 58 | 65 | 75 | 125 | 17 | 03 |
| 3 (300–999 mg/dL) | 27 | 14 | 88.5 | 138 | 222 | 450 | 133 | 01 |
| 4 (>1000 mg/dL) | 00 | 00 | 00 | 00 | 00 | 00 | 00 | 00 |
| CSF Erythrocyte | ||||||||
| 0 (0–4/cumm) | 264 | 00 | 00 | 00 | 00 | 05 | 00 | 18 |
| 1 (5–10/cumm) | 30 | 10 | 10 | 18.5 | 25 | 30 | 15 | 00 |
| 2 (11–25/cumm) | 06 | 34 | 34 | 44.5 | 55 | 55 | 21 | 00 |
| 3 (26–50/cumm) | 21 | 50 | 100 | 175 | 184 | 188 | 84 | 00 |
| 4 (>250/cumm) | 39 | 250 | 250 | 350 | 490 | 1800 | 240 | 00 |
The strip test showed a high sensitivity of 99% (95% CI: 94–99%) but a low specificity of 54% (95% CI: 32–74%) for proteins. For glucose, the strip was highly sensitive (98%, 95% CI: 93–99%) as well as highly specific (92%, 95% CI: 63–99%).It showed a high sensitivity and specificity for leukocytes ≥10 cells/cumm i.e. 100% (95% CI: 90–100%) and 96% (95% CI: 89–99%) respectively. For CSF erythrocytes, both sensitivity (95% CI: 89–100%) and specificity (95% CI: 95–100%) were 100% as shown in Table 2.
According to the ROC curve, CSF erythrocytes had highest AUC of 1.000 followed by AUC of CSF leucocytes i.e. 0.916, than AUC of CSF glucose i.e. 0.887 and lastly of AUC CSF protein i.e. 0.714 as shown in Table 2 and Fig. 3.
4. Discussion
CSF evaluation has been indicated in various diseases like meningitis (Bacterial, viral etc), subarachnoid haemorrhage, demyelinating/degenerative disorders, primary and metastatic tumors of central nervous system (CNS), pressure recordings –pseudotumor cerebri, normal pressure hydrocephalus, head injury, suspected cerebral abscess, access for neuro-radiologic procedures and also for intrathecal administration of drugs [[4], [5], [6]].
Out of all these causes, meningitis is the most common indication for CSF examination. Meningitis is classified into 3 subtypes - bacterial, aseptic and tuberculous meningitis [4].Guillain–Barré syndrome (GBS) is uncommon indication for CSF examination [4].
First step in evaluation of CSF includes total and differential leucocyte count (DLC) with protein and glucose estimation. Depending upon gross appearance, increased or decreased values of protein, glucose and leucocytes count with DLC in CSF, initial diagnosis of various neurological diseases can be made [3]. Routinely, trained technical staff as well as doctor along with well-equipped laboratory are required for determination of above parameters. Urinary reagent strip method could be used as an alternative in hospitals/centre with limited resources and lacking trained manpower [6].
In the present study, Combur-10 urinary reagent strip was used for rapid analysis of the CSF in emergency laboratory for leucocytes, proteins, glucose and erythrocytes in lesser turnaround time. Maximum cases were of meningitis [tuberculous: 66%, bacterial: 22%, aseptic: 8%] followed by GBS (1%) and SSPE (0.8%). Only one case (0.2%) of primary CNS tumor was observed as shown in Fig. 4.
Different CSF findings were encountered in different neurological disorders by reagent strip test in our study. We found raised protein with increased leucocyte count in most of the cases of meningitis. All cases of GBS had high protein with normal leucocyte count which was correlated with clinical symptoms. Acellular CSF with slightly raised CSF protein was observed in sub-acute sclerosing panencephalitis which is a complication of measles. We had also encountered one case of CNS tumor (ependydoma).In this case CSF was grossly mucoid with raised protein and presence of atypical cells on microscopy.
Our study showed high sensitivity and specificity for leucocytes ≥10 cells/cumm i.e. 100% and 96% respectively whereas for CSF erythrocytes, both sensitivity and specificity were 100% by strip method. For CSF proteins; the strip test was more sensitive (99%) and less specific (54%). With respect to glucose, the strip was highly specific (92%) and highly sensitive (98%). Area under curve for leucocytes ≥10 cells/cumm, protein, glucose and erythrocyte were 91%, 71%, 88% and 100% respectively. Lower cut-off for proteins in the urine is 30 mg/dl and for the CSF is 15 mg/dl. So, it can give high false positive results by showing 1 + in reagent strip even when the value of CSF protein is in normal range (15–45 mg/dl).This could be the reason for low specificity for protein in our study.
Observations of our study were similar to some previous studies conducted especially in meningitis as shown in Table 5 [2,4,7,8,10].Moosa et al. and Salvador et al. also used previous version of Combur-10 strips i.e.Combur-9 strips, concluding that this method is also useful in making a rapid bedside diagnosis of CSF analysis [11,12].
Table 5.
Comparison between our study and various other studies.
| Our study | Chikkannaiah et al (2016) | Joshi et al. (2013) | Parmar et al. (2004) | Romanelli et al. (2001) | Maclennan et al. (2004) | |
|---|---|---|---|---|---|---|
| Reference No: | – | 2 | 6 | 3 | 7 | 9 |
| Cases | 360 | 103 | 75 | 63 | 154 | 200 |
| Leucocytes ≥10 cells/cumm | Sensitivity: 100.0% Specificity: 96% AUC: 92% |
Sensitivity: 97% Specificity: 94% AUC: 99% |
Sensitivity: 85% Specificity: 89% AUC: 87%, |
Sensitivity: 97%, Specificity: 96%, PPV: 97% NPV: 96% |
Sensitivity: 91% Specificity: 98% PPV: 95%, NPV: 96% |
NOT DONE |
| CSF Protein | Sensitivity: 99% Specificity: 54% AUC: 89% |
For ≥ 30 mg/dl, Sensitivity: 95% Specificity: 46% AUC: 85% For ≥ 100 mg/dl, Sensitivity: 96% Specificity: 87%. AUC:96% |
For ≥ 30 mg/dl, Sensitivity: 98% Specificity: 57% AUC: 77%, For ≥ 100 mg/dl, Sensitivity: 93% Specificity: 88% AUC: 90%, |
NOT DONE | ||
| CSF Glucose | Sensitivity: 98% Specificity: 92% AUC: 100% |
For ≤ 40 mg/dl Sensitivity: 29% Specificity: 100% AUC: 87% For ≤ 50 mg/dl Sensitivity: 14%. Specificity: 100% AUC: 77% |
For ≤ 40 mg/dl Sensitivity: 61% Specificity: 97% AUC: 78%, For ≤ 50 mg/dl Sensitivity: 46% Specificity: 98% AUC: 72% |
NOT DONE | ||
| CSF Erythrocyte | Sensitivity: 100% Specificity: 100% AUC: 72% |
NOT DONE | NOT DONE | NOT DONE | NOT DONE | NOT DONE |
| Spectrum of cases |
|
Meningitis Bacterial: 43 Viral: 19 No alterations: 83 |
In bacterial meningitis, nitrate patch will become positive because of increased granulocytes. |
One additional parameter i.e. CSF erythrocytes was undertaken in our study which was not included in any of the previous studies. Erythrocytes in CSF are seen in subarachnoid haemorrhage or in traumatic tap. Excessive erythrocytes in CSF artifactually increase leucocyte count and protein levels which could lead to erroneous results. In our study corrected CSF leucocyte count and proteins were calculated in hemorrhagic samples [3].
Diagnostic accuracy of strip test was high in respect to glucose, leucocytes, erythrocytes and protein in CSF when compared with other methods performed on high-end equipments. Other advantages of using urinary reagent strip in analysis of CSF were that it was very simple, rapid and semi quantitive test; it did not require any expertise or any costly instrument and results were dispatched in lesser turnaround time which is need of the hour in emergency laboratory.Disadvantages of the present method were that it was not a fully quantitative method and showed low specificity for proteins. Upper limit for erythrocyte in urinary reagent strip was 250 cells/cumm, so in cases of traumatic tap, it could give erroneous results. To avoid this error CSF was diluted and then analysed.
Limitations of our study were smaller sample size and only few cases of neurological disorders, other than meningitis were noted, so studies with larger sample size are required for elaborative analysis in future. Secondly, despite matching reagent strip very carefully, color grading was subjective, so there could be an inter observer variation in reading the results. However in present study, a designated pathologist and technician interpreted the results which limited interobserver variation. Lastly, reagent strip is required to be stored properly at 2–30 °C in tightly capped container as they are prone to oxidization and they should not be used after exposure to air or after its expiry date.
Henceforth, in our study Combur-10 urinary reagent strips were used for analysis of CSF protein, glucose, leucocytes and erythrocytes in emergency laboratory. The sensitivity and specificity of this method was found to be reasonably high for all parameters when compared with standardized methods performed on advanced equipments. Thereby it is concluded that Combur-10 urinary reagent strips are very efficient in rapid evaluation of CSF in various neurological disorders, especially in emergency settings where turn-around time is crucial. So, that patients can receive treatment at the earliest, this in turn reduces both morbidity as well as mortality. Since this method does not require any expertise, it could be helpful if implemented properly in small laboratories or in rural areas where high end equipments and trained technical staff are lacking.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2019.e00124.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Adhikary M., Chatterjee R.N. Laboratory evaluation of cases of meningitis attending a tertiary care hospital in India: an observational study. Int J Nutr Pharmacol Neurol Dis. 2013;3:282–288. [Google Scholar]
- 2.Chikkannaiah P., Benachinmardi K.K., Srinivasamurthy V. Semi-quantitative analysis of cerebrospinal fluid chemistry and cellularity using urinary reagent strip: an aid to rapid diagnosis of meningitis. Neurol. India. 2016;64:50–55. doi: 10.4103/0028-3886.173641. [DOI] [PubMed] [Google Scholar]
- 3.Nigrovic L.E., Shah S.S., Neuman M.I. Correction of cerebrospinal fluid protein for the presence of red blood cells in children with a traumatic lumbar puncture. J. Pediatr. 2011;159:158–159. doi: 10.1016/j.jpeds.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Parmar R.C., Warke S., Sira P., Kamat J.R. Rapid diagnosis of meningitis using reagent strips. Indian J. Med. Sci. 2004;58:62–66. [PubMed] [Google Scholar]
- 5.Murthy J.M.K. Tuberculous meningitis: the challenges. Neurol. India. 2010;58:716–722. doi: 10.4103/0028-3886.72178. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R. Aseptic meningitis: diagnosis and management. Indian J. Pediatr. 2005;72:57–63. doi: 10.1007/BF02760582. [DOI] [PubMed] [Google Scholar]
- 7.Joshi D., Kundana K., Puranik A., Joshi R. Diagnostic accuracy of urinary reagent strip to determine cerebrospinal fluid chemistry and cellularity. J. Neurosci. Rural Pract. 2013;4:140–145. doi: 10.4103/0976-3147.112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanelli R.M., Thome E.E., Duarte F.M., Gomes R.S., Camargos P.A., Freire H.B. Diagnosis of meningitis with reagent strips. J. Pediatr. 2001;77:203–208. doi: 10.2223/jped.207. [DOI] [PubMed] [Google Scholar]
- 10.Maclennan C., Banda E., Molyneux E.M., Green D.A. Rapid diagnosis of bacterial meningitis using nitrite patch testing. Trop. Doct. 2004;34:231–232. doi: 10.1177/004947550403400417. [DOI] [PubMed] [Google Scholar]
- 11.Moosa A.A., Quortum H.A., Ibrahim M.D. Rapid diagnosis of bacterial meningitis with reagent strips. Lancet. 1995;345:1290–1291. doi: 10.1016/s0140-6736(95)90931-1. [DOI] [PubMed] [Google Scholar]
- 12.Oses Salvador J.M., Zarallo Cortes L., Cardesa Garcia J.J. Usefulness of reactive strips in the diagnosis of suppurative meningitis, at the patient's bedside. An EspPediatr. 1988;29:105–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.