Abstract
Objective
To investigate the impact of health information technology (IT) systems on clinicians’ work practices and patient engagement in the management and follow-up of test results.
Materials and Methods
A search for studies reporting health IT systems and clinician test results management was conducted in the following databases: MEDLINE, EMBASE, CINAHL, Web of Science, ScienceDirect, ProQuest, and Scopus from January 1999 to June 2018. Test results follow-up was defined as provider follow-up of results for tests that were sent to the laboratory and radiology services for processing or analysis.
Results
There are some findings from controlled studies showing that health IT can improve the proportion of tests followed-up (15 percentage point change) and increase physician awareness of test results that require action (24–28 percentage point change). Taken as whole, however, the evidence of the impact of health IT on test result management and follow-up is not strong.
Discussion
The development of safe and effective test results management IT systems should pivot on several axes. These axes include 1) patient-centerd engagement (involving shared, timely, and meaningful information); 2) diagnostic processes (that involve the integration of multiple people and different clinical settings across the health care spectrum); and 3) organizational communications (the myriad of multi- transactional processes requiring feedback, iteration, and confirmation) that contribute to the patient care process.
Conclusion
Existing evidence indicates that health IT in and of itself does not (and most likely cannot) provide a complete solution to issues related to test results management and follow-up.
Keywords: missed test results, diagnostic tests, workflow, patient participation, medical informatics
INTRODUCTION
The results of laboratory tests and medical imaging reports directly impact clinical decision-making contributing to the diagnosis, treatment, prevention, and management of patient care.1 The World Alliance for Patient Safety has identified poor test follow-up as an international priority area for concern in patient care,2 and in 2017 the US Emergency Care Research Institute flagged inadequate test results follow-up as a key patient safety issue.3 Many clinicians, aware of the extent of poor test results management, have expressed concerns about systemic shortcomings in organizational follow-up procedures within and across health care settings.4
Potential strategies to improve test results follow-up include the use of health information technology (IT) for the communication of test results using automated result notifications.2,5,6 The introduction of IT has been supplemented by initiatives to establish guidelines and recommendations for successful implementation, quality improvement, and evaluation.7–12 Attention has also been focused on the patient’s role as a partner in the process of enhancing the safety of care.13,14 This is particularly relevant to situations where the failure to inform patients of their results has been described as legally indefensible in malpractice claims.15 Electronic health records (EHRs) are seen as the basis for greater patient involvement, particularly as they provide the means by which patients can access their own information using a secure electronic patient portal, which, in addition to allowing access to personal information, also facilitates communication with health professionals.16
There is ample evidence that while IT is capable of helping to prevent medical errors, it also has the capacity to introduce its own class of errors.17 This is particularly relevant to test results management, where the way that information is collected, reported, and presented can have major safety consequences.18,19 Despite a growing evidence base of the diffusion of health IT applications,6 their impact on test results follow-up, management, and patient engagement has not been widely appraised and is not well understood.20,21 This systematic review integrates quantitative and qualitative research findings on how health IT has been used to engage with patients. The systematic review thus provides an overview of the current state of evidence about how health IT has been used to address the test results management and follow-up process and contributes to a better understanding of the gaps and challenges as identified by existing research. The aims of this systematic review were to:
Describe the types of health IT systems that are utilized in the management and follow-up of test results,
Investigate the impact of health IT systems on the rate of missed test results and other outcomes,
Identify the impact of health IT systems on clinicians’ test results management work practices, and
Assess the impact of health IT on patient engagement and the follow-up of test results.
MATERIALS AND METHODS
Study identification
We conducted a search for health IT systems and clinician test results management in the following databases: MEDLINE, EMBASE, CINAHL, Web of Science, ScienceDirect, ProQuest, and Scopus for studies published between January 1999 and June 2018 in accordance with PRISMA guidelines.22 Our protocol was registered on the PROSPERO register of systematic reviews (CRD42016043148). Search strategies for all databases are presented in Appendix 1. We reviewed reference lists of all literature identified as potentially relevant. Table 1 provides a complete list of peer-reviewed and gray literature sources that were hand-searched.
Table 1.
List of hand-searched sources for peer-reviewed and gray literature
| Gray literature/Hand-searching |
|---|
| Google Scholar |
| Royal Australasian College of Physicians |
| Royal Australian College of General Practitioners |
| European Federation for Medical Informatics |
| International Medical Informatics Association |
| American Medical Informatics Association |
| World Health Organization |
| Article reference lists |
Study selection
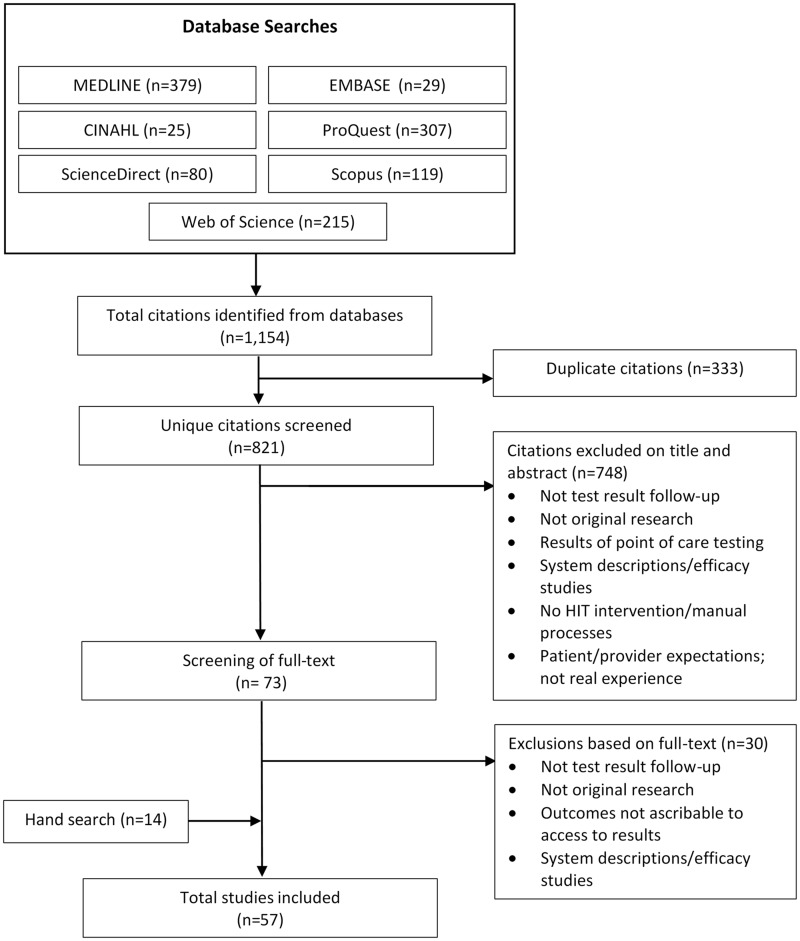
Two authors (JL, JT) independently reviewed titles and abstracts identified from the search. Papers without abstracts were retrieved and reviewed in full. The identification and selection process of studies is detailed in Figure 1. We resolved discrepancies through discussion or referral to a third researcher (AG). The same authors (JL, JT) retrieved and independently reviewed full text articles of all selected papers for inclusion in accordance with our eligibility criteria.
Figure 1.
Study identification and selection process.
Original studies of all types were included if they reported the impact of any health IT system on the test results follow-up process in hospital and ambulatory care settings. Failure to follow up on a test is defined as failing to take the appropriate next steps after the test.23 For the purposes of this review, missed test results and/or failure to follow up test results occurs when there is no evidence that the responsible provider becomes aware of a result (laboratory or radiology). We excluded point of care testing. Studies were also excluded if they did not report original research, if the reported outcomes were not directly ascribable to the follow-up of test results (eg, when patients access their entire electronic medical records [EMRs]), or studies which evaluated the accuracy/specificity of a health IT system (eg, algorithm). Studies which explored provider opinions regarding potential patient access to test results, patient preferences, or expectations for potential future electronic access to test results and results of user testing of patient result access applications were excluded. The study selection process is presented in Figure 1.
Data extraction and synthesis
Information regarding health IT, its impact on the rate of missed test results, its effects on clinician test results follow-up work processes, and the patient’s response to electronic access to their own results was extracted from included papers. Due to significant heterogeneity between studies, a meta-analysis of results was not performed. Descriptive statistics were used to summarize the body of evidence including the number of included studies, country of origin, year of publication, and study design. The details of individual studies including primary author, title, year, country, type of health IT, methodology, impact on missed test results follow-up, results type, study site, department/study population, and study size were extracted. Findings pertaining to each of the 4 objectives of the systematic review were extracted.
Two authors (JL, JT) assessed the quality of each included study using a tool applicable to the study design (Table 2). We used the Critical Appraisal Skills Programme (CASP) Qualitative Checklist24 to appraise qualitative studies. We assessed mixed-methods studies based on the methodological design of the study, or, for studies which included both qualitative and quantitative methods, according to the predominant method. For randomized controlled tests (RCTs) and quasi-experimental study designs, we applied the relevant “Study Quality Assessment Tools” developed by the US Department of Health and Human Services National Institutes of Health (NIH): National Heart, Lung, and Blood Institute.25 The selection of these tools allowed us to assess all RCTs, controlled trials, and quasi-experimental studies using a similar method with the same quality rating approach. For each study we recorded a quality assessment outcome of either poor, fair, or good.
Table 2.
Quality assessment tools
| National Heart, Lung and Blood Institute (Quantitative studies) 25 | Critical Appraisal Skills Programme (CASP) (Qualitative Studies) 24 |
|---|---|
| Quality Assessment of Controlled Intervention Studies | CASP Qualitative Checklist |
| Quality Assessment of Observational Cohort and Cross-Sectional Studies | |
| Quality Assessment of Before-After (Pre-Post) Studies with No Control Group |
RESULTS
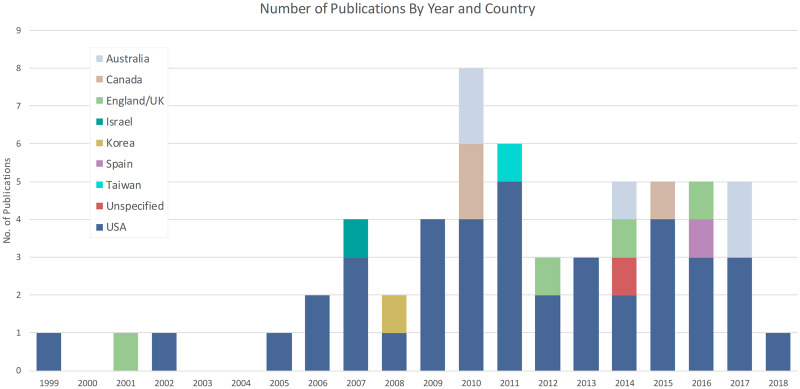
A total of 57 studies were included in the systematic review, 53 (93%) of which were published between 2006 and 2018. The earliest study was published in 1999 with an apparent rise in studies from 2006 onward (see Figure 2). Most studies (72%, n = 41) were conducted in the US. Figure 2 presents a detailed breakdown of studies by year and country. The studies incorporated a range of research methods which included 7 RCTs (12% of studies), 32 observational studies (56%), 12 mixed-methods studies (21%), and 6 qualitative studies (11%). The quality of the evidence presented in this review overall was rated as mostly fair (n = 35) and good (n = 20) with 2 studies rated as poor quality. A summary of included studies and their quality assessment is presented in Table S1.
Figure 2.
Breakdown of included articles by year and country.
Types of health IT systems utilized in the management and follow-up of test results
The literature reported a variety of health IT systems for the clinical management and follow-up of test results:
Electronic alerts (interruptive and non-interruptive) delivered to clinicians about results with abnormal/critical values,26–45
Computerized provider order entry (CPOE) systems with electronic results viewing46,47 and clinical information systems where results are viewed electronically, but orders are placed manually,48–50
Electronic medical record (EMR)/electronic health record (EHR) systems,51–58
Electronic results acknowledgment systems which require physicians to electronically document that they have seen a test result,59–62
Electronic results tracking systems which allow users to track the progress of tests and status of results (eg, viewed, pending at discharge),63,64
EHR-based trigger algorithms which identify patients at risk of diagnostic delays,65 and
Electronic report generation systems for abnormal results.66,67
The categories were based on how each study reported their intervention. The health IT interventions varied according to whether they reported on the impact of an EMR/EHR system (eg, category 3), or as a dedicated feature of an EMR/EHR system (eg, category 1, 2, and 4). In part these differences reflect the development and increasing specialization of health IT systems over time.
Studies of health IT-facilitated patient engagement and follow-up of results featured two types of electronic systems: 1) patient portals and 2) personal health records (PHRs). Patient portals68–79 offer access to personal health information via a secure website,80 while integrated (tethered) PHRs81–83 are institutionally-managed and connected to a health care organization’s EHR system thus offering patients direct access to their medical records.84,85 No studies evaluated patient-managed PHRs (ie, freestanding or untethered and not connected to a health care organization). The majority (n = 13) of systems studied offered patients real-time access to test results as they became available. In the remaining 2 studies, patients were able to view results after a delay to allow prior review of results by clinicians.75,83
Impact of health IT systems on the rate of missed test results
A total of 18 studies reported the impact of electronic results management on the rate of missed test results. The majority of the studies in this section were rated as good30,33,35,48,51,57,59 and fair29,32,38–40,45,47,50,52,66 quality. Of all the studies, 1 was rated as poor quality34 (Table S1).
Alerts
A cluster-RCT of an automated email notification system undertaken by Dalal et al. reported on survey results (152 from hospital physicians and 112 from primary care physicians [PCPs] in the community) which revealed that, compared to the control group, a significantly larger proportion (24–28 percentage point difference) of physicians who used the notification system were aware of actionable test results.29 A prospective cluster-RCT by El-Kareh used an automated email-based alert system which notified physicians of positive culture results not adequately treated at discharge.40 This study reported a 15% increase in the documented follow-up of positive postdischarge culture test results.
A cross-sectional study by Wahls et al. involving 106 PCPs found that, despite use of an EMR with a result-alerting function, 37% of primary care physicians reported seeing at least 1 patient with a missed test result.39 Another cross-sectional survey (of 143 PCP respondents) reported that 30% encountered at least 1 patient with a diagnosis/treatment delay due to a missed test result. The authors noted that the procedures for management of results were not uniform, with only 55% of respondents reporting use of the electronic notification system for results management.38
Observational studies by Singh et al.32,34 and Bhise et al.57 reported a range of between 0.2% and 16.7% failure to follow up test results with the use of an integrated, comprehensive EHR with a test results notification system. A cluster-RCT of an email notification system by Dalal et al. reported no significant difference in the rate of documented evidence of follow-up action for test results initially pending at hospital discharge.45
The relationship between acknowledgment of electronic alerts and subsequent follow-up action was investigated in 2 studies by Singh et al.33,35 Failure to act on abnormal results ranged from 6.4%35 to 7.3%33 overall and did not differ significantly between acknowledged and unacknowledged alerts. A before and after study of a mandatory EHR notification system was undertaken by Laxmisan et al. across 2 sites. Logistic regression uncovered a significant intervention effect (preintervention OR 0.7; 95% CI, 0.5–1.0) after accounting for site-specific differences in follow-up, with a lower likelihood of timely follow-up at 1 site (OR 0.4; 95% CI, 0.2–0.7).30
Computerized provider order entry (CPOE) and clinical information systems
Studies of CPOE and clinical information systems investigated the impact of electronic transmission of results. These studies reported varying levels of follow-up or physician awareness of results. Provider failure to review or follow up results in the emergency department setting ranged from 1.5% of radiology and microbiology results47 to 45% of all emergency biochemistry tests.48 Patient transitions between care settings was also identified as a potential risk factor. It was reported in 1 study that inpatient and primary care physicians were unaware of 61.6% of results pending at hospital discharge.52 Clinicians in this study deemed 37.1% of missed results as actionable with 12.6% requiring urgent action.
Result acknowledgment systems
An Australian study investigated the impact of an electronic results acknowledgment system which incorporated escalation procedures (based on delineated levels of test follow-up responsibility) for dealing with unacknowledged results in an Australian hospital. The system led to the clinical acknowledgment of all results.59
Impact of health IT interventions on clinicians’ test results management work practices
The key findings from studies of the impact of health IT on test results management work practices spanned the following themes: 1) changes in workload, 2) hybrid paper/electronic systems, 3) effect of the organizational context, 4) time to test results follow-up, and 5) implications for patient outcomes.
The quality of studies in this category was rated as either good (n = 11)26,36,37,43,44,53,55,56,60,65,67 or fair quality (n = 13). 27,28,31,41,42,46,49,54,58,61–64
Changes in workload
Respondents in a qualitative study conducted in the US noted the additional time burden for clinicians associated with acknowledging clinically irrelevant alerts from EHR-based test results systems.37 These findings were echoed by a web-based survey of 2590 PCPs, 85.6% of whom reported that they were required to work after hours or over the weekend to address test results notifications.54
Hybrid paper/electronic systems
The use of multiple systems within a mixed-media (paper and electronic) environment for managing test results was reported to have negative impacts on test results follow-up work practices by 2 studies.46,58 Menon et al. found that 43% of 2554 PCPs surveyed used paper or a combination of paper and computer-based workarounds to support results management.56 A mixed-method study by Elder across 4 sites concluded that IT alone was insufficient to achieve the highest levels of safety when no site performed better against test results management measures despite varying levels of health IT adoption (partial CPOE to full EHR).55
Effect of the organizational context
Li et al. undertook a qualitative study that investigated clinician perspectives of the utilization of an electronic results acknowledgment system on radiology and microbiology results follow-up. Their results showed that contextual factors, such as how the health IT system aligns with existing work practices, and the departmental staff mix can affect the success or otherwise of the new system.62 Similarly, Menon et al. undertook a mixed-method study of a view alert system for abnormal results and concluded that context-related vulnerabilities (eg, existing test results follow-up policies and escalation procedures) could lead to missed test results in EHR-based settings and advised that interventions should recognize the influence of organizational factors on outcomes of health IT.53
Time to test results follow-up
The effects of a real-time, automated paging system for critical laboratory values on internalists’ response times was investigated in 1 RCT. The study found no significant difference in median response time to alerts between the control and intervention groups (39.5 mins vs 16 mins, respectively, p = 0.33).27 Park et al. reported on a study which examined the effects of sending SMS messages to doctors in wards, in addition to the hospital laboratory ringing doctors with critical results.42 They found a significant decrease in time to the ordering of treatment in the general wards (249 mins pre to 63 mins post SMS (p < .001) but this combined intervention had no significant effect in changing test results follow-up times in the ICU. Lin et al.’s before and after study in an outpatient department found that episodes of hyperkalemia were more likely to be followed up within 4 days following the introduction of a system which flagged abnormal results and tracked the status of reports (90.0% post vs 62.2% pre; p = 0.003).64
Implications for patient outcomes
Studies measuring the impact on patient outcomes reported mostly positive results following the introduction of health IT systems. Benefits included reductions in time to diagnostic evaluation or completion of follow-up action from availability of results,31,41,44,65 time to receipt of follow-up care for patients requiring referral to other practitioners,28,44 time to diagnostic resolution,41,67 and likelihood of diagnostic resolution.67
Impact of health IT systems on patient engagement in the follow-up of test results
Studies that investigated patient engagement tools used 1 or more of the following methods: qualitative interviews,68,74,75,82,83 surveys/questionnaires,69–71,73,75–77,81–83 or observational data.72,74,77,78 Mixed-methods were employed in 6 studies.74,75,77,79,82,83 While 2 studies were rated as good quality,70,72 12 were of fair quality,68,69,71,73–79,81,83 and 1 was of poor quality.82
Patient utilization of patient portals
Ling et al.’s patient survey involving 429 patients with access to results from a sexually transmitted infection clinic showed that 75% of respondents who accessed results online did so primarily because they could check results at any time of the day.70 Woywodt et al. reported that from a sample of 295 renal patient portal users (predominantly made up of transplant patients), 42% accessed their results after their clinic appointments and 78% accessed the portal on an average of 1–5 times per month.76 Most respondents (93%) felt that the portal assisted them in the management of their condition.
Key considerations related to patient access to results
A survey by Christensen examined patient experiences with the use of a tethered PHR. They found that patients associated electronic access to laboratory results with positive feelings including satisfaction and relief and typically engaged in discussions with family and friends about their results following access.81 Wiljer et al. investigated the clinical, technical, and educational support needs of breast cancer patients with portal access to laboratory and radiology reports.74 The authors reported that 98% (122/150) of user support requirements were technical in nature (eg, difficulties accessing results). Cimino et al.’s mixed-method study reported that all 4 interviewed patients who reviewed and tracked their laboratory test results believed that it improved communication during physician visits and promoted greater ownership of their care.82
Clinician experiences following patient access to results were also positive. A survey exploring the experiences of 508 patients and 48 physicians following direct release of radiology reports to patients83 found that an equal proportion of patient and physician groups (88%) viewed the ability of patients to access radiology reports as important and useful (although almost half of these patients [49%] received communication from physicians about the result prior to report release). Only 8% of physicians ceased releasing reports online due to confusion and anxiety among patients.83
Abnormal or critical test results
Giardina et al.’s qualitative study on the impact of patient access to abnormal test results reported that most patients unequivocally favored access to results electronically.68 Yet, some respondents mentioned that results of high emotional impact or “sensitivity,” such as those involving life-threatening illnesses, cancer diagnoses, genetic testing, and incurable conditions warranted verbal communication prior to electronic release. Winget et al. surveyed the experiences of 82 oncologists following direct release of results which were potentially indicative of disease progression in cancer patients.75 Half (49%) of the oncologists reported that sharing online results had negative impacts on their communication with patients. Oncologists generally believed that sensitive information requiring counseling should be delivered in a face-to-face consultation.75
DISCUSSION
This systematic review identifies evidence across 2 decades incorporating: 1) multiple research methods (eg, qualitative and quantitative), 2) a range of health IT systems and software applications, 3) investigations into clinical work practices, and 4) assessments of the impact on patient engagement. By doing so the review contributes to a more structured picture of how the broader socio-technical system (technology, clinicians, patients, processes, and organization) impacts on the issue of test results follow-up.17 The research findings from RCTs provides some indication that health IT systems can increase the proportion of documented follow-up (15 percentage point change)40 and improve physician awareness of test results that required action (24–28 percentage point improvement).29 However, taken as a whole, the body of evidence on the impact of health IT on the management and follow-up of test results is not strong. The implication of these findings is that health IT, in and of itself, does not (and most likely cannot) provide a single or complete solution to issues surrounding the inadequate follow-up of test results.
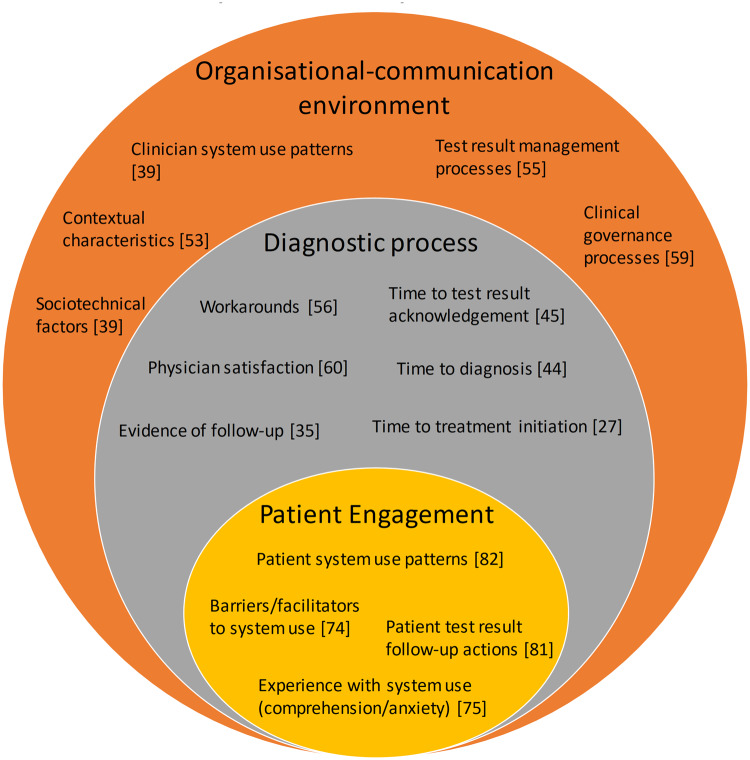
The issue of test results management and follow-up is multi-layered and interwoven. This interconnectedness is conceptualized in Figure 3, which is constructed from the key outcome measures identified within the existing research, as a basis for examining the significance and implications of the research. These layers can be described as: 1) the organizational-communication environment (eg, existing practices about how test results are communicated),55,60 2) the diagnostic process (eg, the numerous tasks among different people and across different clinical settings that need to be coordinated and synchronised for safe and effective test results management),20,86 and 3) patient engagement in the test results follow-up process (eg, how and when do patients access test results, if at all?).68,74,75
Figure 3.
Key conceptual domains identified from existing evidence with examples of study outcome measures.
The organizational-communication environment
The communication of test results is reflective of existing patterns of accountability, responsibility, and authority that are shaped by clinical governance processes and the contextual characteristics present within different health care settings.53,59,62 These communication patterns are not direct one-way processes but multi-transactional processes requiring feedback, iteration, and confirmation.87,88 Communication processes are a critical component of the makeup and function of an organization linking people across space and time. The potential disruptive impact of health IT cannot really be appreciated without attention to its ability to change the role that communication plays in linking people and activities across space and time.37,39,54,62,63
The diagnostic process
The diagnostic process is not a linear set of tasks but rather a series of tasks that involve multiple people (and different clinical settings) across the health care spectrum.7 This is evidenced by the array of outcome indicators reported by the existing literature to explore this issue.27,44,45,56,60 Health IT systems offer different ways to manage the test results follow-up process, including facilitating the access to and sharing of information, test tracking, and the provision of prompts/alerts.89,90 The evidence of the impact of health IT on test results management work practices draws attention to the importance of 1) enhancing the alignment of health IT with the diagnostic process and 2) accounting for the numerous tasks among different professionals and across different clinical settings that need to be coordinated and synchronized for safe and effective test results management.20,86 For instance, the evidence in this review indicates that systems which focus on ensuring physician review of results (via CPOE, alerts, results acknowledgment, and tracking systems) is, in some cases, insufficient in ensuring completion of subsequent steps of the follow-up process (eg, actioning a result).33,35
The failure to properly integrate different electronic systems within and across health care settings and the continued existence of hybrid paper/electronic systems has emerged as a risk to patient safety.91 Health IT systems that are not maximized to work effectively and efficiently have the capacity to hinder correct diagnosis by contributing to health professionals’ work burdens, resulting in less time to communicate with patients and other health care professionals. For instance, a high volume of alerts (not all of which may be clinically relevant) increases the possibility of clinical cognitive overload which can threaten the value of alerts.35
Patient engagement in the test results follow-up process
Many of the studies in this review highlighted the connection between test results follow-up, IT, and patient engagement. This is because attempts to engage patients in the care process invariably involve shared information (enabling patients to read, comment on, and share in decisions about their care) and timely and meaningful communication (enabling consumers to receive, send, and comprehend the information required).
The role of patient-centerd IT systems was investigated across a range (qualitative and quantitative) of studies revealing that patient access to, and ability to check, laboratory results (in real time) is a major reason for patient utilization of portals.69,82 The most important reported benefits included improved communication with physicians and the management of the patient condition.76,81–83 There were no findings that directly reported on issues of privacy and security even though the literature often cites these as key sources of concern.92 In situations involving life-threatening illnesses, cancer diagnoses, and incurable conditions, the evidence highlighted the preference for face-to-face consultations and the initial involvement of the responsible physician.75,93 Patient-managed PHRs provide a set of computer-based tools owned and administered by patients with access to personal clinical information.94,95 No studies in our review evaluated such a system; yet it is such technologies, organized around a person’s own preferences, that may be emblematic of what is meant by IT-enabled patient-centered health care.94,96
Limitations
The aim of this systematic review was to examine the impact of health IT on test results follow-up across a number of dimensions (including clinical workflows and patient engagement) incorporating studies that used different methods (quantitative and qualitative). The results yielded numerous outcome measures that ranged from the rate of test results missed, to patient satisfaction rates and the number of workarounds. Although the heterogeneity of the studies makes it hard to provide 1 definitive result about the effect of health IT, the findings from this systematic review do nevertheless identify several factors that collectively contribute to the delivery of safe and effective test results management.
The publication (or non-publication) of research based on the direction (positive or negative) of the results can affect the validity of review conclusions.97 The scope and variability of findings from this systematic review was accentuated by our incorporation of 1) an array of search engines and databases including of gray literature sources and 2) extensive use of hand searches of relevant research literature.
CONCLUSION
Effective results follow-up is a fundamental part of the diagnostic process, essential to the delivery of quality patient care. Alongside results of laboratory and medical imaging tests, the diagnostic process involves the integration of information (eg, clinical history, physical examination, and consultation) that forms the key to diagnosis and a treatment plan.90 This process involves multiple tasks often incorporating different medical personnel and usually spread out over time.98 The central message of this systematic review is that the construction of safe and effective test results management IT systems should pivot on several axes including 1) patient-centerd engagement (involving shared information and timely and meaningful communication), 2) diagnostic processes (that involve the integration of multiple people and different clinical settings across the health care spectrum), and 3) organizational communications and the myriad of multi-transactional processes10 requiring feedback, iteration, and confirmation that contribute to the patient care process.
FUNDING
This study was supported by an Australian National Health and Medical Research Partnership Grant APP1111925.
AUTHOR CONTRIBUTIONS
AG conceptualized the study and design. AG, JL, and JT were involved with the acquisition, analysis, and interpretation of data. JL and AG drafted the manuscript. All authors made critical revisions to the manuscript for important intellectual content.
Conflict of Interest Statement
None declared.
Supplementary Material
Appendix 1 – detailed search strategies
Table S1.
Summary of included studies
| Database | Search Strategy (4th wk Jun 2018) |
|---|---|
|
|
| CINAHL |
|
| Limiters—Published Date: 19990101-20180618; English Language; Human Search modes—Boolean/Phrase | |
| Web of science |
|
| ScienceDirect | (pub-date > 1998 and ttl (electronic OR computer* OR online)) AND (pub-date > 1998 and ttl (failure OR miss* OR “follow* up” OR lack* OR management OR view* OR access*)) AND (pub-date > 1998 and ttl (test* OR laboratory OR radiolog* OR result*))[All Sources(Biochemistry, Genetics and Molecular Biology, Computer Science, Immunology and Microbiology, Medicine and Dentistry, Neuroscience, Nursing and Health Professions, Pharmacology, Toxicology and Pharmaceutical Science, Psychology)] |
| ProQuest | (ti(electronic OR computer* OR online) AND ti(failure OR miss* OR “follow* up” OR lack* OR management OR view* OR access*) AND ti(test* OR laboratory OR radiolog* OR result*) AND la.exact(“English”) AND pd(>19990101)) NOT stype.exact(“Trade Journals” OR “Wire Feeds” OR “Newspapers” OR “Magazines”) AND pd(>19990101) |
| Scopus | TITLE ( ( electronic OR computer* OR online ) AND ( failure OR miss* OR “follow*up” OR lack* OR management OR view* OR access* ) AND ( test* OR laboratory OR radiology* OR result* ) ) AND PUBYEAR > 1999 AND LANGUAGE ( english ) AND ( LIMIT-TO ( SUBJAREA , “MEDI” ) OR LIMIT-TO ( SUBJAREA , “COMP” ) OR LIMIT-TO ( SUBJAREA , “HEAL” ) OR LIMIT-TO ( SUBJAREA , “NURS” ) OR LIMIT-TO ( SUBJAREA , “BIOC” ) OR LIMIT-TO ( SUBJAREA , “PSYC” ) OR LIMIT-TO ( SUBJAREA , “NEUR” ) OR LIMIT-TO ( SUBJAREA , “IMMU” ) OR LIMIT-TO ( SUBJAREA , “PHAR” ) ) AND ( EXCLUDE ( DOCTYPE , “le” ) ) AND ( EXCLUDE ( LANGUAGE , “Italian” ) OR EXCLUDE ( LANGUAGE , “French” ) OR EXCLUDE ( LANGUAGE , “German” ) OR EXCLUDE ( LANGUAGE , “Turkish” ) ) |
REFERENCES
- 1. Wolcott J, Schwartz A, Goodman C. Laboratory Medicine: A National Status Report;2008. Available at:https://stacks.cdc.gov/view/cdc/30726. Accessed March 2019.
- 2. World Health Organization, World Alliance for Patient Safety, Research Priority Setting Working Group. World Alliance for Patient Safety - Summary of the Evidence on Patient Safety: Implications for Research. Geneva: World Health Organization; 2008. [Google Scholar]
- 3. ECRI Institute. Top 10 Patient Safety Concerns for Healthcare Organizations; 2017. www.ecri.org/PatientSafetyTop10. Accessed July 2017.
- 4. Poon EG, Gandhi TK, Sequist TD, et al. “ I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med 2004; 16420: 2223–8. [DOI] [PubMed] [Google Scholar]
- 5. Callen J, Georgiou A, Li J, et al. The safety implications of missed test results for hospitalized patients: a systematic review. BMJ Qual Saf 2011; 202: 194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slovis BH, Nahass TA, Salmasian H, et al. Asynchronous automated electronic laboratory result notifications: a systematic review. J Am Med Inform Assoc 2017; 246: 1173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanna D, Griswold P, Leape L, et al. Communicating critical test results: safe practice recommendations. Jt Comm J Qual Patient Saf 2005; 312: 68–80. [DOI] [PubMed] [Google Scholar]
- 8. Ash J, Singh H, Sittig D. Test Results Reporting and Follow-Up SAFER Guide; 2014. Available at: https://www.healthit.gov/sites/default/files/safer_test_results_reporting.pdf. Accessed March 2019.
- 9. Singh H, Sittig DF.. Measuring and improving patient safety through health information technology: The Health IT Safety Framework. BMJ Qual Saf 2016; 254: 226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiff GD, Reyes Nieva H, Griswold P, et al. Randomized trial of reducing ambulatory malpractice and safety risk: results of the Massachusetts PROMISES Project. Med Care 2017; 558: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agency for Healthcare Research and Quality. Improving Your Laboratory Testing Process: A Step-by-Step Guide for Rapid-Cycle Patient Safety and Quality Improvement; 2018. Available at:https://www.ahrq.gov/news/improving-lab-testing.html. Accessed March 2019.
- 12. Partnership for Health IT Patient Safety. Closing the Loop: Using Health IT to Mitigate Delayed, Missed, and Incorrect Diagnoses Related to Diagnostic Testing and Medication Changes. ECRI Institute; 2018. Available at: https://www.ecri.org/Resources/HIT/Closing_Loop/Closing_the_Loop_Toolkit.pdf. Accessed March 2019.
- 13. The National Patient Safety Foundation. Safety Is Personal; Partnering with Patients and Families for the Safest Care; 2014. http://www.npsf.org/about-us/lucian-leape-institute-at-npsf/lli-reports-and-statements/safety-is-personal-partnering-with-patients-and-families-for-the-safest-care/. Accessed May 2017.
- 14. Australian Commission on Safety and Quality in Health Care (ACSQHC). National Safety and Quality Health Service Standards. Sydney, Australia: Commonwealth of Australia; 2012. [Google Scholar]
- 15. Bolton P. A doctor's duty to follow up preventable conditions: Young v Central Australian Aboriginal Congress - a bridge too far? NTLJ 2012; 2(3): 154. [Google Scholar]
- 16. Ammenwerth E, Schnell-Inderst P, Hoerbst A.. The impact of electronic patient portals on patient care: a systematic review of controlled trials. J Med Internet Res 2012; 14: e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Committee on Patient Safety and Health Information Technology; Institute of Medicine. Health IT and Patient Safety: Building Safer Systems for Better Care. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 18. Carr S. Health IT and diagnostic safety: promise and peril. Improve Diagnosis 2015; 2: 1–4. [Google Scholar]
- 19. Sittig DF, Murphy DR, Smith MW, et al. Graphical display of diagnostic test results in electronic health records: a comparison of 8 systems. J Am Med Inform Assoc 2015; 22(4): 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitehead N, Williams L, Meleth S, et al. Interventions to improve follow-up of laboratory test results pending at discharge: a systematic review. J Hosp Med 2018. doi: 10.12788/jhm.2944. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darragh PJ, Bodley T, Orchanian-Cheff A, et al. A systematic review of interventions to follow-up test results pending at discharge. J Gen Intern Med 2018; 33(5): 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 67: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graber ML, Franklin N, Gordon R.. Diagnostic error in internal medicine. Arch Intern Med 2005; 16513: 1493–9. [DOI] [PubMed] [Google Scholar]
- 24. Critical Appraisal Skills Progam. CASP Qualitative Checklist. https://casp-uk.net/casp-tools-checklists/. Accessed June 2018.
- 25. National Heart Lung and Blood Institute. Study Quality Assessment Tools: US Department of Health and Human Services. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed June 2018.
- 26. Chen TC, Lin WR, Lu PL, et al. Computer laboratory notification system via short message service to reduce health care delays in management of tuberculosis in Taiwan. Am J Infect Control 2011; 395: 426–30. [DOI] [PubMed] [Google Scholar]
- 27. Etchells E, Adhikari NK, Cheung C, et al. Real-time clinical alerting: effect of an automated paging system on response time to critical laboratory values—a randomised controlled trial. Qual Saf Health Care 2010; 192: 99–102. [DOI] [PubMed] [Google Scholar]
- 28. Humphrey LL, Shannon J, Partin MR, et al. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med 2011; 267: 691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalal AK, Roy CL, Poon EG, et al. Impact of an automated email notification system for results of tests pending at discharge: a cluster-randomized controlled trial. J Am Med Inform Assoc 2014; 213: 473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laxmisan A, Sittig DF, Pietz K, et al. Effectiveness of an electronic health record-based intervention to improve follow-up of abnormal pathology results: a retrospective record analysis. Medical Care 2012; 5010: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staes CJ, Evans RS, Rocha BH, et al. Computerized alerts improve outpatient laboratory monitoring of transplant patients. J Am Med Inform Assoc 2008; 153: 324 [Erratum appears in J Am Med Inform Assoc 2008; 15 (5): 708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh H, Arora HS, Vij MS, et al. Communication outcomes of critical imaging results in a computerized notification system. J Am Med Inform Assoc 2007; 144: 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh H, Thomas EJ, Mani S, et al. Timely follow-up of abnormal diagnostic imaging test results in an outpatient setting: are electronic medical records achieving their potential? Arch Intern Med 2009; 169: 1578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh H, Wilson L, Petersen LA, et al. Improving follow-up of abnormal cancer screens using electronic health records: trust but verify test result communication. BMC Med Inform Decis Mak 2009; 9(1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh H, Thomas EJ, Sittig DF, et al. Notification of abnormal lab test results in an electronic medical record: do any safety concerns remain? American Journal of Medicine 2010; 1233: 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hysong SJ, Sawhney MK, Wilson L, et al. Provider management strategies of abnormal test result alerts: a cognitive task analysis. J Am Med Inform Assoc 2010; 171: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hysong SJ, Sawhney MK, Wilson L, et al. Understanding the management of electronic test result notifications in the outpatient setting. BMC Med Inform Decis Mak 2011; 11: 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wahls T, Haugen T, Cram P.. The continuing problem of missed test results in an integrated health system with an advanced electronic medical record. Jt Comm J Qual Patient Saf 2007; 338: 485–92. [DOI] [PubMed] [Google Scholar]
- 39. Wahls TL, Cram PM.. The frequency of missed test results and associated treatment delays in a highly computerized health system. BMC Fam Pract 2007; 8(1): 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El-Kareh R, Roy C, Williams DH, et al. Impact of automated alerts on follow-up of post-discharge microbiology results: a cluster randomized controlled trial. J Gen Intern Med 2012; 2710: 1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuperman GJ, Teich JM, Tanasijevic MJ, et al. Improving response to critical laboratory results with automation: results of a randomized controlled trial. J Am Med Inform Assoc 1999; 66: 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park H-I, Min W-K, Lee W, et al. Evaluating the short message service alerting system for critical value notification via PDA telephones. Ann Clin Lab Sci 2008; 382: 149–56. [PubMed] [Google Scholar]
- 43. Hayes SA, Breen M, McLaughlin PD, et al. Communication of unexpected and significant findings on chest radiographs with an automated PACS alert system. J Am Coll Radiol 2014; 118: 791–5. [DOI] [PubMed] [Google Scholar]
- 44. Browning T, Kasper J, Rofsky NM, et al. Quality improvement initiative: enhanced communication of newly identified, suspected GI malignancies with direct critical results messaging to surgical specialist. BMJ Qual Saf 2013; 222: 168–75. [DOI] [PubMed] [Google Scholar]
- 45. Dalal AK, Schaffer A, Gershanik EF, et al. The impact of automated notification on follow-up of actionable tests pending at discharge: a cluster-randomized controlled trial. J Gen Intern Med 2018; 33(7): 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Callen J, Georgiou A, Prgomet M, et al. A qualitative analysis of emergency department physicians’ practices and perceptions in relation to test result follow-up. Stud Health Technol Inform 2010; 160 (Pt 2): 1241–5. [PubMed] [Google Scholar]
- 47. Callen J, Paoloni R, Georgiou A, et al. The rate of missed test results in an emergency department: an evaluation using an electronic test order and results viewing system. Methods Inf Med 2010; 49: 37–43. [DOI] [PubMed] [Google Scholar]
- 48. Kilpatrick ES, Holding S.. Use of computer terminals on wards to access emergency test results: a retrospective audit. BMJ 2001; 3227294: 1101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Topol P, Porat N, Zelker R, et al. Quality improvement program to assure the delivery of pathology test results: a systemic intervention in a large general hospital. Dermatol Nurs 2007; 19: 253–7. [PubMed] [Google Scholar]
- 50. Rodriguez-Borja E, Villalba-Martinez C, Barba-Serrano E, et al. Failure to review STAT clinical laboratory requests and its economical impact. Biochem Med 2016; 26: 61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kern LM, Callahan MA, Brillon DJ, et al. Glucose testing and insufficient follow-up of abnormal results: a cohort study. BMC Health Serv Res 2006; 6: 87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005; 1432: 121–8. [DOI] [PubMed] [Google Scholar]
- 53. Menon S, Smith MW, Sittig DF, et al. How context affects electronic health record-based test result follow-up: a mixed-methods evaluation. BMJ Open 2014; 411: e005985.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singh H, Spitzmueller C, Petersen NJ, et al. Primary care practitioners’ views on test result management in EHR-enabled health systems: a national survey. J Am Med Inform Assoc 2013; 204: 727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Elder NC, McEwen TR, Flach JM, et al. Management of test results in family medicine offices. Ann Fam Med 2009; 74: 343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Menon S, Murphy DR, Singh H, et al. Workarounds and test results follow-up in electronic health record-based primary care. Appl Clin Inform 2016; 0702: 543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhise V, Meyer AND, Singh H, et al. Errors in diagnosis of spinal epidural abscesses in the era of electronic health records. Am J Med 2017; 1308: 975–81. [DOI] [PubMed] [Google Scholar]
- 58. Ferris TG, Johnson SA, Co JPT, et al. Electronic results management in pediatric ambulatory care: qualitative assessment. Pediatrics 2009; 123 (Suppl 2): S85–91. [DOI] [PubMed] [Google Scholar]
- 59. Georgiou A, Lymer S, Forster M, et al. Lessons learned from the introduction of an electronic safety net to enhance test result management in an Australian mothers’ hospital. J Am Med Inform Assoc 2014; 216: 1104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dalal AK, Pesterev BM, Eibensteiner K, et al. Linking acknowledgment to action: closing the loop on non-urgent, clinically significant test results in the electronic health record. J Am Med Inform Assoc 2015; 224: 905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Georgiou A, McCaughey EJ, Tariq A, et al. What is the impact of an electronic test result acknowledgement system on emergency department physicians’ work processes? A mixed-method pre-post observational study. Int J Med Inform 2017; 99: 29–36. [DOI] [PubMed] [Google Scholar]
- 62. Li J, Callen J, Westbrook JI, et al. What factors determine the use of an electronic test result acknowledgment system? A qualitative study across two EDs In: Ryan A, Schaper L, Whetton S, eds. Studies in Health Technology & Informatics. Vol 239 Amsterdam:IOS Press; 2017: 70–6. [PubMed] [Google Scholar]
- 63. Dalal AK, Poon EG, Karson AS, et al. Lessons learned from implementation of a computerized application for pending tests at hospital discharge. J Hosp Med 2011; 61: 16–21. [DOI] [PubMed] [Google Scholar]
- 64. Lin JJ, Moore C.. Impact of an electronic health record on follow-up time for markedly elevated serum potassium results. Am J Med Qual 2011; 264: 308–14. [DOI] [PubMed] [Google Scholar]
- 65. Murphy DR, Wu L, Thomas EJ, et al. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. JCO 2015; 3331: 3560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choksi VR, Marn CS, Bell Y, et al. Efficiency of a semiautomated coding and review process for notification of critical findings in diagnostic imaging. AJR Am J Roentgenol 2006; 1864: 933–6. [DOI] [PubMed] [Google Scholar]
- 67. Dupuis EA, White HF, Newman D, et al. Tracking abnormal cervical cancer screening: evaluation of an EMR-based intervention. J Gen Intern Med 2010; 256: 575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Giardina TM, Varsha P, Danielle Singh H.. The patient portal and abnormal test results: An exploratory study of patient experiences. Patient Exp J 2015; 2: 148–54. [PMC free article] [PubMed] [Google Scholar]
- 69. Hazara AM, Bhandari S.. Barriers to patient participation in a self-management and education website Renal PatientView: a questionnaire-based study of inactive users. Int J Med Inform 2016; 87: 10–4. [DOI] [PubMed] [Google Scholar]
- 70. Ling SB, Richardson DB, Mettenbrink CJ, et al. Evaluating a web-based test results system at an urban STI clinic. Sex Transm Dis 2010; 374: 259–63. [DOI] [PubMed] [Google Scholar]
- 71. Mak G, Smith Fowler H, Leaver C, et al. The effects of web-based patient access to laboratory results in British Columbia: a patient survey on comprehension and anxiety. J Med Internet Res 2015; 17: e191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Miles RC, Hippe DS, Elmore JG, et al. Patient access to online radiology reports: frequency and sociodemographic characteristics associated with use. Acad Radiol 2016; 239: 1162–9. [DOI] [PubMed] [Google Scholar]
- 73. Mukoro F, Sweeney G, Mathews B, editors. Providing patients online access to their live test results: an evaluation of usage and usefulness. In: IADIS International Conference e-Health, Lisbon; 2012. [Google Scholar]
- 74. Wiljer D, Urowitz S, Apatu E, et al. Understanding the support needs of patients accessing test results online. PHRs offer great promise, but support issues must be addressed to ensure appropriate access. J Healthc Inf Manag 2010; 24: 57–63. [PubMed] [Google Scholar]
- 75. Winget M, Haji-Sheikhi F, Brown-Johnson C, et al. Electronic release of pathology and radiology results to patients: opinions and experiences of oncologists. JOP 2016; 128: e792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Woywodt A, Vythelingum K, Rayner S, et al. Single-centre experience with Renal PatientView, a web-based system that provides patients with access to their laboratory results. J Nephrol 2014; 275: 521–7. [DOI] [PubMed] [Google Scholar]
- 77. Rodriguez ES, Thom B, Schneider SM.. Nurse and physician perspectives on patients with cancer having online access to their laboratory results. Oncol Nurs Forum 2011; 384: 476–82. [DOI] [PubMed] [Google Scholar]
- 78. Okawa G, Ching K, Qian H, et al. Automatic release of radiology reports via an online patient portal. J Am Coll Radiol 2017; 149: 1219–21. [DOI] [PubMed] [Google Scholar]
- 79. Giardina TD, Baldwin J, Nystrom DT, et al. Patient perceptions of receiving test results via online portals: a mixed-methods study. J Am Med Inform Assoc 2018; 254: 440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Office of the National Coordinator for Health Information Technology. What is a patient portal? https://www.healthit.gov/providers-professionals/faqs/what-patient-portal. Accessed June 2017.
- 81. Christensen KS. Viewing laboratory test results online: patients’ actions and reactions. J Participat Med 2013; 5: e38. [Google Scholar]
- 82. Cimino JJ, Patel VL, Kushniruk AW.. The patient clinical information system (PatCIS): technical solutions for and experience with giving patients access to their electronic medical records. Int J Med Inform 2002; 68 (1–3): 113–27. [DOI] [PubMed] [Google Scholar]
- 83. Henshaw D, Okawa G, Ching K, Garrido T, Qian H, Tsai J.. Access to radiology reports via an online patient portal: experiences of referring physicians and patients. J Am Coll Radiol 2015; 126: 582–6. e1. [DOI] [PubMed] [Google Scholar]
- 84. Institute of Medicine. Health IT and Patient Safety: Building Safer Systems for Better Care. Washington DC: Institute of Medicine of the National Academies; 2011. [PubMed] [Google Scholar]
- 85. Office of the National Coordinator for Health Information Technology. Are there different types of personal health records (PHRs)? https://www.healthit.gov/faq/are-there-different-types-personal-health-records-phrs. Accessed June 2017.
- 86. Georgiou A, Westbrook JI, Braithwaite J.. Time matters - a theoretical and empirical examination of the temporal landscape of a hospital pathology service and the impact of e-health. Soc Sci Med 2011; 7210: 1603.. [DOI] [PubMed] [Google Scholar]
- 87. Georgiou A, Westbrook JI, Braithwaite J.. An empirically-derived approach for investigating health information technology: the elementally entangled organisational communication (EEOC) framework. BMC Med Inform Decis Mak 2012; 12(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kuziemsky CE, Borycki EM, Purkis ME.. An interdisciplinary team communication framework and its application to healthcare ‘e-teams’ systems design. BMC Med Inform Decis Mak 2009; 9(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Schiff GD, Bates DW.. Can electronic clinical documentation help prevent diagnostic errors?. N Engl J Med 2010; 36212: 1066–9. [DOI] [PubMed] [Google Scholar]
- 90. National Academies Of Science Engineering and Medicine Improving Diagnosis in Health Care. Washington, DC: The National Acadamies Press; 2015. [Google Scholar]
- 91. Georgiou A, Prgomet M, Paoloni R, et al. The impact of computerized provider order entry systems on clinical care and work processes in emergency departments: a systematic review of the quantitative literature. Ann Emerg Med 2013; 616: 644–53. [DOI] [PubMed] [Google Scholar]
- 92. Scott P, Rigby M, Ammenwerth E, et al. Evaluation considerations for secondary uses of clinical data: principles for an evidence-based approach to policy and implementation of secondary analysis. IMIA Yearbook 2017; 26(1): 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giardina TD, Callen J, Georgiou A, et al. Releasing test results directly to patients: a multisite survey of physician perspectives. Patient Educ Couns 2015; 986: 788–96. [DOI] [PubMed] [Google Scholar]
- 94. Rigby M, Georgiou A, Hyppönen H, et al. Patient portals as a means of information and communication technology support to patient-centric care coordination–the missing evidence and the challenges of evaluation: a joint contribution of IMIA WG EVAL and EFMI WG EVAL. Yearb Med Inform 2015; 10: 148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hordern A, Georgiou A, Whetton S, et al. Consumer eHealth - an overview of the research evidence and the implications for future policy. Health Inf Manag 2011; 402: 6–14. [DOI] [PubMed] [Google Scholar]
- 96. Otte-Trojel T, de Bont A, Rundall TG, et al. How outcomes are achieved through patient portals: a realist review. J Am Med Inform Assoc 2014; 214: 751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sterne JA, Egger M, Moher D.. Addressing reporting biases In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews. New York: Wiley; 2008: 297–333. [Google Scholar]
- 98. Carayon P, Karsh BT, Cartmill R, et al. Incorporating Health IT into Workflow Redesign: Request for Information Summary Report (Publication No. 10-0098-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.