We discuss recent data and concepts important for development of host-directed therapies for tuberculosis that emerged from the “Strategic Discussion on Repurposing Drugs & Host Directed Therapies for TB” workshop organized by the Stop TB Working Group on New Drugs.
Keywords: drug development, host-directed therapy, macrophage, meningitis, tuberculosis
Abstract
New therapeutics to augment current approaches and shorten treatment duration are of critical importance for combating tuberculosis (TB), especially those with novel mechanisms of action to counter the emergence of drug-resistant TB. Host-directed therapy (HDT) offers a novel strategy with mechanisms that include activating immune defense mechanisms or ameliorating tissue damage. These and related concepts will be discussed along with issues that emerged from the workshop organized by the Stop TB Working Group on New Drugs, held at the Gordon Research Conference for Tuberculosis Drug Development in Lucca, Italy in June 2017, titled “Strategic Discussion on Repurposing Drugs & Host Directed Therapies for TB.” In this review, we will highlight recent data regarding drugs, pathways, and concepts that are important for successful development of HDTs for TB.
MODULATING HOST IMMUNE FUNCTION
Several recent reviews summarized the classes of compounds with promising host-directed therapy (HDT) activity for tuberculosis (TB), including US Food and Drug Administration-approved drugs as well as drugs currently in use for TB [1, 2]. Possible HDTs act on a variety of host targets, and, in many cases, the mechanisms of action are still poorly understood. Some of these aspects are summarized in Figure 1. Within the macrophage, Mycobacterium tuberculosis (Mtb) expresses a plethora of immunomodulatory factors enabling survival and ultimate killing of the macrophage, some of which are overcome as the macrophage undergoes activation. Endogenous antimicrobial peptides (AMPs), such as cathelicidin and LL-37 (a product of cathelicidin cleavage by kallikrein 5 and kallikrein 7), are produced upon cytosolic activation of Toll-like receptors by Mtb ligands [3]. These AMPs mediate killing of tubercle bacteria by targeting the mycobacterial cell wall, and modulation of their activity and/or production provides an attractive option for HDT. The active form of vitamin D, 1,25-dihydroxyvitamin D(3), induces production of cathelicidin and reactive oxygen species (ROS) production, although contrasting results have been documented for the use of vitamin D as adjunctive HDT for TB treatment [4–6]. Due to the possible synergy between phenylbutyrate and vitamin D, clinical trials involving coadministration of sodium 4-phenylbutyrate with active vitamin D (cholecalciferol/vitamin D3/1,25-dihydroxyvitamin D [3]) as adjunctive therapy to TB treatment have been performed (clinical trial studies NCT01580007 and NCT01698476). These studies suggested that adjunctive coadministration of sodium 4-phenylbutyrate with vitamin D3 may contribute to clinical recovery in pulmonary TB patients compared with a placebo group [7]. Although these data are intriguing, the clinical significance is not clear and requires further study.
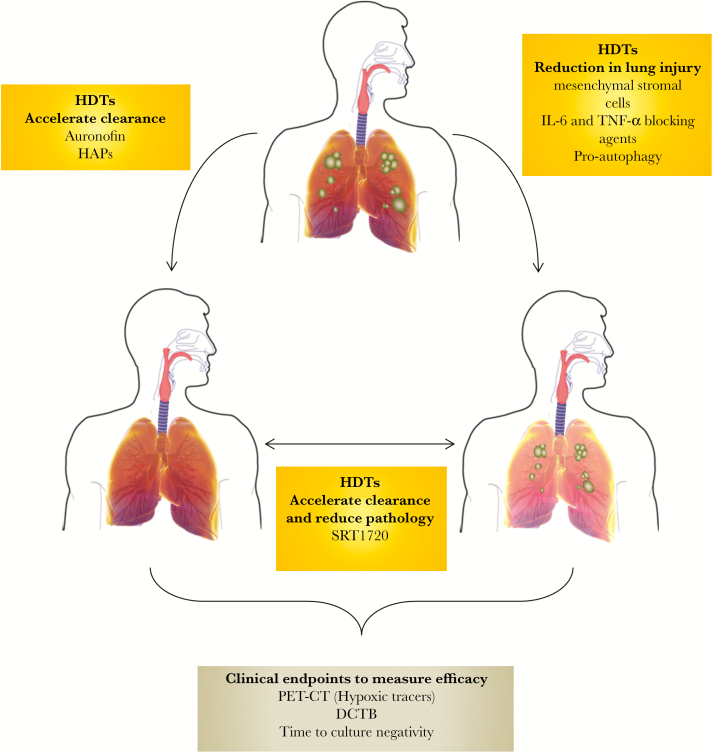
Figure 1.
Possible effects of host-directed therapies (HDTs) on the outcome of tuberculosis (TB) treatment. Improved TB treatment outcomes with HDTs are reliant either on the ability of these agents to accelerate bacterial clearance or reduce the lung damage associated with granulomatous disease. Some agents promise to modulate both these effects to improve treatment outcomes. The use of appropriate outcome measures is critical for ensuring that the best potential HDTs are advanced in clinical trials. In this regard, recent developments such as monitoring changes in pathology, using novel tracers, and assessing the presence of differentially culturable bacteria (DCTB), which resist treatment, may prove important for prioritizing agents for further human studies. HAP, hypoxia-activated prodrug; IL, interleukin; PET-CT, positron emission tomography-computed tomography; TNF, tumor necrosis factor.
It is interesting to note that epigenetic regulation in the macrophage by targeting histone deacetylases (HDACs) with HDAC activators is becoming an area of intense interest for developing HDTs [8]. Mycobacterium tuberculosis downregulates the HDAC sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase in macrophages during infection [8]. Restoration of SIRT1 activity by resveratrol (a natural SIRT1 activator) or SRT1720 (a synthetic SIRT1 activator) contributes to autophagy and phagolysosome-mediated bacterial killing in the macrophage [8]. SRT1720 was found to enhance bacterial clearance in mice when administered with isoniazid (INH), also reducing lung tissue pathology and chronic inflammation [8].
Metformin, an antidiabetic drug, functions by activating the adenosine monophosphate-activate protein kinase (AMPK) in the liver and has also been implicated as effective anti-TB adjunctive therapy in C57BL/6 mice, where it increased host cell ROS production and phagosomal acidification. Furthermore, metformin provides an anti-inflammatory effect through AMPK, which is likely the primary mechanism of anti-TB activity [9], although it failed to show similar effects in the BALB/c mouse model [10]. However, retrospective analysis of TB/diabetes comorbid individuals showed (1) lower mortality during TB treatment and (2) reduced risk to develop active TB disease among patients taking metformin than those taking antidiabetic regimens without metformin [9, 11]. Randomized controlled trials of metformin for adjunctive TB therapy are planned to begin soon.
Inhibition of CD4+ T-cell macrophage signaling by monoclonal antibodies (mAbs) directed against checkpoint receptors, such as programmed cell death protein 1 (PD1) with mAbs such as nivolumab and permbrolizumab, rescues Mtb-specific interferon (IFN)γ-producing CD4+ T cells from apoptosis [12].
Host-directed therapy using regenerative cellular therapy by infusion with bone marrow-derived mesenchymal stromal cells (MSCs) as adjunctive treatment for TB patients with drug-resistant TB (DR-TB) has been shown to be effective in a Phase 1 safety clinical trial [13]. The MSCs augment the proliferation of bronchoalveolar stem cells and contribute to lung tissue repair [14]. Together, these studies indicate that adjunctive HDTs targeting T-cell functionality and/or limiting lung tissue damage caused by excessive inflammation are promising candidates for Phase II clinical trials.
The formation of a granuloma, which is a highly organized structure of immune cells that sequesters infection and localizes bacteria to the lung, is a hallmark feature of TB pathology [15]. Host-directed therapy strategies that modulate granuloma structure and function have been envisaged. An example is the administration of adjunct immunosuppressive drugs with anti-TB drugs. Drugs that modulate cytokine signaling, such as interleukin (IL)-6 blocking agents (siltuximab, tolilizumab) and tumor necrosis factor (TNF)-α blocking agents (despramine, infliximab, adalimumab, and alispovir), can limit inflammation in the granuloma, or drugs may block granuloma angiogenesis (pazopanib, bevacizumab).
CD4+ T-cell responses are essential for anti-TB immunity [16], explaining in part the detrimental synergy between human immunodeficiency virus (HIV) and TB [17]. This is primarily caused by HIV-1-mediated depletion of CD4+ T cells essential for induction of the anti-mycobacterial immune response during HIV-1/TB coinfection [16]. However, even during long-term antiretroviral (ARV) treatment of HIV-1-infected individuals, the risk of Mtb infection remains substantially higher than in the general population, which may be due to incomplete reconstitution of the T-cell profile [17]. Blocking of TNF-α with adjunctive etanercept, which may disrupt the granoluma allowing drug access to the bacilli, during the early stages of TB treatment in a clinical trial study of HIV-1-infected individuals enhanced sputum culture conversion rates. A Phase II clinical trial study (NCT02968927) investigating the potential utility of adjunctive treatment with vitamin D, a TNF-α blocking agent (CC11050), a proautophagy drug (everolimus), or auronofin (a gold-containing compound with anti-TB activity) will investigate the safety of each these interventions as well as their efficacy in shortening TB treatment and/or preventing permanent lung damage in patients with HIV-associated TB.
Intriguingly, solid tumor biology is remarkably like that of the TB lesion. A common feature of both lesions are gradients of oxygen tension [18, 19]. Studies with next generation of hypoxia-activated prodrugs (HAPs) are underway with pharmacological reprogramming of TB lesions to overcome hypoxia or drugs that directly modulate oxygen levels, in combination with conventional anti-mycobacterial therapy [18, 20, 21]. Several HAPs and oxygen modulators are in clinical trials (Table 1). Together, these compounds represent a promising and unique therapeutic strategy for HDTs.
Table 1.
Hypoxia Activated Prodrugs With Potential Anti-TB Applications
| Compound | Mechanism of Action and Clinical Evaluation | Reference |
|---|---|---|
| TH-302 (Evofosfamide) | TH-302 is a 2-nitroimidazole HAP of bromo-isophosphoramide that is reduced via one-electron oxidoreductases to release a potent DNA alkylating agent bromo-isophosphoramide. This HAP showed clear evidence of selective eradication of hypoxic cells and good safety profiles in animals. TH-302 is undergoing several Phase III studies (MAESTRO; NCT01746979 and TH-CR-406/SARC021; NCT01440088). | [18] |
| EO9 (Apaziquone) | EO9 is an indolequinone of mitomycin C and its mechanism of action involves reduction by 1 and/or 2 electron oxidoreductases releasing DNA damaging species under aerobic and hypoxic conditions (Phillips). Although results of Phase II studies were disappointing, an explanation for the poor results was that the presence of hypoxia in the patient’s tumors were not included into the trial design. Two new Phase III trials used a new administration schedule (NCT00598806 and NCT00461591), and further Phase III study has been planned (NCT01410565). | [20] |
| AQ4N (Banoxantrone) | AQ4N is metabolized by cytochrome P450 and inducible nitric oxide synthase (iNOS) to an inhibitor of topoisomerase II. Its mode of action involves an initial 2 electron reduction step followed by further 2 electron reduction steps to generate AQ4. The compound is active in tumor cells, and hypoxic tumor-associated macrophages were induction of iNOS under hypoxic conditions reduces AQ4N, which kill tumor cells via a bystander effect. AQ4N has been evaluated in 3 Phase I studies. | [48] |
| PR-104 | PR-104 undergoes rapid hydrolysis by phosphatases to generate PR-104A, which is metabolized by 1 and/or 2 electron oxidoreductases to facilitate interstrand DNA cross-linking. The compound penetrates severely hypoxic regions of tumors were this metabolized to cytotoxic metabolites. Based on preclinical data, a Phase I/II study has demonstrated good clinical activity. | [49] |
| Tirapazamine (TPZ) | TPZ is reduced by 1 electron oxidoreductases to generate a radical that, in the absence of oxygen, leads to the formation of DNA-damaging radicals. Whereas Phase I and II studies generated positive results, several Phase III clinical trials failed to demonstrate any survival benefit by adding TPZ to chemotherapy in non-small cell lung cancer, head and neck cancer, and cervical cancer. One reason for the failure of TPZ is lack of stratification of patients based on tumor hypoxia levels. | [21] |
| TH-4000 (tarloxotinib bromide) | In contrast to other HAPs that generate metabolites that damage DNA either directly or indirectly, TH-4000 is a hypoxia-activated EGFR tyrosine kinase inhibitor and is currently undergoing Phase II clinical evaluation (NCT02454842 and NCT02449681). | [18] |
| Myo-inositol trispyrophosphate (ITPP) | ITPP is a synthetic derivative of myoinositol hexakisphosphate, which is an allosteric effect of hemoglobin (Hb). By reducing the binding affinity of Hb to oxygen, oxygen is released, enhancing oxygen levels in the hypoxic environment, which inhibit hypoxia-induced angiogenesis. Notably, since ITPP accumulates in erythrocytes, increase oxygen release also occurs in vivo. ITPP display little in vivo toxicity, neither in animals nor humans. Currently, clinical trials are ongoing (NCT02528526). ITPP is exploited as a performance-enhancing doping agent, largely due to its capacity to increase oxygen availability during exercising. | [50] |
Abbreviations: DNA, deoxyribonucleic acid; EGFR, estimated glomerular filtration rate; HAP, hypoxia-activated prodrug.
PRECLINICAL AND CLINICAL ENDPOINTS OF HOST-DIRECTED THERAPIES
The rational for selection of HDT for advancement in clinical trials depends on a variety of objectives, including whether the HDT will be used as monotherapy to limit tissue damage or as adjunctive therapy to standard TB therapy to facilitate bacterial clearance. Depending on the putative mechanism of action of the HDT agent, in vitro models may be first considered when deciding clinical endpoints.
Preclinical study endpoints in animal models should be directed towards the anticipated clinical effect of the HDT agent. For agents expected to accelerate bacterial clearance in the host, microbiological endpoints must quantify the reduction of bacterial burden in lesions of the lungs and other organs when the agent is given alone relative to no treatment, or when the agent is given as adjunctive therapy in combination with 1 or more anti-TB drugs relative to the anti-TB drugs alone. For example, adjunctive treatment with the cholesterol-lowering and immunomodulatory agent simvastatin was found to reduce the lung burden and improve histopathology in Mtb-infected BALB/c mice relative to first-line treatment alone [22]. Relapse studies are critical to show the treatment-shortening potential of adjunctive therapy with the HDT agent of interest relative to the standard regimen alone. Adjunctive treatment with simvastatin reduced the time required to achieve culture-negative lungs by approximately 1 month relative to controls in Mtb-infected BALB/c mice as well as diminish relapse rates 3 months after treatment discontinuation [23].
The effect of HDTs on bacterial clearance can also be measured conventionally by monitoring sputum culture conversion, in addition to other clinical endpoints, such as resolution of respiratory symptoms (cough and dyspnea), radiographic (chest x-ray, computed tomography [CT], or other imaging system) abnormalities, and weight gain [2]. The presence of a subpopulation of bacteria in sputum that are unable to form colonies on plates can adversely affect the readout of treatment efficacy [24]. These noncultivable organisms can be recovered in liquid media, supplemented with culture filtrate from axenic cultures of wild-type Mtb, and have thus been termed differentially culturable tubercle bacteria (DCTB). Because these organisms are difficult to grow, a hallmark feature of dormant bacteria, they are presumed to be drug tolerant and refractory to standard therapy. As a result, the presence of DCTB during pulmonary and extrapulmonary TB may underpin the need for protracted treatment, and researchers attempting to measure treatment-shortening effects of new drugs or HDTs should consider quantification of these organisms.
An alternative quantitative method to evaluate lung (and other organ) inflammation in these models is positron emission tomography (PET)/CT, which allows for (1) serial assessment in the same animal of lesion size and number as well as (2) lesion “activity”. 2-[18F]-fluoro-2-deoxyglucose is the most commonly used tracer, and in animal models it correlates with disease progression and regression [25]. However, human TB lesions are severely hypoxic [19], which affects glucose metabolism as well as blood-borne tracer delivery. Hypoxic PET tracers that have been validated for cancer could potentially be useful in patients with TB.
The guinea pig model of TB chemotherapy offers the advantages of relatively low cost, known human-equivalent doses of the first-line anti-TB drugs and the feasibility to perform relapse studies [26]. Depending on the infecting Mtb strain used, the entire spectrum of human TB, ranging from latent TB infection to cavitary pulmonary disease, may be modeled in New Zealand white rabbits. This model was used to show that adjunctive therapy with a phosphodiesterase-4 inhibitor, which reduces TNF-α production by increasing intracellular cyclic adenosine monophosphate in macrophages, enhances INH-mediated clearance of Mtb and reduces chronic inflammation in rabbit lungs [27]. The nonhuman primate has been advanced as the model that most accurately reproduces the clinical, histological, and microbiological characteristics of human TB. Gleevec (imatinib mesylate) [28], a cancer drug used to treat chronic myelogenous leukemia and gastrointestinal stromal tumors, is currently being investigated as adjunctive HDT in nonhuman primates. Limitations of testing HDT agents (and novel antibiotics) in the nonhuman primate and, to a lesser extent, other large animal models are the significant costs associated with procurement, housing, and veterinary care of the animals, as well as the large quantity of study drugs needed for chemotherapy studies, limiting the number of animals that can be studied and precluding the possibility of assessing relapse. A novel mouse model, the C3Heb/FeJ mouse, which develops hypoxic, necrotic lung granulomas (and occasionally cavities) after Mtb infection, has been used recently to assess the activity of novel anti-TB therapies [29], as well as HDT agents (P. C. K. and N. K. D., unpublished data, 2018). Further work is required to determine (1) the optimal animal model for assessing the potential activity of HDT candidates and (2) the precise microbiological and inflammation outcomes required to justify progression to clinical testing.
TUBERCULOSIS MENINGITIS: A BALANCED CHALLENGE FOR HOST-DIRECTED THERAPY
Tuberculosis meningitis (TBM) is a severe form of TB comprising 1% of cases worldwide [30], with death rates of 18%–40% [31] and disabling neurological deficits in 10%–30% of survivors [31, 32]. Tuberculosis meningitis is one of the few infections where high-quality evidence supports the clinical use of corticosteroids [31]. Further studies of this cohort suggest that the degree of inflammation may impact survival and the response to corticosteroid treatment. Leukotriene A4 hydrolase (LTA4H) regulates the balance of pro- and anti-inflammatory eicosanoids such as LTB4 (which induces TNF) and LXA4. In humans, heterozygosity at an LTA4H single nucleotide polymorphism ([SNP] rs17525495) was associated with protection against pulmonary and meningeal TB and improved survival in TBM patients in Vietnam. The LTA4H SNP encodes for alleles with opposing inflammatory profiles with high cerebrospinal fluid (CSF) inflammation associated with genotype TT and low inflammation with genotype CC (less common allele) [33, 34]. It is interesting to note that dexamethasone was associated with a survival benefit in individuals with the TT genotype but not those with the CC genotype in a retrospective analysis [33]. A separate study from Indonesia did not observe an association of the LTA4H polymorphism with mortality and suggested that some of the observations are population specific [35]. Taken together, these data raise the question of whether corticosteroids should only be used in individuals with genotype TT at rs17525495 who have a high inflammatory profile. This hypothesis is under investigation through an ongoing Phase III randomized controlled noninferiority trial of the effect of dexamethasone in HIV-negative adults with TBM, stratified by LTA4H genotype at rs17525495 (clinical trial study NCT03100786).
Aspirin dampens inflammation by inhibiting cyclooxygenase (COX)-1 and COX-2, which regulate prostaglandin and thromboxane production. Several small studies suggested that aspirin given to patients with TBM on anti-TB therapy was beneficial [36, 37]. In a recent Phase II trial comparing the effects of 2 different aspirin doses to placebo when added to anti-TB therapy, researchers found similar safety across the 3 study arms and risk of death or new brain infarcts that was lower in the aspirin arms, although the difference was not statistically significant [38]. Thalidomide, another drug that inhibits TNF production, is effective in the treatment of leprosy hypersensitivity reactions and has been examined in small TBM studies. Although clinical and radiographic improvement with the addition of thalidomide in children with TBM has been reported [39], high-dose thalidomide was associated with worse outcomes in a small, randomized clinical trial, and teratogenic properties limit its use. Targeting treatment towards specific mediators of TBM pathology has been challenging due to inadequate knowledge about pathogenesis. In HIV-positive individuals, TBM-related immune reconstitution inflammatory syndrome (IRIS) has been shown to have CSF and blood inflammatory signatures that are characterized by neutrophil and inflammasome-mediated inflammatory responses [40]. In addition, elevated levels of TNF and low levels of IFN-γ were associated with a higher risk of developing TBM-IRIS [41]. Possible HDT targets for TBM-IRIS intervention including blockade of IL-1β and TNF-mediated signaling. In HIV-negative subjects, a recent study discovered that low CSF tryptophan levels were associated with increased survival among TBM subjects [42]. The mechanism of this association is unknown but might be secondary to depletion of tryptophan as an energy source for Mtb or effects of tryptophan metabolites on inflammation (eg, kynurenines). Manipulation of tryptophan metabolite levels offers a potential novel HDT strategy for TB and TBM management [43].
In summary, TBM offers unique challenges as closed space infection in that optimal patient outcomes may require a host response that is neither too weak (resulting in TB susceptibility) nor too strong (enhancing pathogenesis, increasing mortality, and neurological impairment). The challenge for HDT for TBM is to identify the appropriate immunologic interventions for an individual patient that balance these competing priorities.
PUBLIC HEALTH IMPLICATIONS
For improved TB control, several potential roles for HDT have been suggested, including reducing the risk of infection or risk of progression from infection to disease, augmenting current first-line and DR-TB treatments to improve potency and lowering the potential for resistance emergence, reducing the risk of relapse after treatment completion and allow for shorter courses of treatment, or reducing immunopathology. In a broad sense, the effect of HDT in these settings may be mediated through enhancing immune system containment of Mtb, or reducing immunopathology, or a combination of the 2 (Figure 1).
Certain potential applications of HDT would be expected to have greater impact on the global TB epidemic than others. Applications aimed at reducing inflammation and/or tissue damage in patients being treated for active TB disease may reduce the long-term sequelae of TB, such as lung function impairment [44]. One challenge is that lung damage may have occurred before patients seek medical attention, although some agents may halt further damage or even reverse it. A mortality benefit has already been demonstrated with dexamethasone as adjunctive therapy in TB meningitis [45], and prednisone has been shown to be effective for both treatment and prevention of TB-associated IRIS, an immune-mediated reaction that may complicate ARV therapy initiation in HIV-infected TB patients [46]. Such applications, altthough potentially improving individual health, are not predicted to have a significant impact in terms of preventing new TB infections or progression to TB disease.
CONCLUSIONS
In terms of substantial epidemic impact, a HDT strategy that involved treating people with latent TB infection to reduce the risk of progression to active disease could have substantial epidemiological impact. It is likely that any such HDT would need to be used as an adjuvant to a microbe-targeted preventive therapy to improve its efficacy and/or shorten the duration of treatment. Ideally, such an HDT would be targeted at individuals at highest risk for progression to active disease, although novel simple biomarkers are required to make this strategy scalable [47]. If such applications were effective, safe, simple, short in duration, and affordable, they could have a substantial epidemiological impact by reducing new cases of TB disease and preventing the secondary infections that occur due to ongoing transmission from such cases.
Notes
Financial support.B. D. K. was supported by funding from an International Early Career Scientist Award from the Howard Hughes Medical Institute, the South African National Research Foundation; the South African Medical Research Council with funds received from the National Department of Health; and the Bill and Melinda Gates Foundation. M. T. S. was supported by the South African National Research Foundation. T. R. H. was supported by National Institutes of Health (NIH) and the Bill and Melinda Gates Foundation. A. J. C. S. was supported by NIH and the South African Medical Research Council. G. M. was supported by the Wellcome Trust, the South African Research Chairs Initiative of the Department of Science and Technology, National Research Foundation of South Africa, and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health.
Potential conflicts of interest.All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: Gordon Research Conference for Tuberculosis Drug Development, June 2017, Lucca, Italy.
References
- 1. Mahon RN, Hafner R.. Immune cell regulatory pathways unexplored as host-directed therapeutic targets for Mycobacterium tuberculosis: an opportunity to apply precision medicine innovations to infectious diseases. Clin Infect Dis 2015; 61(Suppl 3):S200–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallis RS, Hafner R.. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15:255–63 [DOI] [PubMed] [Google Scholar]
- 3. Liu CH, Liu H, Ge B.. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol 2017; 14:963–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wejse C, Gomes VF, Rabna P, et al. . Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2009; 179:843–50 [DOI] [PubMed] [Google Scholar]
- 5. Tukvadze N, Sanikidze E, Kipiani M, et al. . High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr 2015; 102:1059–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ganmaa D, Munkhzul B, Fawzi W, et al. . High-dose vitamin D3 during tuberculosis treatment in mongolia. A randomized controlled trial. Am J Respir Crit Care Med 2017; 196:628–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mily A, Rekha RS, Kamal SM, et al. . Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med 2013; 13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng CY, Gutierrez NM, Marzuki MB, et al. . Host sirtuin 1 regulates mycobacterial immunopathogenesis and represents a therapeutic target against tuberculosis. Sci Immunol 2017; 2:35845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singhal A, Jie L, Kumar P, et al. . Metformin as adjunct antituberculosis therapy. Sci Trans Med 2014; 6:263ra159. [DOI] [PubMed] [Google Scholar]
- 10. Dutta NK, Pinn ML, Karakousis PC.. Metformin adjunctive therapy does not improve the sterilizing activity of the first-line antitubercular regimen in mice. Antimicrob Agents Chemother 2017; 61:e00652–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Degner NR, Wang JY, Golub JE, Karakousis PC.. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin Infect Dis 2018; 66:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh A, Mohan A, Dey AB, Mitra DK.. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis 2013; 208:603–15 [DOI] [PubMed] [Google Scholar]
- 13. Skrahin A, Ahmed RK, Ferrara G, et al. . Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med 2014; 2:108–22 [DOI] [PubMed] [Google Scholar]
- 14. Islam MN, Das SR, Emin MT, et al. . Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012; 18:759–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cadena AM, Fortune SM, Flynn JL.. Heterogeneity in tuberculosis. Nat Rev Immunol 2017; 17:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geldmacher C, Ngwenyama N, Schuetz A, et al. . Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med 2010; 207:2869–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bell LCK, Noursadeghi M.. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol 2018; 16:80–90 [DOI] [PubMed] [Google Scholar]
- 18. Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol 2016; 77:441–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belton M, Brilha S, Manavaki R, et al. . Hypoxia and tissue destruction in pulmonary TB. Thorax 2016; 71:1145–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Connors TA. Bioreductive agents, hypoxic cells and therapy. Eur J Cancer 1996; 32A:1833–4 [DOI] [PubMed] [Google Scholar]
- 21. Hunter FW, Wouters BG, Wilson WR.. Hypoxia-activated prodrugs: paths forward in the era of personalised medicine. Br J Cancer 2016; 114:1071–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skerry C, Pinn ML, Bruiners N, Pine R, Gennaro ML, Karakousis PC.. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother 2014; 69:2453–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dutta NK, Bruiners N, Pinn ML, et al. . Statin adjunctive therapy shortens the duration of TB treatment in mice. J Antimicrob Chemother 2016; 71:1570–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chengalroyen MD, Beukes GM, Gordhan BG, et al. . Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 2016; 194:1532–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malherbe ST, Shenai S, Ronacher K, et al. . Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 2016; 22:1094–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutta NK, Alsultan A, Gniadek TJ, et al. . Potent rifamycin-sparing regimen cures guinea pig tuberculosis as rapidly as the standard regimen. Antimicrob Agents Chemother 2013; 57:3910–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subbian S, Tsenova L, O’Brien P, et al. . Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog 2011; 7:e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Napier RJ, Norris BA, Swimm A, et al. . Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog 2015; 11:e1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lanoix JP, Lenaerts AJ, Nuermberger EL.. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 2015; 8:603–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilkinson RJ, Rohlwink U, Misra UK, et al. . Tuberculous meningitis. Nat Rev Neurol 2017; 13:581–98 [DOI] [PubMed] [Google Scholar]
- 31. Prasad K, Singh MB, Ryan H.. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2016; 4:Cd002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heemskerk AD, Bang ND, Mai NT, et al. . Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016; 374:124–34 [DOI] [PubMed] [Google Scholar]
- 33. Tobin DM, Roca FJ, Oh SF, et al. . Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 2012; 148:434–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thuong NTT, Heemskerk D, Tram TTB, et al. . Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J Infect Dis 2017; 215:1020–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Laarhoven A, Dian S, Ruesen C, et al. . Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J Infect Dis 2017; 215:1029–39 [DOI] [PubMed] [Google Scholar]
- 36. Misra UK, Kalita J, Nair PP.. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 2010; 293:12–7 [DOI] [PubMed] [Google Scholar]
- 37. Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P.. The role of aspirin in childhood tuberculous meningitis. J Child Neurol 2011; 26:956–62 [DOI] [PubMed] [Google Scholar]
- 38. Mai NTH, Dobbs N, Phu NH, et al. . A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife 2018; 7. doi: 10.7554/eLife.33478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Toorn R, du Plessis AM, Schaaf HS, Buys H, Hewlett RH, Schoeman JF.. Clinicoradiologic response of neurologic tuberculous mass lesions in children treated with thalidomide. Pediatr Infect Dis J 2015; 34:214–8 [DOI] [PubMed] [Google Scholar]
- 40. Marais S, Lai RPJ, Wilkinson KA, Meintjes G, O’Garra A, Wilkinson RJ.. Inflammasome activation underlying central nervous system deterioration in HIV-associated tuberculosis. J Infect Dis 2017; 215:677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marais S, Meintjes G, Pepper DJ, et al. . Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013; 56:450–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Laarhoven A, Dian S, Aguirre-Gamboa R, et al. . Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis 2018; 18:526–35 [DOI] [PubMed] [Google Scholar]
- 43. Gautam US, Foreman TW, Bucsan AN, et al. . In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2018; 115:E62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allwood BW, Myer L, Bateman ED.. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013; 86:76–85 [DOI] [PubMed] [Google Scholar]
- 45. Thwaites GE, Nguyen DB, Nguyen HD, et al. . Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351:1741–51 [DOI] [PubMed] [Google Scholar]
- 46. Meintjes G, Wilkinson RJ, Morroni C, et al. . Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zak DE, Penn-Nicholson A, Scriba TJ, et al. . A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; 387:2312–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Albertella MR, Loadman PM, Jones PH, et al. . Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res 2008; 14:1096–104 [DOI] [PubMed] [Google Scholar]
- 49. Konopleva M, Thall PF, Yi CA, et al. . Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica 2015; 100:927–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Limani P, Linecker M, Kachaylo E, et al. . Antihypoxic potentiation of standard therapy for experimental colorectal liver metastasis through myo-inositol trispyrophosphate. Clin Cancer Res 2016; 22:5887–97 [DOI] [PubMed] [Google Scholar]