Endpoints beyond mortality alone elucidate treatment benefits for hospital-acquired/ventilator-associated bacterial pneumonia patients. We developed a “mortality-plus” endpoint using a Medical Dictionary for Regulatory Activities database interrogation tool, incorporating important pneumonia complications (eg, respiratory failure). This endpoint has multiple applications in study analysis/interpretation.
Keywords: hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia, all-cause mortality, mortality-plus endpoint
Abstract
Background
The US Food and Drug Administration solicited evidence-based recommendations to improve guidance for studies of hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP).
Methods
We analyzed 7 HABP/VABP datasets to explore novel noninferiority study endpoints and designs, focusing on alternatives to all-cause mortality (ACM).
Results
ACM at day 28 differed for ventilated HABP (27.8%), VABP (18.0%), and nonventilated HABP (14.5%). A “mortality-plus” (ACM+) composite endpoint was constructed by combining ACM with patient-relevant, infection-related adverse events from the Medical Dictionary for Regulatory Activities toxic/septic shock standardized query. The ACM+ rate was 3–10 percentage points above that of ACM across the studies and treatment groups. Predictors of higher ACM/ACM+ rates included older age and elevated acute physiology and chronic health evaluation (APACHE) II score. Only patients in the nonventilated HABP group were able to report pneumonia symptom changes.
Conclusions
If disease groups and patient characteristics in future studies produce an ACM rate so low (<10%–15%) that a fixed noninferiority margin of 10% cannot be justified (requiring an odds ratio analysis), an ACM+ endpoint could lower sample size. Enrichment of studies with patients with a higher severity of illness would increase ACM. Data on symptom resolution in nonventilated HABP support development of a patient-reported outcome instrument.
(See the Editorial Commentary by Eby on pages 1515–7.)
At the request of the US Food and Drug Administration (FDA), the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium established a project team to advance scientifically rigorous hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP) drug development based on a noninferiority (NI) study design [1].
The multidisciplinary team of representatives from government, academia, and industry with expertise in antibiotic development submitted preliminary recommendations to the FDA docket [2–4]. All-cause mortality (ACM) was acknowledged as an objective, verifiable, and reproducible endpoint, especially for VABP. The team supported utilizing the intention-to-treat (ITT) population as the primary efficacy set, with the microbiological ITT population as a key secondary subset. However, further improvement in HABP/VABP study feasibility, while maintaining scientific validity, was judged desirable.
Important concepts for further exploration included the following [3]:
1. Registrational studies often exclude the most ill patients, exacting costs of reduced enrollment and decreased generalizability. Less restrictive exclusion criteria could increase participation, severity of illness, and generalizability. Identifying prognostic/risk factors for a higher ACM rate would inform study design.
2. An important consideration for a new endpoint is its impact on study sample size. For a given NI margin, utilizing a risk difference analysis approach, the sample size decreases as the endpoint rate decreases from 50%. However, if the endpoint rate drops below 15%, the potential for loss of clinically acceptable efficacy with a 10% NI margin may be judged too great, such that a smaller NI margin is appropriate. If a fixed NI margin of 10% is not supportable, then either a lower fixed NI margin (eg, 7%) or an odds-ratio analysis approach is required [5]. Either approach results in an increased study sample size. For example, a trial design assuming an ACM rate of 15% and a 10% NI margin requires 402 patients for the primary analysis. However, an ACM rate of 10% would most likely require a narrower 7% NI margin to avoid the possibility of an excessive loss of a clinically relevant treatment effect, thereby resulting in an increase in required enrollment to approximately 550 patients. Therefore, a clinically relevant endpoint having an occurrence rate of 15%–20%, which would allow use of a 10% NI margin, would enable the study to have sensitivity to rule out clinically meaningful differences, with a decreased sample size.
3. Differences in ACM among the nonventilated HABP (nv-HABP), ventilated HABP (v-HABP), and VABP groups [6] suggest that pooling their outcomes raises methodological issues. Because a clinically meaningful endpoint of symptom improvement plus survival for nv-HABP is supported by the historical data for community-acquired bacterial pneumonia (CABP) [7], nv-HABP could be studied separately utilizing a patient-reported outcome (PRO) instrument, thereby standardizing symptom collection [8].
Given these considerations, sponsors of recent studies generously provided access to HABP/VABP datasets for further statistically rigorous evaluation. We summarize our findings and recommendations, based on our prior submission to the FDA docket in 2017 [3].
METHODS
A 2-part statistical analysis plan specified: part 1, a descriptive analysis of 3 recent HABP/VABP studies to understand potential endpoints and analysis populations; and part 2, a definitive analysis of the 5 datasets that allowed the most detailed analyses [3]. The FDA generously shared hypothesis-generating analyses [9]. Datasets are identified in the references [10–17]. Although some studies employed the terminology “hospital-acquired pneumonia” and “ventilator-associated pneumonia,” we employ the FDA terminology “HABP” and “VABP” [18]. We defined HABP as “nonventilated” or mechanically “ventilated” (ie, intubated, including tracheostomy patients, and mechanically ventilated at randomization).
Part 1 studies enrolled both HABP and VABP patients: (1) telavancin ATTAIN (Study 0015) [15]; (2) telavancin assessment of telavancin for treatment of hospital-acquired pneumonia (ATTAIN) (Study 0019) [15] (Theravance Biopharma, Inc); (3) tigecycline (Pfizer Study 311) [10] (Pfizer, Inc) Part 2 utilized the following datasets: (1) Pfizer Study 311; (2) doripenem VABP Study-08, (Dori-08) [11]; (3) doripenem HABP/early VABP Study-09, (Dori-09) [13]; (4) doripenem VABP Study-10, (Dori-10) [14] (all Shionogi, Inc); (5) prospective, observational intensive care unit study (non-industry-sponsored study) (Servei de Pneumologia, Hospital Clinic, Institut d'Investigacions Biomèdiques August Pi i Sunyer, Centro de investigación Biomédica en Red en Enfermedades Respiratorias, Universitat de Barcelona, Spain).
Statistical Analysis Plan, Part 1
This first step evaluated whether the datasets included patient demographics; predictor/risk variables for ACM (eg, acute physiology and chronic health evaluation (APACHE) II score; evolution of pneumonia signs and oxygenation (fractional inspired oxgen (FiO2)/arterial oxygen tension (PaO2)) during treatment; pneumonia symptom improvement over time; ACM rates; and adverse events (AEs) that could form a “mortality-plus” (ACM+) endpoint (a composite endpoint of ACM plus selected AEs reflecting how a patient feels or functions). A focused literature review identified predictors of ACM in HABP/VABP patients [3].
Statistical Analysis Plan, Part 2
Part 2 objectives were to determine ACM incidence during days 14–28; baseline characteristics associated with higher ACM rates; incidence and types of AEs/serious AEs (SAEs) relevant to an ACM+ endpoint; potential utility of a symptom-based endpoint in nv-HABP; impact of prior antibiotic therapy on outcome; impact of adjunctive systemic antibiotic therapy on outcome; and utility of Gram stain of respiratory secretions for predicting microbiologically confirmed infection (not performed due to absence of data).
The primary analysis populations were the all-treated (AT, ie, ITT patients receiving any study drug) and microbiological AT (micro-AT; ie, AT with a pathogen isolated from respiratory secretions and/or blood). Analyses were conducted for nv-HABP, v-HABP, and VABP. Each study sponsor performed analyses, except that author A. D. analyzed the Shionogi database, which provided unblinded treatment assignment.
Cox regression analysis of risk/prognostic factors for ACM was performed on Shionogi studies Dori-08, Dori-09, and Dori-10. (Doripenem was found inferior in efficacy to comparator [including a higher ACM rate] in some studies, and the sponsor permitted analyses that included treatment assignment.) Specified baseline variables were treatment assignment (doripenem vs comparator); older age; female sex; elevated APACHE II score; bacteremia; nonfermenting gram-negative pathogen or methicillin-resistant Staphylococcus aureus (MRSA) as the etiological pathogen; impaired oxygenation (FiO2/PaO2 <250 vs ≥250); prior antibiotic use within 48 hours of study drug initiation; inadequate prestudy antimicrobial therapy; and inadequate initial antimicrobial therapy. Study was also assessed (Dori-08 and Dori-09 with Dori-10 as the reference). Bacteremia was defined by the sponsor’s database. “Inadequate” therapy was defined as discordance between the antimicrobial received and the susceptibility profile of the isolated baseline pathogen(s).
Sample size estimates for an NI study based on ACM or ACM+ endpoint rates were calculated, assuming 80% power and a 2-sided α = .05; calculations also were performed for an endpoint of improvement of ≥2 nv-HABP symptoms.
Part 1 Results
The datasets contained patient demographics and relevant predictor/risk variables for ACM. Although oxygenation data were often available in the ATTAIN VABP population, data points decreased rapidly over time. Absent positive end-expiratory pressure settings were another limitation.
For nv-HABP, the baseline frequency and evolution of pneumonia symptoms supported a symptom-based endpoint, as for CABP [7]. In Pfizer Study 311, 95.6% of nv-HABP patients had ≥2 symptoms at baseline (vs 46.8% for VABP). However, the endpoint previously derived for CABP could not be applied to HABP, as the distribution of baseline symptoms between CABP and HABP was not comparable.
ACM rates were reported for nv-HABP, v-HABP, and VABP, differing notably within and across studies: for example, in Pfizer Study 311, ACM rates were 9.8%, 15.2%, and 12.6%, respectively, at day 28, whereas in the ATTAIN studies, rates were 18.8%, 30.2%, and 26.3%, respectively. The number of AEs plausibly related to the underlying pneumonia (eg, respiratory failure, empyema) supported development of an ACM+ endpoint.
Part 2 Results
A summary of major findings by analysis objective follows.
Determine Incidence of All-Cause Mortality Within a Day 14–28 Endpoint Window
ACM rates for days 14 and 28 are shown in Table 1. The Barcelona study had both the highest rates of ACM and a statistically significant difference in ACM for v-HABP > VABP > nv-HABP.
Table 1.
Range of Point Estimates of Percentage All-Cause Mortality at Days 14 and 28
| Sponsor (Analysis Population) |
VABP | Ventilated HABP |
Nonventilated HABP |
|---|---|---|---|
| Study day 14 | |||
| Shionogi (AT) | 6.3%–9.8% | 9.5%–14.9% | 3.1%–9.6% |
| Pfizer (AT) | 7.9% | 6.5% | 6.1% |
| Theravance (AT) | 16.8% | 16.3% | 13.0% |
| Barcelona (AT-ICU) | 19.8% | 24.0% | 17.4% |
| Study day 28 | |||
| Shionogi (AT) | 10.2%–19.9% | 20.8%–23.2% | 11.0%–13.5% |
| Pfizer (AT) | 12.6% | 15.2% | 9.8% |
| Theravance (AT) | 26.3% | 30.2% | 18.8% |
| Barcelona (AT-ICU) | 27.0% | 39.4% | 21.7% |
Abbreviations: AT, all-treated patient analysis population; HABP, hospital-acquired bacterial pneumonia; ICU, intensive care unit; VABP, ventilator-associated bacterial pneumonia.
Identify Baseline Characteristics Associated With Higher Rates of All-Cause Mortality
The analyses focused on the doripenem studies for reasons noted above. In Dori-09 (HABP and early VABP), no significant independent predictor of ACM was identified (AT population). Subsequent analyses performed without the inadequate therapy variables because of low patient numbers in those groups identified impaired oxygenation at baseline as significant for VABP ACM (hazard ratio [HR], 0.356 [95% confidence interval {CI}, .167–.760]). The ratio was in the opposite direction of that expected; that is, impaired oxygenation was associated with lower ACM. For the micro-AT VABP population, older age was a predictor of ACM (HR, 2.4726 [95% CI, 1.254–4.873]), but impaired oxygenation was not.
In VABP studies Dori-08 and -10, analyses without the inadequate therapy variables showed older age as significant (HR, 1.400 [95% CI, 1.182–1.659]). In the Micro-AT VABP population, older age (Dori-10) and baseline nonfermenting gram-negative pathogen/MRSA (Dori-08) were predictors of ACM (HR, 1.313 [95% CI, 1.005–1.715] and 2.038 [95% CI, 1.039–4.000], respectively).
Combining VABP patients from Dori -08, -09, and -10, older age remained a significant predictor of ACM (Table 2); prior antibiotic use within 48 hours of baseline and enrollment in Dori-08 (the latter consistent with sponsor and FDA analyses) also were significant (Table 2).
Table 2.
Cox Regression Analysis of Risk/Prognostic Factors for All-Cause Mortality in Patients With Ventilator-Associated Bacterial Pneumonia, Combined Across Studies (All-Treated Population, Dori-08, -09, and -10)
| VABP | ||
|---|---|---|
| Variable | Hazard Ratio | (95% CI) |
| Treatment group | 0.955 | (.818–1.115) |
| Older age | 1.400 | (1.182–1.659) |
| Female sex | 1.076 | (.904–1.281) |
| Elevated APACHE II score | 0.978 | (.640–1.496) |
| Bacteremia | 0.937 | (.724–1.211) |
| Nonfermenting gram-negative pathogen or MRSA | 1.085 | (.832–1.416) |
| Impaired oxygenation (PaO2/FiO2 <250) | 0.993 | (.828–1.191) |
| Prior antibiotic use within 48 h | 1.198 | (1.006–1.426) |
| Inadequate prestudy therapy | 0.917 | (.713–1.178) |
| Inadequate initial therapy | 0.818 | (.558–1.199) |
| Study-08 | 1.359 | (1.130–1.635) |
| Study-09 | 1.028 | (.791–1.336) |
Abbreviations: APACHE II, xxx; CI, confidence interval; MRSA, methicillin-resistant Staphylococcus aureus; PaO2/FiO2, xxx; VABP, ventilator-associated bacterial pneumonia.
Determine Incidence of Events That Could Form the “Plus” in a Mortality-Plus Endpoint; Identify Specific Patient Baseline Characteristics Associated With Higher Rates of Candidate AEs/SAEs
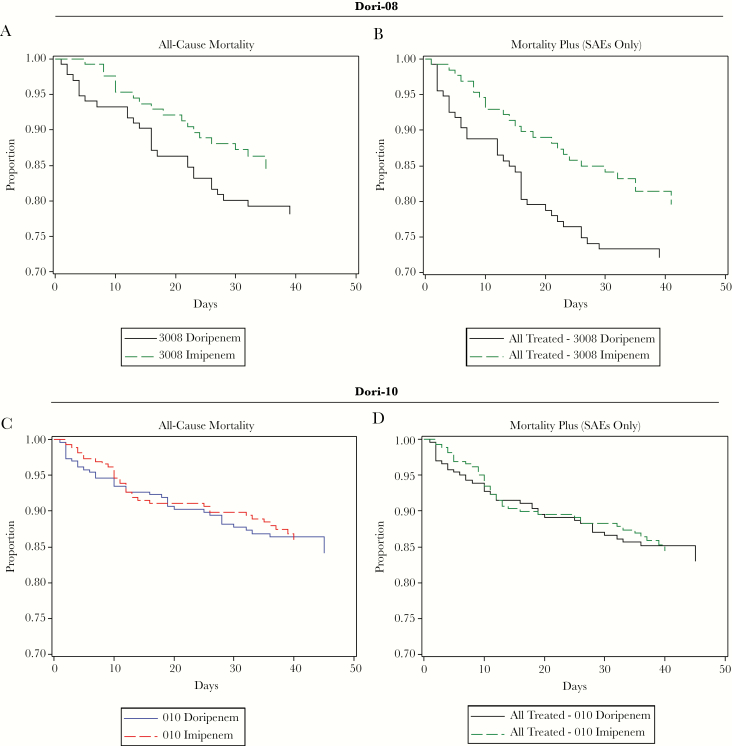
Patient SAEs and AEs as potential components of an ACM+ endpoint were identified via review of the Pfizer Study 311 and Shionogi databases. However, this study-specific approach might miss important patient-relevant events. An alternative approach utilizes the Medical Dictionary for Regulatory Activities (MedDRA) Standardized MedDRA Query (SMQ) tool; SMQs are validated database interrogation tools [19]. The Toxic/Septic Shock SMQ contains multiple, clinically important pneumonia complications (eg, sepsis, respiratory failure). A study database could be interrogated readily using this SMQ to define the “plus” for an ACM+ endpoint. The frequencies of “plus” events were examined for Shionogi studies using nonfatal toxic/septic shock SMQ AEs (including SAEs) and SAEs (Table 3 is an example, using Dori-08 and -10). With variability by study and treatment group, ACM+ demonstrates an increased endpoint event frequency over ACM alone (Table 4). ACM+ rates using toxic/septic shock SMQ SAEs were compared to ACM rates alone for Dori-08 and Dori-10; addition of the “plus” events increased the doripenem event rates in Dori-08 (this study had a significantly higher ACM for doripenem treatment); in contrast, in Dori-10, for which ACM was comparable for doripenem and comparator, the ACM+ analysis did not present any “signal” (compare Figure 1A to Figure 1B and Figure 1C to Figure 1D).
Table 3.
Summary of Serious Adverse Events—Toxic Septic Shock Standardized Medical Dictionary for Drug Regulatory Activities Query (All-Treated Population, Dori-08, -09, -10)
| Dori-08 (VABP) | Dori-010 (VABP) | Dori-09 (HABP) | Dori-09 (VABP) | |||||
|---|---|---|---|---|---|---|---|---|
|
Variable |
Doripenem (n = 135) |
Imipenem- Cilastatin (n = 132) |
Doripenem (n = 262) |
Imipenem- Cilastatin (n = 263) |
Doripenem (n = 160) |
Piperacillin- Tazobactam (n = 160) |
Doripenem (n = 63) |
Piperacillin- Tazobactam (n = 61) |
| MedDRA Preferred Term | ||||||||
| Any SAE—toxic septic shock SMQ | 19 (14.1) | 15 (11.4) | 15 (5.7) | 18 (6.8) | 8 (5.0) | 7 (4.4) | 13 (20.6) | 3 (4.9) |
| General disorders and administration site conditions | ||||||||
| Multiorgan failure | 2 (1.5) | 3 (2.3) | 4 (1.5) | 6 (2.3) | 1 (0.6) | 2 (1.3) | 0 (0.0) | 0 (0.0) |
| Infections and infestations | ||||||||
| Septic shock | 8 (5.9) | 6 (4.5) | 2 (0.8) | 4 (1.5) | 0 (0.0) | 4 (2.5) | 9 (14.3) | 1 (1.6) |
| Renal and urinary disorders | ||||||||
| Acute prerenal failure | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal failure | 4 (3.0) | 1 (0.8) | 0 (0.0) | 1 (0.4) | 1 (0.6) | 0 (0.0) | 1 (1.6) | 0 (0.0) |
| Renal failure acute | 2 (1.5) | 2 (1.5) | 3 (1.1) | 3 (1.1) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Acute respiratory failure | 2 (1.5) | 2 (1.5) | 0 (0.0) | 1 (0.4) | 2 (1.3) | 0 (0.0) | 1 (1.6) | 0 (0.0) |
| Respiratory failure | 4 (3.0) | 3 (2.3) | 6 (2.3) | 6 (2.3) | 4 (2.5) | 1 (0.6) | 1 (1.6) | 2 (3.3) |
| Vascular disorders | ||||||||
| Circulatory collapse | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Shock | 1 (0.7) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Data are presented as No. (%).
Abbreviations: HABP, hospital-acquired bacterial pneumonia; MedDRA, Medical Dictionary for Drug Regulatory Activities; SAE, serious adverse event; SMQ, standardized medical query; VABP, ventilator-associated bacterial pneumonia.
Table 4.
Example of Point Estimates of Rates of All-Cause Mortality (ACM) Versus ACM+ Endpoints (Study Dori-09)
| Nonventilated HABP | VABP | |||
|---|---|---|---|---|
| Endpoint and Timing | Study Drug (n = 160) |
Comparator (n = 160) |
Study Drug (n = 63) |
Comparator (n = 61) |
| Day 14 | ||||
| ACM | 9.6% | 3.1% | 9.5% | 14.9% |
| ACM+ | 14.1% | 6.3% | 19.0% | 19.8% |
| Day 28 | ||||
| ACM | 13.5% | 11.0% | 20.8% | 23.2% |
| ACM+ | 19.4% | 13.5% | 30.4% | 26.5% |
Abbreviations: ACM, all-cause mortality; ACM+, mortality plus (all-cause mortality plus toxic/septic shock standardized Medical Dictionary for Regulatory Activities query adverse events; HABP, hospital-acquired bacterial pneumonia; VABP, ventilator-associated bacterial pneumonia.
Figure 1.
A–D, Kaplan–Meier curves for proportion of patients surviving: all-cause mortality (ACM) vs ACM plus toxic/septic shock standardized MedDRA query adverse events (ACM+) (serious adverse events only) (all-treated population). Abbreviation: SAE, serious adverse event.
In Cox regression analysis for HABP patients in Dori-09 (AT population), older age and elevated APACHE II score were prognostic factors for ACM+. For VABP patients in Dori-09, but not Dori-08 and -10, impaired oxygenation was associated with a reduced ACM+ risk. Therefore, analysis of potential prognostic factors for an ACM+ endpoint gave similar results to that for an ACM endpoint. The “plus” events generally increased the endpoint frequency relative to ACM alone, eg, by 3–10 percentage points in Dori-09. Sample size estimates for an ACM+ (or ACM) endpoint in an NI study, assuming 80% power and a 2-sided α = .05, were calculated. For a 10% NI margin, the required sample sizes for event rates of 15%, 20%, or 30% are 402, 504, and 660 patients, respectively. For a 7% margin at event rates of 10% or 12%, they are 578 and 678 patients, respectively.
Determine the Potential Utility of a Symptom-Based Endpoint in nv-HABP
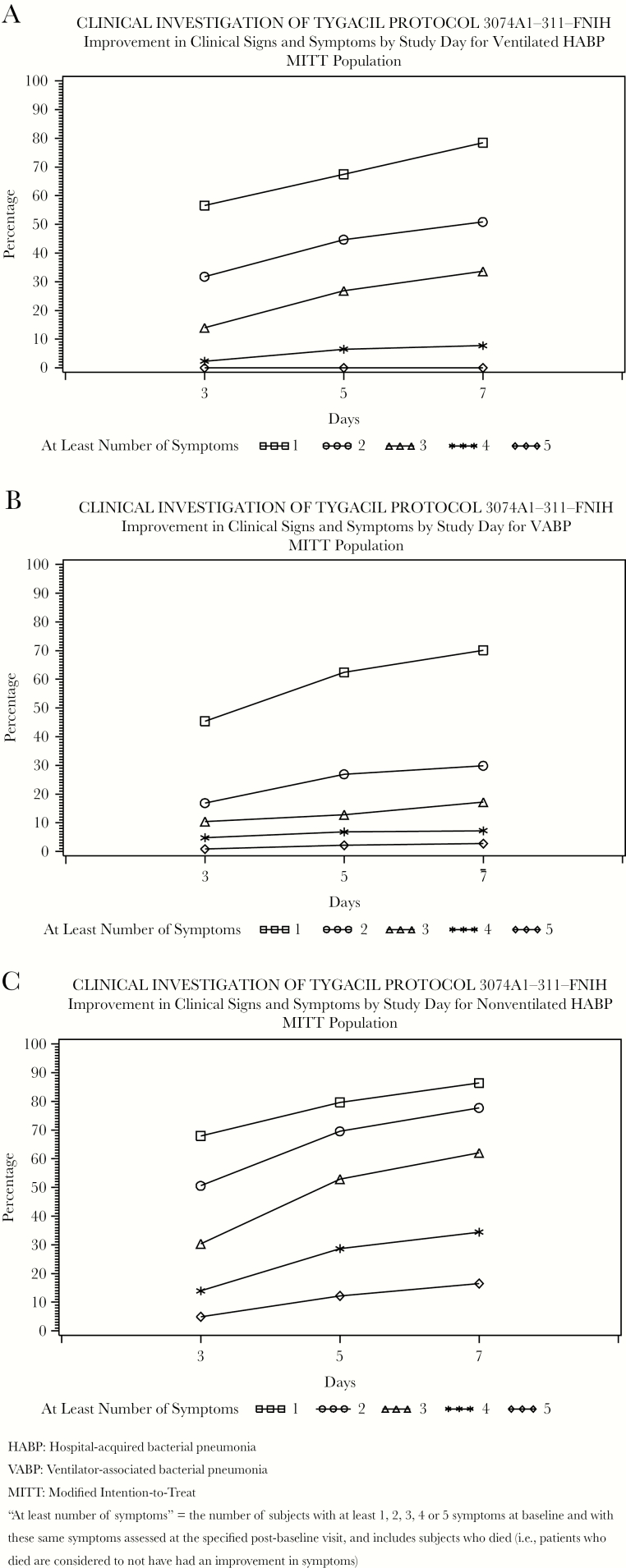
In the Pfizer database, 78.9% of all patients had 2 or more baseline pneumonia symptoms: 95.6% for nv-HABP vs 72.1% for v-HABP and 46.8% for VABP. Symptom improvement was observed at days 5 and 7. Approximately 75% of nv-HABP patients with at least 2 baseline symptoms had improvement or resolution by day 7 (Figure 2).
Figure 2.
A–C, Improvement in clinical symptoms by study day (Pfizer Study 311).
“At least number of symptoms” indicates the number of subjects with at least 1, 2, 3, 4, or 5 symptoms at baseline and with these same symptoms assessed at the specified postbaseline visit, and includes subjects who died (ie, patients who died are considered to not have had an improvement in symptoms). Abbreviations: FNIH, Foundation for the National Institutes of Health; HABP, hospital-acquired bacterial pneumonia; MITT, modified intention-to-treat; VABP, ventilator-associated bacterial pneumonia.
In the Shionogi database, 72 % of nv-HABP patients had ≥2 baseline symptoms, with 83% showing improvement in ≥2 of these at day 7 (Table 5). In the Theravance database, comparable findings were noted. Sample size estimates for an NI study based on improvement of 2 or more nv-HABP symptoms at day 5 or day 7 are presented in the docket.
Table 5.
Improvement/Resolution of Symptoms by Study Day (Shionogi Database)
| Nonventilated HABP | ||||
|---|---|---|---|---|
| Improvement | Doripenem, % (no./No.) | Piperacillin-Tazobactam, % (no./No.) | ||
| Day 5 | Day 7 | Day 5 | Day 7 | |
| Improvement in at least 2 symptoms | 71.4 (80/112) | 83.8 (78/93) | 77.6 (90/116) | 82.1 (78/95) |
| Improvement in 3 symptoms | 69.7 (23/33) | 65.5 (19/29) | 79.1 (34/43) | 89.2 (33/37) |
No. indicates the number of subjects with 2 or 3 symptoms at baseline and with these same symptoms assessed at the specified postbaseline visit, and includes subjects who died (ie, patients who died are considered to not have had an improvement in symptoms).
Abbreviation: HABP, hospital-acquired bacterial pneumonia.
DISCUSSION
Our analyses explore design elements for future HABP/VABP clinical studies for either regulatory authority review or clinical practice.
A notable observation is the difference in ACM rate across the 3 disease groups comprising the HABP/VABP indications. The mean VABP ACM rate was 18%, whereas the mean v-HABP ACM rate was 28%. ACM numerical directionality was v-HABP > VABP > nv-HABP.
The higher ACM rates for v-HABP and VABP suggest the possibility of enrolling these patients in the same study, while studying nv-HABP separately. A study of all 3 disease groups should define a priori the optimal/allowable proportion of patients in each group. Study sample size should be based on the proportion of patients and associated mortality in each group. Other options are to not combine these groups, or to statistically power each group so that there is sensitivity to treatment effect within each.
Justification for including nv-HABP and v-HABP in a single study may depend on effect modifiers, for example the effect of antibiotics on the endpoint. If a relatively constant treatment effect on the endpoint odds ratio can be assured across disease groups, they could be combined in one study. In that case, the inclusion of v-HABP, with the highest ACM, enriches the cohort and allows wider enrollment. FDA is further analyzing this issue.
Baseline characteristics associated with higher ACM and/or ACM+ rates vary by study and indication, but often include older age and an elevated APACHE II score, confirming literature observations. Enriching HABP/VABP clinical studies with such patients should increase the ACM rate. The Barcelona study, conducted without commonplace registrational trial exclusions, reported the highest ACM, highlighting the potential limited generalizability of some industry trial results, as well as opportunities to increase enrollment and enrich for ACM.
If the anticipated ACM rate is so low as to dictate a smaller NI margin (to ensure the relative loss of efficacy at the extreme of the NI margin is not too large), then an ACM+ approach can be employed to increase the endpoint frequency. Defining SAEs and/or AEs post hoc is unacceptable. A better approach is employing the MedDRA toxic/septic shock SMQ to define the “plus” in an ACM+ endpoint. This endpoint could also add value as a sensitivity analysis, informing treatment effects on survivors. A weakness of the ACM endpoint is the exclusion of a majority of patients from analysis. Finally, use of an ACM+ endpoint could increase event rates at day 14 to a level compatible with a 10% NI margin.
Is it methodologically appropriate to use ACM+ instead of ACM to increase the outcome event rate? The answer depends on whether the “plus” is important to how a patient feels or functions and would be impacted by antibiotic therapy. For example, an AE of gunshot wound after hospital discharge arguably should not be part of an ACM+ endpoint in a HABP/VABP study. However, “plus” events need not be rigidly pneumonia-related (eg, empyema) but, rather, should include general infection-related events such as those in the toxic/septic shock SMQ.
Does an NI margin justification for ACM apply to an ACM+ endpoint: so-called “margin bridging”? Our analyses for the HABP/VABP indications support the propositions that events from the toxic/septic shock SMQ can boost the endpoint event rate and that NI margin bridging allows the same NI margin for an ACM+ endpoint as for ACM.
A symptom-based endpoint could be useful for studies of nv-HABP patients, who can report symptoms of their pneumonia at baseline and on therapy. Study days 5–7 are a suitable window to capture symptom resolution. These data support the ongoing development of a PRO measure for nv-HABP [20], similar to what is being done for CABP [21].
Study strengths include the diverse databases explored, their recent vintages, and the novel hypotheses explored. Limitations of these analyses include their retrospective nature and the interrogation of selected study datasets. Pneumonia symptom data may not have been elicited as actively as with administration of a PRO instrument. Additionally, data analysis was performed within the constraints of the sponsor’s original study design and data capture, including the inability for comprehensive baseline pathogen identification or specific bacterial resistance phenotype. These considerations do not impact conclusions about the utility of an ACM+ endpoint or the development of an nv-HABP PRO.
In summary, these analyses produced useful insights into future HABP/VABP study design options that could provide flexibility to sponsors, improve study feasibility, and maintain scientific rigor.
Supplementary Material
APPENDIX
HABP/VABP Project Team members. George H. Talbot, MD (Co-Chair; Talbot Advisors); Jeff Alder, PhD (Bayer Healthcare); Mari Ariyasu (Shionogi); Helen Boucher, MD (Tufts University, Infectious Diseases Society of America); Sue Cammarata, MD (Melinta); Lynn Connolly, MD (Achaogen); Stephanie Cush, PhD (Foundation of the NIH [FNIH]); Aaron Dane, MSc (DaneStat); Anita Das, PhD (AdStat Consulting); Carisa De Anda, PharmD (Merck); Dennis Dixon, PhD (NIH/National Institute of Allergy and Infectious Diseases [NIAID]); Mike Dudley, PharmD (The Medicines Company); Roger Echols, MD (Infectious Disease Drug Development Consulting); Janet Ehlert, RAC (Shionogi); Barry Eisenstein, MD (CARB-X); Mark Eisner, MD (Genentech); Thomas File, MD (Summa Health System); Thomas R. Fleming, PhD (University of Washington); Dean Follmann, PhD (NIH/NIAID); H. David Friedland, MD (Cerexa); Ian Friedland, MD (Achaogen); Steve Hoffmann, M.S. (FNIH); Maria Arantxa Horga, MD (Roche); Kellee Howard, M.S. (ICON); Nicholas A. Kartsonis, MD (Merck); Achim Kaufhold, MD, PhD (Basilea Pharmaceutica International); Amy Kindrick (Roche/Genentech); Lily Llorens, PhD (Cerexa); Jeff Loutit, MD (The Medicines Company); Paul McGovern (Paratek Pharma); John H. Powers, MD (George Washington University School of Medicine); Philippe Prokocimer, MD (Cubist/Merck); John H. Rex, MD (F2G Ltd); Elyse Seltzer, MD (Nabriva Therapeutics); Claire Sherman, PhD (Theravance); Anthony Suffredini, MD (Clinical Center, NIH); Antoni Torres, MD (University of Barcelona; Centro de investigación Biomédica en Red en Enfermedades Respiratorias and Hospital Clinic); Larry Tsai, MD (Tetraphase); Michele Wible, MS (Pfizer); and Richard Wunderink, MD (Northwestern University).
Food and Drug Administration nonvoting observers: Joseph Toerner, MD, MPH, Daniel Rubin, PhD. Partners (*contributed clinical study data and statistical analysis): Achaogen, Inc; Actelion Pharmaceuticals Ltd; American Thoracic Society; AstraZeneca Pharmaceuticals; LP Basilea Pharmaceutica International Ltd; Bayer HealthCare Pharmaceuticals, Inc; Cubist Pharmaceuticals, Inc; ICON plc; InClin; Infectious Diseases Society of America; Johnson and Johnson*; The Medicines Company; Melinta Therapeutics; Merck Sharp & Dohme; Nabriva Therapeutics; AG; NIAID; Pfizer Inc*; Roche Shionogi Inc*; Tetraphase Pharmaceuticals; Theravance Biopharma, Inc*; Universitat de Barcelona*.
Presented in part: 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 21–24 April 2018 and American Society for Microbiology Microbe, Atlanta GA, 4–7 June 2018. In addition, details of the work were submitted to the US Food and Drug Administration docket: “Considerations for Clinical Trial Design for the Study of Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacteria Pneumonia. Foundation for the National Institutes of Health Biomarkers Consortium HABP/VABP Project Team” for HABP/VABP draft guidance, 13 June 2017, submission to docket number FDA-2010-D-0589.
Notes
Acknowledgments. The work of the Project Team (see Appendix) is gratefully acknowledged, especially because it depends upon the generous contributions of personal time and corporate resources in this public–private endeavor. We give special thanks to Anita Das, Aaron Dane, Michele Wible, and Antoni Torres for data analysis.
Financial support. This work was supported by the Foundation of the National Institutes of Health (NIH) Biomarkers Consortium Project “Developing Endpoints for Clinical Trials of Drugs for Treatment of HABP and VABP”. In addition to the NIH and FDA , funding organizations included Achaogen, Actelion, Basilea Pharmaceutica, Bayer, Cubist Pharmaceuticals, The Medicines Company, Melinta, Merck, Roche, and Tetraphase. Clinical trial data were generously contributed to the project by Johnson & Johnson, Pfizer Inc, Shionogi Inc, Theravance Biopharma, Inc, and Hospital Clinic and University of Barcelona.
Potential conflicts of interest. G. H. T. has served as a board member for Nabriva Therapeutics; as a consultant for Adynxx and Meiji Seika; as a scientific advisor (review panel or advisory committee) for Actelion, Arsanis, Polyphor, Ltd, Recida Therapeutics, and Zavante; as a shareholder (excluding diversified mutual funds) for AN2 Therapeutics, Calixa Therapeutics, Nabriva Therapeutics, Recida Therapeutics, and Tripex. A. D. has served as a consultant for Paratek, Nabriva Therapeutics, Achaogen, AntibioTx, Cempra, Contrafect, Iterum Therapeutics, Tetraphase, Wockhardt, and Zavante; and as a data and safety monitoring board member for Theravance. S. C. is an employee and shareholder (excluding diversified mutual funds) of Melinta Therapeutics. J. H. P. has served as a consultant for AbbVie, Cardeas, Cempra, ContraFect, Gilead, JNJ, Lilly, MedImmune, Otsuka, and Roche. A. D. has served as a consultant for F2G, Zavanate, Geom, Achaogen, Allecra, Cidara, ContraFect, Spero, Phico Therapeutics, TenNor, Roche, GSK, Nabriva Therapeutics, and Davolterra; and as a shareholder (excluding diversified mutual funds) for Geom. M. W. is an employee and shareholder (excluding diversified mutual funds) of Pfizer. R. E. has served as a consultant for Shionogi, KBP Biosciences, Aradigm Corp, and Motif Bio. A. T. has served as a scientific advisor (review panel or advisory committee) for Bayer, Roche, Arsanis, Polyphor Ltd, and Pfizer. J. H. R. has served as a board member for F2G Ltd and Adenium Biotech ApS; as a consultant for Adenium Biotech ApS, Advent Life Sciences, Phico Therapeutics, ABAC Therapeutics, Polyphor, Heptares Therapeutics, Gangagen, Basilea Pharmaceutica International, Allecra Therapeutics GmbH, Forge Therapeutics, AtoxBio, and Peptilogics; as a scientific advisor (review panel or advisory committee) for Wellcome Trust, Macrolide Pharmaceuticals, Bugworks Research, Basilea Pharmaceutica, Forge Therapeutics, and Novo Holdings; and as a shareholder (excluding diversified mutual funds) for AstraZeneca Pharmaceuticals, F2G, Advent Life Sciences, Macrolide Pharmaceuticals, Bugworks Research, and Adenium Biotech ApS. J. L. is an employee and shareholder (excluding diversified mutual funds) of The Medicines Company. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rex JH, Talbot GH, Goldberger MJ, et al. . Progress in the fight against multidrug-resistant bacteria 2005-2016: modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis 2017; 65:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foundation for the National Institutes of Health Biomarkers Consortium HABP/VABP Working Group. Interim considerations for clinical trial design for the study of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. July 15, 2013, submission to docket #FDA-2013-N-0556 https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Pink%20Sheet/75/36/fnihcommentsantibacterial.pdf. Accessed 2 April 2018.
- 3. Biomarkers Consortium of the Foundation for the National Institutes of Health HABP-VABP Project Team. Considerations for clinical trial design for the study of hospital-acquired bacterial pneumonia and ventilator-associated bacteria pneumonia to the FDA docket for HABP/VABP draft guidance, June 13, 2017, submission to docket number FDA-2010-D-0589 https://www.regulations.gov/document?D=FDA-2010-D-0589-0027. Accessed 2 April 2018.
- 4. Talbot GH, Powers JH, Hoffmann SC; Biomarkers Consortium of the Foundation for the National Institutes of Health CABP-ABSSSI and HABP-VABP Project Teams Developing outcomes assessments as endpoints for registrational clinical trials of antibacterial drugs: 2015 update from the biomarkers consortium of the foundation for the National Institutes of Health. Clin Infect Dis 2016; 62:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaul S, Diamond GA. Good enough: a primer on the analysis and interpretation of noninferiority trials. Ann Intern Med 2006; 145:62–9. [DOI] [PubMed] [Google Scholar]
- 6. Esperatti M, Ferrer M, Theessen A, et al. . Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med 2010; 182:1533–9. [DOI] [PubMed] [Google Scholar]
- 7. Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H; CABP-ABSSSI Project Team Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis 2012; 55:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powers JH 3rd, Howard K, Saretsky T, et al. . Patient-reported outcome assessments as endpoints in studies in infectious diseases. Clin Infect Dis 2016; 63(Suppl 2):S52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rubin D. HABP/VABP issues and efficacy endpoints. FNIH biomarkers consortium HABP/VABP project team meeting, April 1, 2014. [Google Scholar]
- 10. Freire AT, Melnyk V, Kim MJ, et al. . 311 Study Group Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 2010; 68:140–51. [DOI] [PubMed] [Google Scholar]
- 11. Kollef MH, Chastre J, Clavel M, et al. . A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 2012; 16:R218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013; 57:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Réa-Neto A, Niederman M, Lobo SM, et al. . Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr Med Res Opin 2008; 24:2113–26. [DOI] [PubMed] [Google Scholar]
- 14. Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit Care Med 2008; 36:1089–96. [DOI] [PubMed] [Google Scholar]
- 15. Rubinstein E, Lalani T, Corey GR, et al. . ATTAIN Study Group Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 2011; 52:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wunderink RG, Niederman MS, Kollef MH, et al. . Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54:621–9. [DOI] [PubMed] [Google Scholar]
- 17. Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–97. [PubMed] [Google Scholar]
- 18. US Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER) guidance for industry. hospital-acquired bacterial pneumonia and ventilator- associated bacterial pneumonia: developing drugs for treatment. May 2014 clinical/antimicrobial revision 2 https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM234907.pdf. Accessed 2 April 2018.
- 19. Medical Dictionary for Regulatory Activities. Standardized MedDRA Queries (SMQs) https://www.meddra.org/how-to-use/tools/smqs. Accessed 17 October 2017.
- 20. Howard K, Clifford S, Saretsky T, et al. . Content validation of a new patient-reported outcome (PRO) instrument in hospital-acquired bacterial pneumonia (HABP). In: PIN53. ISPOR 22nd Annual International Meeting, Boston, MA, 20–24 May 2017. [Google Scholar]
- 21. Howard K, Clifford S, Powers JH, et al. . Community-acquired bacterial pneumonia (CABP): development of a new patient-reported outcome (PRO). In: PIN84. ISPOR 20th Annual International Meeting, Philadelphia, PA, 16–20 May 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.