The participation rate in an Ebola vaccine protocol among eligible contacts in a small Liberian Ebola outbreak was low, and vaccination occurred relatively late after the case diagnosis, resulting in a low likelihood of benefit. Significant resources expended on vaccination might be more effectively allocated to traditional response activities.
Keywords: Ebola virus, vaccine, Ebola virus disease, PREVAIL, cluster vaccination, ring vaccination, rVSVΔG-ZEBOV-GP vaccine, Liberia, outbreak response
Abstract
Objective
In November 2015, a 15-year-old boy received a diagnosis of Ebola virus disease (EVD) at the John F. Kennedy Medical Center in Monrovia, Liberia. Two additional family members received a diagnosis of EVD. The protocol for a phase 2 placebo-controlled trial of 2 Ebola vaccines was amended and approved; in 4 days, a single-arm cluster vaccination trial using the Merck rVSVΔG-ZEBOV-GP vaccine was initiated. Here, we evaluate the safety and immunogenicity of the vaccine and discuss challenges for its implementation in a small Ebola outbreak.
Method
We conducted a ring vaccination study among contacts and contacts of close contacts of EVD cases a in Monrovia. Participants were evaluated 1 and 6 months after vaccination.
Results
Among 650 close contacts and contacts of close contacts of EVD cases, 210 (32%) consented and were vaccinated with rVSVΔG-ZEBOV-GP. Of those vaccinated, 189 (90%) attended the month 1 follow-up visit; 166 (79%) attended the month 6 visit. No serious adverse events were reported. Among 88 participants without an elevated antibody level at baseline, 77.3% (95% confidence interval, 68.5–86.1) had an antibody response at 1 month.
Conclusions
The Merck rVSVΔG-ZEBOV-GP vaccine appeared to be safe and immunogenic among the vaccinated individuals. However, fewer than one third of eligible individuals consented to vaccination. These data may help guide implementation decisions for of cluster vaccination programs in an Ebola cluster outbreak response situation.
Liberia was first declared free of Ebola virus transmission by the World Health Organization (WHO) in May 2015. Transmission resurfaced 3 additional times: in June 2015, November 2015, and March–April 2016. In August 2015, Guinea announced interim results from the rVSVΔG-ZEBOV-GP ring vaccination study (Ebola Ca Suffit!) [1], which showed that the rVSVΔG-ZEBOV-GP was safe and suggested clinical efficacy, based on the number of cases in individuals >10 days after vaccination as compared to the number of cases in those not vaccinated. Thereafter, Guinea and Sierra Leone introduced the rVSVΔG-ZEBOV-GP vaccine as part of their efforts to end Ebola virus transmission.
In November 2015, a 15-year-old boy received a diagnosis of Ebola virus disease (EVD) while hospitalized in the pediatric ward at the John Fitzgerald Kennedy (JFK) Memorial Hospital in Monrovia, Liberia. Subsequently, the boy’s father and younger brother also had EVD diagnosed. The source of the infections has been linked subsequently by molecular sequencing to the mother, who was an unrecognized survivor of EVD in 2014; owing to either Ebola virus persistence or recurrent disease, she transmitted the virus to her family [2]. Immediately following their diagnoses, as part of the response effort, the Liberian Ministry of Health requested that the National Institutes of Health access the investigational supply of the rVSVΔG-ZEBOV-GP vaccine (Merck Sharp Dohme, NJ), which was being stored in Liberia. This vaccine had been previously studied in a phase 2 trial in Liberia through the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) [3]. The PREVAIL 1 study examined the safety and immunogenicity of the rVSVΔG-ZEBOV-GP vaccine but was not able to provide any data on efficacy, owing to the decline in the number of cases in Liberia.
To fulfill this request for access to the experimental vaccine, the PREVAIL I trial protocol was amended to conduct a cluster (ie, “ring”) vaccination study in which close contacts and contacts of close contacts of EVD cases could be rapidly vaccinated using the expanded access ring protocols used in Guinea and Sierra Leone after the interim results of the Ebola Ca Suffit! study were announced. In this article, we report the safety and immunogenicity of the vaccine used in the cluster vaccination study and discuss the challenges and lessons learned in implementing this type of study during an outbreak.
MATERIALS AND METHODS
Research Protocol
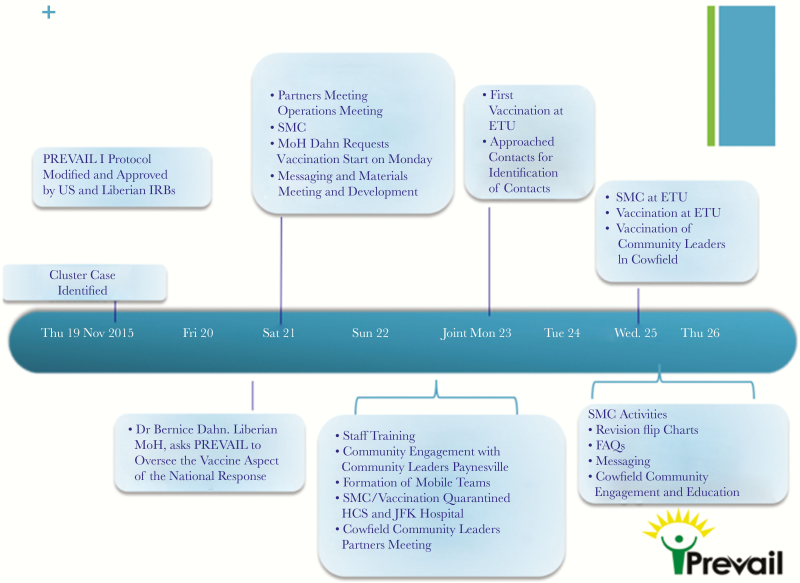
A research protocol to conduct an Ebola cluster vaccination study was quickly developed and implemented by amending the PREVAIL I protocol (clinical trials registration NCT02344407). Ethical clearance from the National Institutes of Health Institutional Review Board was obtained on 20 November 2015. The study was approved by the National Research Ethics Board and the Liberia Medicine and Health Regulatory Authority on 23 November 2015, and thus the protocol was ready for implementation 4 days after the first EVD case in the cluster was identified (Figure 1).
Figure 1.
Initial vaccination response time line. ETU, Ebola treatment unit; FAQ, frequently asked question; IRB, institutional review board; JFK Hospital, John F. Kennedy Medical Center; MoH, Minister of Health; SMC, Social Mobilization, Community Engagement, and Communications.
EVD Cases and Contacts
The index case for this cluster was a 15-year-old boy for whom EVD was diagnosed on 19 November 2015; on 20 November, the boy’s father and younger brother also received a diagnosis of EVD.
Following the diagnoses, the Centers for Disease Control and Prevention, the WHO, and the Liberian Ministry of Health worked together to identify the close contacts and contacts of the close contacts of the EVD cases. These contacts formed the cohort eligible for immunization. Close contacts were defined as those who lived in the same households as the cases, those who had visited the cases since the onset of their illnesses, and those who were in close physical contact with the cases’ body or bodily fluids, linens, clothes, or dishes. These contacts included people who lived in the households in a ring around the family with EVD, healthcare workers at the JFK Memorial Hospital and the associated Ebola treatment unit, and patients being cared for at the JFK Memorial Hospital at the time of the cases’ diagnoses. The geographical boundary for the ring was defined with the assistance of community leaders. Ultimately, >2000 households were visited to identify 650 possible close contacts over approximately 10 days.
Because contacts were reluctant to have vaccination activities next to their homes, community leaders identified a school about 0.5 km from the affected community, where vaccinations could be performed. Close contacts, contacts of close contacts from the neighborhood, healthcare workers, and patients being seen at the JFK Memorial Hospital at the time the cases had EVD diagnosed were invited to the school to learn about the study, be assessed for vaccination eligibility, provide consent for the study, and receive vaccine, where applicable. A mobile vaccination team was also created to vaccinate consenting healthcare workers from the JFK Memorial Hospital and those caring for patients at the Ebola treatment unit.
The 20-member vaccination team included a number of skilled, experienced individuals from the PREVAIL 1 study, including but not limited to a social mobilization expert, a nurse with experience obtaining informed consent, a phlebotomist, and a physician (Table 2). The team had prior experience with the PREVAIL 1 vaccine study and required only 1 day of training on the new protocol. The team provided information about the vaccine and the protocol, including that the vaccine was still investigational, to interested individuals who visited the school compound and to eligible healthcare workers. The informed consent process included a group information session and an individual consent session. If the person consented to vaccination, an informed consent form was signed and 1 copy was given to the volunteer, with a second copy retained by the study team for its records. The process ensured that potential participants understood the possible risks and benefits of the vaccine and their right to refuse to be vaccinated without having to disclose a reason for refusal.
Table 2.
Mobile Team Composition
| Role | Individuals, No. |
| Team lead | 1 |
| General information provider (nurse) | 1 |
| Triage nurse | 2 |
| Consent nurse | 2 |
| Vaccinator | 2 |
| Tracker | 2 |
| Pharmacist | 2 |
| Social mobilization and communications provider | 6 |
| Hygienist | 2 |
| Overall | 20 |
Those consenting to vaccination were then screened for eligibility, which was based on the expanded access ring protocol used in Sierra Leone and Guinea after August 2015. Individuals aged ≥6 years who consented to vaccination were eligible for vaccination. Criteria for exclusion included a history (self-reported or laboratory confirmed) of EVD, fever (temperature, >38°C), verbal report of pregnancy or breast-feeding, a history (self-reported) of anaphylaxis to a vaccine or vaccine component, a severe illness that rendered the person bed bound or that required hospitalization at the time of the vaccination, and any other condition that, in the judgment of the investigator or the caregiver, negatively impacted the person’s ability to provide informed consent.
Social Mobilization, Communication, and Community Engagement
Social mobilization, communication, and community engagement in the vaccination areas were crucial and robust. A community engagement team, including PREVAIL staff, the Liberian Ministry of Health, the United Nation’s Children Fund, the WHO, community leaders, and country health officers, mobilized key decision makers and political leaders, including traditional medicine practitioners and religious leaders, to explain the study and secure support for advocacy and mobilization. Flyers that explained the rationale for vaccination were distributed throughout the affected community. Numerous community meetings were held to review frequently asked questions, to answer additional questions, and to exchange information with community members, to ensure a transparent and meaningful participatory process. Some leaders volunteered to be the first vaccinated and became advocates for mobilizing the communities for vaccination.
Managing and Administering Vaccine
The rVSVΔG-ZEBOV-GP vaccine was stored in a secure freezer (temperature, −80°C) and transported to the vaccine site daily, with signature-confirmed handoff and the use of cold chain equipment that maintained temperatures between 2°C and 8°C. All movements of the vaccine were documented using electronic monitoring systems. Vaccine accountability, storage, shipment, and handling were conducted in accordance with the standard operating procedures of the manufacturer.
On the day of vaccination, vaccine vials were allowed to thaw at ambient temperature. The vaccine was then brought from the storage facility to the Cowfield vaccination site and reconstituted according to the manufacturer’s guidelines. Because this was a response to an active EVD outbreak, triage was required before people entered the vaccination site, including measurement of body temperature and assessment for EVD symptoms. The vaccinator prepared the vaccine and then, after donning basic personal protective equipment, administered the vaccine. The rVSVΔG-ZEBOV-GP vaccine (dose, 2 × 107 plaque-forming units) was administered intramuscularly in the deltoid muscle of either arm, avoiding broken skin or injuries. Participants were observed at the vaccination site for 30 minutes after the vaccine was administered, to monitor for any immediate reactions. During these 30 minutes, a small snack and water were provided. Each vaccine recipient received a unique identifier showing that they had received vaccine. Every effort was made to vaccinate eligible and consenting participants within 72 hours but no later than 21 days after potential exposure to an individual with confirmed EVD.
Data Collection
Before vaccination, we collected demographic data and information about the nature of each individual’s potential exposure to EVD. A blood specimen was collected from consenting volunteers, to measure the concentration of immunoglobulin G (IgG) antibody against the Ebola virus surface glycoprotein. Participants were given the contact details of the medical monitors, to whom they were asked to report any conditions experienced after the vaccine.
Participants were seen 1 month after vaccination, to assess injection site reactions, targeted symptoms, and any serious adverse events that occurred. Targeted symptoms included feverishness, fatigue, muscle pain, headache, nausea, abnormal sweating, rash, mouth ulcers, unexplained bleeding/bruising, and joint pain. Injection site reactions and targeted symptoms were graded on a 4-point scale as described in the PREVAIL 1 protocol [3]. A blood specimen was also collected only at the 1-month visit, for IgG antibody testing. Participants were seen 6 months after vaccination, for assessment of serious adverse events. IgG antibody levels against the Ebola virus surface glycoprotein were measured in serum at baseline and at 1 month, using the Filovirus Animal Non-Clinical Group assay [3].
Statistical Analysis
Injection site reactions and targeted symptoms were summarized as the percentage of participants with an event of any severity grade and by grade. Antibody levels were log10 transformed and summarized using geometric means. Median fold increases from baseline and the percentage of participants with a positive antibody response at 1 month were also determined. A response at 1 month was considered positive, using the same criteria used in PREVAIL 1 study: if participants did not have positive antibody responses at entry (the baseline level was <607 EU/mL), they were considered to have a positive antibody response at a follow-up visit if the increase from baseline was >0.60 log10 (a 4-fold increase) [3]. Distributions of antibody levels at baseline and at 1 month were displayed using box plots. Change in average antibody response was assessed using a paired t test to compare the log10 antibody levels at baseline and 1 month. Statistical analyses were performed using SAS, version 9.4 (SAS Institute).
RESULTS
Study Sample
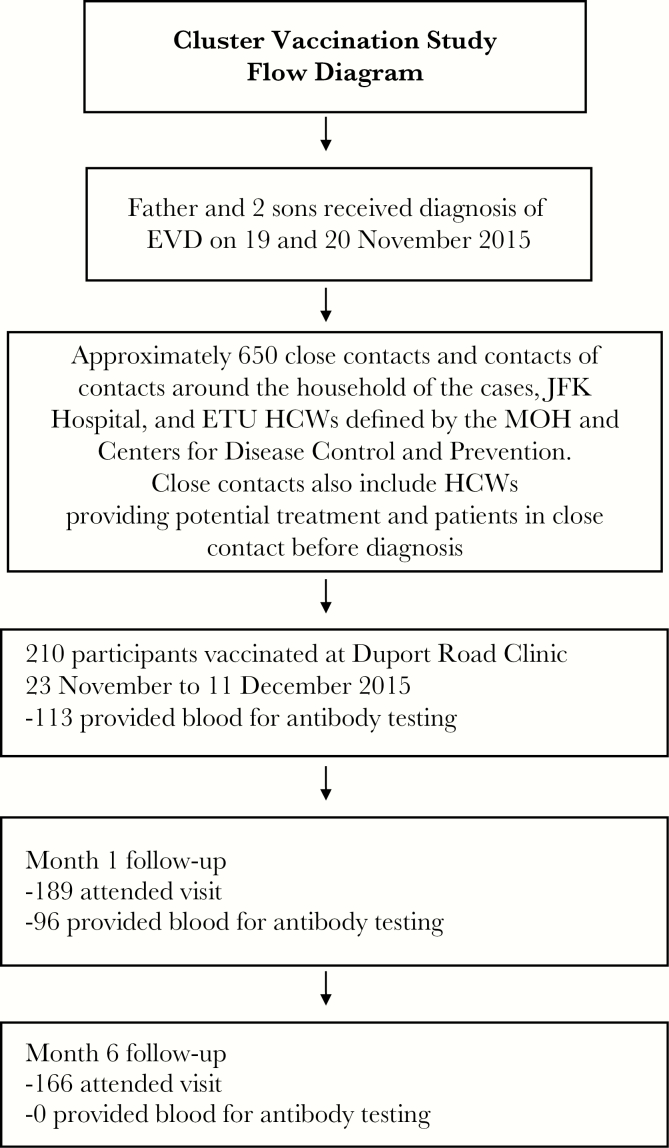
Approximately 650 people were identified as close contacts, as illustrated in a flow diagram (Figure 2). Most of these individuals (approximately 500) lived within a ring around the household of the cases. The contacts included 57 healthcare workers at the JFK Memorial Hospital and Ebola treatment unit and 4 patients being seen at the JFK Memorial Hospital at the time EVD was diagnosed in cases. Of the estimated 650 close contacts or contacts of close contacts, 210 (32%) consented to participate and were vaccinated during the study.
Figure 2.
Flow diagram for cluster vaccination study in Liberia. ETU, Ebola treatment unit; EVD, Ebola virus disease; HCW, healthcare worker; JFK Hospital, John Fitzgerald Kennedy Memorial Hospital; MoH, Ministry of Health.
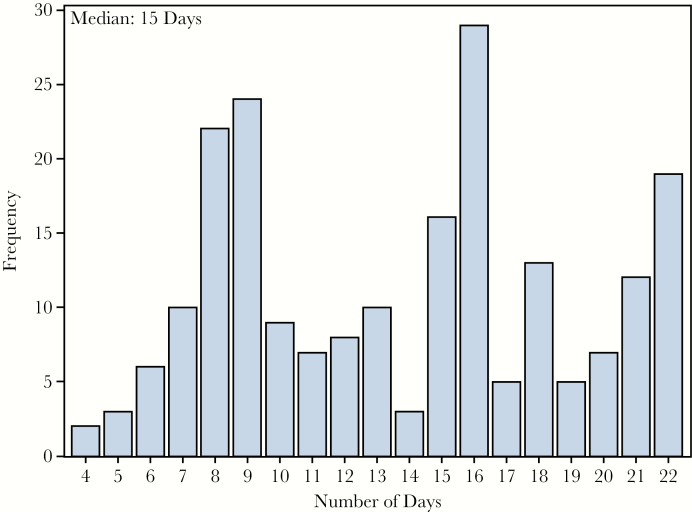
The median number of days from laboratory confirmation of EVD in the index case, on 19 November, until vaccination of his contacts was 15 days (range, 4–22 days; Figure 3). Vaccination ended on 11 December 2015, which was 21 days after the last contact was isolated. Provision of a blood sample was optional for the study: 113 of 210 participants (53.8%) provided a baseline blood sample.
Figure 3.
Distribution of days from case diagnosis to participant vaccination.
The average age of participants was 33 years (range, 18–70 years); the sex of 14% of participants was female. Seven participants (3.3%) reported contact with bodily fluids; 1 had contact with skin, linen, clothes, or dishes; 194 (92.4%) reported contact with close contacts; and 15 (7.1%) reported being healthcare workers at a facility visited by one of the cases.
Of the 210 participants vaccinated, 189 (90%) attended the month 1 follow-up visit, and 96 provided a blood specimen for antibody testing; 166 participants (79%) attended the month 6 visit, and none provided a blood sample.
Vaccine Safety
The most common targeted symptoms were headache (40%), feverishness (31%), fatigue (13%), and muscle pain (13%); 56% of participants reported at least 1 symptom (Table 1). Most symptoms were mild (grade 1); 5% reported at least 1 grade 2 or higher symptom, and 1% (2 participants) reported a least 1 grade 3 or 4 symptom. One participant reported grade 3 feverishness, fatigue, headache, and nausea; a second participant reported grade 4 feverishness and joint pain and grade 3 fatigue and muscle pain. No participants reported a serious adverse event during the study.
Table 1.
Symptoms Targeted During the PREVAIL Cluster Vaccination Study During the First Month After Vaccination
| Symptom | Participants, No. (%) (n = 189) |
| Any severity grade | |
| Feverishness | 59 (31.2) |
| Fatigue | 25 (13.2) |
| Muscle pain | 25 (13.2) |
| Headache | 76 (40.2) |
| Nausea | 6 (3.2) |
| Abnormal sweating | 10 (5.3) |
| Rash | 4 (2.1 |
| Mouth ulcer | 1 (0.5) |
| Unexplained bleeding/bruising | 0 (0.0) |
| Joint pain | 18 (9.5) |
| Other symptoms | 12 (6.3) |
| Overall | 106 (56.1) |
| Severity grade 2 or higher | 9 (4.8) |
| Severity grade 3 or higher | 2 (1.1) |
Antibody Levels
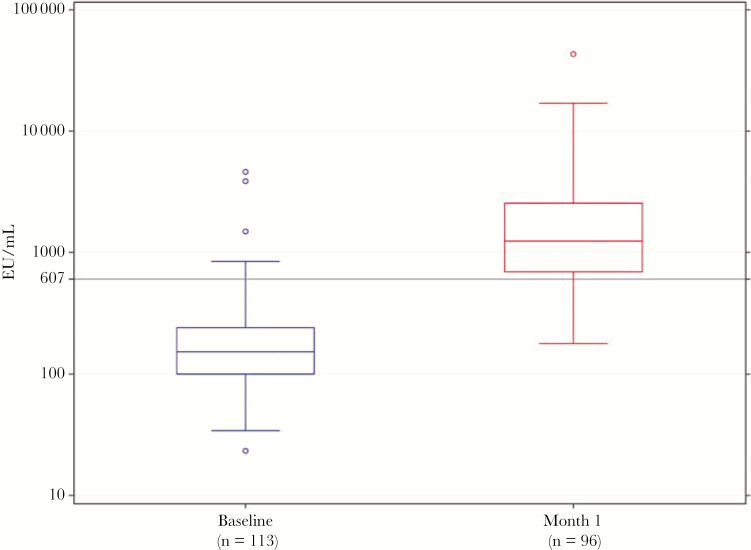
Five participants (4.4%) had elevated antibody levels (>607 EU/mL) at baseline; 3 had values >1000 EU/mL. The median baseline antibody level for all participants was 151 EU/mL (interquartile range [IQR], 99–241 EU/mL). The geometric mean level was 161 EU/mL. Significant increases in antibody levels were observed at 1 month (Figure 4), with an increase in the median titer from 151 to 1238 EU/mL (IQR, 694–2549 EU/mL). The median fold increase from baseline was 8.6 (IQR, 4.1–8.6). Among those without an elevated antibody level at baseline, 77.3% (95% confidence interval, 68.5%–86.1%) had an antibody response. The geometric mean titer at 1 month for these participants was 1357 EU/mL (95% confidence interval, 1122–1641 EU/mL).
Figure 4.
Distribution of antibody levels before and 1 month after vaccination. Median antibody levels before vaccination were 151 EU/mL (interquartile range, 99–241 EU/mL). One month after vaccination, these antibody levels were 1339 EU/mL (interquartile range, 1103–1626 EU/mL).
DISCUSSION
We successfully implemented a cluster vaccination study, designed on the basis of the Ebola Ca Suffit! Guinea ring vaccination trial [4], of the investigational rVSVΔG-ZEBOV-GP vaccine as part of the public health Ebola response for a small, 3-person EVD outbreak cluster in Liberia. With the presence of established PREVAIL clinical research capacity, an existing protocol was amended and implemented within 4 days of identification of a new confirmed EVD case, demonstrating that a prepared clinical research platform can quickly pivot to response research. There were no additional EVD cases identified during the outbreak response.
As in the PREVAIL 1 study and most phase 1 studies conducted with the rVSVΔG-ZEBOV-GP vaccine [4–7], the vaccine was generally well tolerated, without serious adverse events. Common symptoms were headache, feverishness, fatigue, and muscle pain; almost all were grade 1. Only 2 participants reported grade 3 or 4 adverse events in the month following vaccination.
Seventy-seven percent of participants in the cluster vaccination study had an antibody response after 1 month. In the PREVAIL I study, use of a similar definition for responders revealed that 84% of rVSVΔG-ZEBOV-GP vaccine recipients had an antibody response at 1 month [3].
Approximately one third of participants identified as close contacts or contacts of close contacts were vaccinated. The median time from case diagnosis until vaccination of contacts was 15 days (range, 4–22 days). In the Guinea ring vaccination trial, the time from the EVD cases’ initial symptoms to randomization of clusters was about 11 days. Also, EVD in many cases occurred within the first 10 days of vaccination. If this cluster or ring vaccination strategy is to be used in future outbreaks, it will be important to continue research on the rapidity of the immune response associated with other vaccines.
Conducting the integrated response research described here as part of the public health response to the Cowfield outbreak illustrated the strengths of research preparedness. Because the rVSVΔG-ZEBOV-GP vaccine is an investigational vaccine, its use under a research protocol was mandatory. Since the PREVAIL 1 study was ongoing in Liberia, it was possible to quickly amend the PREVAIL 1 protocol, enabling approvals and initiation of the protocol in a 4-day window. The ongoing work on the PREVAIL 1 study also provided the necessary infrastructure with which the cluster vaccination study could be performed. Furthermore, sufficient rVSVΔG-ZEBOV-GP vaccine already existed in the country, with an established cold chain. The public health response, which initially involved visiting thousands of households to identify close contacts and contacts of close contacts, was substantial and time consuming. This would have occurred even if a vaccine was not available to use, but the research was able to leverage these outbreak surveillance efforts. The research team worked closely with the contact tracing team to ensure that the team was aware of who was vaccinated. As a result, if a vaccinated contact developed fever, they could undergo testing with the GeneXpert, which can distinguish between vaccine reactogenicity, those with a single positive glycoprotein target, and a new case of Ebola virus infection, those with both a glycoprotein and nucleoprotein gene targets. The PREVAIL research platform was able to address challenges previously identified from outbreak research, ensuring a timely response; robust social mobilization, communication, and community engagement; maintenance of high scientific standards; and adherence to ethical requirements for research [8–11].
Additional challenges resulted from conducting research with an experimental product during a cluster outbreak. A major challenge was getting eligible close contacts and contacts of contacts to participate in the study. Some of the study population had suspicions about the vaccine, including fear that it might cause Ebola and fear of stigmatization. Generating a list of eligible persons proved impossible owing to the unwillingness of contacts to share the names of their contacts. This unwillingness was attributed in part to distrust of authorities and to potential abuse of name-based rosters in postconflict settings. Thus, a geographical ring strategy was used. Further, although studies such as PREVAIL 1 had established the safety and immunogenicity of the vaccine in adults, safety data were not yet available for children. Thus, no one aged <18 years volunteered for the vaccine, even though children older than 6 years were eligible. Similarly, women were less likely to volunteer. The reluctance of women to be vaccinated was also seen in the PREVAIL 1 study. A possible explanation is that Liberian men are considered the head of the home. As such, men are expected to take the lead at all times. In addition, there was a community rumor that the vaccine would prevent women from becoming pregnant. Healthcare workers also expressed a high level of mistrust for the experimental vaccine, perhaps in part because of the reemergence of Ebola in Cowfield after Liberia had been declared Ebola free. The public health response required comprehensive community engagement to explain the epidemiology of the disease, as well as the risks and potential benefits of the vaccine. In the recent 2018 ebolavirus outbreaks in the Democratic Republic of the Congo, vaccine uptake has been reported by the WHO to be close to 100%. This suggests that uptake of an Ebola vaccine will be higher in a large outbreak with ongoing transmission than in a small cluster outbreak with absence of ongoing transmission.
A final challenge was defining the cluster in an urban environment. Plans were immediately developed and implemented to identify close contacts and contacts of close contacts of the 3 cases in the Cowfield region where the family lived, as in the trial in Guinea [1, 4]. As described above, it was difficult to obtain a list of contacts of contacts from close contacts owing to fears and suspicion associated with the normal contact-tracing framework. In addition, the definition of the cluster had to be broadened to include (1) healthcare workers at the JFK Memorial Hospital and Ebola treatment unit who initially cared for the EVD cases, (2) patients in the clinic and hospital floor where the cases were initially identified, and (3) visitors to the neighborhood where the family lived. The eventual cluster size in Liberia was therefore much larger than 80 people, the median size of clusters in Guinea.
Our study design precludes determination of whether the vaccinations contributed to containing this outbreak. Challenges in conducting this study are likely to be relevant to other small-outbreak settings where licensed vaccines and therapeutic agents are not available and response research is deemed desirable. Although this study was initiated 4 days after the recognition of the outbreak, the average time of vaccination after identification of the index case was 15 days (range, 4–22 days). In the ring study, approximately two thirds of EVD episodes (41 of 60) occurred within the first 10 days and did not contribute to the efficacy analysis [1, 4]. Even if data were available for postexposure prophylaxis, broad population-based use in this setting with unlicensed and intravenous agents is not practical. Despite rapid initiation of the cluster response vaccination protocol, the opportunity to influence protection in this small 3 person family cluster was limited. For future outbreak-response research in which the Merck rVSVΔG-ZEBOV-GP vaccine is used, it would be prudent for modelers to inform the size or transmission characteristics of an outbreak where use of the vaccine would likely influence control and containment efforts. In small outbreaks such as the Cowfield outbreak described in this article, the significant effort to deploy a response-research platform to evaluate a vaccine risks distracting responders from efficacious control efforts, with potentially little added benefit. However, there are several valuable lessons regarding the manner in which the PREVAIL team addressed challenges in executing the PREVAIL cluster study that may help guide future research efforts during disease outbreaks.
Presented in part: Meeting of the World Health Organization Strategic Advisory Group of SAGE Working Group on Ebola Vaccines and Vaccination, Geneva, Switzerland, 14-15 March 2017.
Notes
Acknowledgments. We thank the Liberian Minister of Health, Dr Bernice Dahn, and the Incident Management System Chair and Deputy Chair, the Honorable Tolbert Nyenswah and Dr Francis Kateh, respectively, for support and leadership; the Liberian Centers for Disease Control and Prevention Country Director, Dr Desmond Williams, and the World Health Organization Country Director, Dr Alex Gasasira, for supporting the integration of the clustered vaccination study; Jennifer Mann, for developing the geographic ring approach; the Cowfield community leaders; the study participants; the many PREVAIL staff, particularly Dr Mark Kieh, who conducted the study; the United Nations Children’s Fund, Liberia, for graciously providing 2 tents for the mobile teams; and Dr Nancy Touchette, for providing editorial assistance.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health the Liberian Ministry of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Henao-Restrepo AM, Longini IM, Egger M, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. [DOI] [PubMed] [Google Scholar]
- 2. Dokubo EK, Wendland A, Mate SE, et al. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis 2018; 18(9):1015–24. [DOI] [PubMed] [Google Scholar]
- 3. Kennedy SB, Bolay F, Kieh M, et al. . Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henao-Restrepo AM, Camacho A, Longini IM, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huttner A, Dayer JA, Yerly S, et al. . The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Santis O, Audran R, Pothin E, et al. . Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16:311–20. [DOI] [PubMed] [Google Scholar]
- 7. Regules JA, Beigel JH, Paolino KM, et al. . A recombinant vesicular stomatitis virus ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macklin R, Cowan E. Conducting research in disease outbreaks. PLoS Negl Trop Dis 2009; 3:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snider DE Jr., Stroup DF. Defining research when it comes to public health. Public Health Rep 1997; 112:29–32. [PMC free article] [PubMed] [Google Scholar]
- 10. Lane HC, Marston HD, Fauci AS. Conducting clinical trials in outbreak settings: Points to consider. Clin Trials 2016; 13:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Academies of Sciences, Engineering, and Medicine. Integrating clinical research into epidemic response: the Ebola experience. Washington, DC: National Academies Press,2017. doi:10.17226/24739. [PubMed] [Google Scholar]