Highlights
-
•
Oxytocin act as a neuromodulator, its mechanism depends upon the distribution of OTR which vary in different brain region.
-
•
Oxytocin receptor coupled with heterotrimeric Gq/11 protein which activate phospholipase Cβ pathway (PLCβ), causing release of Ca2+ from intracellular store and increase neuronal excitation, which enhance GABA release from interneuron.
-
•
Oxytocin induced inhibition of sensory neurotransmission indirectly via excitation of inhibitory GABA interneuron.
Keywords: Neuropeptide, Neurotransmitter, Receptor, Neuromodulation, Alteration
Abstract
Gamma amino butyric acid (GABA) is the primary inhibitory neurotransmitter in the vertebral central nervous system. It functions by altering the membrane conductance of Cl− ions, maintaining the membrane potential close to the resting potential. The hormone oxytocin (OT) has a central action where it acts as a neuromodulatory peptide and exerts its action depending upon the distribution of OT receptors (OTR) in the target site. OTRs are G-protein-coupled receptors (GPCRs) comprising different subunits (Gq, Gi, and Gs). The G- protein isoforms have the ability to activate different pathways, but specific agonists and antagonists may show different affinities to OTRs, depending on the specific G-protein isoform to which they are coupled. It is well documented that OTR distribution varies with age and species and in regions of the brain. In this study, we attempted to observe the impact of OT and atosiban (OTA), an OT antagonist, on GABA levels in different regions of the brain. Study animals were exposed intraperitoneally (i.p.) to normal saline (0.89%), OT 0.0116 mg/kg, and OTA 1 mg/kg in different combinations, for 30days. It was observed that OT and OTA administration modulated GABA levels in different regions of brain, while normal saline had no effect. It may be due to OTR receptor expression in different regions of the brain.
This is significant because region-specific expression of different receptors could be important in the development of new drugs targeting specific neuropsychiatric disorders.
1. Introduction
Oxytocin (OT) is a neuropeptide synthesized in the hypothalamus by neurosecretory cells (magnocellular neurons) of the hypothalamic paraventricular (PVN) and supraoptic nuclei (SON), and secreted by the posterior pituitary lobe into the blood (Bargmann, 1949). OT neurons are also present in the parvocellular neurons of the PVN, superchiasmatic nucleus, bed nucleus of stria terminalis (BST), medial amygdalae, dorsomedial hypothalamus, vertical diagonal band of Broca, and olfactory bulb nuclei in rats (Buijs, 1978; Caffé and Leeuwen, 1983). However, OT neurons are absent from the dorsomedial hypothalamus, vertical diagonal band of Broca, and olfactory bulb of mice (Caffé and Leeuwen, 1983; Tobin et al., 2010). It is possible that this is related to species-dependent differences in social behavior. OT is a hormone involved in different physiological and pathological functions like sexual activity, penile erection, ejaculation, pregnancy, uterus contraction, milk ejection, maternal behavior, and social bonding among others (Stoop, 2012). In addition, OT acts centrally as a neurotransmitter and the release of OT within the brain occurs from dendrites, axons, and somata of magnocellular neurons of the PVN in different regions of the brain (Dumais and Veenema, 2016; Moghadam et al., 2018). Furthermore, OT plays an important role in the brain by interacting with specific receptors in different regions of the brain and helps in neuromodulations. It has been shown that receptor distribution varies with age (Elizabeth and Hammock, 2015) and species of animal (Dumais and Veenema, 2016).
In a recent study, it was also shown that OT protects against inflammation and oxidative stress, which is due to OT and GABAA receptor interaction in the CNS (Kaneko et al., 2016). Gamma amino butyric acid (GABA) is the principal inhibitory neurotransmitter synthesized by decarboxylation of glutamate through the action of glutamic acid decarboxylase (GAD) and binds to three receptors namely GABAA, GABAB, and GABAC (Kaneko et al., 2016; Roberts, 1960).
GABAA receptors are ionotropic Cl− channels gated by the major inhibitory neurotransmitter γ-aminobutyric acid and are widely expressed throughout CNS. They play a major role in synaptic inhibition in the CNS (Kaneko et al., 2016; Wisden and Seeburg, 1992). OT modulates GABAA receptor subunit expression, which mediates the hyperpolarization of the membrane potential and reduces neuronal excitability due to chloride ion influx (Kaneko et al., 2016). This suggests that perturbations in GABAergic inhibition have the potential to result in neurodegenerative disorders (Piantadosi and Floresco, 2014).
Numerous studies in humans and animals have established that OT affects the social life of mammals and reduces anxiety (Sabihi et al., 2017). Various brain regions have been identified as the site of action for the anxiolytic effects of OT, including the hypothalamic PVN (Blume et al., 2008; Smith et al., 2016), amygdale (Bale et al., 2001; Neumann, 2002), raphe nucleus (Yoshida et al., 2009), and prelimbic (PL) region of the medial prefrontal cortex (mPFC) (Sabihi et al., 2014a,b). Apart from this, evidence also suggests that OT interacts with GABA to reduce anxiety (Nuss, 2015; Smith et al., 2016). Therefore, interest is growing towards the study of neuropeptides and their region-specific receptors that may be important when designing new drugs targeting specific neuropsychiatric diseases (Busnelli et al., 2013). The mouse, which is widely used in neuroscience research, has a rich social life. Like other rodents, their social communication and behavior depends upon their sex, age, and hormonal status, among other factors. The important of OT on different regions of the mouse brain is less well-documented. Therefore, in the present investigation we have tried to evaluate the impact of exogenous OT and atosiban (OTA) on GABAergic transmission in different regions of the brain.
2. Results
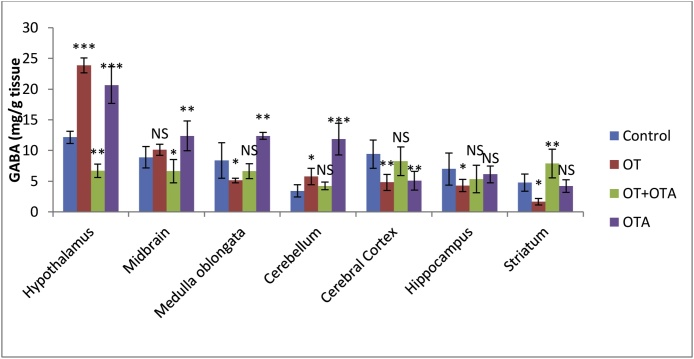
OT and OTA treated animals showed significant changes in GABA contents in different regions of the brain relative to the control group. After 30 days exposure to OT and OTA, GABA levels were significantly higher (p < 0.01, one-way analysis of variance [ANOVA]) in the hypothalamus and cerebellum of the brain of Mus musculus than those of the control group. However, these values were significantly lower (p < 0.01, one-way analysis of variance [ANOVA]) when OTA was administered along with OT for 30 days than in the exclusively OT-or OTA-treated groups (Fig. 1). OT reduced the GABA levels in the hippocampus, cerebral cortex, medulla oblongata, and striatum of the brain of Mus musculus relative to the control groups. However, when OTA was administered along with OT, out of the seven regions of the brain considered, only four regions of the brain i.e., the hippocampus, cerebral cortex, medulla oblongata, and striatum, showed an increase in the GABA level in comparison to those in OT exposed animals. Three regions, i.e., the hypothalamus, cerebellum, and midbrain, showed greater lowering of the GABA levels when we administered OT along with OTA for up to 30 days.
Fig. 1.
Gamma Amino Butyric Acid (GABA) estimation (mg/g tissue) in the brain of female animal, Mus musculus after 30 days of treatment with OT, OT + OTA, OTA, and Control.
Values are mean ± SEM of 6 animals.
* Difference p < 0.05 in control vs study deduced by one-way ANOVA test.
**Significant difference p < 0.01 in control vs study deduced by one-way ANOVA test.
*** Highly Significant difference p < 0.001 in control vs study deduced by one-way ANOVA test.
3. Discussion
Numerous studies on humans as well as animals have shown that OT reduces anxiety (Ayers et al., 2011; Bale et al., 2001; Blume et al., 2008; Oliveira et al., 2012), and leads to expressions of social behavior (Stoop, 2012) by interacting with GABA in the CNS, effects which vary with species and sex (Elizabeth and Hammock, 2015). The mouse, which is widely used in neuroscientific research, has a rich social life. Similar to other rodents, their social communication and behavior depends upon their sex, age, and hormonal status, among other factors. The present study revealed a novel mechanism underlying the effect of interaperitoneal (i.p.) administration of OT and OTA on GABAergic transmission in different regions of the brain. Intraperitoneal administration of OT crosses blood brain barrier (Mizuno et al., 2015; Neumann et al., 2013; Peñagarikano et al., 2015) and modulate social behavior buffer anxiety in autism, schizophrenia as well as recovery of neurodegenerative disorder. In present study OT significantly increased (p < 0.01) GABAergic neuronal activity in the hypothalamus, midbrain, and cerebellum in the experimental group.
OT activates GABAergic neurons by interacting with the GABAA receptor in the hypothalamo-pituitary-adrenal (HPA) axis in the hypothalamic PVN, and reduces anxiety, increases social buffering, and calmness in females (Blume et al., 2008; Smith et al., 2016). Apart from this, exogenous OT increases GABA in the midbrain. Central OT plays an important role in the reward system through its effects on social behavior like social reward, social learning, pair bonding, parenting, and mating (Choe et al., 2015; Dölen et al., 2013; Gimpl and Fahrenholz, 2001; Love, 2014; Marlin et al., 2015). A number of studies have demonstrated the modulatory action of OT and dopamine (DA) in the CNS and characterized axonal projection from OT neurons of the hypothalamic PVN to midbrain DA regions (Charlet and Grinevich, 2017; Xiao et al., 2017). OT neurons are exclusively present in the hypothalamus, but DA neurons are present in the ventral tegmental area (VTA) and substantia nigra (SN). However OTR are present in the VTA as well as the SN. OT activates two pathways; it directly activates VTA neurons and indirectly inhibits SN neurons through local GABAergic interneurons (Charlet and Grinevich, 2017; Xiao et al., 2017).
In this experiment, it was also found that GABA activity was higher in the cerebellum. The cerebellum is known to play an important role in classical conditioned reflex responses, mental imagery, affective behavior, and control of sensory data acquisition (Fatemi et al., 2012). Many of these functions are disturbed in autism. GABA is the principal inhibitory neurotransmitter in several brain regions including the cerebellum and is synthesized by decarboxylation of glutamate through the action of GAD. GABA binds to three receptors, namely, GABAA, GABAB and GABAC (Kaneko et al., 2016; Roberts, 1960). GABAA receptors help in the opening of chloride ion channels in the cell membrane that are gated by GABA, causing hyperpolarization and inhibition of neuronal excitation (Bing et al., 2018).
The GABAergic neuromodulatory mechanism of OT depends upon the distribution of OTRs, which varies in different regions of the brain. OTRs are members of the G-protein-coupled receptor (GPCR) superfamily. The structure of GPCRs is characterized by seven transmembrane (7-TM) α-helices connected by three intracellular (IL-1 to IL-3) and three extracellular loops (EL-1 to EL-3). These receptors can be coupled to different G-proteins and exhibit different functions. An OTR coupled with the heterotrimeric Gq/11 protein activates the phospholipase Cβ pathway (PLCβ), causing the release of Ca2+ from intracellular stores, increasing neuronal excitation (Gimpl and Fahrenholz, 2001) and thereby enhancing GABA release from interneurons (Breton et al., 2008; Lara et al., 2009; Piloni et al., 2006). The Gq/11 family of proteins consists of four members, two of which (Gq and G11) are almost solely expressed in the CNS (Tanaka et al., 2000). These appear responsible for maternal behavior after parturition in females (Wettschureck et al., 2004). Furthermore, OT can also activate the inward rectifying current through the Gi/o protein, which is also responsible for its antiproliferative effect (Gravati et al., 2010). In addition, the Gs protein of the OTR can also increase cAMP production by activating adenylate cyclase, which opens sodium channels (Stoop, 2012).
In fetal rats, OT increased the intracellular chloride concentration in GABA neurons, thereby reducing neuronal excitation; this process was thought to protect the neonate from anoxic injury (Tyzio et al., 2006, 2014).
Apart from this OTA activates different signaling pathways by coupling with different OTR subunits. For example, OTA is used in the treatment of preterm labor due to its antagonistic ability to block Gq/PLC/calcium signaling pathway in myometrial cells (Stoiber et al., 2018). However, OTA shows biased agonist properties by coupling with OTR Gi, inhibiting the proliferation of some cancer cells (Reversi et al., 2005), therefore its use limited to only 48 h in patients with a high risk of preterm delivery (Kim et al., 2017). Recent evidence indicates the antagonistic properties of OTA in the CNS (Abdullahi et al., 2018), OT is well known for its action on social memory and memory consolidation when identifying novel objects in the nucleus basalis of Meynert; OTA also impaired memory consolidation in the CNS (Gard et al., 2012). Our finding also supported the antagonistic properties of OTA in the hypothalamus, midbrain, and cerebellum of the experimental group. However, in certain regions, i.e., the medulla oblongata, cerebral cortex, striatum, and hippocampus, OTA does not show an inhibitory action on OTRs, indicating that OTA activates different signaling pathways in the CNS by binding with different receptor subunits.
Apart from this OT and vasopressin (VP) are two important neuropeptide in CNS and plays an important role in the control of social, cognitive, and neuroendocrine function (Sala et al., 2011). OT and VP have high degree of similarity in their structure and structure of receptor i.e. OTR and V1a receptor (Gimpl and Fahrenholz, 2001; Maybauer et al., 2008; Zhimin et al., 2016). Evidences from previous study support that OT and VP control social behavior and physiological responses by activating each other’s receptor i.e. OTR and V1a, in CNS (Zhimin et al., 2016). In summary, the findings of the current study demonstrated that long term exposure to exogenous OT and OTA activate different pathways in different region by binding with different receptor subunits in female mice. This represents a very valuable tool for investigating the molecular basis of neuropeptide action in the CNS.
3.1. Conclusion
From the above study we can conclude that G protein isoforms have the ability to activate different pathways, but specific agonists and antagonists may show different affinities to OTRs because, their action depends on the specific G protein (Gq, Gi or Gs) to which they are coupled. For example, the OTR antagonist, OTA does not affect receptor internalization, possibly due to the selective activation of only those OTRs that are coupled to a Gi protein (Busnelli et al., 2013). We can also conclude that region-specific expression of different receptors could be important for the development of new drugs targeting specific neuropsychiatric disorders.
4. Experimental procedure
4.1. Animals
The present experiment was performed on mature female mice, Mus musculus, weighing 25 ± 5 g. All animals were acclimatized to laboratory conditions, i.e., 25 ± 3 °C, and light and dark photoperiod (12 h light:12 h dark) in the animal house of the Laboratory of Endocrinology, Bioscience Department, Barkatullah University, Bhopal, India. Hygienic conditions were maintained with rice husk bedding in separate polypropylene cages. Animals were provided with standard feed and water ad libitum. The study was performed with the approval of the ethical committee, i.e., Institutional Animal Ethics Committee (IAEC) of CPCSEA (Ref No.1885/GO/S/16/CPCSEA/IAEC/B.U./04).
4.2. Chemical
OT (brand name Pitocin) was purchased from a local medical shop in Bhopal, Madhya Pradesh, and the OT antagonist i.e., OTA, was obtained from Sigma –Aldrich, for this experimental study.
4.3. Preparation of dose
OT 0.0116 mg/kg and OTA 1 mg/kg was prepared in 0.89% normal saline. The dose of OT and OTA was finalized after reviewing various previous studies and confirmed through experimental investigation (Han et al., 2016; Mizuno et al., 2015; Teng et al., 2016).
4.4. Experimental design
The mice were divided into four groups of six each. The control group received a balanced diet, water ad libitum, and normal saline (0.89%) for 30 days. Group 2 received a balanced diet, water ad libitum and was treated daily with OT 0.0116 mg/kg i.p. for 30-days. Group 3 received a balanced diet, water ad libitum, and was treated daily with OT 0.0116 mg/kg and OTA 1 mg/kg i.p. for 30-days. Group 4 received a balanced diet, water ad libitum, and was treated daily only with OTA 1 mg/kg i.p. for 30-days.
4.5. Dissection
After 30 days of treatment, all animals from the different groups were killed by cervical dislocation. The brains were carefully removed, washed in normal saline, and kept at −20 °C. The dissection was performed on an ice-cooled glass plate. The frozen tissue was divided into seven regions, the medulla oblongata, midbrain, cerebellum, cerebral cortex, striatum, hippocampus, and hypothalamus, adopting the methodology of (Glowinski and Iversen, 1966) as follows.
First, the rhombencephalon (A) was separated by a transverse section from the rest of the brain and dissected into two parts, the cerebellum and medulla oblongata. A transverse section was made at the level of the optic chiasma, which delimits the anterior part of the hypothalamus, and through the anterior commissure. This section separated the cerebrum into two parts, B and C. Part B was divided into five fractions. First, the hypothalamus was dissected by taking the anterior commissure as a horizontal reference and the line between the posterior hypothalamus and mammillary bodies as the caudal limit. The striatum was dissected with the external walls of the lateral ventricles as the internal limits and corpus callosum as the external limits. The frontal parts of the striatum, which are in section, were dissected separately from the remaining parts of the brain. The hippocampus was then dissected. The remainder of part B was combined with the remainder of part C to form the cortex. An example of the reproducibility of this procedure is given in Table 1.
Table 1.
Reproducibility of dissection procedure.
| Sr. No. | Brain Region | Mean weight mg ± Error |
|---|---|---|
| 1. | Hypothalamus | 7.6 ± 0.72 |
| 2. | Hippocampus | 49.92 ± 0.51 |
| 3. | Striatum | 59.6 ± 0.91 |
| 4. | Cerebellum | 37.46 ± 0.72 |
| 5. | Cerebral cortex | 55.68 ± 0.99 |
| 6. | Midbrain | 22.18 ± 0.85 |
| 7. | Medulla oblongata | 23.08 ± 1.01 |
Mean weights for various brain regions dissected from five animal’s ± error.
4.6. Assay
Different regions of tissue were blotted, weighed, and placed in different tubes containing 5 ml ice cold TCA (10%W/V), homogenized, and centrifuged at 10,000 rpm for 10 min at 0 °C. Next, 0.1 ml of supernatant was dissolved in 0.2 ml of ninhydrin solution (0.15 M) in 0.5 M carbonate-bicarbonate buffer (pH 9.95). This mixture was kept in a water bath maintained at 60 °C for 30 min and then cooled. The cooled mixture was treated with 5 ml of copper tartarate reagent. After 10 min, florescence was observed at 377–455 nm by using a spectrofluorometer (Lowe et al., 1958).
4.7. Data analysis
Results are expressed as the mean and standard error of mean of different groups. The intergroup variation was measured using a one way analysis of variance (ANOVA) followed by Tukey’s test (Tukey, 1949). Statistical analysis was performed using the Sigma Stat Statistical Software version 3.5. p < 0.05 was considered statistically significant.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We are very thankful to the Madhya Pradesh Council of Science and Technology (MPCST), Bhopal for financial support. We are also grateful to Prof. Vinod Kumar Singh (Director IISER) and Dr. Sunando Datta, Associate Professor, Department of Biological Science, Indian Institute of Science Education and Research (IISER), Bhopal, for their excellent instrument facilities and assistance.
References
- Abdullahi R., Eskandarian S., Ghanbari A., Rashidy-Pour A. Oxytocin receptor antagonist atosiban impairs consolidation, but not reconsolidation of contextual fear memory in rats. Brain Res. 2018;1695:31–36. doi: 10.1016/j.brainres.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Ayers L.W., Missig G., Schulkin J., Rosen J.B. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacology. 2011;36(12):2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T.L., Davis A.M., Auger A.P., Dorsa D.M., McCarthy M.M. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 2001;21(7):2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann W. The neurosecretory connection between the hypothalamus and the neurohypophysis. Z. Zellforsch. Mikrosk. Anat. 1949;34(5):610–634. [PubMed] [Google Scholar]
- Bing W., Summary H., Xiaojing Y., Feiyong J., Du L. Advances in the role of γ-aminobutyric acid signaling pathway in autism spectrum disorders. Chin. J. Contemp. Pediatrics. 2018;20(11):974–979. doi: 10.7499/j.issn.1008-8830.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A., Bosch O.J., Miklos S., Torner L., Wales L., Waldherr M., Neumann I.D. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur. J. Neurosci. 2008;27(8):1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Breton J.-D., Veinante P., Uhl-Bronner S., Vergnano A.M., Freund-Mercier M.J., Schlichter R., Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I-II which amplify GABAergic inhibition. Mol. Pain. 2008;4 doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs R. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192(3):423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Busnelli M., Bulgheroni E., Manning M., Kleinau G., Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J. Pharmacol. Exp. Ther. 2013;346(2):318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffé A.R., Leeuwen V.F.W. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res. 1983;233(1):23–33. doi: 10.1007/BF00222229. [DOI] [PubMed] [Google Scholar]
- Charlet A., Grinevich V. Oxytocin mobilizes midbrain dopamine toward sociality. Neuron. 2017;95:236–237. doi: 10.1016/j.neuron.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Choe H.K., Reed M.D., Benavidez N., Montgomery D., Soares N., Yim Y.S., Choi G.B. Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron. 2015;87(1):152–163. doi: 10.1016/j.neuron.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G., Darvishzadeh A., Huang W.K., Malenka C.R. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais K., Veenema A. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth A.D., Hammock Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2015;40(1):24–42. doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S.H., Aldinger A.K., Ashwood P., Bauman L.M., Blaha D.C., Blatt J.G. Pathological role of the cerebellum in autism. Cerebellum. 2012;11(3) doi: 10.1007/s12311-012-0355-9. 777-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard R.P., Naylor C., Ali S., Partington C. Blockade of pro-cognitive effects of angiotensin IV and physostigmine in mice by oxytocin antagonism. Eur. J. Pharmacol. 2012;683(1–3):155–160. doi: 10.1016/j.ejphar.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L.L. Regional studies of catecholamines in the rat brain-1 the disposition of norepinephrine, dopamine and DOPA in various regions of the brain. J. Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gravati M., Busnelli M., Bulgheroni E., Reversi A., Spaiardi P., Parenti M. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J. Neurochem. 2010;114(5):1424–1435. doi: 10.1111/j.1471-4159.2010.06861.x. [DOI] [PubMed] [Google Scholar]
- Han T.R., Lee H., Lee J., Lee S.-B., Kim J.H., Back K.S., Na S.H. Brief isolation changes nociceptive behaviour and compromises drug tests in mice. World Inst. Pain. 2016;16(6):749–757. doi: 10.1111/papr.12325. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Pappas C., Tajiri N., Boriongan C.V. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci. Rep. 2016;6(35659) doi: 10.1038/srep35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Pohl O., Chollet A., Gotteland J.-P., Fairhurst D.J.A., Bennett R.P., Terzidou V. Differential effects of oxytocin receptor antagonists, Atosiban and Nolasiban, on oxytocin receptor–mediated signaling in human amnion and myometrium. Mol. Pharmacol. 2017;91(4):403–415. doi: 10.1124/mol.116.106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara C.M., Piloni R.G., Lorenzana M.G., Hidalgo L.M., Jiménez R.J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Love M.T. Oxytocin, motivation and the role of dopamine. Pharmacol. Biochem. Behav. 2014;119:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe I.P., Robins E., Eyerman G.S. The fluorometric measurement of glutamic decarboxylase and its distribution in brain. J. Neurochem. 1958;3(1):8–18. doi: 10.1111/j.1471-4159.1958.tb12604.x. [DOI] [PubMed] [Google Scholar]
- Marlin B.J., Mitre M., D’Amour J.A., Chao M.V., Froemke R.C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520(7548) doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybauer M.O., Maybauer D.M., Enkhbaatar P., Traber D.L. Physiology of the vasopressin receptors. Best Pract. Res. Clin. Anaesthesiol. 2008;22(2):253–263. doi: 10.1016/j.bpa.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Mizuno A., Cherepanov M.S., Kikuchi Y., Fakhrul A.A., Akther S., Deguchi K. Lipo-oxytocin-1, a novel oxytocin analog conjugated with two palmitoyl groups, has long-lasting effects on anxiety-related behavior and social avoidance in CD157 knockout mice. Brain Sci. 2015;5(1):3–13. doi: 10.3390/brainsci5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam S.E., Tameh A.A., Vahidinia Z., Atlasi M.A., Bafrani Hassani H., Naderian H. Neuroprotective effects of oxytocin hormone after an experimental stroke model and the possible role of Calpain-1. J. Stroke Cerebrovasc. Dis. 2018;27(3):724–732. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Neumann I.D. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo–pituitary–adrenal axis. Prog. Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann D.I., Maloumby R., Beiderbeck I.D., Lukas M., Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D.C., De, Chagas M.H., Garcia L.V., Crippa J.A., Zuardi A.W. Oxytocin interference in the effects induced by inhalation of 7.5% CO(2) in healthy volunteers. Hum. Psychopharmacol. 2012;27(4):378–385. doi: 10.1002/hup.2237. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O., Lázaro T.M., Lu X.-H., Gordon A., Dong H., Lam A.H. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015;7(271) doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi T.P., Floresco B.S. Prefrontal cortical GABA transmission modulates discrimination and latent inhibition of conditioned fear: relevance for schizophrenia. Neuropsychopharmacology. 2014;39(10):2473–2484. doi: 10.1038/npp.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piloni R.G., López-Hidalgo M., Martínez-Lorenzana G., Rodríguez-Jiménez J., Condés-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2006;1137(1):69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Reversi A., Rimoldi V., Marrocco T., Cassoni P., Bussolati G., Parenti M., Chini B. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J. Biol. Chem. 2005;280(16):16311–16318. doi: 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- Roberts E. Some aspects of biochemistry and physiology of gamma aminobutyric acid in the central nervous sytem. Am. J. Orthopsychiatery. 1960;30(22) doi: 10.1111/j.1939-0025.1960.tb03004.x. [DOI] [PubMed] [Google Scholar]
- Sabihi S., Dong S.M., Durosko N.E., Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci. 2014;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S., Durosko N.E., Dong S.M., Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S., Dong M.S., Maurer D.S., Post C., Leuner B. Oxytocin in the medial prefrontal cortex attenuates anxiety: anatomical and receptor specificity and mechanism of action. Neuropharmacology. 2017;125:1–12. doi: 10.1016/j.neuropharm.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M., Braida D., Lentini D., Busnelli M., Bulgheroni E., Capurro V. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Smith A.S., Tabbaa M., Lei K., Eastham P., Butler M.J., Linton L. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016;63:50–58. doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber B., Haslinger C., Schäffer M.K., Zimmermann R., Schäffer L. Effect of dual tocolysis with fenoterol and atosiban in human myometrium. J. Perinat. Med. 2018;47:190–194. doi: 10.1515/jpm-2018-0010. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76(1):142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Nakagawa S., Kushiya E., Yamasaki M., Fukaya M., Iwanaga T. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur. J. Neurosci. 2000;12(3):781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Teng L.B., Nikolova D.V., Riddick V.N., Agste L.K., Crowley J.J., Baker K.L. Reversal of social deficits by subchronic oxytocin in two autism mouse models. Neuropharmacology. 2016;105:61–71. doi: 10.1016/j.neuropharm.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin V.A., Hashimoto H., Wacker D.W., Takayanagi Y., Langnaese K., Caquineau C. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;454(7287):413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey J. Comparing individual mean in the analysis of variance. Biometrics. 1949;5(2):99–114. [PubMed] [Google Scholar]
- Tyzio R., Cossart R., Khalilov I., Minlebaev M., Hubner C.A., Represa A. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Tyzio R., Nardou R., Ferrari D.C., Tsintsadze T., Shahrokhi A., Eftekhari S. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343(6171):675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- Wettschureck N., Moers A., Hamalainen T., Lemberger T., Schütz G., Offermanns S. Heterotrimeric g proteins of the Gq/11 family are crucial for the induction of maternal behavior in mice. Mol. Cell. Biol. 2004;24(18):8048–8054. doi: 10.1128/MCB.24.18.8048-8054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. GABAA receptor channels: from subunits to functional entities. Curr. Opin. Neurobiol. 1992;2:263–269. doi: 10.1016/0959-4388(92)90113-y. [DOI] [PubMed] [Google Scholar]
- Xiao L., Priest F.M., Nasenbeny J., Lu T., Kozorovitskiy Y. Biased oxytocinergic modulation of midbrain dopamine system. Neuron. 2017;95:368–384. doi: 10.1016/j.neuron.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Takayanagi Y., Inoue K., Kimura T., Young L.J., Onaka T., Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhimin S., Tony E.L., Malley M.O., Alber H.E. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus) Horm. Behav. 2016 doi: 10.1016/j.yhbeh.2016.02.004. [DOI] [PubMed] [Google Scholar]