Abstract
There are no specific structural neuropathological hallmarks found in the brain of mood disorders. Instead, there are molecular, functional and structural alterations reported in many brain areas. The neurodevelopmental underpinning indicated the presence of various genetic and developmental risk factors. The effect of genetic polymorphisms and developmental sequalae, some of which may start in the womb, result in functional changes in a network mediated by neurotransmitters and neuropeptides, which make the emotion- and stress-related brain systems more vulnerable to stressful events. This network of stress-related neurocircuits consists of, for instance, brainstem nuclei, the amygdala, habenula, prefrontal cortex and hypothalamus. Various nuclei of the hypothalamus form indeed one of the crucial hubs in this network. This structure concerns not only the hypothalamo-pituitary-adrenal (HPA) axis that integrate the neuro-endocrine-immune responses to stress, but also other hypothalamic nuclei and systems that play a key role in the symptoms of depression, such as disordered day-night rhythm, lack of reward feelings, disturbed eating, sex, and disturbed cognitive functions. The present review will focus on the changes in the human hypothalamus in depression, with the HPA axis in the center. We will discuss the inordinate network of neurotransmitters and neuropeptides involved, with the hope to find the most vulnerable neurobiological systems and the possible development of tailor-made treatments for mood disorders in the future.
Keywords: The hypothalamo-pituitary-adrenal axis, Glutamate, GABA, Neuropeptide, Sex differences
Introduction
Structural and functional brain alterations have been found in mood disorders in a network of stress-related systems, such as the brainstem nuclei, the locus coeruleus (LC) and raphe nuclei (Bao et al., 2012), which are the first relay stations for many physiological stressors. The amygdala processes fear and anxiety responses (Wang et al., 2014); the hippocampus mediates learning and memory and encodes the importance of a stimulus to the organism (Aihara et al., 2007; Wang et al., 2012); and the prefrontal cortex (PFC), which not only holds cognitive and executive functions but also regulates the stress axis (see below) (Aihara et al., 2007; Qi et al., 2013, 2015; Qi et al., 2017; Zhao et al., 2016; Lu et al., 2017; Price and Drevets, 2012). The lateral habenula is a relatively new structure known to be involved in depression (Yang et al., 2018). This review deals with one of the important hubs in this network, which is involved in the symptoms of depression, i.e. a number of human hypothalamic neuropeptide-containing nuclei, from which recent evidence of their alterations in depression is accumulating. It should be noted that not all hypothalamic nuclei are discussed, partly because of a lack of information, such as for the anterior part of the hypothalamus with the exception of the suprachiasmatic nucleus (SCN, see below); and partly because of no important alterations were observed in depression, such as in the histaminergic tuberomammillary nucleus in the posterior hypothalamus (Shan et al., 2013). In the present review, we put the hypothalamo-pituitary-adrenal (HPA) axis in the center, because of its intensive interaction with other stress-related neuropeptide-, amine- and amino acid systems in depression. It should be noted though, that a causal role of the human HPA axis in general, and of corticotropin-releasing hormone (CRH) in particular, in mood disorders, is still in discussion (Spierling and Zorrilla, 2017). Changes in molecular neuropathology in the hypothalamus, i.e. the neurotransmitter and neuromodulator alterations, are due to a multitude of different genetic and developmental causes, which lead to changes in the stress- and reward-related network (Lucassen et al., 2014; Menezes et al., 2018). These changes may in different ways cause individuals to be at risk for a mood disorder. In case of the occurrence of stressful environmental events, they may over-react to these events and develop a depression.
The HPA axis
The first time the HPA axis became the focus of depression studies was by the correlation found by Hans Selye in the 1930s between the dysregulation of the stress response and mood disorders, especially major depression (Selye, 1998). It has since then become well accepted that, although the stress response is necessary to maintain homeostasis, long-term activation of the stress system may bring hazardous or even lethal effects, and increases the risk of depression (Selye, 1998). Abnormalities in the ‘stress system’ have been documented in mood disorders in various stress related systems. The neural network that encodes and evaluates the stressful event comprises e.g. the HPA axis, the arginine vasopressin (AVP) systems, and the noradrenergic system, which are the very same brain circuits that, when they are hyperactive due to a combination of a genetic background, developmental sequelae and life events, underlies negative emotions and moods. Major depressive disorder (MDD) thus arises from the interaction of vulnerability genes and developmental and environmental factors (Lucassen et al., 2014). Prenatal environmental stressors such as placental insufficiency, food shortage or nicotine exposure due to smoking of the pregnant mother may sensitize the child for developing depression in later life (Clark, 1998; Swaab, 2004). Psychosocial stress such as early maternal separation, child abuse or neglect may also program the HPA axis by epigenetic means permanently into a higher activity, while death or loss of a spouse, or personal injury or illness form risk factors that may trigger the early episodes of depression (Lucassen et al., 2014; Bao et al., 2012).
When activated, the HPA axis stimulates the synthesis and release of cortisol, which normally has broad biological effects throughout the body that are adaptive but can become damaging when chronically elevated. Chronic elevation of cortisol secretion in some depressive subtypes is thought to be responsible for mitochondrial dysfunction, neuropathological changes, elevated temperature, osteoporosis and aging, and other medical causes of individuals with mood disorders (Gold et al., 2002). Cortisol exerts negative feedback at the pituitary and the hypothalamic level (Wang et al., 2008) and acts on other brain areas such as the PFC and the hippocampus via two types of receptors, i.e. the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR), to shut down the stress responses after the threat has passed (Aihara et al., 2007; Qi et al., 2013). The termination of the stress response is as important as its initiation, since superfluous cortisol can cause broad endocrine dysregulation (Lucassen et al., 2014). Moreover, the high proportion of depressed patients in cases of Cushing’s disease and during the treatment with high dosage of synthetic corticosteroids point to a possible causal role of glucocorticoids in a subset of mood disorders (Bao et al., 2012). However, it should be noted here that certainly not all studies on peripheral blood samples show elevated levels of cortisol in MDD patients (see for instance (Sigurdsson et al., 2014). Several points should be taken into consideration here. In the first place, morning salivary and serum cortisol levels were reported to be reduced in depression (Strickland et al., 2002), while such observations may well be related to the alterations in the circadian rhythms in mood disorder patients (Zhou et al., 2001; Wu et al., 2017) rather than pointing to the lack of activation of the HPA axis. Secondly, if cortisol is not elevated in mood disorder patients, it does not necessarily mean that the HPA axis is not activated. The classic CRH neurons in the paraventricular nucleus (PVN) project to the portal blood vessels of the median eminence and regulate adrenocorticotropic hormone (ACTH) production in the pituitary. However, there are also CRH neurons that are activated in the stress response and depressive disorders, that project to different brain areas and may induce at least a part of the mood disorder symptoms. It is important, in this respect, that central administration of CRH in the brain can mimic the signs and symptoms of major depression (Holsboer et al., 1992). Activity changes in a subgroup of centrally projecting CRH neurons may not be directly monitored by measuring hormonal alterations in the periphery, but might be reflected better in CRH cerebrospinal fluid (CSF) levels. Thirdly, enhanced sensitivity for cortisol may also be a reason for low peripheral cortisol levels, while CRH expression is high, as shown in posttraumatic stress disorder (PTSD), a disorder with an enhanced risk for mood disorder (Morris et al., 2012). A meta-analysis showed that plasma or serum cortisol levels in PTSD did not differ from controls. When controls that were not exposed to trauma were compared to PTSD patients, the cortisol levels in the PTSD group were even lower in the latter (Meewisse et al., 2007). The hypersensitivity of the cortisol feedback is at least partly due to single nuclear polymorphisms (SNPs) in the GR gene, FKBP5 gene or CRH receptor (CRH-R)-1 gene (Castro-Vale et al., 2016). The genetic basis of the hypersensitivity means that this will be a general phenomenon in the brain of patients carrying such a SNP. Finally, an opposite case of low levels of CRH should also be mentioned, i.e. the atypical depression, which shows high levels of corticosteroids, accompanied by low levels of CRH (Gold et al., 2002; Erkut et al., 1998).
Concluding, during development the HPA axis may be permanently activated by genetic or environmental factors and make people vulnerable to environmental stress that can further activate the HPA axis on different levels. The activated HPA axis may affect mood by the central projections of CRH and/or enhanced circulating levels of corticosteroids, while SNPs on the different levels of the HPA axis may be the basis of variability in sensitivity of the brain for cortisol.
Interaction between the HPA axis, neurotransmitters and neuromodulators
Monoamines
The HPA axis is innervated and regulated by a large number of neurotransmitters and neuropeptides that show alterations in mood disorders. Over the past four decades, the focus on the brain monoaminergic systems, which contain serotonin ( = 5-hydroxytryptamine, 5-HT), noradrenalin (NA) and dopamine (DA), has contributed significantly to our knowledge of the pathophysiology and treatment of depression. In addition, the focus on amines has contributed to the development of a growing number of antidepressants, including the tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors and monoamine receptor antagonists (Hirschfeld, 2012).
Stress activates not only the HPA axis, but also 5-HT neuronal activity and increases the extracellular 5-HT levels in the dorsal raphe nucleus (Waselus et al., 2011). A large body of evidence shows a remarkable effect of an altered 5-HT system on the activity of the HPA axis involving the 5-HT (serotonin) 2C receptor. In particular, acute administration of 5-HT receptor ligands increased the plasma levels of ACTH and cortisol in both animals and humans (Heisler et al., 2007). A light and electron microscopic immunocytochemical study in the rat brain demonstrated that serotonin-containing terminals formed axo-dendritic and axo-somatic synapses with CRH-immunoreactive (ir) neurons in the hypothalamic PVN, which indicates that the central serotonergic system can influence the function of the HPA axis via a direct action upon the CRH synthesizing neurons (Liposits et al., 1987). Indeed, 5-HT1 A and 5-HT2A receptors with a high degree of co-localization in the CRH neurons were found in the PVN, while microinjection of 5-HT1 A agonist (8−OH-DPAT) into the PVN triggered ACTH secretion through local 5-HT1 A receptor activation (Osei-Owusu et al., 2005; Zhang et al., 2004).
It should be noted, however, that antidepressants aiming at the aminergic systems appear to be effective only in 40–60% of the patients. Moreover, there is a huge discrepancy between the pharmacological/biochemical function of these antidepressants (a few minutes) and their clinical mood-altering responses (10–15 days or longer) (Leonard, 2007), which is a serious problem in understanding what mechanisms these antidepressants modify in their effects to relieve depression. It is now also clear that there is no simple direct relationship between changes in the aminergic systems and the occurrence of depression (Ruhe et al., 2007).
Communication between NA and the HPA axis has also been extensively reported during stress responses and in depression (Gold, 2015). Electrical stimulation of the ventral noradrenergic ascending bundle, the principal pathway of NA fibers to the PVN, was found to increase CRH release to the portal system, an effect that could be blocked by an α1-adrenergic antagonist. Norepinephrine (NE = NA) microinjection into the PVN of conscious rats induces a rapid increase of CRH heteronuclear RNA expression in the parvocellular division of the PVN. In addition, immobilization stress has been observed not only to enhance NE release, reuptake, metabolism, and synthesis in the PVN and the medial PFC, but also to trigger transcriptional activation of the genes encoding catecholamine biosynthetic enzymes in the LC, a target for CRH neurons. Moreover, local CRH infusion showed that CRH serves as an excitatory neurotransmitter in the LC, and suggest that its actions on LC neurons are translated into enhanced NE release, which has an impact on cortical targets. Furthermore, NA-receptor sensitivity is altered by the circulating level of glucocorticoids (Bao et al., 2012). Under normal circumstances, noradrenergic systems can influence the magnitude of the HPA axis response to stress. However, in MDD subjects the HPA axis activation appears autonomous of noradrenergic influence (Young et al., 2005).
DA, too, plays a role in the stress response and is dysregulated in mood disorders. Stress exposure and elevated levels of glucocorticoids enhance DA release and influence the activity or synthesis of tyrosine hydroxylase in the nucleus accumbens (Belujon and Grace, 2017). At the anatomical level, a large number of DA receptor D-1 subtype positive neurons exist in both magnocellular and parvocellular parts of the hypothalamic PVN, and there is evidence that corticosterone may regulate the expression of D-1 while DA via D-1 receptors may regulate the amount of circulating corticosterone(Czyrak et al., 2003). Human brain imaging studies have provided further evidence that stress-related increases in cortisol are associated with DA accumulation in the ventral striatum (Oswald et al., 2005; Pruessner et al., 2004). DA was found to be produced not only in the substantia nigra and ventral tegmentum, but also in the neurons of the PVN and supraoptic nucleus (SON) (Panayotacopoulou et al., 2005; Dudas et al., 2010), although the role of DA in these nuclei in physiology and mood disorders is not clear at present. It seems that not only glucocorticoids, acting through GRs, may regulate the function of the dopaminergic systems, but that DA, especially via D-1 receptor, found in the hypothalamic PVN, SON, and SCN (Fremeau et al., 1991), may participate in a wide variety of hypothalamic functions involved in the integration of endocrine, autonomic, and behavioral processes. Expression of D-1 receptors in the SCN, the biological clock of the hypothalamus, suggests that D-1 receptors may influence the regulation of circadian rhythms that are disturbed in depression (Czyrak et al., 2000, 2003; Wu et al., 2017).
Concluding, the HPA axis is innervated by the serotoninergic, noradrenalinergic and dopaminergic systems, while during the stress response the interplay between the HPA axis and these aminergic systems forms a concerted action.
Glutamate and GABA
Amino acid neurotransmitters are also presumed to play an important role in the pathogenesis of depression. Glutamate and GABA are, respectively, the principal excitatory and inhibitory neurotransmitters in the central nervous system (Lener et al., 2017). Unlike the monoamine transmitters that occupy about 5% of the total synapses in the brain, glutamate and GABA are thought to account for at least 50% of the synapses (Leonard, 2007). In addition, excitatory glutamatergic and inhibitory GABAergic synaptic inputs have been identified on hypothalamic CRH-expressing neurons. Glutamate administration in the PVN of rat causes a rise in ACTH and corticosterone serum levels by promoting CRH secretion (Herman et al., 2004), while glutamate modulators have shown therapeutic effects in depression (Henter et al., 2018). Moreover, a reduced GABAergic tone of the parvocellular neurons in the PVN, indicating hyperactivity of this nucleus, was observed during chronic stress (Gao et al., 2017). This implies a diminished inhibition to CRH-producing cells that may lead to an activation of the HPA axis (Verkuyl et al., 2004). A 43% significant reduction of the density of glutamic acid decarboxylase (GAD)65/67-ir was found in the hypothalamic PVN of MDD patients (Gao et al., 2013). In fact, it is well accepted now that there are two major inhibitory mechanisms which serve to constrain the basal and stress-induced activity of the HPA axis: 1) the corticosteroid feedback and 2) the inhibition of the PVN by the neurotransmitter GABA, the two of which work together and influence each other (Gao et al., 2013; Schmidt et al., 2005).
Neuropeptides
There is strong evidence implying that neuropeptides, e.g. CRH and AVP and oxytocin (OXT), do not only play an important role in the integration of endocrine, autonomic, and higher brain functions, but also contribute to the symptoms of depression (Bao et al., 2012).
CRH
The number of CRH neurons, the number of CRH neurons co-expressing AVP, and the amount of CRH-mRNA in the PVN are significantly increased in the postmortem brain of subjects with a long history of mood disorders, independent of whether they die during a depressive state or not (Bao et al., 2005; Raadsheer et al., 1994a, 1995; Wang et al., 2008). A recent study showed that the CRH plasma levels in MDD patients are significantly higher than those in healthy controls (Lu et al., 2018). In addition, epigenetic DNA methylations of the CRH gene were found to be risk factors for psychiatric illness in adolescents (Jokinen et al., 2018). Intracerebral injection of CRH in rodents induces depression-like changes including decreased food intake, decreased sexual activity, disturbed sleep and motor behavior, and increased anxiety (Holsboer, 2001).
Parvocellular PVN neurons secrete both, CRH and AVP, as neurohormones in the median eminence into the portal capillaries, that transport them to the anterior lobe of the pituitary. AVP strongly potentiates the ACTH-releasing activity and, eventually, the production of corticosteroids from the adrenal gland (Engelmann et al., 2004). Thus, there is a close interaction between CRH, AVP and HPA axis activity. During postnatal development, CRH controls the activity of the HPA axis and mediates the effects of early disturbances such as maternal deprivation through the CRH-R1 (Schmidt et al., 2006). In addition, SNPs in the CRH-R1 and CRH-R2 genes were found to be associated with increased susceptibility to MDD, indicating a possible primary role for the CRH-R at least in some cases of depression (Liu et al., 2006b; Ishitobi et al., 2012).
GR-mRNA concentration and glucocorticoid binding activity were increased in brain tissues of animals chronically treated with antidepressants. The time course of the antidepressant effects on GRs coincides with their long-term actions on the HPA activity and follows closely that of clinical improvement of depression (Barden, 1996). In addition, decreased methylation of the GR gene promoter was observed in leukocytes of patients with anxiety and depressive disorder (Tyrka et al., 2016). The clinical observation that patients with treatment-resistant depression noticed a significant improvement in mood after receiving dexamethasone while remaining on their antidepressant (sertraline or fluoxetine) further supports the concept that hyperactive CRH neurons play a causal role in the symptomatology of depression (Dinan et al., 1997). Moreover, the CRH concentrations in CSF in healthy volunteers and depressed patients decrease due to prolonged treatment with antidepressant drugs (Heuser et al., 1998), although it should be noted that CSF-CRH can also be derived from other brain areas, such as the thalamus (Bao et al., 2005; Hsu et al., 2001).
Lastly, in depressed patients significantly increased CRH-mRNA levels in the PVN were found to be accompanied by a significantly increased expression of genes involved in the activation of CRH neurons, such as CRH-R1, MR, estrogen receptor-alpha (ER-α), and AVP receptor (AVPR) subunit AVPR1a, together with a significantly decreased expression of genes involved in the inhibition of CRH neurons, such as the androgen receptor (AR) mRNA (Wang et al., 2008). These findings obtained by laser microdissection of the PVN further raise the possibility that a disturbed receptor balance in the PVN may contribute to the activation of the HPA axis in depression. Together, the arguments mentioned above support the CRH-hypothesis of depression (Holsboer, 1988): hyperactivity of CRH neurons, and thus of the HPA axis, is of crucial importance for the induction of the symptoms of depression, at least in a subgroup of patients.
AVP
Besides the role of AVP in the regulation of osmolality, blood pressure, temperature, and corticosteroid secretion, the centrally-projecting vasopressinergic fibers are involved in the stress response, cognition, social attachment and emotionality. Intranasal AVP differentially affects social communication in men and women (Caldwell and Albers, 2016). The human brain contains AVPR in many areas (Freeman et al., 2017; Loup et al., 1991). There are at least 4 different vasopressinergic systems intimately involved in the symptoms of depression (for reviews see (Swaab, 2003, 2004)). First, AVP is produced as a neurohormone by the magnocellular neurons of the hypothalamic SON and PVN, whose axons run to the neurohypophysis where it is released into the general circulation. Circulating AVP has an influence on the anterior pituitary and high levels of circulating AVP also affect mood. In the second place, parvocellular neurons of the PVN secrete CRH and AVP also as neurohormones from their axons in the median eminence into the portal capillaries that transport them to the anterior lobe of the pituitary. AVP strongly potentiates ACTH-releasing activity (Engelmann et al., 2004). Third, additional vasopressinergic fibers are found to project from the hypothalamus to subregions of the hippocampus, septum, amygdala, and brainstem areas, where AVP serves as a neurotransmitter/neuromodulator via AVPR1a and AVPR1b receptors that are widely distributed (Surget and Belzung, 2008). AVP works through AVPR1b on pituitary corticotropes and the AVPR1b seems to be more reactive in depression (Dinan et al., 2004). In contrast, a major SNP haplotype of the AVPR1b has been found to protect against recurrent MDD (van West et al., 2004), while also evidence was found for the involvement of SNPs of AVPR1b in childhood-onset mood disorders, particularly in females (Dempster et al., 2007). These observations support the possibility of a direct involvement of AVP in the pathogenesis of depression, at least in some subjects. Moreover, particularly the magnocellular hypothalamic neurons release AVP from their dendrites and somata, subsequently diffusing through the brain’s extracellular fluid to act as neuromodulators on receptors at some distance from their site of release (Ludwig et al., 2005). Forth, AVP is also released into the brain with a circadian rhythm by neurons of the SCN, which shows significant changes in depression (see below). Once over-expressed and over-released, AVP may contribute to hyper-anxiety and depression-like behaviors, while AVP deficit, in turn, may cause signs of both diabetes insipidus, hypo-anxiety (Inder et al., 1997; Landgraf et al., 2007; Mlynarik et al., 2007; Iovino et al., 2018) and disturbed rhythmicity (Zhou et al., 2001; Wu et al., 2017).
As mentioned before, a multiple misbalance of receptor genes involved in the regulation of the HPA axis activity has been proposed in depression (Wang et al., 2008), in which the increased AVPR1a suggests a role of enhanced somato-dendritic AVP release in the PVN (Surget and Belzung, 2008). In addition, circulating AVP from the SON may induce ACTH release from the pituitary (Gispen-de Wied et al., 1992). Transient activation of the HPA axis following a single exposure to a stressor may induce delayed and long-lasting hyperproduction, hyperstorage, and hypersecretion of AVP, e.g. from hypothalamic CRH neurons, which result in hyperresponsiveness of the HPA axis to subsequent stimuli (Schmidt et al., 1995, 1996). The sensitization of neuronal processes is proposed to be an important feature in promoting depressive-like states. It was suggested that with each stressor experience and with each successive episode of depression, neuronal sensitization becomes more pronounced, and hence the stressor severity necessary to elicit the neurochemical changes (and thus to induce a depressive episode) becomes progressively smaller. This is in line with clinical observations that the relation between life-events and recurrences of depressive episodes is less clear in those patients who have highly recurrent MDD (Ten Have et al., 2018). It is also important to note that, whereas in acute stress CRH is the main cause of increased ACTH release, animal experiments show that in chronic stress there is a switch from CRH to AVP stimulation of ACTH release (Scott and Dinan, 1998). Since depression is a chronic disorder, the AVP-driven HPA hyperactivity in depression is receiving more and more attention (Meynen et al., 2006). In case of chronic depression, AVP is proposed to be persistently increased within CRH neurons, so that even minor day-to-day annoyances might trigger excessive CRH/AVP release. This, in turn, would favor the presentation of dysphoric symptoms, and might even be a factor responsible for triggering MDD and dysthymia (Griffiths et al., 2000). The number of CRH and AVP-colocalizing neurons is indeed increased in the human PVN in depression and during the course of aging, at least in males (Bao and Swaab, 2007; Raadsheer et al., 1993, 1994b).
Feedback of corticosteroids takes place on the PVN, SON and SCN. Following different types of corticosteroid treatment in different disorders or during the presence of high levels of endogenous corticosteroids produced by a tumor, we found - in postmortem tissue - not only that CRH-expressing neurons are strongly down-regulated, but also that AVP expression in the SON and PVN is strongly decreased. On the other hand, OXT neurons were not affected. Therefore, in the human brain, the negative-feedback of corticosteroids is acting selectively on CRH cells and cells that (co-)express AVP but not on OXT cells. A glucocorticoid-induced suppression of AVP-synthesis has been proposed to occur at the posttranscriptional level (Erkut et al., 1998, 2002). In the human SCN we found a diminished AVP mRNA following administration of corticosteroids (Liu et al., 2006a), which may be one explanation for the disturbed circadian rhythms in depression.
In the PVN of mood disorder patients, the number of AVP and OXT expressing neurons is increased (Purba et al., 1996). Using radioactive in situ hybridization, our group determined the amount of AVP mRNA in the PVN and SON in formalin-fixed, paraffin-embedded archival postmortem brain tissues of depressed subjects and their controls. In the SON, a 60% increase of AVP mRNA expression was found in depressed subjects but AVP mRNA expression was only significantly increased in both the SON and the PVN in the melancholic subgroup (Meynen et al., 2006). Enhanced AVP mRNA production in the SON of depressed patients (Meynen et al., 2006) leads to increased plasma levels of AVP (van Londen et al., 1997, 1998b; van Londen et al., 2001) that are also reported to be related to an enhanced suicide risk in depression (Inder et al., 1997), as well as to an anxious-retarded type of depression (de Winter et al., 2003; Goekoop et al., 2006). It has also been proposed that AVP may not only be important in mediating psychomotor retardation but also in affecting memory processes in depressed patients, possibly by altering arousal and attention (Van Londen et al., 1998a; van Londen et al., 1998b). The possibility that circulating AVP may also induce symptoms of depression is supported by the case-story of a man displaying chronically elevated plasma AVP levels due to AVP secretion by an olfactory neuroblastoma, which induced his first episode of MDD. Depressive symptoms improved markedly after surgical resection of the tumor and subsequent normalization of plasma AVP levels. In this patient the HPA axis was suppressed (Muller et al., 2000), indicating a primary role of AVP in the pathogenesis of depression.
In addition, in depression and suicide, alterations in centrally released AVP from the PVN may play a role, since immunoreactive AVP was elevated in the dorsomedial prefrontal cortex and reduced in the dorsal vagal complex (Merali et al., 2006).
OXT
In contrast to AVP, OXT inhibits ACTH and glucocorticoid secretion in various species including humans (Legros, 2001; Iovino et al., 2018). When OXT is released from the neurohypophysis it acts as a neurohormone and plays a role in uterine contractions and milk ejection during parturition and lactation (Carson et al., 2013). In recent decades, however, PVN OXT was shown to be synaptically released into different brain areas and to play a central role as neuromodulator (Knobloch et al., 2012). OXT receptors are present in many brain areas (Freeman et al., 2017; Loup et al., 1991; Boccia et al., 2013). Central effects of OXT include affective social behaviors, such as maternal care and pair bonding (Smith et al., 2010), the attenuation of fear and of the stress response (Quirin et al., 2011), and the modulation of the symptoms of psychiatric disorders such as MDD and bipolar disorder (BD) (Cochran et al., 2013). Since the observations of the social and/or clinical effects of OXT have been generally performed by intranasal OXT administration, the belief that all effects of intranasal OXT can be explained by a direct OXT action on brain systems has been challenged (Leng and Ludwig, 2016).
The literature tends to remain equivocal on the direction of OXT changes in the circulation in depression. For instance, both enhanced (Heinrichs and Domes, 2008; Turan et al., 2013) and weakened (Ozsoy et al., 2009) serum OXT levels have been reported in mood disorders. These considerations rendered it crucial to do postmortem brain research on the OXT system in the brain in clinically and neuropathologically well-characterized patients with mood disorders. Activation of OXT neurons has indeed been found in depression and may be related to decreased appetite and weight loss in this disease, due to the central effects of this neuropeptide as a satiety hormone (Gimpl and Fahrenholz, 2001; Meynen et al., 2007; Purba et al., 1996; Dai et al., 2017). Enhanced OXT production in the PVN was indeed found in postmortem material of melancholic depressed patients (Meynen et al., 2007). There is, in addition, an interesting difference in the effect of sex hormones on this system: androgens inhibit and estrogens stimulate OXT transcription (Dai et al., 2017). This observation explains, at least partly, the reason why there were opposing effects of OXT and testosterone in cognitive and behavioral functions and in various neuropsychiatric disorders including depression (Crespi, 2016).
It deserves further study whether the increased PVN OXT may decrease ACTH and glucocorticoid secretion and thus diminishes the negative feedback on hypothalamic CRH expression, which will cause further hyperactivity of CRH and depression symptoms. It should be noted that in contrast to the central activation of the OXT system, decreased peripheral serum OXT levels were observed in female depressed patients both pre- and posttreatment (Ozsoy et al., 2009). This indicates that neuropeptides such as OXT, AVP and CRH may independently be secreted into the circulation and to brain areas, and that by measuring circulating hormonal alterations in the periphery we do not necessarily get the right information on its central release.
Orexin (hypocretin)
A relatively novel neuropeptide, i.e. orexin (=hypocretin), is produced in the hypothalamus and has reciprocal connections with the aminergic system, the cholinergic system and the CRH neurons. Orexin stimulates CRH neurons (Lu et al., 2017). Recently, we have observed that the amount of orexin-ir neurons was significantly increased in female but not in male depression patients. In addition, hypothalamic orexin showed a clear diurnal fluctuation, which was absent in depression. Moreover, male depressive patients who had committed suicide showed significantly increased orexin-receptor-2-mRNA expression in the anterior cingulate cortex (Lu et al., 2017). These data showed a clear sex-related change in the hypothalamic orexin-ir in depression. In summary, these observations support the concept that the HPA axis holds a prominent position in a network of stress- (and reward-) related neurotransmitter and neuromodulator systems, all of which are also crucially involved in depression.
Sex difference in the HPA axis and depression
The sex difference found in depression might provide additional support for the CRH-hypothesis of depression. Since MDD is twice as prevalent in women of reproductive age as in men, organizing and/or activating effects of sex hormones, directly or indirectly on the HPA axis, are proposed as risk factors for depression. The possible importance of fluctuating levels of sex hormones as a risk factor for depression is underlined by the higher prevalence of premenstrual depression, antepartum or postpartum depression, and depression during the transition to menopause (Bao et al., 2004, 2012). Studies in rodents have found that the female brain's innate strategy to handle stress differs from that of the male brain (Ter Horst et al., 2009). In addition, organizing effects of estrogen-like compounds during fetal life may also be responsible for a higher prevalence of mood disorders, as appeared in children exposed in utero to diethylstilbestrol (Meyer-Bahlburg and Ehrhardt, 1987). Furthermore, age-related sex differences have been found in CRH -expressing neurons in the human hypothalamic PVN, which illustrates a relationship between sex hormones and CRH. First, from the age of 24 years onward, men had significantly more CRH-expressing neurons than women, while in the second place there was a significant age-related increase of CRH-expressing neurons in men, but not in women (Bao and Swaab, 2007). It is of interest to note here that AVP neurons in the hypothalamic SON, which is the major source for circulating AVP, showed the opposite pattern. These neurons were only activated during the course of aging in women, and not in men (Ishunina et al., 1999). Moreover, an abnormal hormone status, induced by castration because of prostate cancer, ovariectomy or a sex hormone-producing tumor, was accompanied by changes in the number of CRH-expressing neurons in the human brain (Bao and Swaab, 2007).
It should be noted that sex hormones can also be synthesized locally in the brain as ‘neurosteroids’ (Qi et al., 2017), which may alter in mood disorders and may interact with circulating sex hormones by as yet unknown mechanisms. Sex hormones act by binding to sex hormone receptors that are expressed by CRH neurons in the hypothalamus and by corticotropes in the adenohypophysis (Bao et al., 2005, 2006; Pereira-Lima et al., 2004; Scheithauer et al., 2008; Stefaneanu et al., 1994). We have found co-localization of CRH and sex hormone receptors, indicating a direct effect of sex hormones on CRH neurons. Both nuclear ERα (Bao et al., 2005) and nuclear AR (Bao et al., 2006) were present in CRH expressing neurons in the human hypothalamic PVN. In addition, there was a significantly positive correlation between the increased number of CRH-expressing neurons containing nuclear ERα and the increased number of CRH neurons in mood disorders, both in males and females (Bao et al., 2005). The human CRH gene promoter contains 5 perfect, half-palindromic estrogen-responsive elements (EREs) (Vamvakopoulos and Chrousos, 1993), and animal studies have shown that estrogens stimulate CRH expression (Lund et al., 2004). We have also identified an androgen-responsive element (ARE) in the CRH gene promoter region, but that appeared to initiate a repressing effect of AR on CRH transcription (Bao et al., 2006), which is in agreement with an animal study showing that androgens inhibit CRH production (Lund et al., 2004). Interestingly, the human OXT gene promoter was also found to be up-regulated by estrogens when ER was present (Richard and Zingg, 1990) and down-regulated by testosterone via its combination with nuclear AR (Dai et al., 2017). The changes in the orexin system in depression were also sexually dimorphic. The number of orexin expressing neurons was only significantly increased in females, while male depressive patients who had committed suicide showed significantly increased orexin-receptor-2-mRNA expression in the anterior cingulate cortex (Lu et al., 2017). A change in the AVP level in depression in the biological clock, the SCN, was only present in female- and not in male depression patients (Wu et al., 2017). Sex plays, in addition, an important role in determining whether functional genetic variation in MR is beneficial or detrimental in the etiology of depression following childhood maltreatment (Vinkers et al., 2015).
Thus, there are sex differences in in stress systems that may be a basis for the sex difference in the prevalence of depression. Whether they are due to organizing or activating/inhibiting effects of sex hormones on these neuropeptide stress systems should be further investigated.
Interaction between the HPA axis and the SCN
HPA activity is modulated by the hypothalamic SCN, the biological clock. The SCN is of interest in relation to depression for various reasons. Light is directly influencing the activity of the SCN, and light therapy is effective in depression. In addition, seasonal affective disorder (SAD) is more prevalent in the northern states of the US than in southern states (Miller, 2005). Moreover, the symptoms of depression fluctuate over the day and sometimes the season, and the stress response is also strongly influenced by the time of the day via the SCN. The human SCN shows not only circadian but also circannual variations in neuronal activity (Hofman and Swaab, 1992, 1993), which may be related to circadian and circannual fluctuations in mood and to sleeping disturbances in depression (van Londen et al., 2001). Polymorphisms in the clock genes and an altered epigenetic regulation of a clock gene have been linked to a dysfunctional circadian rhythm and susceptibility for mood disorders, in particular in SAD and BD (Johansson et al., 2003; Kishi et al., 2009; Shi et al., 2008; Oliveira et al., 2018; Dallaspezia and Benedetti, 2015; Bengesser et al., 2016), indicating that the SCN may also play a causal role in depression at least in a subgroup of patients. Moreover, alterations in circadian rhythms and sleep are characterizing both mania and depression (Dallaspezia and Benedetti, 2015). Finally, lithium, the most effective and well established medication for BD is acting on the SCN (Bengesser et al., 2016; Dallaspezia and Benedetti, 2015; Etain et al., 2011; Oliveira et al., 2018; McCarthy et al., 2013; Malhi and Outhred, 2016).
A disorder of SCN function, characterized by an increased number of AVP-expressing neurons, a decreased amount of AVP mRNA in this nucleus, and diminished circadian fluctuation of AVP mRNA has been found in the postmortem brain of depressed patients (Zhou et al., 2001). Recently, this finding was confirmed by Wu et al., with the new finding that a significant increase of SCN-AVP protein levels was only present in female and not in male depression patients (Wu et al., 2017). It is also of interest to note, that most SCN neurons are GABAergic. Therefore, Wu et al. studied the expression of GABA, manifested as SCN GAD65/67-ir and GAD67-mRNA levels in depression, and observed significantly increase, both in SCN GAD65/67-ir and in SCN GAD67-mRNA in the depression group. Given the crucial role of GABA in mediating SCN function, the increased SCN GABA expression may significantly contribute to the disordered circadian rhythms in depression (Wu et al., 2015).
The daily fluctuations of saliva cortisol is flatter in mood disorder patients than in non-depression controls (Wichers et al., 2008; Doane et al., 2013). This may give confusion about the presence or absence of a difference between controls and mood disorder patients if studies are based upon one or a few time points instead of 24-hours sampling.
Melatonin is regulating SCN function and the melatonine-1 receptor appeared to be specifically increased in the SCN of mood disorder patients, and may increase during the course of the disease. This may contribute to the circadian disorders in depression and be the basis for the efficacy of melatonin or melatonin receptor agonists in depression (Wu et al., 2013).
It should be noted that corticosteroids inhibit SCN function (Liu et al., 2006a), which also indicates a close interaction between the SCN and the HPA activity. Light therapy is effective not only in SAD but also for MDD, while - as an adjuvant to conventional antidepressants in MDD patients, or lithium in BD patients -, morning light hastens and potentiates the antidepressant response (Wirz-Justice et al., 2005). We propose, therefore, that light therapy may not only reset the SCN activity in depression, but also diminish CRH activity in the PVN.
Conclusions
This review focused on the molecular neuropathology of mood disorders in the hypothalamus, and elucidated in particular the role of the HPA axis, and its interaction with monoamines, glutamate and GABA, neuropeptides, and an other hypothalamic nucleus, i.e. the SCN, in the pathogenesis of mood disorders (Fig. 1). All these changes are based upon variable alterations in genetic background, developmental history, sex, and environmental stressors. One may hope that in the future these data will allow a better prediction for the vulnerable neurobiological system in an individual depressed patient, and that this will lead, ultimately, to an optimal tailor-made anti-depressive therapy.
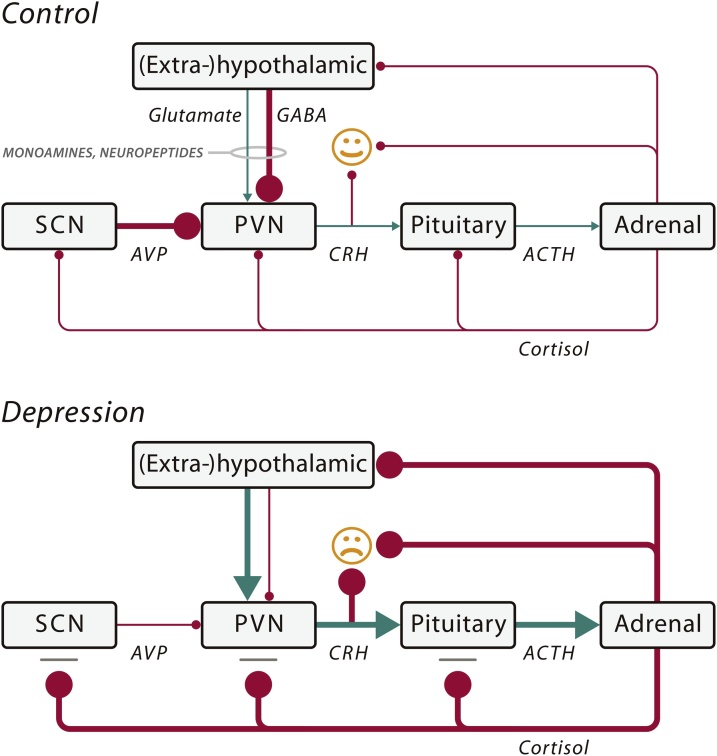
Fig. 1.
Schematic illustration of some of the key mechanisms that may cause a mood disorder by impaired input of neurotransmitters (monoamines and amino acids) and/or neuropeptides (arginine vasopressin (AVP), oxytocin (OXT)) on the hypothalamo–pituitary– adrenal (HPA) axis. In the control situation (normal mood), the corticotropinreleasing hormone (CRH) neurons of the stress axis (HPA axis) are inhibited by a γ-aminobutyric acid (GABA)-ergic input from (extra-)hypothalamic areas and by an AVP input from the suprachiasmatic nucleus (SCN). Some monoamines and neuropeptides (e.g., OXT) also inhibit the HPA axis. When there is depression, the HPA axis is activated by: (1) diminished GABAergic input; and/or (2) increased glutaminergic input from (extra-)hypothalamic sites; and/or (3) diminished inhibition by the SCN; and/or (4) stimulatory influence on the HPA axis by alterations in the monoamine or neuropeptide input; and/or (5) a deficient cortisol feedback effect due to the presence of glucocorticoid resistance. The resulting disinhibition of the paraventricular nucleus (PVN) causes a chronic rise in CRH and cortisol levels in depression, which causes mood changes through their action on the brain. The hyperactivity of the HPA axis may be due to a multitude of risk factors such as genetic polymorphisms, development sequelae, and environmental factors. A decreased amount of AVP mRNA of the SCN was found in depression, which seems to be the basis of the impaired circadian regulation of the HPA system in depression and a decreased inhibition of CRH neurons. Arrow: stimulation; Bar with ball-head: inhibition. The thickness of the lines indicates the strength of the effects. ACTH, adrenocorticotropic hormone. Modified from Fig. 1 of (Bao et al., 2012).
Conflicts of interest
The Authors declare no conflict of interest.
Acknowledgements
Prof. Ai-Min Bao was supported by the National Key Research and Development Program of China (2016YFC1306700), the National Natural Science Foundation of China (91332102). Prof. Dick Swaab was supported by Programme of Introducing Talents of Discipline to Universities of China (B13026) and Stichting Vrienden van het Herseninstituut.
References
- Aihara M., Ida I., Yuuki N., Oshima A., Kumano H., Takahashi K., Fukuda M., Oriuchi N., Endo K., Matsuda H., Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Swaab D.F. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85:27–36. doi: 10.1159/000099832. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Ji Y.F., Van Someren E.J., Hofman M.A., Liu R.Y., Zhou J.N. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Horm. Behav. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Hestiantoro A., Van Someren E.J., Swaab D.F., Zhou J.N. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Fischer D.F., Wu Y.H., Hol E.M., Balesar R., Unmehopa U.A., Zhou J.N., Swaab D.F. A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol. Psychiatry. 2006;11:567–576. doi: 10.1038/sj.mp.4001800. [DOI] [PubMed] [Google Scholar]
- Bao A.M., Ruhe H.G., Gao S.F., Swaab D.F. Neurotransmitters and neuropeptides in depression. Handb. Clin. Neurol. 2012;106:107–136. doi: 10.1016/B978-0-444-52002-9.00008-5. [DOI] [PubMed] [Google Scholar]
- Barden N. Modulation of glucocorticoid receptor gene expression by antidepressant drugs. Pharmacopsychiatry. 1996;29:12–22. doi: 10.1055/s-2007-979536. [DOI] [PubMed] [Google Scholar]
- Belujon P., Grace A.A. Dopamine system dysregulation in Major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengesser S.A., Reininghaus E.Z., Lackner N., Birner A., Fellendorf F.T., Platzer M., Kainzbauer N., Tropper B., Hormanseder C., Queissner R., Kapfhammer H.P., Wallner-Liebmann S.J., Fuchs R., Petek E., Windpassinger C., Schnalzenberger M., Reininghaus B., Evert B., Waha A. Is the molecular clock ticking differently in bipolar disorder? Methylation analysis of the clock gene ARNTL. World J. Biol. Psychiatry. 2016:1–9. doi: 10.1080/15622975.2016.1231421. [DOI] [PubMed] [Google Scholar]
- Boccia M.L., Petrusz P., Suzuki K., Marson L., Pedersen C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Caldwell H.K., Albers H.E. Oxytocin, vasopressin, and the motivational forces that drive social behaviors. Curr. Top. Behav. Neurosci. 2016;27:51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- Carson D.S., Guastella A.J., Taylor E.R., Mcgregor I.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. 2013;27:231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Castro-Vale I., Van Rossum E.F., Machado J.C., Mota-Cardoso R., Carvalho D. Genetics of glucocorticoid regulation and posttraumatic stress disorder--what do we know? Neurosci. Biobehav. Rev. 2016;63:143–157. doi: 10.1016/j.neubiorev.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Clark P.M. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. Eur. J. Pediatr. 1998;157(Suppl 1):S7–S10. doi: 10.1007/pl00014289. [DOI] [PubMed] [Google Scholar]
- Cochran D.M., Fallon D., Hill M., Frazier J.A. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry. 2013;21:219–247. doi: 10.1097/HRP.0b013e3182a75b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi B.J. Oxytocin, testosterone, and human social cognition. Biol. Rev. Camb. Philos. Soc. 2016;91:390–408. doi: 10.1111/brv.12175. [DOI] [PubMed] [Google Scholar]
- Czyrak A., Chocyk A., Mackowiak M., Fijal K., Wedzony K. Distribution of dopamine D1 receptors in the nucleus paraventricularis of the hypothalamus in rats: an immunohistochemical study. Brain Res. Mol. Brain Res. 2000;85:209–217. doi: 10.1016/s0169-328x(00)00240-0. [DOI] [PubMed] [Google Scholar]
- Czyrak A., Mackowiak M., Chocyk A., Fijal K., Wedzony K. Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol. J. Pharmacol. 2003;55:667–674. [PubMed] [Google Scholar]
- Dai D., Li Q.C., Zhu Q.B., Hu S.H., Balesar R., Swaab D., Bao A.M. Direct involvement of androgen receptor in oxytocin Gene expression: possible relevance for mood disorders. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaspezia S., Benedetti F. Chronobiology of bipolar disorder: therapeutic implication. Curr. Psychiatry Rep. 2015;17:606. doi: 10.1007/s11920-015-0606-9. [DOI] [PubMed] [Google Scholar]
- De Winter R.F., Van Hemert A.M., Derijk R.H., Zwinderman K.H., Frankhuijzen-Sierevogel A.C., Wiegant V.M., Goekoop J.G. Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology. 2003;28:140–147. doi: 10.1038/sj.npp.1300002. [DOI] [PubMed] [Google Scholar]
- Dempster E.L., Burcescu I., Wigg K., Kiss E., Baji I., Gadoros J., Tamas Z., Kennedy J.L., Vetro A., Kovacs M., Barr C.L. Evidence of an association between the vasopressin V1b receptor gene (AVPR1B) and childhood-onset mood disorders. Arch. Gen. Psychiatry. 2007;64:1189–1195. doi: 10.1001/archpsyc.64.10.1189. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Lavelle E., Cooney J., Burnett F., Scott L., Dash A., Thakore J., Berti C. Dexamethasone augmentation in treatment-resistant depression. Acta Psychiatr. Scand. 1997;95:58–61. doi: 10.1111/j.1600-0447.1997.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., O’brien S., Lavelle E., Scott L.V. Further neuroendocrine evidence of enhanced vasopressin V3 receptor responses in melancholic depression. Psychol. Med. 2004;34:169–172. doi: 10.1017/s0033291703001004. [DOI] [PubMed] [Google Scholar]
- Dudas B., Baker M., Rotoli G., Grignol G., Bohn M.C., Merchenthaler I. Distribution and morphology of the catecholaminergic neural elements in the human hypothalamus. Neuroscience. 2010;171:187–195. doi: 10.1016/j.neuroscience.2010.08.050. [DOI] [PubMed] [Google Scholar]
- Engelmann M., Landgraf R., Wotjak C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front. Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Erkut Z.A., Pool C., Swaab D.F. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J. Clin. Endocrinol. Metab. 1998;83:2066–2073. doi: 10.1210/jcem.83.6.4881. [DOI] [PubMed] [Google Scholar]
- Erkut Z.A., Gabreels B.A., Eikelenboom J., Van Leeuwen F.W., Swaab D.F. Glucocorticoid treatment is associated with decreased expression of processed AVP but not of proAVP, neurophysin or oxytocin in the human hypothalamus: are PC1 and PC2 involved? Neuro Endocrinol. Lett. 2002;23:33–44. [PubMed] [Google Scholar]
- Etain B., Milhiet V., Bellivier F., Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur. Neuropsychopharmacol. 2011;21(Suppl 4):S676–82. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Freeman S.M., Smith A.L., Goodman M.M., Bales K.L. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Soc. Neurosci. 2017;12:113–123. doi: 10.1080/17470919.2016.1156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau R.T., Jr, Duncan G.E., Fornaretto M.G., Dearry A., Gingrich J.A., Breese G.R., Caron M.G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.F., Klomp A., Wu J.L., Swaab D.F., Bao A.M. Reduced GAD(65/67) immunoreactivity in the hypothalamic paraventricular nucleus in depression: a postmortem study. J. Affect. Disord. 2013;149:422–425. doi: 10.1016/j.jad.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhou J.J., Zhu Y., Kosten T., Li D.P. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology. 2017;104:194–208. doi: 10.1159/000446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gispen-De Wied C.C., Westenberg H.G., Koppeschaar H.P., Thijssen J.H., Van Ree J.M. Stimulation of the pituitary-adrenal axis with a low dose [Arg8]-vasopressin in depressed patients and healthy subjects. Eur. Neuropsychopharmacol. 1992;2:411–419. doi: 10.1016/0924-977x(92)90003-q. [DOI] [PubMed] [Google Scholar]
- Goekoop J.G., De Winter R.P., De Rijk R., Zwinderman K.H., Frankhuijzen-Sierevogel A., Wiegant V.M. Depression with above-normal plasma vasopressin: validation by relations with family history of depression and mixed anxiety and retardation. Psychiatry Res. 2006;141:201–211. doi: 10.1016/j.psychres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gold P.W., Gabry K.E., Yasuda M.R., Chrousos G.P. Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol. Metab. Clin. North Am. 2002;31:37–62. doi: 10.1016/s0889-8529(01)00022-6. vi. [DOI] [PubMed] [Google Scholar]
- Griffiths J., Ravindran A.V., Merali Z., Anisman H. Dysthymia: a review of pharmacological and behavioral factors. Mol. Psychiatry. 2000;5:242–261. doi: 10.1038/sj.mp.4000697. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Heisler L.K., Pronchuk N., Nonogaki K., Zhou L., Raber J., Tung L., Yeo G.S., O’rahilly S., Colmers W.F., Elmquist J.K., Tecott L.H. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J. Neurosci. 2007;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henter I.D., De Sousa R.T., Zarate C.A., Jr Glutamatergic modulators in depression. Harv. Rev. Psychiatry. 2018 doi: 10.1097/HRP.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Mueller N.K., Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann. N. Y. Acad. Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Heuser I., Bissette G., Dettling M., Schweiger U., Gotthardt U., Schmider J., Lammers C.H., Nemeroff C.B., Holsboer F. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress. Anxiety. 1998;8:71–79. [PubMed] [Google Scholar]
- Hirschfeld R.M. The epidemiology of depression and the evolution of treatment. J. Clin. Psychiatry. 2012;73(Suppl 1):5–9. doi: 10.4088/JCP.11096su1c.01. [DOI] [PubMed] [Google Scholar]
- Hofman M.A., Swaab D.F. Seasonal changes in the suprachiasmatic nucleus of man. Neurosci. Lett. 1992;139:257–260. doi: 10.1016/0304-3940(92)90566-p. [DOI] [PubMed] [Google Scholar]
- Hofman M.A., Swaab D.F. Diurnal and seasonal rhythms of neuronal activity in the suprachiasmatic nucleus of humans. J. Biol. Rhythms. 1993;8:283–295. doi: 10.1177/074873049300800402. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Implications of altered limbic-hypothalamic-pituitary-adrenocortical (LHPA)-function for neurobiology of depression. Acta Psychiatr. Scand. Suppl. 1988;341:72–111. doi: 10.1111/j.1600-0447.1988.tb08556.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J. Affect. Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F., Spengler D., Heuser I. The role of corticotropin-releasing hormone in the pathogenesis of cushing’s disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog Brain Res. 1992;93:385–417. doi: 10.1016/s0079-6123(08)64586-0. [DOI] [PubMed] [Google Scholar]
- Hsu D.T., Lombardo K.A., Herringa R.J., Bakshi V.P., Roseboom P.H., Kalin N.H. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neuroscience. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Inder W.J., Donald R.A., Prickett T.C., Frampton C.M., Sullivan P.F., Mulder R.T., Joyce P.R. Arginine vasopressin is associated with hypercortisolemia and suicide attempts in depression. Biol. Psychiatry. 1997;42:744–747. doi: 10.1016/s0006-3223(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Iovino M., Messana T., De Pergola G., Iovino E., Dicuonzo F., Guastamacchia E., Giagulli V.A., Triggiani V. The role of neurohypophyseal hormones vasopressin and oxytocin in neuropsychiatric disorders. Endocr. Metab. Immune Disord. Drug Targets. 2018 doi: 10.2174/1871530318666180220104900. [DOI] [PubMed] [Google Scholar]
- Ishitobi Y., Nakayama S., Yamaguchi K., Kanehisa M., Higuma H., Maruyama Y., Ninomiya T., Okamoto S., Tanaka Y., Tsuru J., Hanada H., Isogawa K., Akiyoshi J. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B:429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- Ishunina T.A., Salehi A., Hofman M.A., Swaab D.F. Activity of vasopressinergic neurones of the human supraoptic nucleus is age- and sex-dependent. J. Neuroendocrinol. 1999;11:251–258. doi: 10.1046/j.1365-2826.1999.00318.x. [DOI] [PubMed] [Google Scholar]
- Johansson C., Willeit M., Smedh C., Ekholm J., Paunio T., Kieseppa T., Lichtermann D., Praschak-Rieder N., Neumeister A., Nilsson L.G., Kasper S., Peltonen L., Adolfsson R., Schalling M., Partonen T. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- Jokinen J., Bostrom A.E., Dadfar A., Ciuculete D.M., Chatzittofis A., Asberg M., Schioth H.B. Epigenetic changes in the CRH Gene are related to severity of suicide attempt and a General psychiatric risk score in adolescents. EBioMedicine. 2018;27:123–133. doi: 10.1016/j.ebiom.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T., Kitajima T., Ikeda M., Yamanouchi Y., Kinoshita Y., Kawashima K., Okochi T., Okumura T., Tsunoka T., Inada T., Ozaki N., Iwata N. Association study of clock gene (CLOCK) and schizophrenia and mood disorders in the Japanese population. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259:293–297. doi: 10.1007/s00406-009-0869-4. [DOI] [PubMed] [Google Scholar]
- Knobloch H.S., Charlet A., Hoffmann L.C., Eliava M., Khrulev S., Cetin A.H., Osten P., Schwarz M.K., Seeburg P.H., Stoop R., Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Kessler M.S., Bunck M., Murgatroyd C., Spengler D., Zimbelmann M., Nussbaumer M., Czibere L., Turck C.W., Singewald N., Rujescu D., Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Legros J.J. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Lener M.S., Niciu M.J., Ballard E.D., Park M., Park L.T., Nugent A.C., Zarate C.A., Jr Glutamate and gamma-aminobutyric acid systems in the pathophysiology of Major depression and antidepressant response to ketamine. Biol. Psychiatry. 2017;81:886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Ludwig M. Intranasal oxytocin: myths and delusions. Biol. Psychiatry. 2016;79:243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Leonard B.E. Psychopathology of depression. Drugs Today (Barc) 2007;43:705–716. doi: 10.1358/dot.2007.43.10.1122223. [DOI] [PubMed] [Google Scholar]
- Liposits Z., Phelix C., Paull W.K. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- Liu R.Y., Unmehopa U.A., Zhou J.N., Swaab D.F. Glucocorticoids suppress vasopressin gene expression in human suprachiasmatic nucleus. J. Steroid Biochem. Mol. Biol. 2006;98:248–253. doi: 10.1016/j.jsbmb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhu F., Wang G., Xiao Z., Wang H., Tang J., Wang X., Qiu D., Liu W., Cao Z., Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci. Lett. 2006;404:358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Loup F., Tribollet E., Dubois-Dauphin M., Dreifuss J.J. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhao J., Balesar R., Fronczek R., Zhu Q.B., Wu X.Y., Hu S.H., Bao A.M., Swaab D.F. Sexually dimorphic changes of hypocretin (orexin) in depression. EBioMedicine. 2017;18:311–319. doi: 10.1016/j.ebiom.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.R., Zhang Y., Rao Y.B., Chen X., Lou H.F., Zhang Y., Xie H.Y., Fang P., Hu L.W. The changes in, and relationship between, plasma nitric oxide and corticotropin-releasing hormone in patients with major depressive disorder. Clin. Exp. Pharmacol. Physiol. 2018;45:10–15. doi: 10.1111/1440-1681.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen P.J., Pruessner J., Sousa N., Almeida O.F., Van Dam A.M., Rajkowska G., Swaab D.F., Czeh B. Neuropathology of stress. Acta Neuropathol. 2014;127:109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Bull P.M., Tobin V.A., Sabatier N., Landgraf R., Dayanithi G., Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J. Physiol. 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T.D., Munson D.J., Haldy M.E., Handa R.J. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Outhred T. Therapeutic mechanisms of lithium in bipolar disorder: recent advances and current understanding. CNS Drugs. 2016;30:931–949. doi: 10.1007/s40263-016-0380-1. [DOI] [PubMed] [Google Scholar]
- Mccarthy M.J., Wei H., Marnoy Z., Darvish R.M., Mcphie D.L., Cohen B.M., Welsh D.K. Genetic and clinical factors predict lithium’s effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl. Psychiatry. 2013;3:e318. doi: 10.1038/tp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse M.L., Reitsma J.B., De Vries G.J., Gersons B.P., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Menezes I.C., Von Werne Baes C., Lacchini R., Juruena M.F. Genetic biomarkers for differential diagnosis of major depressive disorder and bipolar disorder: a systematic and critical review. Behav. Brain Res. 2018 doi: 10.1016/j.bbr.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Merali Z., Kent P., Du L., Hrdina P., Palkovits M., Faludi G., Poulter M.O., Bedard T., Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol. Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg H.F., Ehrhardt A.A. Raven Press; New York, USA: 1987. A Prenatal-Hormone Hypothesis for Depression in Adults With a History of Fetal DES Exposure. Hormones and Depression. [Google Scholar]
- Meynen G., Unmehopa U.A., Van Heerikhuize J.J., Hofman M.A., Swaab D.F., Hoogendijk W.J. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biol. Psychiatry. 2006;60:892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Meynen G., Unmehopa U.A., Hofman M.A., Swaab D.F., Hoogendijk W.J. Hypothalamic oxytocin mRNA expression and melancholic depression. Mol. Psychiatry. 2007;12:118–119. doi: 10.1038/sj.mp.4001911. [DOI] [PubMed] [Google Scholar]
- Miller A.L. Epidemiology, etiology, and natural treatment of seasonal affective disorder. Altern. Med. Rev. 2005;10:5–13. [PubMed] [Google Scholar]
- Mlynarik M., Zelena D., Bagdy G., Makara G.B., Jezova D. Signs of attenuated depression-like behavior in vasopressin deficient brattleboro rats. Horm. Behav. 2007;51:395–405. doi: 10.1016/j.yhbeh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Morris M.C., Compas B.E., Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.B., Landgraf R., Keck M.E. Vasopressin, major depression, and hypothalamic-pituitary-adrenocortical desensitization. Biol. Psychiatry. 2000;48:330–333. doi: 10.1016/s0006-3223(00)00886-6. [DOI] [PubMed] [Google Scholar]
- Oliveira T., Marinho V., Carvalho V., Magalhaes F., Rocha K., Ayres C., Teixeira S., Nunes M., Bastos V.H., Pinto G.R. Genetic polymorphisms associated with circadian rhythm dysregulation provide new perspectives on bipolar disorder. Bipolar Disord. 2018 doi: 10.1111/bdi.12624. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P., James A., Crane J., Scrogin K.E. 5-hydroxytryptamine 1A receptors in the paraventricular nucleus of the hypothalamus mediate oxytocin and adrenocorticotropin hormone release and some behavioral components of the serotonin syndrome. J. Pharmacol. Exp. Ther. 2005;313:1324–1330. doi: 10.1124/jpet.104.082073. [DOI] [PubMed] [Google Scholar]
- Oswald L.M., Wong D.F., Mccaul M., Zhou Y., Kuwabara H., Choi L., Brasic J., Wand G.S. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Ozsoy S., Esel E., Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009;169:249–252. doi: 10.1016/j.psychres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Panayotacopoulou M.T., Malidelis Y., Van Heerikhuize J., Unmehopa U., Swaab D. Individual differences in the expression of tyrosine hydroxylase mRNA in neurosecretory neurons of the human paraventricular and supraoptic nuclei: positive correlation with vasopressin mRNA. Neuroendocrinology. 2005;81:329–338. doi: 10.1159/000088760. [DOI] [PubMed] [Google Scholar]
- Pereira-Lima J.F., Marroni C.P., Pizarro C.B., Barbosa-Coutinho L.M., Ferreira N.P., Oliveira M.C. Immunohistochemical detection of estrogen receptor alpha in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology. 2004;79:119–124. doi: 10.1159/000077269. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Champagne F., Meaney M.J., Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba J.S., Hoogendijk W.J., Hofman M.A., Swaab D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Qi X.R., Kamphuis W., Wang S., Wang Q., Lucassen P.J., Zhou J.N., Swaab D.F. Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology. 2013;38:863–870. doi: 10.1016/j.psyneuen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Qi X.R., Zhao J., Liu J., Fang H., Swaab D.F., Zhou J.N. Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb. Cortex. 2015;25:75–83. doi: 10.1093/cercor/bht203. [DOI] [PubMed] [Google Scholar]
- Qi X.R., Luchetti S., Verwer R.W.H., Sluiter A.A., Mason M.R.J., Zhou J.N., Swaab D.F. Alterations in the steroid biosynthetic pathways in the human prefrontal cortex in mood disorders: a post-mortem study. Brain Pathol. 2017 doi: 10.1111/bpa.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M., Kuhl J., Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Raadsheer F.C., Sluiter A.A., Ravid R., Tilders F.J., Swaab D.F. Localization of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus of the human hypothalamus; Age-dependent colocalization with vasopressin. Brain Res. 1993;615:50–62. doi: 10.1016/0006-8993(93)91113-7. [DOI] [PubMed] [Google Scholar]
- Raadsheer F.C., Hoogendijk W.J., Stam F.C., Tilders F.J., Swaab D.F. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raadsheer F.C., Tilders F.J., Swaab D.F. Similar age related increase of vasopressin colocalization in paraventricular corticotropin-releasing hormone neurons in controls and Alzheimer patients. J. Neuroendocrinol. 1994;6:131–133. doi: 10.1111/j.1365-2826.1994.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Raadsheer F.C., Van Heerikhuize J.J., Lucassen P.J., Hoogendijk W.J., Tilders F.J., Swaab D.F. Corticotropin-releasing hormone mRNA levels in the paraventricular nuc leus of patients with Alzheimer’s disease and depression. Am. J. Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Richard S., Zingg H.H. The human oxytocin gene promoter is regulated by estrogens. J. Biol. Chem. 1990;265:6098–6103. [PubMed] [Google Scholar]
- Ruhe H.G., Mason N.S., Schene A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Scheithauer B.W., Kovacs K., Zorludemir S., Lloyd R.V., Erdogan S., Slezak J. Immunoexpression of androgen receptor in the nontumorous pituitary and in adenomas. Endocr Pathol. 2008;19:27–33. doi: 10.1007/s12022-007-9012-0. [DOI] [PubMed] [Google Scholar]
- Schmidt E.D., Janszen A.W., Wouterlood F.G., Tilders F.J. Interleukin-1-induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH)--neurons and hyperresponsiveness of the hypothalamus-pituitary-adrenal axis. J. Neurosci. 1995;15:7417–7426. doi: 10.1523/JNEUROSCI.15-11-07417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.D., Binnekade R., Janszen A.W., Tilders F.J. Short stressor induced long-lasting increases of vasopressin stores in hypothalamic corticotropin-releasing hormone (CRH) neurons in adult rats. J. Neuroendocrinol. 1996;8:703–712. [PubMed] [Google Scholar]
- Schmidt M.V., Levine S., Oitzl M.S., Van Der Mark M., Muller M.B., Holsboer F., De Kloet E.R. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Deussing J.M., Oitzl M.S., Ohl F., Levine S., Wurst W., Holsboer F., Muller M.B., De Kloet E.R. Differential disinhibition of the neonatal hypothalamic- pituitary-adrenal axis in brain-specific CRH receptor 1-knockout mice. Eur. J. Neurosci. 2006;24:2291–2298. doi: 10.1111/j.1460-9568.2006.05121.x. [DOI] [PubMed] [Google Scholar]
- Scott L.V., Dinan T.G. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci. 1998;62:1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. 1936. J. Neuropsychiatry Clin. Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Shan L., Qi X.R., Balesar R., Swaab D.F., Bao A.M. Unaltered histaminergic system in depression: a postmortem study. J. Affect. Disord. 2013;146:220–223. doi: 10.1016/j.jad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Shi J., Wittke-Thompson J.K., Badner J.A., Hattori E., Potash J.B., Willour V.L., Mcmahon F.J., Gershon E.S., Liu C. Clock genes may influence bipolar disorder susceptibility and dysfunctional circadian rhythm. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1047–1055. doi: 10.1002/ajmg.b.30714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson B., Palsson S.P., Aevarsson O., Olafsdottir M., Johannsson M. Saliva testosterone and cortisol in male depressive syndrome, a community study. The Sudurnesjamenn study. Nord. J. Psychiatry. 2014;68:579–587. doi: 10.3109/08039488.2014.898791. [DOI] [PubMed] [Google Scholar]
- Smith A.S., Agmo A., Birnie A.K., French J.A. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierling S.R., Zorrilla E.P. Don’t stress about CRF: assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl) 2017;234:1467–1481. doi: 10.1007/s00213-017-4556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaneanu L., Kovacs K., Horvath E., Lloyd R.V., Buchfelder M., Fahlbusch R., Smyth H. In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J. Clin. Endocrinol. Metab. 1994;78:83–88. doi: 10.1210/jcem.78.1.8288720. [DOI] [PubMed] [Google Scholar]
- Strickland P.L., Deakin J.F., Percival C., Dixon J., Gater R.A., Goldberg D.P. Bio-social origins of depression in the community. Interactions between social adversity, cortisol and serotonin neurotransmission. Br. J. Psychiatry. 2002;180:168–173. doi: 10.1192/bjp.180.2.168. [DOI] [PubMed] [Google Scholar]
- Surget A., Belzung C. Involvement of vasopressin in affective disorders. Eur. J. Pharmacol. 2008;583:340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Swaab D.F. The human hypothalamus. Basic and clinical aspects. Part I: nuclei of the hypothalamus. In: AMINOFF M.J., F. B, SWAAB D.F., editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2003. [Google Scholar]
- Swaab D.F. The human hypothalamus. Basic and clinical aspects. Part II: neuropathology of the hypothalamus and adjacent brain structures. In: AMINOFF M.J., F. B, SWAAB D.F., editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2004. [Google Scholar]
- Ten Have M., De Graaf R., Van Dorsselaer S., Tuithof M., Kleinjan M., Penninx B. Recurrence and chronicity of major depressive disorder and their risk indicators in a population cohort. Acta Psychiatr. Scand. 2018 doi: 10.1111/acps.12874. [DOI] [PubMed] [Google Scholar]
- Ter Horst G.J., Wichmann R., Gerrits M., Westenbroek C., Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Turan T., Uysal C., Asdemir A., Kilic E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38:2890–2896. doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Tyrka A.R., Parade S.H., Welch E.S., Ridout K.K., Price L.H., Marsit C., Philip N.S., Carpenter L.L. Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: associations with early adversity and depressive, anxiety and substance-use disorders. Transl. Psychiatry. 2016;6:e848. doi: 10.1038/tp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamvakopoulos N.C., Chrousos G.P. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J. Clin. Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Londen L., Goekoop J.G., Van Kempen G.M., Frankhuijzen-Sierevogel A.C., Wiegant V.M., Van Der Velde E.A., De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Van Londen L., Goekoop J.G., Zwinderman A.H., Lanser J.B., Wiegant V.M., De Wied D. Neuropsychological performance and plasma cortisol, arginine vasopressin and oxytocin in patients with major depression. Psychol Med. 1998;28:275–284. doi: 10.1017/s0033291797006284. [DOI] [PubMed] [Google Scholar]
- Van Londen L., Kerkhof G.A., Van Den Berg F., Goekoop J.G., Zwinderman K.H., Frankhuijzen-Sierevogel A.C., Wiegant V.M., De Wied D. Plasma arginine vasopressin and motor activity in major depression. Biol. Psychiatry. 1998;43:196–204. doi: 10.1016/S0006-3223(97)80433-7. [DOI] [PubMed] [Google Scholar]
- Van Londen L., Goekoop J.G., Kerkhof G.A., Zwinderman K.H., Wiegant V.M., De Wied D. Weak 24-h periodicity of body temperature and increased plasma vasopressin in melancholic depression. Eur. Neuropsychopharmacol. 2001;11:7–14. doi: 10.1016/s0924-977x(00)00124-3. [DOI] [PubMed] [Google Scholar]
- Van West D., Del-Favero J., Aulchenko Y., Oswald P., Souery D., Forsgren T., Sluijs S., Bel-Kacem S., Adolfsson R., Mendlewicz J., Van Duijn C., Deboutte D., Van Broeckhoven C., Claes S. A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol. Psychiatry. 2004;9:287–292. doi: 10.1038/sj.mp.4001420. [DOI] [PubMed] [Google Scholar]
- Verkuyl J.M., Hemby S.E., Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., Joels M., Milaneschi Y., Gerritsen L., Kahn R.S., Penninx B.W., Boks M.P. Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology. 2015;54:90–102. doi: 10.1016/j.psyneuen.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Kamphuis W., Huitinga I., Zhou J.N., Swaab D.F. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol. Psychiatry. 2008;13(786-99):741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- Wang Q., Joels M., Swaab D.F., Lucassen P.J. Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology. 2012;62:527–533. doi: 10.1016/j.neuropharm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Wang Q., Verweij E.W., Krugers H.J., Joels M., Swaab D.F., Lucassen P.J. Distribution of the glucocorticoid receptor in the human amygdala; Changes in mood disorder patients. Brain Struct. Funct. 2014;219:1615–1626. doi: 10.1007/s00429-013-0589-4. [DOI] [PubMed] [Google Scholar]
- Waselus M., Valentino R.J., Van Bockstaele E.J. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A., Benedetti F., Berger M., Lam R.W., Martiny K., Terman M., Wu J.C. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–944. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wu Y.H., Ursinus J., Zhou J.N., Scheer F.A., Ai-Min B., Jockers R., Van Heerikhuize J., Swaab D.F. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J. Affect. Disord. 2013;148:357–367. doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Wu X.Y., Hu Y.T., Guo L., Lu J., Zhu Q.B., Yu E., Wu J.L., Shi L.G., Huang M.L., Bao A.M. Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav. 2015;145:118–121. doi: 10.1016/j.physbeh.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Wu X., Balesar R., Lu J., Farajnia S., Zhu Q., Huang M., Bao A.M., Swaab D.F. Increased glutamic acid decarboxylase expression in the hypothalamic suprachiasmatic nucleus in depression. Brain Struct. Funct. 2017 doi: 10.1007/s00429-017-1442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang H., Hu J., Hu H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Young E.A., Abelson J.L., Cameron O.G. Interaction of brain noradrenergic system and the hypothalamic-pituitary-adrenal (HPA) axis in man. Psychoneuroendocrinology. 2005;30:807–814. doi: 10.1016/j.psyneuen.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gray T.S., D’souza D.N., Carrasco G.A., Damjanoska K.J., Dudas B., Garcia F., Zainelli G.M., Hanley Sullivan, Battaglia N.R., Muma G., N. A, Van De Kar L.D. Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J. Pharmacol. Exp. Ther. 2004;310:59–66. doi: 10.1124/jpet.103.062224. [DOI] [PubMed] [Google Scholar]
- Zhao J., Verwer R.W., Van Wamelen D.J., Qi X.R., Gao S.F., Lucassen P.J., Swaab D.F. Prefrontal changes in the glutamate-glutamine cycle and neuronal/glial glutamate transporters in depression with and without suicide. J. Psychiatr. Res. 2016;82:8–15. doi: 10.1016/j.jpsychires.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Zhou J.N., Riemersma R.F., Unmehopa U.A., Hoogendijk W.J., Van Heerikhuize J.J., Hofman M.A., Swaab D.F. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch. Gen. Psychiatry. 2001;58:655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]