Abstract
Study Design:
Retrospective cohort study.
Objective:
Anterior approaches are often used during lumbar interbody fusion procedures. Visceral injuries (bowel injuries) are rare but represent a primary risk during anterior approaches to the lumbar spine. Left untreated, these injuries can result in significant complications. The aim of this study was to investigate the presentation and management of bowel injury cases following anterior approaches to the lumbar spine to raise the surgeon’s awareness of this rare complication.
Methods:
All direct anterior, oblique anterior, and transpsoas lumbar interbody fusion surgeries performed at our institution between 2012 and 2016 were analyzed retrospectively. Charts were screened for cases requiring return to the operating room owing to a suspected bowel injury and details of the case were extracted for illustrative purposes.
Results:
A total of 775 anterior lumbar surgeries were conducted at a single tertiary care institution between July 2012 and June 2017. A total of 590 transpsoas lumbar interbody fusion (TPIF) surgeries were performed. Four patients, each having undergone TPIF, were suspected of bowel injury and underwent an exploratory laparotomy. At surgery, 3 patients were confirmed to have a bowel injury, giving a procedure-specific incidence of 0.51% and overall incidence of 0.39%. Among the 3 confirmed bowel injury cases, average delay between surgery and visceral injury diagnosis was 4.7 days (range 3-7 days).
Conclusions:
We noted abdominal pain, distention, and fever as the most common findings in the setting of a visceral injury. A high index of suspicion and computed tomography imaging remain critical for identifying postoperative bowel injuries.
Keywords: bowel injury, spine surgery, complication, lateral approach, peritonitis
Introduction
Interbody fusions of the lumbar spine are performed widely throughout the United States and rates of anterior lumbar interbody fusions have risen in recent years.1-3 Indications include trauma, infection, and degenerative disorders.4 While most commonly performed posteriorly, the lumbar spine may also be accessed through a direct anterior, oblique anterior, or transpsoas approach.1,5,6 Different approaches including minimal invasive can be used based on pathology and surgeons preference.7 Evidence suggests an outcome advantage for anterior over posterior approach in lumbar interbody fusion in appropriately selected patients.6,8-10 Goz et al2 found that anterior lumbar interbody fusions accounted for 15% of lumbar fusions in 2010. While rare, each of these approaches is associated with the risk of vascular and visceral (bowel) injury.11-24
Given the low incidence rates of bowel injury, there is a paucity of reports in the literature examining details of bowel perforations following anterior approaches to the lumbar spine, with most of the literature consisting of case reports.25-31 The often-unclear clinical symptoms (abdominal tenderness and bloating) can lead to a diagnostic delay resulting in severe sepsis.25,32
We seek to build on the extant literature by examining our experience with this rare complication of anterior approaches to the lumbar spine to enhance the surgeon’s awareness of its presentation and management.
Patients and Methods
We retrospectively reviewed all lumbar interbody fusion surgeries performed at a single tertiary care institution between July 2012 and June 2017 that used an anterior approach (direct anterior, oblique anterior, or transpsoas). The Swedish Medical Center Institutional Review Board approved the study and a waiver of informed consent was granted. Institutional medical records were examined for all cases of direct anterior lumbar interbody fusion (ALIF), oblique anterior lumbar interbody fusion (OAIF), and transpsoas lumbar interbody fusion (TPIF). For all included cases, surgical and general demographic data (sex, age) were collected for analysis (Table 1). Patient charts were further reviewed to identify cases requiring a return to the operating room for suspected bowel perforations (Figure 1). Data surrounding the presentation and management of these cases was collected. We extracted the following data from patient charts: surgery details (type of surgery, side of incision, number of incisions, level of surgery, type of implanted device), patient history (major medical comorbidities, history of previous abdominal surgery, opioid dependence), postoperative conditions (nausea/vomiting, abdominal distention, bowel sounds, abdominal pain, opioid use, oral intake status), postoperative day on which bowel injury was discovered, laparotomy findings, type of corrective surgery, culture type, antibiotic use, and patient outcome.
Table 1.
Patient Demographics (N = 775).
| Surgery type, n (%) | |
| TPIF | 590 (76.12) |
| ALIF | 171 (22.06) |
| OAIF | 14 (1.8) |
| Gender, n (%) | |
| Male | 418 (53.93) |
| Female | 357 (46.07) |
| Age, years | |
| Mean | 64.8 |
| Range | 32-82 |
Abbreviations: ALIF, anterior lumbar interbody fusion; OAIF, oblique anterior lumbar interbody fusion; TPIF, transpsoas lumbar interbody fusion.
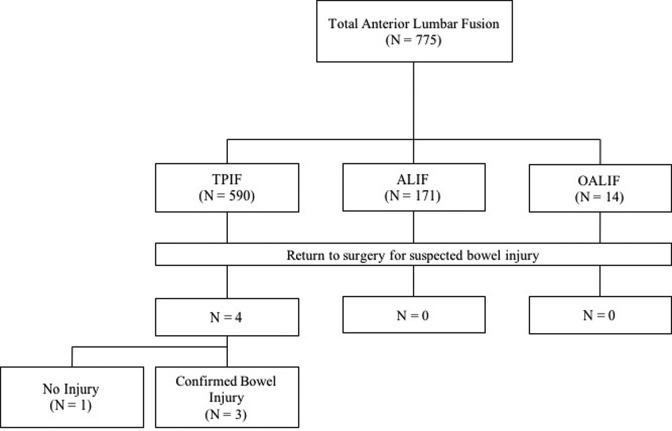
Figure 1.
Flowchart for final patient selection in this study.
Results
During the review period, 775 anterior approach lumbar interbody fusion surgeries were conducted at our institution. These included 171 ALIF, 590 TPIF, and 14 OAIF procedures (Table 1). Over this period, 4 cases of suspected visceral injury (bowel perforation) were recorded. All these cases had undergone TPIF and underwent emergency laparotomies for suspected visceral injury. The visceral injuries were confirmed by laparotomy in 3 cases.
The 3 patients with confirmed bowel injuries were all female with a mean age of 74.3 years (range 72-78 years). The average time between surgery and diagnosis of bowel injury was 4.7 days (range 3-7 days). The most consistent clinical finding was abdominal pain, distention, and fever. Case details, including surgical and case management information, are presented in Table 2.
Table 2.
Bowel Perforation Case Details.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age, years | 73 | 78 | 72 |
| Gender | Female | Female | Female |
| Surgery | TPIF | TPIF | TPIF and posterior fusion |
| Side of approach | Right | Right | Right |
| No. of incisions | 1 | 2 | 2 |
| Level of fusion | L4-5 | L2-3, L3-4 | L2-3, L3-4, L4-5 |
| Cage/plate | Cage and plate | Cage, planned for stage 2 posteriorly | Cage, underwent a posterior fusion after 2 days |
| Major medical comorbidities | Grave’s disease, constipation | Hypertension, irritable bowel syndrome, constipation, breast cancer | Hepatitis C, liver cirrhosis |
| Previous abdopelvic surgery | None | Hysterectomy | None |
| Opioid dependence | No | No | No |
| Nausea/vomiting | Yes | Yes | Yes |
| Abdominal distension | Severe | Severe | Yes, significant |
| Bowel sounds | Hypoactive | Hypoactive | Hypoactive |
| Abdominal pain | Yes, 4 quadrants | Significant | Significant |
| Opioid use | Yes | Yes | Yes |
| Status oral intake following TPIF | Tolerating solids | Tolerating oral liquids | Tolerating Solids |
| Sepsis | Yes | Yes | Yes |
| Day of bowel injury identification | Day 4 | Day 3 | Day 7 |
| Finding at laparotomy | Retroperitoneal abscess; perforation of the ascending colon; fecal contamination | Retroperitoneal fecal contamination; two separate colon perforations in ascending colon | Retroperitoneal extensive fecal contamination; bowel perforation involving the ascending colon; colon was stuck to the L3-4 interbody cage |
| Surgery | Ileocecectomy and side to side anastomosis | Colectomy and diverting ileostomy | Colectomy and end anastomosis |
| Growth | GNR, GPC | GNR | GNR, GPR |
| Antibiotic used | Piperacillin/tazobactam, vancomycin, fluconazole | Cefepime, metronidazole, micafungin | Cefipime, metronidazole |
| Outcome | Good | Good | Good |
Abbreviations: DNR, gram-negative rods; GPC, gram-positive cocci; TPIF, transpsoas lumbar interbody fusion.
Case 1
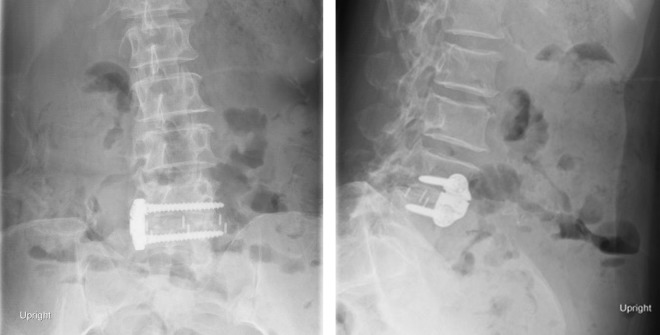
A 73-year-old woman presented with severe L4-5 stenosis, grade 1 degenerative listhesis, and severe neurogenic claudication. Comorbidities included Grave’s disease and chronic constipation, with no history of abdominal or pelvic surgery. A L4-5 TPIF was performed with use of a PEEK (polyetheretherketone) cage and a lateral buttress plate through a single right-side incision (Figure 2A and B). No intraoperative complications were noted.
Figure 2.
Patient 1: (left) anterior-posterior and (right) lateral x-ray images showing L4-5 lateral interbody fusion with a lateral plate.
The patient remained hemodynamically and neurologically stable overnight after surgery. She was on narcotic medication for pain and could tolerate a solid diet. On postoperative day (POD) 2, she had not passed flatus and had abdominal distension with mild abdominal pain. Bowel sounds were hypoactive in all 4 quadrants. On POD 4, she developed a high fever of 42.2°C with hypotension.
An abdominal CT revealed a large complex collection of air and fluid in the right ilio-psoas region with extension of air along fascial planes superiorly to the diaphragmatic crus and inferiorly into the right anterior thigh along the right groin, raising concern for early fasciitis (Figure 3). A small amount of pericolonic fluid and extraluminal foci of air in the right lower quadrant, together with loss of bowel continuity and thickening of the bowel wall observed on imaging.
Figure 3.
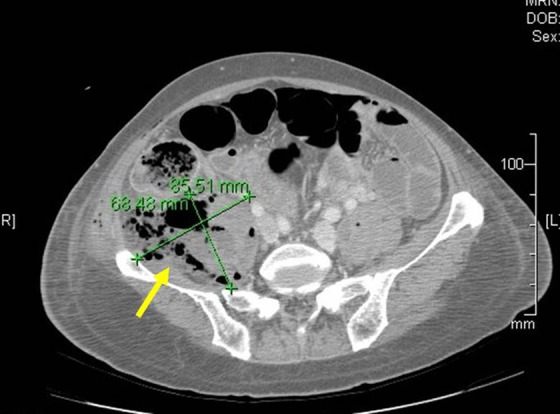
Patient 1: postoperative computed tomography (CT) scan of the abdomen showing presence of extraluminal air and pericolic abscess formation (yellow arrow).
An emergency exploratory laparotomy was performed, which confirmed a retroperitoneal abscess with feculent contamination due to a single perforation in the retroperitoneal ascending colon. Following a thorough lavage, an ileocecectomy and a side-to-side anastomosis was performed. Peritoneal culture grew gram-negative rods (GNR) and gram-positive cocci (GPC) and the patient was started on piperacillin-tazobactam, vancomycin, and fluconazole. Hospital length of stay was extended to 2 weeks because of these complications.
Case 2
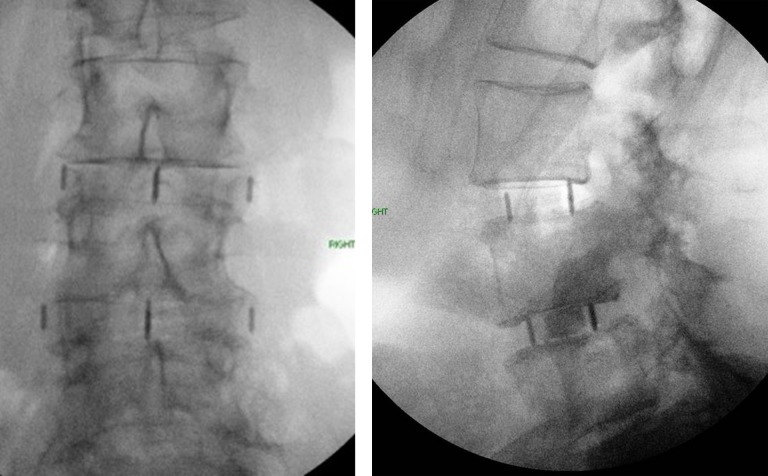
A 78-year-old woman presented to clinic with L2-4 moderate to severe stenosis and L4-5 grade 1 degenerative listhesis. Comorbidities included hypertension, irritable bowel syndrome, lactose and gluten intolerance, constipation, a history of breast cancer, and a prior hysterectomy. A 2-stage L2-4 TPIF and posterior L2-S1 fusion was planned. An uneventful L2-4 TPIF was performed through 2 right-side incisions (Figure 4A and B). No buttress plate was used, and no intraoperative complications were noted.
Figure 4.
Patient 2: (left) anterior-posterior and (right) lateral intraoperative images showing L3-5 interbody fusion.
The patient’s immediate postoperative period was uneventful. On POD 1, she was tolerating an oral diet but complained of abdominal distension that evening. By POD 3, she had developed a fever of 38.9°C to 39.4°C. The second-stage operation was postponed due to suspicion of a urinary tract infection, and a course of intravenous antibiotics was started. The patient became unresponsive that evening and was determined to be in sepsis. An abdominal CT showed large amounts of gas and debris within the right retroperitoneal cavity. There was a loss of continuity of the ascending colon and the ascending colon was suspected to be leaking into the retroperitoneal cavity adjacent to the gas collection (Figure 5). There was also a small amount of free intraperitoneal gas.
Figure 5.
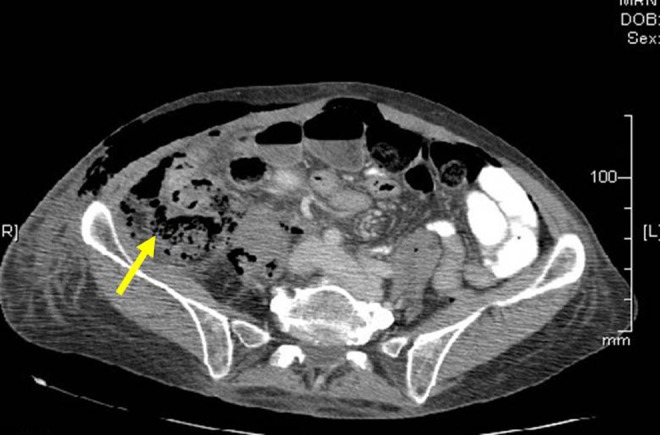
Patient 2: postoperative computed tomography scan of the abdomen showing inflamed ascending colon showing focal extraluminal air collection suggestive of site of perforation (yellow arrow).
An emergency exploratory laparotomy was performed. Surgical findings included 2 separate colonic perforations in the ascending colon and a large amount of stool in the right retroperitoneal cavity. Following debridement of the cavity, a right colectomy and diverting ileostomy was performed. Her ascetic culture grew GNR and she was started on cefepime, metronidazole, and micafungin. The patient did well after the laparotomy and her ileostomy was closed after 4 months. At her last follow-up (2 years), she was ambulatory with improvement of her preoperative symptoms and did not wish to have additional spine surgery.
Case 3
A 72-year-old woman presented with scoliosis and sagittal and coronal imbalance with severe lumbar canal stenosis. Comorbidities included hepatitis C with liver cirrhosis. There was no history of previous abdominal or pelvic surgery. The patient underwent a L2-5 TPIF with use of PEEK cages through 2 right-side transverse incisions. No intraoperative complications were noted.
The patient remained stable overnight and was scheduled for the posterior stage of her surgery in 2 days. Complaints of abdominal distension and nausea were managed with medication. On POD 2, the patient underwent an uneventful posterior fusion. She was started on a patient-controlled analgesia for pain management. On the second day following the posterior surgery, she was hypotensive and septic. This was believed to be the result of Escherichia coli urosepsis. Revision surgery to correct a malpositioned screw observed on routine postoperative CT imaging was postponed and she was started on antibiotics. Clinical findings included poor bowel sounds and mild abdominal distension, but she could tolerate solid food. On day 3 following the stage-2 surgery, the abdominal distension worsened, accompanied by abdominal tenderness with no obvious peritoneal signs.
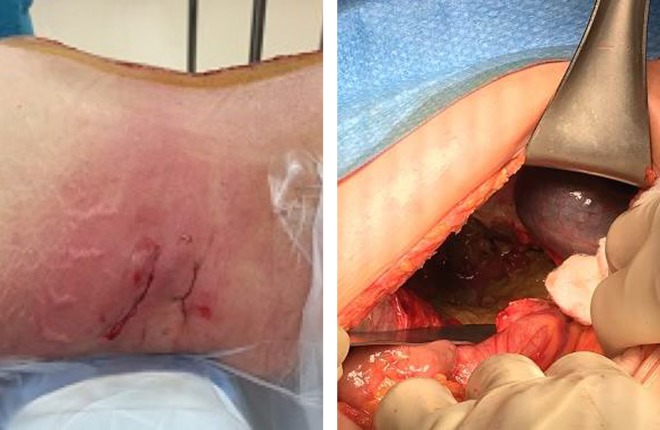
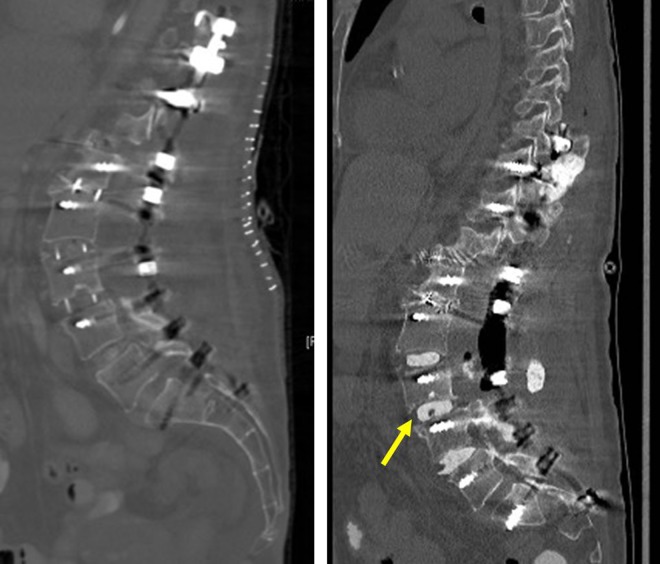
An abdominal CT revealed right retroperitoneal air and fluid collection extending from the mid-abdomen to the pelvis. No extraluminal air entrapment, intraperitoneal free air or loss of bowel continuity was noted. Four days following the second stage surgery, her clinical condition failed to improve and induration was seen over the right flank. It was decided to take her to the operating room for wound washout and revision of the malpositioned screw, observed during routine postoperative imaging. The posterior hardware revision surgery was performed, and the screw was replaced without complications. After the posterior incision was closed, a lateral incision was opened and explored, whereupon feces were encountered (Figure 6A and B). An exploratory laparotomy revealed a perforation of the ascending colon. The colon was found to be adherent to the L3-4 cage. The patient underwent a hemicolectomy followed by an end to end anastomosis after a peritoneal lavage. Her cultures grew GNR and GPR and she was started on cefepime and metronidazole. Despite the antibiotic administration, the patient had persistently elevated white blood cell, C-reactive protein, and erythrocyte sedimentation rate levels. She returned to the operating room where the PEEK cages were removed and cement with tobramycin and vancomycin was inserted into the L2-L4 disc spaces with subsequent improvement of her inflammatory markers and resolution of her leukocytosis (Figure 7A and B). She had a repeat abdominal wound wash following a leak in the anastomosis. At her last follow-up, she was independently ambulatory.
Figure 6.
Patient 3: (left) transpsoas lumbar interbody fusion (TPIF) incision with marked redness and induration, (right) intraoperative image of the lateral incision showing the bowel contents seeping through the lateral incision.
Figure 7.
Patient 3: (left) computed tomography (CT) scan sagittal image obtained after lateral surgery showing polyetheretherketone (PEEK) cages at L2-5 levels. The bowel was stuck to the L3-4 PEEK. (right) CT scan sagittal image showing removal of the PEEK cage and use of polymethylmethacrylate (PMMA) antibiotic cement spacers (yellow arrow).
Discussion
While uncommon, visceral injury is a primary risk of anterior approach procedures, carrying a reported incidence of 0% to 5%.11-24 Because of this low incidence, there is little literature surrounding visceral injuries during interbody fusions involving an anterior approach to the lumbar spine. We therefore conducted a review of all anterior approach lumbar interbody fusion surgeries (ALIF, TPIF, OAIF) performed at our institution between July 2012 and June 2017. Of 775 procedures, 4 cases were postoperatively suspected of a bowel perforation and returned to the operating room for an exploratory laparotomy. All cases suspected of bowel perforation had undergone TPIF. Bowel perforation was confirmed in 3 cases by laparotomy.
TPIF is a transpsoas approach that can be used as an alternative to anterior approach surgeries and has been associated with lower complication rates.33-35 A 13 000-patient multisurgeon survey revealed a 0.08% incidence of visceral injuries following TPIF.32 Our series saw a 0.51% incidence of visceral injury following TPIF.
Postoperative recognition of a visceral injury occurred at an average of 4.7 days (range, 3-7 days) in our series. This is similar to the postoperative diagnostic delays of 2 to 5 days (mean, 3.2 days) reported by Uribe and Deukmedjian.32 Presentation may be delayed if a bowel laceration leaks bowel contents only after few days or in quantities too small to result in an immediately alarming presentation. However, the most likely reason for a delayed presentation is a retroperitoneal leak that accumulates in a “walled off” or contained abscess. The immediate postoperative period in all three cases was rather uneventful and the patients tolerated oral intake. Though hypoactive, bowel sounds were present in all the 3 cases. The fact that they all tolerated oral intakes and had bowel sounds presumably created a low index of suspicion for a major bowel injury.
Comorbidities associated with bowel injuries include previous renal failure, gall bladder disease, chronic, mega colon, previous bowel surgery, diverticulosis, and prior caesarian section.32 We find nausea and vomiting to be of little clinical value in raising the suspicion of a significant bowel injury, especially when the patient is receiving pain medications including narcotics. Abdominal pain, distension, and fever were the most consistent findings in our series associated with visceral injury. Balsano et al25 also reported abdominal tenderness and bloating as the key clinical findings. Because of the risk of bowel injury, surgeons should monitor and closely observe patients with these clinical findings and additional imaging should be obtained.
The classical findings on abdominal CTs after a perforated bowel include discontinuity of the bowel wall and intra-/extraperitoneal air. Extraluminal air entrapment is highly specific and predicts the site of perforation.36,37 This was seen in cases 1 and 2 of our series, indicating a bowel perforation was very likely. Indirect imaging findings include bowel wall thickening, abnormal bowel wall enhancement, abscess, inflammatory mass adjacent to the bowel, mesenteric fat stranding, and oral contrast extravasation (Table 3).38-45 Perhaps the most important anatomical relationship for surgeons performing lateral retroperitoneal approaches to recognize is the relationship between the ascending and descending colon and the peritoneum. Perforation of the anterior wall results in intraperitoneal air while perforation of the posterior wall leads to extraperitoneal and retroperitoneal air extravasation.
Table 3.
Pointers to Bowel Perforation.
| Clinical Findings | Postoperative X-Ray | Computed Tomography Scan |
|---|---|---|
| Abdominal distension, abdominal pain, fever | Lateral abdominal x-ray showing presence of entrapped air | Presence of extraluminal entrapped air, intraperitoneal air, loss of bowel continuity, increased bowel wall thickness |
The isolated presence of air/fluid in the retroperitoneal space is easily confounded by the presence of expected postoperative findings following TPIF. In contrast, imaging for cases 1 and 2 revealed loss of bowel wall continuity, bowel wall thickness, and extraluminal gas collection, which strongly pointed toward perforation, in addition to retroperitoneal air and fluid collection. We consider it important for spine surgeons to be aware of the peritoneal reflections of the bowel and to discuss specifically with the radiologist when necessary.
In cases 1 and 2 of our series, infection was controlled without removing the lumbar interbody cages. In case 3, clinical signs and laboratory parameters suggested ongoing inflammation, despite repeated wound wash and antibiotics. The PEEK cages were eventually removed and replaced with antibiotic-loaded cement spacers that led to marked improvement in the infection control and the patient’s clinical condition. Antibiotic spacers have been used frequently in orthopedics for osteomyelitis, infected joints, and open fractures with infected bone.46-48 We feel that if the inflammatory markers are not controlled with parenteral antibiotics, the cage acts as a nidus for infection.
Emergency exploratory laparotomies revealed colonic perforations on the side of the surgery in each of our 3 cases. The colon, being a fixed structure, is more likely to be injured either during placement of the K-wire or dilators, or secondary to pressure necrosis.25 Case 1 in our series had perforation following an approach to the L4-5 disc space. While the L4-5 disc space is frequently fused using a TPIF approach, the high riding crest sometimes requires the retractors to be placed anteriorly and angled posteriorly and inferiorly toward that space. This could potentially violate the bowel contents, especially while sharp instruments such as K-wires are being passed into the disc space. It would be interesting for future studies to explore the possibility of association between the level of TPIF and bowel injuries.
Cases 2 and 3 underwent TPIF at the level L2/3. Yilmaz et al31 reported in their anatomical cadaver study a higher risk for bowel injuries at the level L2/3 and L3/4.
All perforations in our series occurred on the right side. Uribe and Deukmedjian32 found that 78% of visceral injuries followed a left-side approach, though associated spinal deformities were not mentioned. The side of approach in scoliosis is mainly dependent on surgeon preference and the spinal anatomy. In a case series of 8 spinal deformity patients undergoing TPIF, Tormenti et al26 reported 1 bowel perforation. Although the authors suggested the rotatory component of the scoliotic spine increases the likelihood of injury to the retroperitoneal structures, they did not discuss this in detail. Uribe and Deukmedjian32 found that 70% of bowel injury cases were associated with single-incision lateral procedures. However, the authors were unable to support this as a significant finding because of a lack of data regarding the number of incisions in the comparative group without visceral injuries.32 A single-incision for a multilevel interbody fusion might lead to higher angulation and different retractor placement. This could lead to an impaired intraoperative view/vision and contribute to injuries of retroperitoneal organs.
This study is subject to several limitations. The small cohort and the retrospective design as well as the generally low incidence of visceral injury makes it difficult to draw strong conclusions about procedure-specific visceral injury risk.
Conclusions
Bowel injuries following interbody fusion procedures involving lateral approaches to the lumbar spine are rare. We note abdominal pain, distention, and fever as the most common findings in the setting of a visceral injury and emphasize the importance of maintaining a high index of suspicion postoperatively for bowel perforation. Owing to anatomical variability in the site of injury, TPIF can cause injury in the posterior wall of the colon, leading to retroperitoneal abscess formation and a delayed presentation. A high index of suspicion and CT imaging remain critical for identifying postoperative bowel injuries.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tarush Rustagi, MD  https://orcid.org/0000-0001-9950-3780
https://orcid.org/0000-0001-9950-3780
Emre Yilmaz, MD  https://orcid.org/0000-0002-1492-1201
https://orcid.org/0000-0002-1492-1201
Sarah Hopkins  https://orcid.org/0000-0003-4896-7344
https://orcid.org/0000-0003-4896-7344
References
- 1. Pannell WC, Savin DD, Scott TP, Wang JC, Daubs MD. Trends in the surgical treatment of lumbar spine disease in the United States. Spine J. 2015;15:1719–1727. [DOI] [PubMed] [Google Scholar]
- 2. Goz V, Weinreb JH, Schwab F, Lafage V, Errico TJ. Comparison of complications, costs, and length of stay of three different lumbar interbody fusion techniques: an analysis of the Nationwide Inpatient Sample database. Spine J. 2014;14:2019–2027. [DOI] [PubMed] [Google Scholar]
- 3. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- 4. Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: intractable low-back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2005;2:670–672. [DOI] [PubMed] [Google Scholar]
- 5. Yoshihara H, Yoneoka D. National trends in the surgical treatment for lumbar degenerative disc disease: United States, 2000 to 2009. Spine J. 2015;15:265–271. [DOI] [PubMed] [Google Scholar]
- 6. Kerolus M, Turel MK, Tan L, Deutsch H. Stand-alone anterior lumbar interbody fusion: indications, techniques, surgical outcomes and complications. Expert Rev Med Devices. 2016;13:1127–1136. [DOI] [PubMed] [Google Scholar]
- 7. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glassman S, Gornet MF, Branch C, et al. MOS short form 36 and Oswestry Disability Index outcomes in lumbar fusion: a multicenter experience. Spine J. 2006;6:21–26. [DOI] [PubMed] [Google Scholar]
- 9. Udby PM, Bech-Azeddine R. Clinical outcome of stand-alone ALIF compared to posterior instrumentation for degenerative disc disease: a pilot study and a literature review. Clin Neurol Neurosurg. 2015;133:64–69. [DOI] [PubMed] [Google Scholar]
- 10. Strube P, Hoff E, Hartwig T, Perka CF, Gross C, Putzier M. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J Spinal Disord Tech. 2012;25:362–369. [DOI] [PubMed] [Google Scholar]
- 11. Baker JK, Reardon PR, Reardon MJ, Heggeness MH. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976). 1993;18:2227–2230. [DOI] [PubMed] [Google Scholar]
- 12. Brau SA, Delamarter RB, Schiffman ML, Williams LA, Watkins RG. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409–412. [DOI] [PubMed] [Google Scholar]
- 13. Fantini GA, Pappou IP, Girardi FP, Sandhu HS, Cammisa FP., Jr Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976). 2007;32:2751–2758. [DOI] [PubMed] [Google Scholar]
- 14. Fantini GA, Pawar AY. Access related complications during anterior exposure of the lumbar spine. World J Orthop. 2013;4:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flynn JC, Price CT. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976). 1984;9:489–492. [DOI] [PubMed] [Google Scholar]
- 16. Garg J, Woo K, Hirsch J, Bruffey JD, Dilley RB. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51:946–950. [DOI] [PubMed] [Google Scholar]
- 17. Hamdan AD, Malek JY, Schermerhorn ML, Aulivola B, Blattman SB, Pomposelli FB., Jr Vascular injury during anterior exposure of the spine. J Vasc Surg. 2008;48:650–654. [DOI] [PubMed] [Google Scholar]
- 18. Inamasu J, Guiot BH. Vascular injury and complication in neurosurgical spine surgery. Acta Neurochir (Wien). 2006;148:375–387. [DOI] [PubMed] [Google Scholar]
- 19. Kulkarni SS, Lowery GL, Ross RE, Ravi Sankar K, Lykomitros V. Arterial complications following anterior lumbar interbody fusion: report of eight cases. Eur Spine J. 2003;12:48–54. [DOI] [PubMed] [Google Scholar]
- 20. Lindley EM, McBeth ZL, Henry SE, et al. Retrograde ejaculation after anterior lumbar spine surgery. Spine (Phila Pa 1976). 2012;37:1785–1789. [DOI] [PubMed] [Google Scholar]
- 21. Penta M, Fraser RD. Anterior lumbar interbody fusion. A minimum 10-year follow-up. Spine (Phila Pa 1976). 1997;22:2429–2434. [DOI] [PubMed] [Google Scholar]
- 22. Rajaraman V, Vingan R, Roth P, Heary RF, Conklin L, Jacobs GB. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91(1 suppl):60–64. [DOI] [PubMed] [Google Scholar]
- 23. Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976). 2003;28:1023–1026. [DOI] [PubMed] [Google Scholar]
- 24. Wood KB, Devine J, Fischer D, Dettori JR, Janssen M. Vascular injury in elective anterior lumbosacral surgery. Spine (Phila Pa 1976). 2010;35(9 suppl):S66–S75. [DOI] [PubMed] [Google Scholar]
- 25. Balsano M, Carlucci S, Ose M, Boriani L. A case report of a rare complication of bowel perforation in extreme lateral interbody fusion. Eur Spine J. 2015;24(suppl 3):405–408. [DOI] [PubMed] [Google Scholar]
- 26. Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. [DOI] [PubMed] [Google Scholar]
- 27. Krieger RH, Wojcicki KM, Berry AC, Reuther WL, 3rd, McArthur KD. Small bowel perforation as a postoperative complication from a laminectomy. Case Rep Surg. 2015;2015:378218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoff-Olsen P, Wiberg J. Small bowel perforation as a complication of microsurgical lumbar diskectomy. A case report and brief review of the literature. Am J Forensic Med Pathol. 2001;22:319–321. [DOI] [PubMed] [Google Scholar]
- 29. Cases-Baldó MJ, Soria-Aledo V, Miguel-Perello JA, Aguayo-Albasini JL, Hernández MR. Unnoticed small bowel perforation as a complication of lumbar discectomy. Spine J. 2011;11:e5–e8. [DOI] [PubMed] [Google Scholar]
- 30. Shakir AJ, Paterson HM. Small bowel perforation—an unusual complication of microdiscectomy: a case report. Acta Chir Belg. 2011;111:36–37. [DOI] [PubMed] [Google Scholar]
- 31. Houten JK, Frempong-Boadu AK, Arkovitz MS. Bowel injury as a complication of microdiscectomy: case report and literature review. J Spinal Disord Tech. 2004;17:248–250. [DOI] [PubMed] [Google Scholar]
- 32. Uribe JS, Deukmedjian AR. Visceral, vascular, and wound complications following over 13,000 lateral interbody fusions: a survey study and literature review. Eur Spine J. 2015;24(suppl 3):386–396. [DOI] [PubMed] [Google Scholar]
- 33. Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28(3):E8. [DOI] [PubMed] [Google Scholar]
- 34. Deukmedjian AR, Le TV, Baaj AA, Dakwar E, Smith DA, Uribe JS. Anterior longitudinal ligament release using the minimally invasive lateral retroperitoneal transpsoas approach: a cadaveric feasibility study and report of 4 clinical cases. J Neurosurg Spine. 2012;17:530–539. [DOI] [PubMed] [Google Scholar]
- 35. Le TV, Uribe JS. The minimally invasive retroperitoneal transpsoas approach In: Chung KJ, ed. Spine Surgery. Rijeka, Croatia: IntechOpen; 2014:79–96. [Google Scholar]
- 36. Hainaux B, Agneessens E, Bertinotti R, et al. Accuracy of MDCT in predicting site of gastrointestinal tract perforation. AJR Am J Roentgenol. 2006;187:1179–1183. [DOI] [PubMed] [Google Scholar]
- 37. Zissin R, Hertz M, Osadchy A, Even-Sapir E, Gayer G. Abdominal CT findings in nontraumatic colorectal perforation. Eur J Radiol. 2008;65:125–132. [DOI] [PubMed] [Google Scholar]
- 38. Borofsky S, Taffel M, Khati N, Zeman R, Hill M. The emergency room diagnosis of gastrointestinal tract perforation: the role of CT. Emerg Radiol. 2015;22:315–327. [DOI] [PubMed] [Google Scholar]
- 39. Cadenas Rodríguez L, Martí de Gracia M, Saturio Galán N, Pérez Dueñas V, Salvatierra Arrieta L, Garzón Moll G. Use of multidetector computed tomography for locating the site of gastrointestinal tract perforations [in Spanish]. Cir Esp. 2013;91:316–323. [DOI] [PubMed] [Google Scholar]
- 40. Elton C, Riaz AA, Young N, Schamschula R, Papadopoulos B, Malka V. Accuracy of computed tomography in the detection of blunt bowel and mesenteric injuries. Br J Surg. 2005;92:1024–1028. [DOI] [PubMed] [Google Scholar]
- 41. Faggian A, Berritto D, Iacobellis F, Reginelli A, Cappabianca S, Grassi R. Imaging patients with alimentary tract perforation: literature review. Semin Ultrasound CT MR. 2016;37:66–69. [DOI] [PubMed] [Google Scholar]
- 42. LeBedis CA, Anderson SW, Soto JA. CT imaging of blunt traumatic bowel and mesenteric injuries. Radiol Clin North Am. 2012;50:123–136. [DOI] [PubMed] [Google Scholar]
- 43. Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR Am J Roentgenol. 2001;176:1105–1116. [DOI] [PubMed] [Google Scholar]
- 44. Maniatis V, Chryssikopoulos H, Roussakis A, et al. Perforation of the alimentary tract: evaluation with computed tomography. Abdom Imaging. 2000;25:373–379. [DOI] [PubMed] [Google Scholar]
- 45. Sherck J, Shatney C, Sensaki K, Selivanov V. The accuracy of computed tomography in the diagnosis of blunt small-bowel perforation. Am J Surg. 1994;168:670–675. [DOI] [PubMed] [Google Scholar]
- 46. Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882. [DOI] [PubMed] [Google Scholar]
- 47. Kelm J, Anagnostakos K, Regitz T, Schmitt E, Schneider G, Ahlhelm F. MRSA-infections-treatment with intraoperatively produced gentamycin-vancomycin PMMA beads [in German]. Chirurg. 2004;75:988–995. [DOI] [PubMed] [Google Scholar]
- 48. Thonse R, Conway J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21:258–268. [DOI] [PubMed] [Google Scholar]