Abstract
A structural study about the changes induced by plasticization of native corn starch was carried out in this work. The influence of talc nanoparticles presence during starch thermal processing was also evaluated. Macroscopic observation of the granules appearance evolution during melt-mixing and thermo-compression was supported by a theoretical description related to these processing methods. Melt-mixing induced a polymorphic transformation from A- to Vh-type and a reduction in the degree of crystallinity. Homogenous appearance of the plasticized starch was in accordance to the disruption of granules integrity, evidenced by SEM. This observation agreed to the distinctive XRD pattern of plasticized starch from unprocessed granules. Talc incorporation did not require the adjustment of processing parameters in order to obtain a homogenous thermoplastic material, with an adequate particles distribution within the matrix. Regardless talc presence, plasticized starch presented a Vh-type crystalline structure. Thermo-compression led to particles alignment promoted by talc laminar morphology.
Keywords: Materials science, Native corn starch, Talc nanoparticles, Thermal processing, Polymorphic transformations, Structural changes
1. Introduction
One of the most polysaccharides found in the nature, considered as a biodegradable polymer, is starch. This energy storage compound, produced by plant cells, is the major component of the harvestable parts of many food crops [1, 2]. Native starch is naturally found as semicrystalline and water-insoluble granules, which are constituted by two glucose polymers: amylose and amylopectin [3, 4]. The ratio of these two components, as well as, granules shapes, sizes, and compositions mainly depend on starch botanical origin [5]. The most common natural sources of starches are cereals (corn, wheat, rice, etc.) and tuberous roots (potato, tapioca, etc.).
Starch is one of the most promising candidates for partially replacing the use of synthetic and non-biodegradable plastics. This polysaccharide has several advantageous characteristics that have caught the attention of the packaging industry, which still employs synthetic polymers. In this sense, low cost and worldwide availability of starch, as well as, its high purity, non-toxicity, and ecofriendly character are valuable features, which impelled the research and use of this biopolymer [6]. Despite all these beneficial aspects, starch has a drawback compared to synthetic polymers to be processed since granules do not possess thermoplastic character in their pristine form. To overcome this drawback, granules should be destructured. The most commonly method used to alter starch crystalline structure is the gelatinization process, which involves the incorporation of water as destructuring agent [7]. During starch gelatinization, the disruption of crystalline structure is achieved by granules swelling and ulterior breaking. However, gelatinized suspensions regain starch crystalline nature during the drying stage. This recrystallization could be prevented by the incorporation of plasticizers to starch formulations. The plasticizers usually employed for the processing of starch based materials are polyols such as glycerol. The main role of this kind of plasticizers is to reduce the intermolecular hydrogen bonds and entanglements among starch chains. Besides, due to the high plasticizers hydrophilic character, they increase the amount of absorbed water by starch, which exerts an additional plasticizing effect [6]. In order to select the proper plasticizer, its efficiency and compatibility with starch should be considered.
Starch processing at industrial scale requires that this biopolymer would be converted into a thermoplastic material. The use of a plasticized starch would allow its processing by employing the conventional technology developed for synthetic polymers. This advantage would imply a low economic inversion and a few modifications on plastics production lines. Thermoplasticization of starch granules occurs during their processing under high temperature and shear fields, in the presence of plasticizers [8]. Thus, starch is converted into a homogeneous material constituted by a hard-elastic network and soft amorphous regions, called as thermoplastic starch (TPS) [9]. It is well-known that there is a strong relationship processing-structure-properties of polymeric materials and its comprehension allows inferring their possible applications. Thus, it is relevant a deep study about the structural changes that starch granules suffer during their thermal processing. These physicochemical transformations of starch granules during thermoplasticization derive from different phenomena such as fragmentation, destructuration, plasticization, and melting [9, 10]. The disruption of granular organization induces modifications on morphological characteristics, crystalline architecture, and chemical structure of native starch. Besides, TPS performance is completely different from the corresponding to the granular polysaccharide. In this manner, starch applications fields and its potential are widened.
Even though starch thermoplasticization leads to obtain materials which can be processed in conventional equipment for synthetic polymers, they have yet many disadvantages associated to the starch hydrophilic character. These drawbacks can be sorted by adding inorganic particles as fillers into TPS matrices. Among these minerals, talc qualifies as good reinforcement agent because it is a layered mineral with a high aspect ratio (particle diameter/thickness ≈20:1). This is a consequence of its platy nature, having micron-sized dimensions on length and width, with nanometric thicknesses [11]. It is important to comprehend the influence of talc presence on starch thermoplasticization in order to establish processing parameters of composites and understand processing-microstructure-properties relationship.
Several works related to development and characterization of materials based on TPS using native and modified starches from different botanical sources, studies reporting the structural and morphological changes on starch structure during thermal processing in the presence of particles are scarce. In this sense, the aim of this work was to study the microstructural changes of corn starch granules during melt-mixing and thermo-compression process. Within this context, modifications on the polymorphism of starch crystallites, granules morphology evolution, and changes on chemical interactions were evaluated through different characterization techniques. Moreover, the effect of talc nanoparticles incorporation on starch structural changes during the thermal processing and consequently of these final properties was also reported [12, 13, 14, 15].
2. Materials and methods
2.1. Materials
Native corn starch was provided by Misky-Arcor (Tucumán, Argentine), which was previously characterized by López et al. [16]. Talc sample was supplied by Dolomita SAIC (Argentine). This mineral comes from an Australian ore, having a high purity, median size d50 of 5.9 ± 3.8 μm and a nanometric thickness of 79 ± 16 nm [12]. Glycerol (Anedra, Argentine) was used as plasticizer in nanocomposites melt-mixing.
2.2. Composite processing
Mixtures of native corn starch, glycerol (30 wt %), distilled water (45 wt %) and talc nanoparticles (0, 8.7 wt %) were prepared. Composition of these formulations was expressed in grams per 100 g of starch, which implies that they were used 0 and 5 wt % of talc, respect to TPS. Talc was premixed with starch to achieve good particle dispersion between both powders. Then, glycerol and distilled water were added and samples were mixed and conditioned at 25 °C during 24 h. Nanocomposite processing was carried out in a Brabender Plastograph (Brabender, Germany) at 140 °C and 50 rpm for 15 min, using roller blades [12]. Samples obtained after melt-mixing were named as TPS-M (melt mixed thermoplastic starch) and TPS/T-M (melt mixed thermoplastic starch with talc particles). From torque-time curves, plasticization energy was determined as it was proposed by Córdoba et al. [9]. After melt-mixing, samples were conditioned at 25 °C and 60 % relative humidity (RH). The required time to reach the equilibrium was optimized by the following of specimens weight gain during their conditioning. Samples weight was registered at different time intervals and the corresponding gravimetrical curves were recorded. Films were obtained by thermo-compression at 150 kg cm-2 and 140 °C, during 6 min. The temperature to thermo-compress TPS was selected based on the results from Thermogravimetric Analysis, reported in a previous work [15]. The onset degradation temperature for TPS and TPS-5T was 275 and 255 °C, respectively. Films obtained after thermo-compression were named as TPS-F (film of thermoplastic starch) and TPS/T-F (film of thermoplastic starch with talc particles).
Before characterization, films were conditioned at 25 °C and 60 % relative humidity (RH).
2.3. X-ray diffraction (XRD)
Crystal structure identification and degree of crystallinity (CD) were studied by XRD. Diffractograms were obtained in an X-ray diffractometer Philips PW1710 (Philips, Holland), provided with a tube, a copper anode, and a detector operating at 45 KV and 30 mA within 2θ from 3 to 60°. Degree of crystallinity was calculated using the method reported by Soliman and Furuta [17].
2.4. Scanning electronic microscopy (SEM)
This study was performed in a JEOL JSM-35 CF electron microscope (Japan). All samples were mounted on bronze stubs. Particularly, powdered starch was air-aided dispersed to avoid granules agglomeration; meanwhile films were previously cryofractured by immersion in liquid nitrogen. In the case of melt processed samples, they did not require any specific preparation. Specimens were coated with a gold layer (∼30 Å), using an argon plasma metallizer (sputter coater PELCO 91000). To determine size distribution of native starch, mean diameter of the projected area of granules was determined, considering around 300 particles. Data were analyzed using a free software (GNU Image Manipulation Program, GIMP 2.8.20).
2.5. Fourier transform infrared spectroscopy (FTIR)
Spectra were obtained using a Thermo Nicolet Nexus spectrophotometer (USA). Pressed discs were prepared from fine powder composed by KBr (Sigma–Aldrich, 99 %) and samples (3 wt %). Spectra were obtained from 100 accumulated scans at 4 cm−1 resolution in the range 4000–400 cm−1.
2.6. Statistical analysis
Analysis of variance (ANOVA) was used to compare mean differences on samples properties, considering at least triplicates for each assay. Besides, comparison of mean values was performed by Fisher's least significant difference test conducted at a significance level p = 0.05.
3. Results and discussion
3.1. Thermal processing
Since starch undergoes phase transition during its thermal processing, it is important to understand the transformation of this polysaccharide before to be processed. Starch plasticization is carried out applying shear fields in the presence of plasticizers, being polyols the more conventional additives [18]. Along with promoting plasticization, the addition of glycerol improves the final properties of starch based materials. However, it is noteworthy that at low concentrations (<10–15 wt %), glycerol may exhibit an anti-plasticization effect due to its higher affinity with water molecules than with starch ones [19].
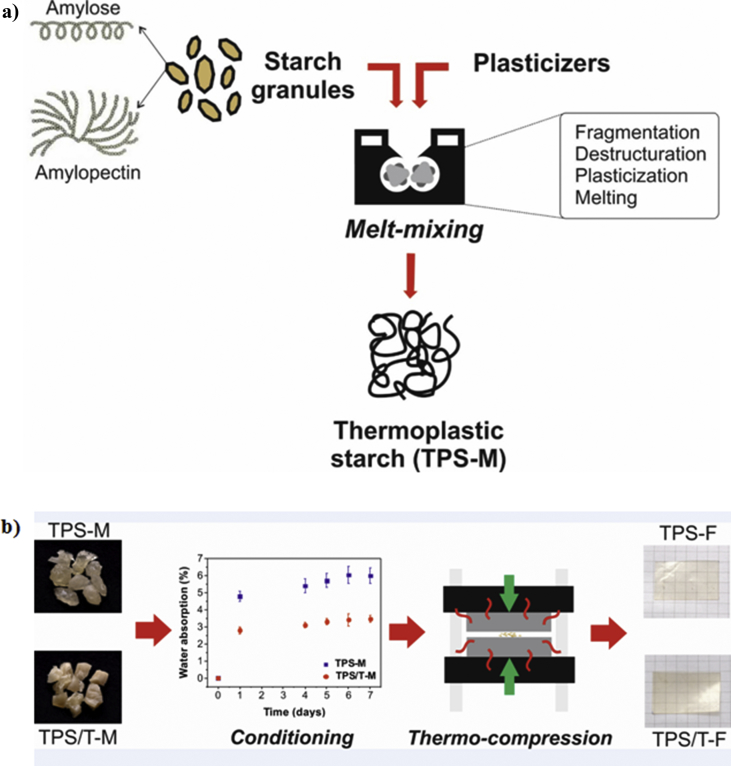
A schematic representation of starch thermal processing by is shown in Fig. 1. Starch melt-mixing is described in the scheme included in Fig. 1a. Starch is organized in a granular structure, which is composed by two types of polysaccharides: amylose and amylopectin [20]. Along starch transformation, granular structure is disrupted, releasing some of these carbohydrate polymer molecules, mainly amylose chains [9]. Several physicochemical processes take place during starch thermoplasticization such as fragmentation, destructuration, plasticization, and melting [10]. Consequently, TPS is constituted by a highly plasticized amorphous phase and a crystalline region associated to the remaining starch granules. It is important to highlight that optimal processing conditions allow obtaining a high proportion of the TPS plasticized phase. Undesirable effect during starch thermal processing is the occurrence of a partial chains depolymerization, being amylopectin molecules more susceptible than amylose ones [21]. From studies about starch degradation during its thermo-mechanical processing, it was concluded that the involved mechanisms depend not only on molecule size but also on branching level [22].
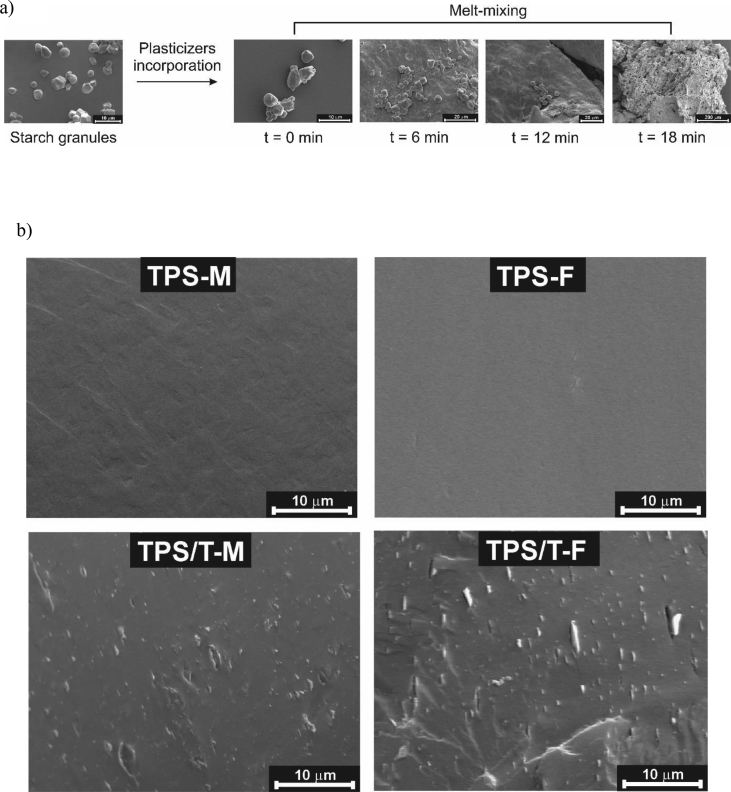
Fig. 1.
Schematic representation of starch thermal processing: a) melt-mixing and b) thermo-compression. Ref: TPS-M and TPS-F (melt-mixtures and films of thermoplastic corn starch) and TPS/T-M and TPS/T-F (melt-mixtures and films of nanocomposites containing talc particles).
Fig. 1b shows photographs corresponding to the resulting materials after melt-mixing, as well as, those of films obtained by thermo-compression. Concerning starch mixtures after thermoplasticization process, they seemed to be vitreous materials. This feature could be related to the partial samples dehydration during melt-mixing. Macroscopically, these processed materials were translucent, with a homogeneous appearance and a slight amber hue. Talc presence did not affect significantly the visual appreciation of TPS. Due to the thermoplastic character achieved by melt-mixing using plasticizers under high temperature and high shear stress, they can be processed by different methodologies such as injection molding, thermocompression, and extrusion, among others. This advantage allows tailoring starch based materials with diverse shapes, sizes, and properties. In order to facilitate the mixtures reprocessing is suitable their conditioning under controlled temperature and relative humidity. In this work, the chosen parameters of the surrounding environment were 25 °C and 60 % RH. The required time to reach the equilibrium was established from gravimetrical assays and the corresponding curves are also included in Fig. 3. Regardless talc presence, 7 days under conditioning assured the starch mixtures stabilization, evidenced by a time-constant weight.
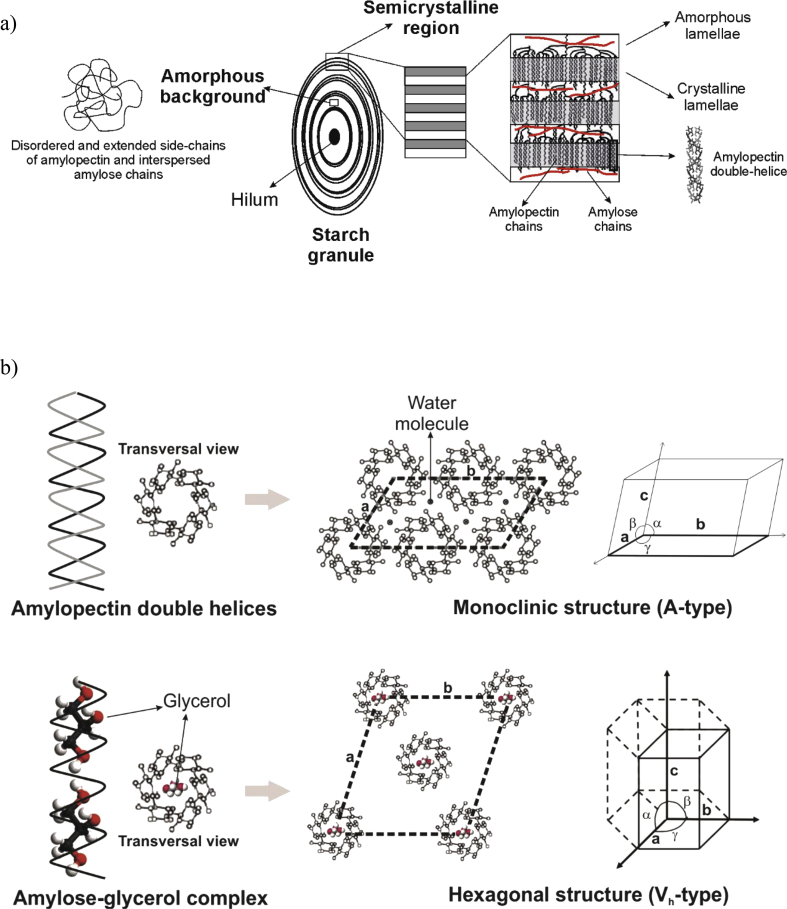
Fig. 3.
a) Architecture of corn starch granules and b) A- and Vh-type crystalline structures.
After the conditioning stage, mixtures became more mouldable, favoring their ulterior processing. From these conditioned thermoplastic mixtures, flexible films were obtained by thermo-compression. A critical step during TPS film obtaining is the specimen cooling after their pressing. Thus, in order to conserve film flexibility, samples should be cooled under pressure. Carrying out this procedure, starch constituent molecules are free to accommodate within film microstructure, avoiding the occurrence of internal stresses. A similar methodology was reported by Thunwall et al. [23].
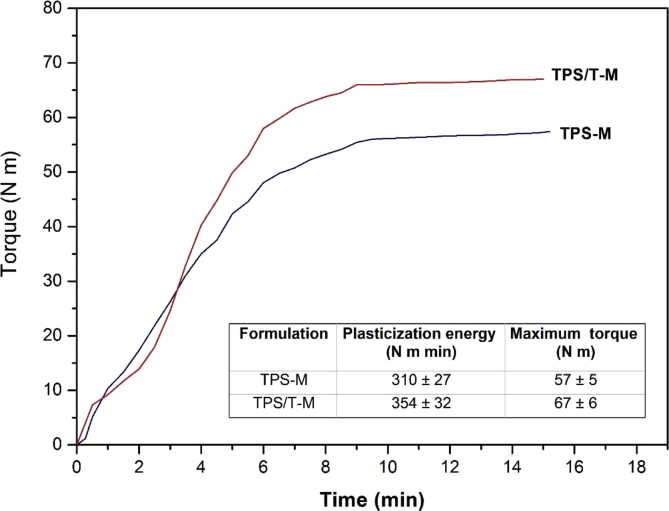
In order to design the starch processing at industrial scale, torque curves should be considered since they provide information about the viscosity changes and the time needed to achieve the steady molten starch phase. Fig. 2 shows torque-time curves corresponding to melt-mixing of TPS and the composite with talc nanoparticles (TPS/T). This figure also includes a table with the values of plasticization energy and the reached maximum torque. A relevant advantage related to thermal processing of starch based materials is their capability to be processed using the technology developed for synthetic polymers such as extrusion, blown film extrusion, and injection molding, among others. However, starch melt-mixing needs specific adjustment of the operating conditions, mainly based on the rheological behavior of this biopolymer. TPS and nanocomposites showed a different pattern than those reported for synthetic polymers (Fig. 2). Generally, for conventional polyolefins, torque rises up to a maximum in the initial processing stages and then decrease, reaching a plateau with an almost constant value [24]. In the case of the starch based materials, torque increased to a maximum value and then remained stable. In accordance to Qiao et al. [25], the notable initial torque increment is mainly attributed to the energy required to weak hydrogen bonds among starch molecules. The processing time to reach a complete homogenization of the starch melt was ∼9 min, evidenced by the torque stabilization. During this first stage of the mixing process, the plasticizer diffusion inside the starch molecules takes place. Regarding to talc particles presence, it was not evidenced any change on plasticization profile of TPS (Fig. 2). However, the slight increase in maximum torque induced by talc was in accordance with the resistance to the flux that particles offer during the melt-mixing. Even though the plasticization energy required to process TPS/T nanocomposites was higher than those corresponding to TPS, both homogenization times resulted similar.
Fig. 2.
Torque-time curves and processing parameters of thermoplastic corn starch (TPS-M) and nanocomposites based on TPS containing talc particles (TPS/T-M).
3.2. Starch structural changes induced by thermal processing and talc presence
In order to comprehend the influence of thermal processing on starch structure, it is indispensable a deep knowledge about the molecular architecture of the polysaccharide granules. Fig. 3a describes the main starch constituents, as well as, their distribution within granules structure. Starch biosynthesis starts from the hilum by the successive layers stacking [26]. Granules are characterized by the occurrence of intercalating semicrystalline and amorphous regions, conforming ‘growth rings’. Starch crystallinity is due to the presence of amylopectin chains organized as double helices. On the other hand, amorphous character is attributed to the granules core, amorphous background, and amorphous lamellae within semicrystalline rings. These last regions are conformed mainly by amylopectin branch points, amylopectin chains not organized into helical configurations, and long linear amylopectin chains. Regarding to amylose chains, their spatial distribution within starch granules are not yet well understood. One of the hypothesis proposes that amylose chains are disposed in the amorphous lamellae within semicrystalline rings, as well as, in the amorphous background, interspersed with disordered and extended side-chains of amylopectin [27].
Cereal starch granules present a spatial arrangement of amylopectin double helices within semicrystalline region in accordance to A-type polymorphism. Fig. 3b shows this crystalline structure, where double helices are packed in monoclinic unit cells (a ≠ b ≠ c; α = γ = 90° ≠ β) with four water molecules per unit cell [28]. During thermal processing of starch based materials, crystalline structure of granules is disrupted, becoming in a predominantly amorphous material. TPS can recrystallize in different polymorphic cells depending on the conditioning variables such as temperature, relative humidity, and time, among others. Thus, it is relevant the knowledge of the material glass transition temperature (Tg). Particularly, TPS presents two Tg values due to this material is constituted by two domains: plasticizer- and starch-rich phases. In a previous work, Tg of the glycerol-rich phase was determined for nanocomposites based on TPS and talc, being the mean value around -49 °C. Regarding Tg of starch-rich domain, it was only detected for nanocomposites with 3 and 5 wt % talc, registering values of ∼46 °C [12]. Since TPS nanocomposites were conditioned under a temperature above Tg of the glycerol-rich phase, they recrystallized in a different structure than the corresponding to the native polysaccharide. This new phase mainly involved the crystallization of amylose in single helices containing glycerol molecules. In Fig. 3b is shown a simplified representation of plasticizer molecules entrapped into an amylose single helix (frontal and transversal views). These amylose-glycerol complexes are organized in a V-type structure, in accordance with the results discussed by Corradini et al. [24]. Crystals of intra-helical inclusion complexes can be developed as V-hydrated (Vh) or V-anhydrous (Va) subtypes. The main difference between these two V-crystalline phases is the presence of water molecules within crystalline structure. Hydrated subtype can transform into anhydrous one by losing water molecules between the amylose helices [29]. Particularly, glycerol-amylose complex usually crystallizes in Vh-type structure. The unit cell associated to this crystal morphism has a hexagonal geometry (a = b ≠ c; α = β = 90° and γ = 120°).
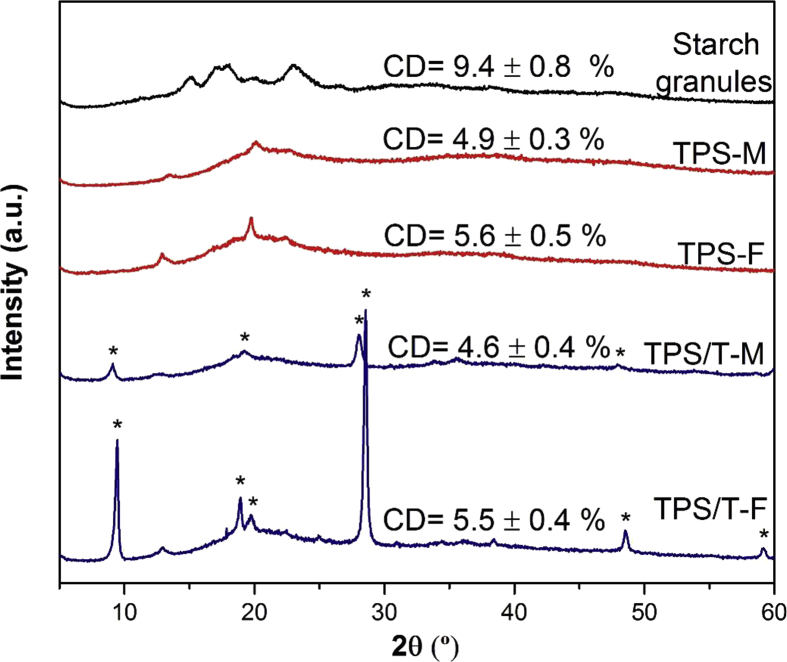
Fig. 4 shows XRD diffractograms of starch granules, as well as, of TPS and nanocomposites, both for melt processed samples and films. Characteristics reflection peaks corresponding to A-type pattern were distinguished for native starch, which were located at 2θ = 15, 17, 18 and 23° [4]. Meanwhile, XRD spectra of TPS and nanocomposites were in accordance to V-type structure, regardless the processing (melt-mixing and thermo-compression). This polymorphic change revealed that starch granules suffered many physical transformations such as fragmentation, destructuration, plasticization, and melting. During melt-mixing, mechanical and thermal energy induces starch granules fragmentation, which involves breakdown of the main and secondary valence bonds, and the hydrogen bonds between neighboring starch molecules [30, 31]. In accordance with Mohammadi Nafchi et al. [32], starch destructuration in TPS production is carried out adding limited quantities of plasticizers, favored by high temperatures. Plasticizer molecules penetrate starch granules and destroy the inner hydrogen bonds, replacing starch–starch interactions by starch–plasticizer ones [33]. The melting process comprises in starch destructuration leads to the loss of crystalline morphology and the melt mass can recrystallize in a new structure. This crystalline transformation was corroborated by the change in XRD pattern from A- to V-type (Fig. 5). The occurrence of reflections at 2θ = 12.6 and 19.4° revealed that not only the thermal processing but also the conditioning variables, allowed the recrystallization of TPS based materials in Vh subtype. Talc presence during starch processing did not affect XRD pattern of TPS, demonstrating that mineral particles did not interfere in the amylose recrystallization process. However, the presence of talc particles was evidenced by the occurrence of mineral characteristics peaks, which were identified in Fig. 4 [34]. Thermo-compression induced a slight shift of talc peaks located at 2θ = 9.1, 28.0, and 47.9° to higher values. Besides, an increase in intensity of mineral signals was also detected because of particles alignment during thermo-compression. Regarding degree of crystallinity (CD), the values corresponding to melt processed samples were lower than the one of starch granules. In accordance with Liu et al. [30], decrease in CD is a consequence of the mechanical disruption of molecular bonds due to the intense shear fields. This result is an indicative that this thermal processing induced a higher degree of amorphization, regardless talc addition. Ulterior thermo-compression of the melt processed samples favored the recrystallization stage, allowing a slight increase of the CD values.
Fig. 4.
XRD spectra and degree of crystallinity (CD) of starch granules, melt-mixtures and films of thermoplastic corn starch (TPS-M and TPS-F) and nanocomposites containing talc particles (TPS/T-M and TPS/T-F). Ref: * indicates talc peaks.
Fig. 5.
SEM micrographs of: a) time-evolution of starch morphology during melt-mixing and b) melt-mixtures and films of thermoplastic corn starch (TPS-M and TPS-F) and nanocomposites containing talc particles (TPS/T-M and TPS/T-F).
SEM micrographs included in Fig. 5a show the transformation of starch granules into a thermoplastic material along their thermal processing under the presence of glycerol and water as plasticizers. Morphological characteristics of starch granules such as structure, size, and shape mainly depend not only on the botanical source but also on the cultural practices [35]. Corn starch presented angular-shaped granules, revealing a predominant polyhedral morphology. Through a detailed examination of granules appearance, smooth surfaces without holes and furrows were detected. Corn starch presented unimodal size distribution with a media value of 10–12 μm. When glycerol and water were incorporated to starch powder, granules were impregnated by the plasticizers and, consequently, they partially lost the initial polyhedral shape. Plasticizers presence also induced a more sticky texture which was corroborated by the occurrence of granules agglomerates in SEM micrographs of the mixture after being processed (Fig. 5a, t = 0 min). Starch granules integrity was gradually disappearing along melt-mixing up to a complete loss of their morphology was reached. This observation is a clue of starch fragmentation and destructuration, previously described. In SEM micrographs corresponding to mixture processed during 6 and 12 min, isolated granules populations were detected onto the surface of the incipient plasticized starch (Fig. 5a). This physical transformation occurs under high shear fields and temperature, involving plasticizers molecules diffusion among starch chains. As it is well-known, plasticizers such as glycerol contain many –OH groups which can form hydrogen bonds with those of starch, reducing intermolecular interactions and entanglements among polymer chains [6]. Thus, the mobility of the polysaccharide molecules is increased, allowing starch mouldability by its ulterior processing by thermo-compression. After 15 min melt-mixing, a material with a homogenous appearance was achieved (TPS-M), without the presence of structural defects at microscopic level (Fig. 5b). In this sense, no pores, cracks, bubbles, nor phases separation in the processed starch samples were detected. Fracture surface of the thermo-compressed films (TPS-F) is also shown in Fig. 5b. The mobility of starch chains achieved by the melt-mixing and ulterior conditioning allowed obtaining films with uniform microstructure, without glycerol migration nor imperfections. SEM micrographs of TPS composites containing talc particles are also included in Fig. 5b (TPS/T-M and TPS/T-F). Plasticized starch matrix can be visualized as a grey background, meanwhile talc particles are distinguished as white short lines. Talc laminar morphology is revealed, as well as, particle micrometric length and nanometric thickness. In general, particles lateral section is observed in SEM micrographs. In processed samples, particles were well-distributed within the matrix and a good TPS-talc interfacial adhesion was detected. Besides, a hinted particle orientation was distinguished, attributed to the laminar talc morphology which allow particle alignment in the flow direction. This microscopic observation is in accordance to the previous discussion about the slight increase in the TPS maximum torque value, induced by talc particles presence (Fig. 2). During melt-mixing, particles are forced to be oriented in the flow direction, increasing this processing parameter. Once, starch plasticization is already reached, and most particles have been aligned, torque value remained constant, as it was appreciated in Fig. 2. Particles easiness to be oriented could be considered as a beneficial concern since it contributes to the minimization of viscous heating during melt-mixing, reducing the starch thermal degradation. The absence of particles aggregates corroborated that a good talc delamination was achieved during nanocomposite processing. Thermo-compression induced a greater particle orientation within the matrix. During this processing, thermoplastic material melts allowing the particles mobility within a viscous system and the ulterior alignment parallel to the film surface. Despite the film was cryofractured to be observed by SEM, no particles pull-out phenomenon was detected. This is mainly attributed to the good TPS-talc compatibility. Moreover, no additional microstructural changes induced by the thermo-compression process were distinguished.
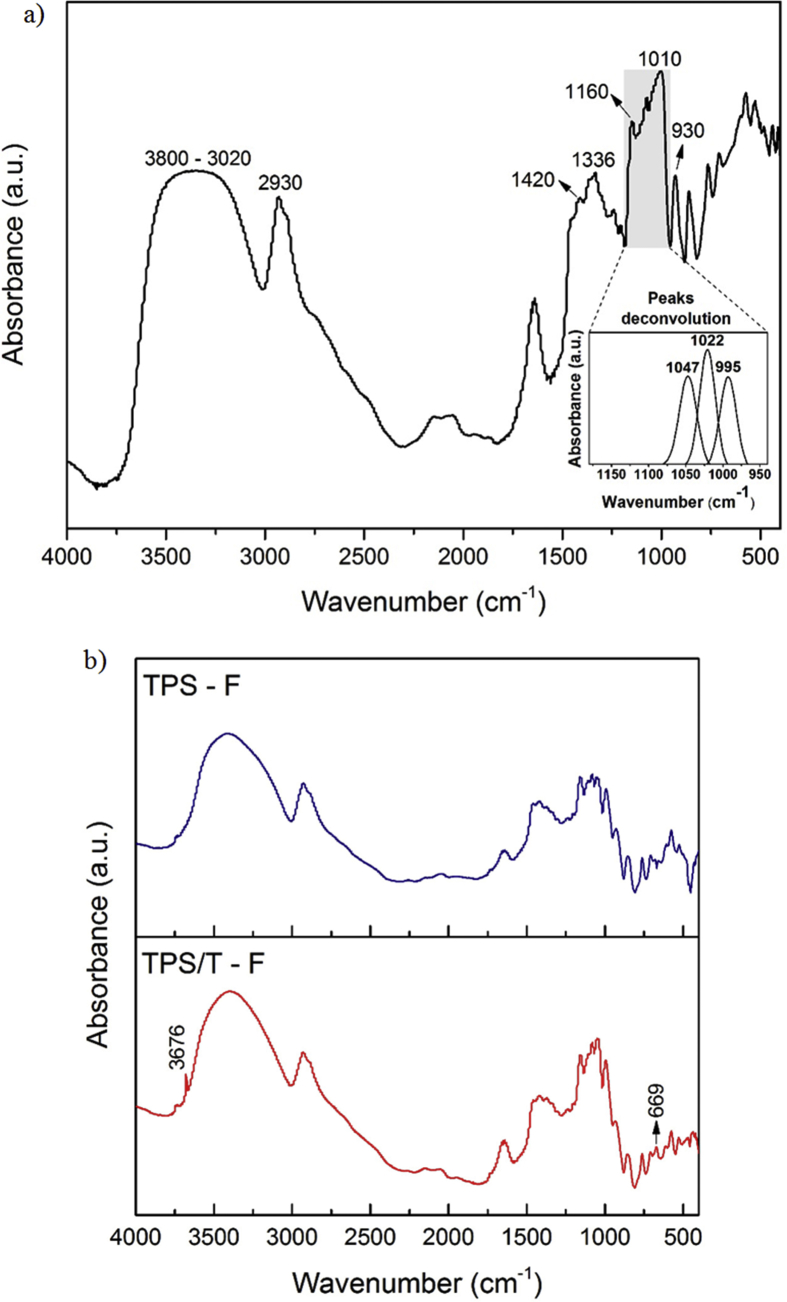
Structural transformations of starch granules by thermal processing, in the presence of talc nanoparticles, was also evaluated by FTIR spectroscopy. This characterization technique is sensitive to changes in starch structure at molecular level (short-range order), such as chain conformation, helicity, crystallinity, and retrogradation processes, as well as, water content [4]. Besides, FTIR allows detecting potential interactions between the composites components. Fig. 6 presents FTIR spectra of starch granules and films based on TPS and nanocomposites containing talc particles. Regarding starch granules spectrum, a band located at 3800 - 3020 cm−1 was detected which corresponds to O–H stretching mode (Fig. 6a). This signal resulted broad because of the high amount of these associated functional groups. In the region comprised between 3020 and 2830 cm−1 it was registered the absorption of C–H stretching, detecting a maximum absorption at 2930 cm−1. Bands corresponding to O–H and C–H bending were found at 1650 and 1420 cm−1, respectively. The absorption bands located in the region between 1500 and 800 cm−1 are highly overlapped, hindering an accurate assignment. Despite the complexity of this region, some signals could be distinguished. Thus, at 1336 cm−1 it was detected a band, which was related to –CH2 bending [36]. The weak signal occurred at 1240 cm−1 was attributed to –OH bending [37]. Absorption peak at 1160 cm−1 was probably associated to C–O and C–C stretching. Bands at 1080 and 1010 cm−1 were assigned to C–O–C bending mode [38]. Starch glycosidic linkages were the responsible of the band observed at 930 cm−1 [36]. Several authors reported that the absorbance bands at 1047 and 995 cm−1 are sensitive to the amount of crystalline structure and the band at 1022 cm−1 is characteristic of the amorphous starch. Therefore, the ratio of the heights of crystalline bands (1047 and 995 cm−1) and 1022 cm−1 expresses the relationship between the amount of crystalline to amorphous structures [37, 38, 39]. Higher values for this ratio indicate a greater structural organization of starch granules [40]. In order to determine this ratio, it was necessary to study the spectral range between 1200 and 940 cm−1. Deconvoluted peaks associated to crystalline and amorphous phases of starch granules are shown inside Fig. 6a. The 1047/1022 and 995/1022 ratios were 0.78 and 0.76, respectively. These values are in agreement with those reported by other authors [38, 41, 42]. Thermal processing induced some changes on starch structure, favored by high shear stress and temperature, as well as, the presence of plasticizers. Thus, the bands located at 1147 and 1074 cm−1 shifted towards to higher wavenumbers. Besides, it was observed the presence of a new signal at 1102 cm−1 which corresponds to glycerol functional groups [43]. FTIR of starch granules presented a band at 856 cm−1 attributed to C–O–C ring vibration of carbohydrate [44]. After thermal processing this signal was broadened and shifted to 862 cm−1, probably due to structural changes in starch molecules (Fig. 6b). TPS also showed an additional peak at 678 cm−1. Regarding composites containing talc, the presence of mineral particles was corroborated by the occurrence of bands at 3676 and 669 cm−1 [34].
Fig. 6.
FTIR spectra of: a) starch granules, and b) films of thermoplastic corn starch (TPS-F) and nanocomposites containing talc particles (TPS/T-F).
4. Conclusions
Native corn starch was processed by melt-mixing and thermo-compression in the presence of plasticizers (water and glycerol) and talc nanoparticles. Complementary techniques allowed corroborating that starch granules suffered irreversible structural changes during their thermal processing. Melt-mixing caused a disruption of crystalline structure of starch granules, from A- to Vh-crystal type. Moreover, a decrease in the degree of crystallinity was observed as a consequence of granules thermal processing under high shear stresses in the presence of plasticizers. At microscopic level, the optimal melt-mixing allowed a complete loss of starch granules integrity, leading to a homogenous thermoplastic material. Flexible films were obtained by an ulterior thermo-compression of plasticized starch. On the other hand, the influence of talc nanoparticles incorporation during starch thermal processing was also analyzed. Even though an increase in torque values during melt-mixing was detected, talc presence did not hinder the plasticization starch. Besides, modified processing parameters were not required when talc nanoparticles were added to starch formulations. Regarding to nanocomposite structure, these mineral fillers did not affect crystalline morphology nor degree of crystallinity of thermoplastic starch. A proper melt-mixing was achieved since the microstructural analysis of the composites revealed that talc nanoparticles were well distributed within starch matrix. Besides, a good particle-TPS adhesion and talc preferential orientation were observed. Particularly, talc laminar morphology and thermo-compression favored particle alignment parallel to film surface.
An integral analysis of starch structural changes induced by thermal processing is relevant to comprehend the materials properties obtained by melt-mixing and thermo-compression. In this manner, it is possible to tailor final materials for specific applications, optimizing not only starch formulations but also their thermal processing.
Declarations
Author contribution statement
Luciana Castillo, Olivia Lopez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Garcia María Alejandra, Silvia Barbosa, Marcelo Villar: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina, grant PIP 0428) and Universidad Nacional del Sur (UNS, Argentina, grant PGI 24/M135).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Xie X., Liu Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch Staerke. 2004;56(8):364–370. [Google Scholar]
- 2.Wang S., Zhang X., Wang S., Copeland L. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez A., Cyras V.P., Alvarez V.A., Moran J.I. Starch/clay nano-biocomposites. In: Avérous L., Pollet E., editors. Environmental Silicate Nano-Biocomposites. Springer; London: 2012. pp. 287–321. [Google Scholar]
- 4.Yu H., Cheng L., Yin J., Yan S., Liu K., Zhang F., Xu B., Li L. Structure and physicochemical properties of starches in lotus (Nelumbo nucifera Gaertn.) rhizome. Food Sci. Nutr. 2013;1(4):273–283. doi: 10.1002/fsn3.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez S., Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch Staerke. 2010;62(8):389–420. [Google Scholar]
- 6.Jiang X., Jiang T., Gan L., Zhang X., Dai H., Zhang X. The plasticizing mechanism and effect of calcium chloride on starch/poly(vinyl alcohol) films. Carbohydr. Polym. 2012;90(4):1677–1684. doi: 10.1016/j.carbpol.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Ayana B., Supratim S., Khatua B.B. Highly exfoliated eco-friendly thermoplastic starch (TPS)/poly (lactic acid) (PLA)/clay nanocomposites using unmodified nanoclay. Carbohydr. Polym. 2014;110:430–439. doi: 10.1016/j.carbpol.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Chen B., Evans J.R.G. Thermoplastic starch-clay nanocomposites and their characteristics. Carbohydr. Polym. 2005;61(4):455–463. [Google Scholar]
- 9.Córdoba A., Cuéllar N., González M., Medina J. The plasticizing effect of alginate on the thermoplastic starch/glycerin blends. Carbohydr. Polym. 2008;73:409–416. [Google Scholar]
- 10.Avérous L. Biodegradable multiphase systems based on plasticized starch: a review. J. Macromol. Sci. C Polym. Rev. 2004;C4(3):231–274. [Google Scholar]
- 11.Castillo L., Barbosa S., Maiza P., Capiati N. Surface modifications of talcs. Effects of inorganic and organic acid treatments. J. Mater. Sci. 2011;46:2578–2586. [Google Scholar]
- 12.Castillo L.A., López O.V., López C., Zaritzky N.E., García M.A., Barbosa S.E., Villar M.A. Thermoplastic starch films reinforced with talc nanoparticles. Carbohydr. Polym. 2013;95:664–674. doi: 10.1016/j.carbpol.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 13.López O.V., Castillo L.A., García M.A., Villar M.A., Barbosa S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocolloids. 2015;43:18–24. [Google Scholar]
- 14.Castillo L.A., López O.V., Ghilardi J., Villar M.A., Barbosa S.E., García M.A. Thermoplastic starch/talc bionanocomposites. Influence of particle morphology on final properties. Food Hydrocolloids. 2015;51:432–440. [Google Scholar]
- 15.López O.V., Castillo L.A., Barbosa S.E., Villar M.A., García M.A. Processing–properties–applications. Relationship of nanocomposites based on thermoplastic corn starch and talc. Polym. Compos. 2018;39(4):1331–1338. [Google Scholar]
- 16.López O.V., García M.A., Zaritzky N.E. Film forming capacity of chemically modified corn starches. Carbohydr. Polym. 2008;73:573–581. doi: 10.1016/j.carbpol.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Soliman E., Furuta M. Influence of phase behavior and miscibility on mechanical, thermal and microstructure of soluble starch-gelatin thermoplastic biodegradable blend films. Food Nutr. Sci. 2014;5:1040–1055. [Google Scholar]
- 18.Xie F., Luckman P., Milne J., McDonald L., Young C., Yang Tu C., Di Pasquale T., Faveere R., Halley P.J. Thermoplastic starch: current development and future trends. J. Renew. Mater. 2014;2(2):95–106. [Google Scholar]
- 19.Shanks R., Kong I. Thermoplastic starch. In: El-Sonbati P.A., editor. Thermoplastic Elastomers. Croatia. InTech; 2012. pp. 95–106. [Google Scholar]
- 20.Tester R.F., Karkalas J., Qi X. Starch-composition, fine structure and architecture. J. Cereal Sci. 2004;39(2):151–165. [Google Scholar]
- 21.Li M., Hasjim J., Xie F., Halley P.J., Gilbert R.G. Shear degradation of molecular, crystalline, and granular structures of starch during extrusion. Starch Staerke. 2014;66(7-8):595–605. [Google Scholar]
- 22.Liu W.-C., Halley P.J., Gilbert R.G. Mechanism of degradation of starch, a highly branched polymer, during extrusion. Macromolecules. 2010;43:2855–2864. [Google Scholar]
- 23.Thunwall M., Boldizar A., Rigdahl M. Compression molding and tensile properties of thermoplastic potato starch materials. Biomacromolecules. 2006;7:981–986. doi: 10.1021/bm050804c. [DOI] [PubMed] [Google Scholar]
- 24.Corradini E., de Carvalho A.J.F., da Silva Curvelo A.A., Marcondes Agnelli J.A., Capparelli Mattoso L.H. Preparation and characterization of thermoplastic starch/zein blends. Mater. Res. 2007;10(3):227–231. [Google Scholar]
- 25.Qiao X., Tang Z., Sun K. Plasticization of corn starch by polyol mixtures. Carbohydr. Polym. 2011;83(2):659–664. [Google Scholar]
- 26.Gott B., Barton H., Samuel D., Torrence R. Biology of starch. In: Torrence R., Barton H., editors. Ancient Starch Research. Left Coast Press; Walnut Creek: 2006. pp. 35–45. [Google Scholar]
- 27.Wang S., Copeland L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: a review. Food & Function. 2013;4:1564–1580. doi: 10.1039/c3fo60258c. [DOI] [PubMed] [Google Scholar]
- 28.Buléon A., Véronèse G., Putaux J.-L. Self-association and crystallization of amylose. Aust. J. Chem. 2007;60:706–718. [Google Scholar]
- 29.Kong L., Ziegler G.R. Molecular encapsulation of ascorbyl palmitate in preformed V-type starch and amylose. Carbohydr. Polym. 2014;111:256–263. doi: 10.1016/j.carbpol.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Liu H., Xie F., Yua L., Chen L., Li L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009;34:1348–1368. [Google Scholar]
- 31.Klingler R.W., Meuser F., Niediek E.A. Effect of the form of energy transfer on the structural and functional characteristics of starch. Starch Staerke. 1986;38:40–44. [Google Scholar]
- 32.Mohammadi Nafchi A., Moradpour M., Saeidi M., Alias A.K. Thermoplastic starches: properties, challenges, and prospects. Starch Staerke. 2013;65:61–72. [Google Scholar]
- 33.Mekonnen T., Mussone P., Khalilb H., Bressler D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A. 2013;1:13379–13398. [Google Scholar]
- 34.Castillo L.A., Barbosa S.E., Maiza P., Capiati N.J. Surface modifications of talcs. Effects of inorganic and organic acid treatments. J. Mater. Sci. 2011;46(8):2578–2586. [Google Scholar]
- 35.Singh N., Singh J., Kaur L., Singh Sodhi N., Singh Gill B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81(2):219–231. [Google Scholar]
- 36.Deeyai P., Suphantharika M., Wongsagonsup R., Dangtip S. Characterization of modified tapioca starch in atmospheric argon plasma under diverse humidity by FTIR spectroscopy. Chin. Phys. Lett. 2013;30(1):1–4. [Google Scholar]
- 37.Mina J., Valadez-González A., Herrera-Franco P., Zuluaga F., Delvasto S. Physicochemical characterization of natural and acetylated thermoplastic cassava starch. Dyna. 2011;78(166):166–173. [Google Scholar]
- 38.Schmiele M., Sehn G.A.R., da Silva Santos V., de Souza Rocha T., Lopes Almeida E., Nabeshima E.H., Chang Y.K., Steel C.J. Physicochemical, structural and rheological properties of chestnut (Castanea sativa) starch. Adv. J. Food Sci. Technol. 2015;3(4A):1–7. [Google Scholar]
- 39.Chung H.J., Liu Q., Hoover R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009;75(3):436–447. [Google Scholar]
- 40.Ji Y., Zhu K., Zhou H., Qian H. Study of the retrogradation behaviour of rice cake using rapid visco analyser, Fourier transform infrared spectroscopy and X-ray analysis. Int. J. Food Sci. Technol. 2010;45:871–876. [Google Scholar]
- 41.Warren F.J., Perston B.B., Royall P.G., Butterworth P.J., Ellis P.R. Infrared spectroscopy with heated attenuated total internal reflectance enabling precise measurement of thermally induced transitions in complex biological polymers. Anal. Chem. 2013;85:3999–4006. doi: 10.1021/ac303552s. [DOI] [PubMed] [Google Scholar]
- 42.Yang Q., Yang Y., Luo Z., Xiao Z., Ren H., Li D., Yu J. Effects of lecithin addition on the properties of extruded maize starch. J. Food Process. Preserv. 2016;40:20–28. [Google Scholar]
- 43.Musa M.B., Yoo M.J., Kang T.J., Kolawole E.G., Ishiaku U.S., Yakubu M.K., Whang D.J. Characterization and thermomechanical properties of thermoplastic potato starch. R. & R.: J. Eng. Technol. 2013;2(4):9–16. [Google Scholar]
- 44.Abdullah A.H.D., Chalimah S., Primadona I., Hanantyo M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. Earth Environ. Sci. 2018;160 [Google Scholar]