Highlights
-
•
We examined the optimal length of actigraphic recording in patients with narcolepsy type 1.
-
•
No differences were detected between the mean values of 7-day and 14-day analyzed sets for the different sleep parameters.
-
•
One week of actigraphic recording in patients with narcolepsy type 1 appears to be sufficient.
Keywords: Actigraphy, Motor activity, Narcolepsy, Sleep, Sleep latency
Abstract
Objective
The aim of the present study was to assess the optimal length of actigraphic recordings in patients with narcolepsy type 1.
Methods
A secondary analysis was carried out with the previously collected data in eleven patients with narcolepsy type 1. Ten of the 11 patients were medicated at the time of actigraphic recording. Each patient originally wore an Actiwatch AW64 actigraph for at least 28 consecutive days. Overall, the patients were analyzed for 308 nights.
Results
No significant differences were observed between the mean values of the 7-day and 14-day analyzed sets for the parameters sleep efficiency, fragmentation index, sleep onset latency, wake after sleep onset, and total sleep time.
Conclusions
Our data suggest that 7 days of actigraphic recording could be sufficient for these patients.
Significance
Our results for the optimal length of actigraphic recording could be useful for both physicians and patients.
1. Introduction
According to the third edition of the International Classification of Sleep Disorders (ICSD-3), narcolepsy type 1 (NT1) is a central disorder of hypersomnolence (American Academy of Sleep Medicine, 2014). The diagnostic criteria for NT1, based primarily on clinical issues, include excessive diurnal sleepiness, cataplexy, abnormalities in rapid eye movement (REM) sleep, and a low concentration of hypocretin-1 in the cerebrospinal fluid.
Recent studies have shown that actigraphic monitoring of sleep–wake behavior could be useful in screening NT1 (Filardi et al., 2015, Alakuijala et al., 2016, Leger et al., 2018) and also documenting the changes in sleep quality and daytime napping after treatment with sodium oxybate (Filardi et al., 2018). A recent systematic review comprising a meta-analysis and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process recommends the use of actigraphy to assess total sleep time (TST) before the multiple sleep latency test (MSLT) in patients suspected of central disorders of hypersomnolence (Smith et al., 2018). Indeed, MSLT could be potentially influenced by chronic sleep deprivation, which could be excluded through actigraphic monitoring. Importantly, these authors have also reported that “In practical terms, actigraphy studies can be obtained over a period of 7–14 days, though currently there is no available data to determine the optimal length of study prior to MSLT” (page 1224). Furthermore, this observation also highlights the paucity of actigraphic data in narcolepsy research (Smith et al., 2018).
To date, the optimal length of actigraphic recording in patients with NT1 remains unknown, even though this information could help to obtain objective and reliable data on the amount and quality of sleep in these patients. This topic was previously investigated in a report on other sleep disorders such as primary insomnia, which also examined patients with dementia (Van Someren, 2007). Although it should be recorded for at least 7 days, the author recommended 14 days as the optimal duration for actigraphic monitoring. More recently, Briscoe et al. (2014) examined a sample of patients with different sleep disorders (primarily insomnia) who had been referred to a sleep disorder center. Contrary to the 14 days proposed by Van Someren (2007), these authors recommended 7 days of actigraphic recording as the standard.
As no such data are currently available for patients with NT1, we chose to carry out a secondary analysis of previously collected data in patients with NT1 (Leger et al., 2018, Tonetti et al., 2019) that originally wore an actigraph for at least 28 consecutive days. The aim of our study was thus to assess whether the optimal length of monitoring should be 7 or 14 days to address the deficiency in knowledge recently identified by Smith et al. (2018). Although actigraphy is not a mandatory tool for diagnosing NT1, it has been considered useful for understanding the disease severity and evaluating the impact of treatments, as well as for monitoring TST before MSLT in patients suspected of a central disorder of hypersomnolence (Smith et al., 2018). The aim of our survey was thus to better understand what time period should be recommended to doctors and patients.
2. Methods
2.1. Participants
Only patients with NT1 who had previously used an actigraph for at least 28 consecutive days were included in the present study, which resulted in the exclusion of two participants from the initial sample of 13 patients with NT1. Thus, the final sample was composed of eleven patients (7 females and 4 males), and the total number of analyzed nights was 308. The mean age of the overall sample was 40.55 ± 12.18 years. The mean age of males (42.75 ± 20.52) did not show significant difference when compared to that of females (39.29 ± 5.62) (t9 = −0.44; p = 0.67). At the time of actigraphic recording, 10 out of the 11 patients were taking a medication (e.g., modafinil or sodium oxybate) to treat narcolepsy. Actigraphic records of these patients were retrieved at the Centre du Sommeil et de la Vigilance, Hôtel-Dieu de Paris (France).
2.2. Actigraphy
The Actiwatch AW64 actigraph model (Cambridge Neurotechnology Ltd; Cambridge, UK) was used in this study, which can directly assess motor activity and indirectly assess sleep through a validated algorithm (Oakley, 1997). The device was equipped with a light sensor. According to the algorithm, each epoch was scored as sleep when the total number of activity counts (A) was equal to or less than the low threshold sensitivity value, i.e., 80 counts per epoch (Tonetti et al., 2008, Natale et al., 2014). A was computed as an2*(1/25) + an1*(1/5) + a + a1*(1/5) + a2*(1/25). In the formula, “an2” and “an1” correspond to the activity counts in the previous 2 epochs, while “a” is the activity count in the current epoch, and “a1” and “a2” are the activity counts in the next 2 epochs. The actigraphs were originally initialized using Actiwatch Activity & Sleep Analysis 5 software (version 5.32, Cambridge Neurotechnology Ltd; Cambridge, UK) to collect data in 1-minute epochs. The battery life of this actigraph model is 6 months.
2.3. Actigraphic parameters
The following actigraphic parameters were computed: sleep efficiency (SE), fragmentation index (FI), sleep onset latency (SOL), wake after sleep onset (WASO), and TST. We chose to examine these actigraphic parameters, as they are useful markers of sleep quality (SE, FI, SOL, and WASO) and quantity (TST). The parameters were calculated as follows:
SE=(TST/time in bed)*100.
FI = [(number of minutes moving/TST)*100] + [(immobility phases of 1 min/number of immobility phases)*100]. FI is an indicator of restlessness.
SOL = sleep onset time-bedtime. Sleep onset is defined as the start of a period of at least 10 min of consecutively recorded immobile data, with no>1 epoch of movement within that time.
WASO = [time in bed-(SOL + TST)].
TST = [time in bed-(SOL + WASO)].
2.4. Procedure
All patients originally wore the actigraph around the wrist of their nondominant hand for at least 28 consecutive days. Patients were instructed to push the event marker button on the top of the actigraph to signal their bedtime and wake-up time, which were used by an experienced scorer to set the time in bed window. If patients forgot to push the event marker button, the scorer referred to the bedtime and wake-up time reported in the sleep diary that was filled in every day. Each participant provided written informed consent before their inclusion in the original study, which received approval by the ethical committee CPP Ile de France 1 (file number 2016 Mars 14180ND).
We split the entire 28-day recording period in half, which allowed us to repeatedly compute the investigated parameters for two separate blocks of 14 days each. We thus computed the actigraphic parameters for two separate blocks of 1 day, 2 days, 3 days, etc., up to two separate blocks of 14 days (i.e., days 1–14 and 15–28).
We then computed the percentage of the mean absolute difference between two estimates of an increasing number of days (Fig. 1). For example, in the case of a 7-day analyzed set, we computed the difference between the mean values from day 1 through day 7 and from day 15 through day 21. In the case of a 14-day analyzed set, we calculated the difference between the mean values from day 1 through day 14 and from day 15 through day 28. These differences were transformed into absolute terms and finally into percentages according to the following formula: (mean absolute difference*100)/mean of the two mean estimates.
Fig. 1.
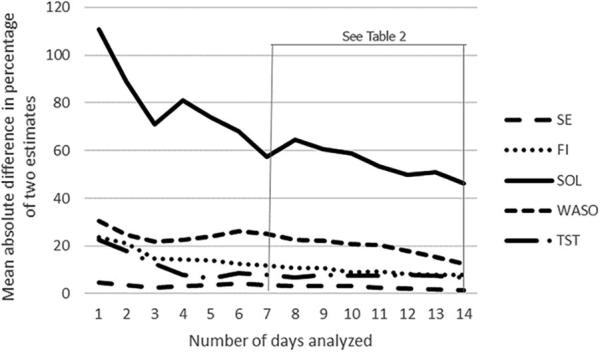
Percentage of the mean absolute difference between two estimates with an increasing number of days analyzed for the parameters SE (sleep efficiency), FI (fragmentation index), SOL (sleep onset latency), WASO (wake after sleep onset), and TST (total sleep time). For the number of days analyzed, “1” refers to day 1 vs. day 15, “2” refers to days 1–2 vs. days 15–16, “3” refers to days 1–3 vs. days 15–17, etc. The mean absolute differences between a 7-day and a 14-day analyzed set were statistically compared, and the results are shown in Table 2.
We also computed the average between two estimates of an increasing number of days (Fig. 2). For example, for a 7-day analyzed set, we computed the average between the mean value from day 1 through day 7 and that from day 15 through day 21. For a 14-day analyzed set, we calculated the average between the mean value from day 1 through day 14 and that from day 15 through day 28.
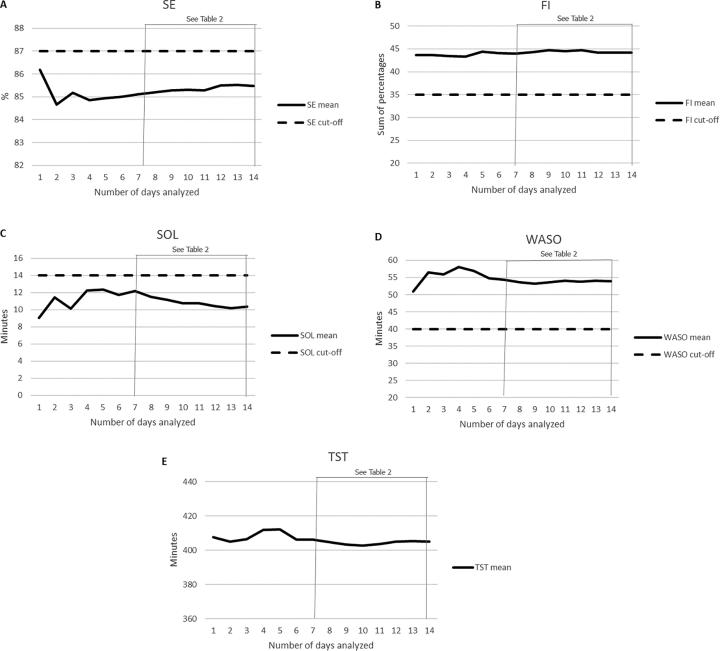
Fig. 2.
Mean between two mean estimates with an increasing number of days analyzed, for the parameters SE (sleep efficiency; normal if >87; A), FI (fragmentation index; normal if <35; B), SOL (sleep onset latency; normal if <14; C), WASO (wake after sleep onset; normal if <40; D), and TST (total sleep time; no cutoff available; E). The cutoff value reported by Natale et al. (2014) to differentiate patients with primary insomnia from healthy controls is provided on the figure. Cutoff values for patients with narcolepsy type 1 are not available. For the number of days analyzed, “1” refers to day 1 vs. day 15, “2” refers to days 1–2 vs. days 15–16, “3” refers to days 1–3 vs. days 15–17, etc. The mean values of a 7-day set and a 14-day analyzed set were statistically compared, and the results are shown in Table 2.
2.5. Statistical analyses
For the percentage of the mean absolute difference and the mean of each actigraphic parameter, we performed a set of paired sample t-tests comparing a 7-day analyzed set with a 14-day analyzed set. As we performed multiple comparisons (n = 10), the significance level was set to 0.005, applying the Bonferroni correction.
The rationale for a 7-day vs. a 14-day analyzed set was based on previous mixed reports on the recommended length of actigraphic monitoring in patients with sleep disorders that differed from NT1, i.e., 14 days according to Van Someren (2007) vs. 7 days, in line with the results of Briscoe et al. (2014). Furthermore, this rationale was also based on a recent work positing that TST actigraphic monitoring should last for 7–14 days before MSLT in patients suspected of central disorders of hypersomnolence (Smith et al., 2018), although the optimal length of such a recording remains unknown.
3. Results
Table 1 presents the row mean values and standard deviations of the actigraphic parameters observed during the whole recording period (i.e., 28 days), as well as the corresponding cutoff values (Natale et al., 2014). These cutoff values were originally proposed to discriminate patients with primary insomnia from healthy controls using the same actigraphic device. As no cutoff values are available for patients with NT1, the values for primary insomniacs were used to discriminate good-quality sleep from poor-quality sleep. It should be noted that no cutoff values were proposed for the TST parameter.
Table 1.
Row mean values (M) and standard deviations (SD) for the various actigraphic parameters recorded during the whole recording period (i.e., 28 days), accompanied by their cutoff values.
| M (SD) | Cutoff valuesa | |
|---|---|---|
| SE | 85.48 (7.98) | >87 |
| FI | 44.15 (16.80) | <35 |
| SOL | 10 (7) | <14 |
| WASO | 54 (33) | <40 |
| TST | 404.96 (51.98) | NA |
Note. SE = sleep efficiency; FI = fragmentation index; SOL = sleep onset latency; WASO = wake after sleep onset; TST = total sleep time; NA = not available.
The cutoff values were originally proposed by Natale et al. (2014) to discriminate patients with primary insomnia from healthy controls. No specifically developed cutoff values for patients with narcolepsy type 1 are currently available.
In Fig. 1, the percentage of the mean absolute difference between two estimates is displayed relative to an increasing number of days analyzed, for each actigraphic parameter. As reported in Table 2, we did not observe any significant differences between the percentage of the mean absolute difference of the estimate for a 7-day set as compared to that for a 14-day set, with regard to the parameters SE, FI, SOL, and TST. However, we observed a difference approaching significance for WASO, in which there was a lower percentage of the mean absolute difference for a 14-day set than for a 7-day set (Table 2).
Table 2.
Mean values (M) and standard deviations (SD) for the percentage of mean absolute difference and the mean values of the different actigraphic parameters, for both 7-day and 14-day analyzed sets.
| 7 days | 14 days | Statistics |
||
|---|---|---|---|---|
| M (SD) | M (SD) | t(10) | pa | |
| Percentage of mean absolute difference | ||||
| SE | 3.47 (3.32) | 1.57 (1.57) | 2.48 | 0.03 |
| FI | 11.90 (8.79) | 7.83 (4.98) | 2.41 | 0.04 |
| SOL | 57.41 (52.21) | 46.40 (42.06) | 1.03 | 0.33 |
| WASO | 24.91 (14.36) | 12.36 (10.20) | 3.45 | 0.006 |
| TST | 8.03 (6.60) | 6.87 (5.60) | 0.50 | 0.63 |
| Mean | ||||
| SE | 85.10 (8.70) | 85.48 (7.98) | −1.46 | 0.18 |
| FI | 43.96 (17.21) | 44.15 (16.80) | −0.43 | 0.67 |
| SOL | 12.18 (10.83) | 10.27 (7.21) | 1.64 | 0.13 |
| WASO | 54.45 (34.23) | 54 (32.66) | 0.55 | 0.59 |
| TST | 406.31 (54.40) | 404.96 (51.98) | 0.33 | 0.75 |
The t-statistics are also provided.
Note. SE = sleep efficiency; FI = fragmentation index; SOL = sleep onset latency; WASO = wake after sleep onset; TST = total sleep time.
Significance level set to p < 0.005 after Bonferroni correction.
Fig. 2 shows the mean values between two estimates relative to an increasing number of days analyzed for each parameter, as well as the corresponding cutoff value (Natale et al., 2014). As shown in Table 2, no significant differences between the mean values of a 7-day and a 14-day set were observed for any of the investigated actigraphic parameters.
4. Discussion
Our study assessed for the first time the optimal duration of actigraphic recording in patients with NT1, thereby addressing the deficiency in knowledge that was previously identified by Smith et al. (2018).
When the whole recording period (i.e., 28 days) was considered, the row mean actigraphic values are outside the normal limits according to the cutoff values (Natale et al., 2014) for all parameters except for SOL (Table 1). Overall, these data are in line with the data previously reported by Filardi et al. (2015) in drug-naïve patients with NT1, in which a different actigraph model (Motionlogger® Micro Watch), with its own specific cutoff values for primary insomnia (Natale et al., 2009), was used. Indeed, based on observations by Filardi and colleagues (cf. Table 2 in Filardi et al., 2015), all of the sleep measures were outside of the normal limits. Taken together, the data observed in the present study indicate a reduced sleep quality in patients, although most of them were under medication.
With regard to the percentage of mean absolute difference between estimates of an increasing number of days analyzed, we observed a reduction in the variability of actigraphic sleep estimates with increases in length of actigraphy monitoring (Fig. 1). Statistically, we did not observe any significant differences between a 7-day and a 14-day analyzed set for the different actigraphic parameters (Table 2).
When the mean value between two estimates was analyzed with an increasing number of days, the mean values for all parameters except SOL were observed to be clinical according to the cutoff scores reported by Natale et al. (2014) for this specific actigraph model (Fig. 2), for the whole period of actigraphy monitoring. Statistically, no significant differences were observed for any parameter between a 7-day and a 14-day analyzed set (Table 2).
Taken together, the data reported in Table 2 on the percentage of mean absolute difference and the mean between the estimates of an increasing number of days analyzed suggest that 7 days of actigraphic recording could be sufficient to reliably describe the sleep features of patients with NT1. However, it is well recognized that NT1 is not diagnosed by actigraphy but by well-defined clinical features detailed in the ICSD-3 (American Academy of Sleep Medicine, 2014). Nevertheless, actigraphy may provide support in understanding the daily lives and sleep patterns of patients, in particular in the assessment of TST before MSLT in patients suspected of this disorder (Smith et al., 2018). Moreover, actigraphy could be easily proposed as a means to understand the impact of treatments on sleep amount and fragmentation, under real conditions.
This study is not without limitations, such as the reduced sample size and the fact that most patients were under medication. However, the shortcoming of the low number of participants is counterbalanced by the high number of analyzed nights. Moreover, there is some precedent for a reduced sample size, as one previous study examined a similar number of patients (n = 10), albeit with a different sleep disorder, primary insomnia (Van Someren, 2007). Finally, evidence that NT1 symptoms can be reduced but not eliminated by drug treatment (Leger et al., 2018) mitigates the limitation of the ongoing therapy.
Acknowledgments
Acknowledgments
The original survey was funded by the Fondation Maladies Rares, France: Somnopro Project 2017. The funding source had no role in the study design, collection, data analysis and interpretation, writing of the report, or the decision to submit the article for publication.
Declaration of Competing Interest
Damien Léger declares that during the past 5 years, he has been employed as an investigator or a consultant by Actellion, Agence Spatiale Européenne, Bioprojet, iSommeil, ESAI, Jansenn, Jazz, Vanda, Merck, Philips, Rythm, Sanofi, Vitalaire, and Resmed. He declares no conflict of interest regarding this study. Lorenzo Tonetti, Caroline Gauriau, Brice Faraut, Maxime Elbaz, Fabien Sauvet, and Vincenzo Natale also declare no conflict of interest.
Authors contributions
DL, CG, BF, ME, and FS conceived and collected data for the original survey; VN conceived the present study and analyzed the data files; LT performed the statistics and wrote the original draft; and DL, LT, CG, BF, ME, FS, and VN revised the manuscript. All authors have approved the final manuscript.
References
- Alakuijala A., Sarkanen T., Partinen M. Hypocretin-1 levels associate with fragmented sleep in patients with narcolepsy type 1. Sleep. 2016;39:1047–1050. doi: 10.5665/sleep.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Westchester: 2014. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- Briscoe S., Hardy E., Pengo M.F., Kosky C., Williams A.J., Hart N. Comparison of 7 versus 14 days wrist actigraphy monitoring in a sleep disorders clinic population. Chronobiol. Int. 2014;31:356–362. doi: 10.3109/07420528.2013.858163. [DOI] [PubMed] [Google Scholar]
- Filardi M., Pizza F., Martoni M., Vandi S., Plazzi G., Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 2015;16:126–130. doi: 10.1016/j.sleep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Filardi M., Pizza F., Antelmi E., Ferri R., Natale V., Plazzi G. In-field assessment of sodium oxybate effect in pediatric type 1 narcolepsy: an actigraphic study. Sleep. 2018;41:zsy050. doi: 10.1093/sleep/zsy050. [DOI] [PubMed] [Google Scholar]
- Leger D., Gauriau C., Tonetti L., Lantin M., Filardi M., Philip P. Using actigraphy to assess sleep and wake rhythms of narcolepsy type 1 patients: a comparison with primary insomniacs and healthy controls. Sleep Med. 2018;52:88–91. doi: 10.1016/j.sleep.2018.07.024. [DOI] [PubMed] [Google Scholar]
- Natale V., Léger D., Martoni M., Bayon V., Erbacci A. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 2014;15:111–115. doi: 10.1016/j.sleep.2013.08.792. [DOI] [PubMed] [Google Scholar]
- Natale V., Plazzi G., Martoni M. Actigraphy in the assessment of insomnia: a quantitative approach. Sleep. 2009;32:767–771. doi: 10.1093/sleep/32.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley N.R. Mini-Mitter; Bend: 1997. Validation with Polysomnography of the Sleepwatch Sleep/Wake Scoring Algorithm Used by the Actiwatch Activity Monitoring System. Technical report. [Google Scholar]
- Smith M.T., McCrae C.S., Cheung J., Martin J.L., Harrod C.G., Heald J.L. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis and GRDE assessment. J. Clin. Sleep Med. 2018;14:1209–1230. doi: 10.5664/jcsm.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti L., Natale V., Gauriau C., Faraut B., Philip P., Leger D. Prospective memory in narcolepsy type 1 patients. J. Psychosom. Res. 2019;117:30–31. doi: 10.1016/j.jpsychores.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Tonetti L., Pasquini F., Fabbri M., Belluzzi M., Natale V. Comparison of two different actigraphs with polysomnography in healthy young subjects. Chronobiol. Int. 2008;25:145–153. doi: 10.1080/07420520801897228. [DOI] [PubMed] [Google Scholar]
- Van Someren E.J.W. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J. Sleep Res. 2007;16:269–275. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]