Abstract
Nonmetastatic hepatic dysfunction known as Stauffer's syndrome is a rare entity. It is commonly associated with clear cell carcinoma of kidney. Rarely it has been reported in cases of prostatic carcinoma, bronchogenic tumor and lymphoproliferative diseases. Stauffer s syndrome presents as elevated alkaline phosphatase, aminotransferases, and prolonged prothrombin time without jaundice. However a very rare variant of Stauffer's syndrome presenting with jaundice has been reported in few case reports of clear cell carcinoma of kidney. But such a presentation in cases of renal sarcomas has not been reported so far. Here we report a unique case of primary synovial sarcoma of kidney presenting as nonmetastatic cholestatic jaundice.
Keywords: Renal synovial sarcoma, Jaundice, Paraneoplastic, Stauffers syndrome
Introduction
Paraneoplastic hepatopathy known as Stauffer's syndrome was first reported by Stauffer1 in 1961. It commonly presents as abnormal liver function tests and/or hepatospleenomegaly.1 Jaundice is not generally a feature of Stauffer's syndrome. Morla et al.2 reported a case of clear cell carcinoma of kidney presenting with paraneoplastic cholestatic jaundice. Similar case reports are available in the literature in patients of renal cell carcinoma.3 The occurrence of paraneoplastic hepatic dysfunction with jaundice was very rarely reported in soft tissue sarcomas and undifferentiated renal tumors. We present a case of primary synovial sarcoma of kidney presenting with paraneoplastic hepatic dysfunction with jaundice in a young male.
Case report
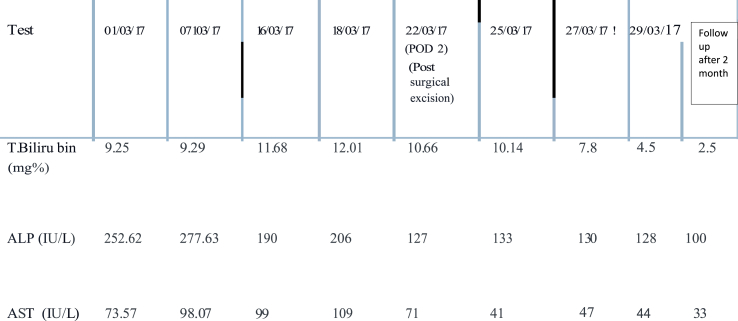
A 32-year male presented to our center with single episode of gross painless hematuria 6 months back. He had fullness in right flank and yellowish discoloration of eyes and itching all over the body for the past 20 days. Further, history revealed loss of appetite and significant weight loss in the past 2 months. He denied any history of fever or altered bowel habits, past history of jaundice, viral hepatitis, blood transfusion or intravenous drug abuse. General physical examination showed presence of deep icterus with scratch marks over arms and abdomen. Vitals were normal with good performance status. Abdominal examination revealed a huge bimanually palpable firm to hard mass occupying right hypochondrium and lumbar region. The routine blood investigations reported hemoglobin of 9.0 gm/dl, serum creatinine of 1.5 mg/dl, total bilirubin of 9.25 mg/dl with direct bilirubin of 6.27 mg/dl, prothrombin time of 45 seconds and alkaline phosphatase, alanine and aspartate transferase levels of 253IU, 30 IU and 74 IU respectively. MRI (Magnetic resonance imaging) abdomen revealed a heterogeneous mass of size 19× 14.3 × 14.4 cm with soft tissue attenuation which was hyperintense on T2 and hypointense on T1 weighted images and occupying the right half of abdomen arising from the right kidney (figure-1A). Hepatobiliary system ultrasound showed no evidence of intra or extra hepatic bile duct dilatation and no evidence of metastasis. Hepatic veins and splenoportal confluence were normal. Further evaluation was done to rule out other causes of hepatic dysfunction. Serology tests for Hepatitis A, B and C were negative. Serum ceruloplasmin and procalcitonin levels were normal. Hemolytic workup and serum ANA (anti-nuclear antibody), SMA (Smooth muscle antigen), AMA (Anti-mitochondrial antibody), LKM (liver kidney microsome) and PCA (Principle component analysis) were also normal. A fibroscan for liver fibrotic changes was also normal. After counseling the patient, right radical nephrectomy was planned. Due to large size of the mass and dubious relation with right renal vessels, renal artery angioembolization was done preoperatively (figure-1B). Perioperative care involved adequate hydration of patient, carbohydrate loading, correcting coagulopathy with vitamin K injections and fresh frozen plasma (FFP) and avoidance of hepatotoxic medications. Patient was laid supine under general anesthesia and bilateral subcostal incision was placed for adequate exposure. Intraoperatively, the tumor was adhered to the undersurface of liver. The inferior vena cava (IVC) was distorted, stretched and pushed medially by the large tumor bulk. There was no tumor or bland thrombus in the renal vein and or IVC. The texture of the liver was normal. The mass was removed and abdomen closed in layers. Postoperative stay in the hospital was uneventful. The serum bilirubin was decreasing gradually and patient was discharged on day 7. Cut section of the specimen showed large variegated mass replacing the entire kidney (figure-2). Final histopathology revealed spindle shaped cells suggestive of synovial cell sarcoma of kidney. On immunohistochemistry (IHC) staining, it showed vimentin positivity, focal positivity for EMA (Epithelial Membrane Antigen) and negative pan CK(Cytokeratin) markers (figure-3). At 2 months follow up, serum bilirubin and liver enzymes were close to normal range (Table 1). Patient was planned for adjuvant chemotherapy; however, he succumbed due to massive myocardial infarction in the follow up period. Autopsy was requested, though patient relatives refused for any further test.
Fig. 1.
A: MRI Whole abdomen (T2 image) showing large lobular heterogeneous signal intensity mass (size 19 × 14.3 × 14.4cm) completely filling and causing marked distension of right pelvicalyceal system. Superiorly mass is indenting the liver and gall bladder, medially indenting the IVC, portal vein, SMV and abdominal aorta. 1B: Angiographic films showing highly vascular tumor with multiple feeding vessels and post embolization film after renal artery coiling.
Fig. 2.
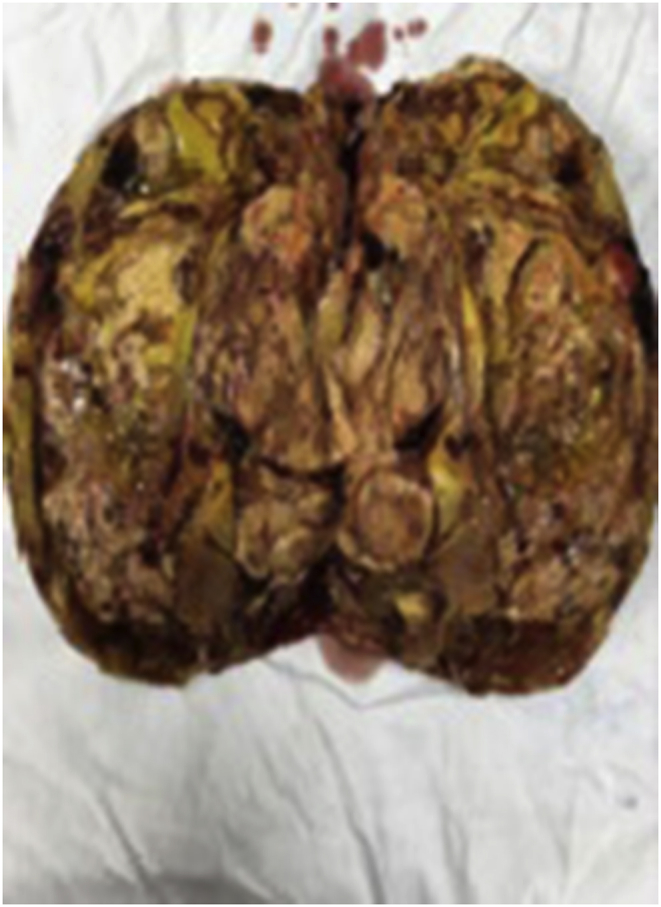
Cut section of the tumor with kidney replaced by tumor mass with variegated appearance.
Fig. 3.
(A) Microscopic picture depicting spindle shaped cells; Immunohistochemical staining EMA focal positive (B), Vimentin positive (C), and pan CK negative (D).
Table 1.
Serial liver function test of the patient.
Discussion
Nephrogenic hepatic dysfunction syndrome (Stauffer's syndrome) was considered a unique paraneoplastic manifestation of renal cell carcinoma that is usually manifested as anicteric cholestasis. This syndrome was originally described in 1961 by M. H. Stauffer, and is characterized by elevated alkaline phosphatase, erythrocyte sedimentation rate, α-2-globulin, and γ-glutamyl transferase, thrombocytosis, prolongation of prothrombin time, and hepatosplenomegaly in the absence of hepatic metastasis.1. In general this syndrome is not associated with jaundice.1,2 However, few case reports of clear cell carcinoma of kidney has presented with paraneoplastic cholestatic jaundice.3 Nevertheless, this variant is seen very rarely in soft tissue and undifferentiated renal sarcomas like in our index case. The underlying pathophysiology of Stauffer's syndrome is not fully understood. Reports have suggested the possible role of interleukin-6 over expression by the primary tumor and even liver biopsy done in such cases reveal only nonspecific hepatitis.4
Presence of jaundice with raised liver enzymes in the presence of a suspected malignancy needs through evaluation to rule out liver metastasis and look for causes for hepatic dysfunction. Work up includes excluding any obstructive cause for jaundice like lymph nodes or malignant mass5 at the porta hepatis, infectious causes like viral hepatitis A, B, C, relevant drug history, alcohol consumption. Involving a hepatologist for thorough evaluation in such cases and optimizing them prior to surgery is of paramount importance. Correction of coagulopathy as far as possible by giving vitamin K and FFP before surgery and performing surgery under adequate FFP cover is necessary to prevent bleeding complications.
The hepatic dysfunction in the form of raised liver enzymes often improves following resection of the primary tumor. The improvement in liver function is however reported in up to 70% of the patients and it might take up to 3 months following definite management of the primary tumor.1,2 Re-appearance of liver dysfunction after initial improvement is a poor prognostic sign and it warrants repeat evaluation to look for recurrence of the primary tumor. Even jaundice may improve slowly after surgical excision of the malignant mass, which was also observed in our case.
Conclusion
Our case highlights a unique and a very rare presentation of renal synovial sarcoma with cholestatic jaundice variant of Stauffer's syndrome. Paraneoplastic syndrome with cholestatic jaundice has not been reported in the primary synovial sarcoma of kidney to best of our knowledge.
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eucr.2019.100841.
Funding
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Stauffer M.H. Nephrogenic hepatomegaly. Gastroenterology. 1961;40:69. [Google Scholar]
- 2.Morla D., Alazemi S., Lichtstein D. Stauffer's Syndrome variant with cholestatic jaundice: a case report. J Gen Intern Med. 2006;21:C11–C13. doi: 10.1111/j.1525-1497.2006.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomadoni A., Garcia C., Marquez M., Ayala J.C., Prado F. Stauffer's syndrome with Jaundice, a paraneoplastic manifestation of renal cell carcinoma: a case report. Arch Esp Urol. 2010;63:154–156. [PubMed] [Google Scholar]
- 4.Blay J.Y., Rossi J.F., Wijdenes J. Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer. 1997;72:424–430. doi: 10.1002/(sici)1097-0215(19970729)72:3<424::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Karakatsanis A., Vezakis A., Fragulidis G., Staikou C., Carvounis E.E., Polydorou A. Obstructive jaundice due to ampullary metastasis of renal cell carcinoma. World J Surg Oncol. 2013;11:262. doi: 10.1186/1477-7819-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.