Abstract
A case of fulminant type 1 diabetes mellitus secondary to administration of pembrolizumab in a patient with urothelial carcinoma is presented. Eight days after the third infusion of pembrolizumab, the patient presented with complaints of malaise and anorexia. The patient's laboratory data showed a blood glucose level of 1092mg/dl with ketonuria and negative for glutamic acid decarboxylase antibody. As leaving ketoacidosis by insulin therapy, pembrolizumab therapy was continued without delay. After administration of another eight infusions of pembrolizumab, the patient's disease was stable without new severe side effects.
Keywords: Urothelial carcinoma, Pembrolizumab, Fulminant type 1 diabetes mellitus
Introduction
Pembrolizumab is an immune checkpoint inhibitors widely used for various cancers. It is an IgG4 monoclonal antibody targeting the programmed cell death-1 (PD-1) receptor. While it is effective for some patients, it may also be associated with immune-related adverse events (irAE), such as fulminant type 1 diabetes mellitus, which is rare but lifethreatening. Herein, we report a case of fulminant type 1 diabetes mellitus secondary to pembrolizumab in a patient with urothelial carcinoma, which is the first case of its kind, to our knowledge.
Case presentation
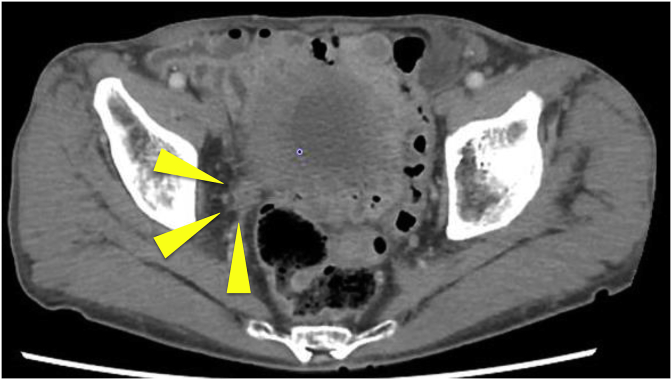
A 75-year-old Japanese man was diagnosed with right lower ureteral cancer with para-aortic lymph node metastasis above the diaphragm (Urothelial carcinoma, Grade2/high grade, cT3N2M1). The patient had no medical or family history of diabetes mellitus and previous glucose tests had been normal. Chemotherapy (Gemcitabine/Cisplatin, three weeks per cycle) was initially administered, but after eight cycles, it was discontinued because of disease progression (Fig. 1). Pembrolizumab (200mg/body) was administered every three weeks as the second line of the treatment.
Fig. 1.
Computed tomography before pembrolizumab administration shows 30 mm right lower ureteral cancer.
Eight days after the third infusion of pembrolizumab, the patient presented at our emergency room with complaints of malaise and anorexia. The patient's level of consciousness was clear. Laboratory data showed a blood glucose level of 1092mg/dl with ketonuria, pH of arterial blood gas was 7.324, bicarbonate was 21.4 mmol/L. The patient's HbA1c was 6.7% (normal range 4.6–6.2%), C-peptide level was undetectably low and glutamic acid decarboxylase (GAD) antibody was negative. Adrenocorticotropic hormone (ACTH) was normal at 13.3 pg/mL and cortisol was slightly high at 31 μg/dL. Pancreatic disease was ruled out by CT. The diagnosis was fulminant type 1 diabetes mellitus associated with pembrolizumab. The patient was managed with intravenous insulin infusion and subsequently switched to regular subcutaneous insulin infusion.
After leaving ketoacidosis, pembrolizumab was continued without delay. Further eight infusions of pembrolizumab were administered resulting in stable disease and no new severe side effects (Fig. 2). Fig. 3 shows the transition graph of HbA1c during pembrolizumab therapy.
Fig. 2.
Computed tomography after eleven cycles of pembrolizumab administration shows 17 mm right lower ureteral cancer.
Fig. 3.
Transition graphs of glucose and HbA1c during pembrolizumab therapy.
Discussion
We present a case of fulminant type 1 diabetes mellitus induced by pembrolizumab for urothelial carcinoma, characterized by a rapid onset of malaise and anorexia, with high blood glucose level and ketonuria. Type 1 diabetes mellitus is a rare irAE of the PD-1 inhibitors, which occurs in 0.2% of cases.1 There were several reports about type 1 diabetes mellitus associated with pembrolizumab in other cancer types,2 but not in urothelial carcinoma, to our knowledge.
Fulminant type 1 diabetes mellitus is a relatively new disease concept of type 1 diabetes mellitus.3 It is a syndrome characterized by extremely rapid and almost complete destruction of pancreatic β-cells. It shows a sudden onset and rapid course leading to hyperglycemia with symptoms of dry mouth, polydipsia and polyuria and ketoacidosis with symptoms of malaise, nausea and vomiting. HbA1c is characterized by a mild increase from normal, and insulin secretion is often depleted at the time of diagnosis. Generally, in fulminant type 1 diabetes mellitus, islet-specific autoantibodies, such as the GAD antibody, are negative. The early detection point for type 1 diabetes mellitus is monitoring of blood glucose and urinalysis at each hospital visit and educating the patient about the symptoms of hyperglycemia. This may diagnose it the hyperglycemia stage and avoid ketoacidosis.
Type 1 diabetes mellitus is caused by destruction of pancreatic, insulin-producing β-cells. Programmed cell death-1 ligand (PD-L1) is expressed on pancreaticβ-cells. A non-obese diabetic mice model demonstrated that the inflammation of pancreatic islet cells deteriorates as the PD-1—PD-L1 pathway gets blocked, and triggers type 1 diabetes mellitus.4 With a similar mechanism, pembrolizumab induces type 1 diabetes mellitus.
Glucocorticoids can improve many of the irAEs. First, managements of grade 3 irAEs, such as colitis and interstitial pneumonia, are immunosuppressive corticosteroids at 1–2 mg/kg/day methylprednisolone intravenously before tapering to oral prednisolone upon improvement of symptoms. However, for type 1 diabetes mellitus, glucocorticoids are not effective because 80–90% pancreaticβ-cells are irreversibly destroyed. Alsksova et al. reported that glucocorticoids administered for type 1 diabetes mellitus associated pembrolizumab in a patient with malignant melanoma resulted in worsened glucose control and no benefit was observed.5 To date as in until today, there was no consensus for the treatment of fulminant type 1 diabetes mellitus due to immune checkpoint inhibitors other than insulin therapy. As stated above, due to the specificity of the disease, when glycemic status improves, pembrolizumab can be continued. For our patient, resuming pembrolizumab did, not worsen glycemic control to a severe degree. As the number of cases is too few, there is no conclusion on the antitumor response of patients who developed type 1 diabetes mellitus.2
Conclusion
During pembrolizumab therapy, it is important to remember that fulminant type 1 diabetes mellitus is a life-threatening irAE. Simple monitoring of ketonuria and blood glucose is essential. After glycemic recuperation, pembrolizumab can be safely continued without delay.
Consent
This study was approved by the institutional review board of Kobe City Medical Center General Hospital. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Conflicts of interest
None.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors thank Dr. Koji Inoue, Dr. Yoshio Sugino, Dr. Noriyuki Makita and Dr. Shiori Murata at Kobe City Medical Center General Hospital for their kind support for the study.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- CT
Computed tomogramphy
- GAD
Gulutamic acid decarboxylase
- irAE
Immune-related event
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-1 ligand 1
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eucr.2019.100849.
Contributor Information
Yoichiro Tohi, Email: yoto716yotoyoto@gmail.com.
Kanta Fujimoto, Email: kantachi@kcho.jp.
Ryosuke Suzuki, Email: 12.ryosuke.19@gmail.com.
Issei Suzuki, Email: i-suzuki@dokkyomed.ac.jp.
Masashi Kubota, Email: kubotamasahi@gmail.com.
Mutsushi Kawakita, Email: m22k74@kcho.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Food and Drug Administration. Pembrolizumab prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf. Accessed August 18. 2018..
- 2.Clotman K., Janssens K., Specenier P. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:3144–3154. doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]
- 3.Ansari M.J., Salama A.D., Chitnis T. The programed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imagawa A., Hanafusa T., Miyagawa J. A novel subtype of diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med. 2000;342:301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 5.Aleksova J., Lau P.K., Soldatos Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-217454. bcr2016217454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.