Abstract
Trypanosoma cruzi is the causative agent of Chagas disease, a parasitic infection endemic in Latin America. Currently there are no effective treatments for the chronic phase of the disease, when most patients are diagnosed, therefore the development of new drugs is a priority area. Several triazoles, used as fungicides, exhibit trypanocidal activity both in vitro and in vivo. The mechanism of action of such drugs, both in fungi and in T. cruzi, relies in the inhibition of ergosterol biosynthesis affecting the cell viability and growth. Among them, terconazole was the first triazole antifungal drug for human use. In this work, the trypanocidal activity of terconazole was evaluated using in vitro assays. In epimastigotes of two parasites strains from different discrete typing units (Y and Dm28c) the calculated IC50 were 25.7 μM and 21.9 μM, respectively. In trypomastigotes and amastigotes (the clinically relevant life-stages of T. cruzi) a higher drug susceptibility was observed with IC50 values of 4.6 μM and 5.9 μM, respectively. Finally, the molecular docking simulations suggest that terconazole inhibits the T. cruzi cytochrome P450 14-α-demethylase, interacting in a similar way that other triazole drugs. Drug repurposing to Chagas disease treatment is one of the recommended approach according to the criterion of international health organizations for their application in neglected diseases.
Keywords: Computational biology, Microbiology
1. Introduction
Chagas disease is caused by infection with the protozoan parasite Trypanosoma cruzi (Chagas, 1909). Although it has a broad geographic distribution, it is an important cause of disability and mortality mainly in Latin America with approximately 7 million infected, more than 70 million people at risk and about 14,000 deaths per year. Endemic Chagas disease is strictly associated to poverty and socioeconomic development and as a consequence of these factors only less than 1% of infected people have access to diagnosis and current treatments (https://www.dndi.org/diseases-projects/chagas/). Concerning the available drugs to treat Chagas disease, the situation is also critical; at present there are only two drugs available, benznidazole and nifurtimox, which were developed more than 50 years ago. These drugs, in addition to present severe side effects, are not efficient in the chronic phase of the disease when most patients are diagnosed, highlighting the urgent need for the development of new treatments (Perez-Molina and Molina, 2018).
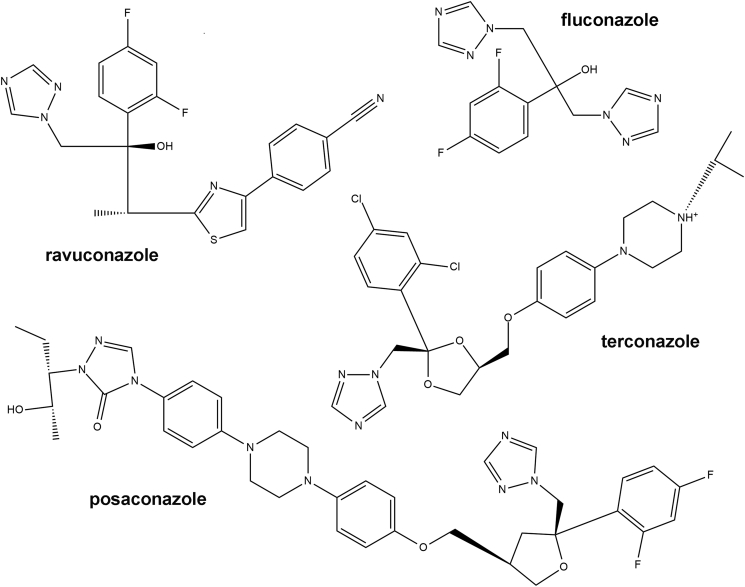
In contrast to mammalian cells, trypanosomatids synthesize mostly ergosterol instead of cholesterol, resembling the fungi sterol metabolism. In fungal cells, disruption of ergosterol biosynthesis alters the membrane structure and function producing an inhibition of cell growth. Azole-based drugs have been used as antifungal agents since the 1980s (Ghannoum and Rice, 1999). The mechanism of action of such compounds is mediated by the inhibition of the cytochrome P450 14-α-demethylase (CYP51) which produces a critical decrease in ergosterol concentration. In T. cruzi azoles have been successfully tested as trypanocidal compounds in the last decades (Buckner, 2008) demonstrating that ergosterol biosynthesis pathway is an interesting target for the development of new drugs for Chagas disease treatment. Recently, many triazole derivatives, including posaconazole and ravuconazole (Fig. 1) were tested in clinical trials for the treatment of chronic Chagas disease (Urbina, 2009).
Fig. 1.
Chemical structure of triazole derivatives. Structures of four antifungal drugs with trypanocidal activity, including terconazole.
Terconazole, a synthetic triazole derivative structurally related to fluconazole (Fig. 1), is an antifungal medication primarily used to treat vaginal fungal infections which also acts inhibiting de novo sterol biosynthesis through CYP51. In this work the trypanocidal activity of terconazole was evaluated in different stages of the T. cruzi life cycle using parasites from two discrete typing units (DTU) and the mechanism of action was predicted by molecular docking.
2. Materials and methods
2.1. Parasites and cells
Epimastigotes (5 × 106 cells/mL) of the Y and Dm28c strains (DTU II and I, respectively) were cultured at 28 °C in plastic flasks (25 cm2), containing 5 mL of BHT (brain-heart infusion-tryptose) medium supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin and 20 μg/mL hemin. Vero cells (African green monkey kidney) and L929 cells (murine fibroblasts) were cultured in MEM medium supplemented with 10% heat inactivated FCS, 0.15% (w/v) NaHCO3, 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in 5% CO2. Trypomastigotes and amastigotes of the Y strain were obtained from Vero infected cells as previously described (Andrews and Colli, 1982).
2.2. Trypanocidal activity assays
T. cruzi epimastigotes (Y and Dm28c strains) were cultured as described above, in 24-wells plate at a start density of 1 × 107 cells/mL in BHT medium. Parasites were treated with different concentrations of terconazole and the control drug benznidazole, and parasite proliferation was determined after 48 h. The drugs were dissolved in dimethyl sulfoxide (DMSO). Trypanocidal activity in trypomastigotes and amastigotes (Y strain) was performed using 1 × 106 cells/mL in 96-well plates and incubating at 37 °C for 24 h in the presence of the corresponding drug. Growth was determined by counting in a Neubauer chamber or by viability assays using “Cell Titer 96® Aqueous One Solution Cell Proliferation Assay (MTS)” (Promega, Madison, WI, USA) according to the manufacturer's instructions.
2.3. Cell viability assay
Cytotoxicity against L929 cells was determined by the crystal violet staining assay. The cells (104 cells/well) were incubated in 96-well plates with the corresponding concentrations of terconazole or DMSO only as negative control and maintained at 37 °C in a 5% CO2 atmosphere for 24 h. At the end of treatment, cells were fixed for 15 min, and stained with 0.5 % crystal violet. After washing with water and drying, the absorbance of stained cells was measured at 570 nm.
2.4. Docking simulations and structure analysis
The x-ray structure of T. cruzi sterol 14 α-demethylase cytochrome P450 (CYP51) was obtained at the Protein Data Bank (https://www.rcsb.org/; PDB ID: 2WX2) (Reigada et al., 2017) and the software AutoDock 4.2.6 (Morris et al., 2009) was employed for docking simulations. A grid that includes the heme group that binds to the antifungal azoles such as fluconazole, and the adjacent residues were used to calculate the optimal energy conformations for the binding of terconazole to CYP51. The program was run using a Lamarckian Genetic Algorithm 100 times, with a population size of 300, and 2.7 × 104 as maximum number of generations.
Shared chemical features between structures were identified by feature-based structure alignments using the LigandScout algorithm (Wolber and Langer, 2005).
2.5. Statistics and data analysis
IC50 values were obtained by non-linear regression of dose-response logistic functions, using GraphPad Prism 6.01 for Windows. All experiments were performed in triplicate and the data are presented as mean ± standard deviation (SD).
3. Results
3.1. Effect of terconazole on epimastigotes
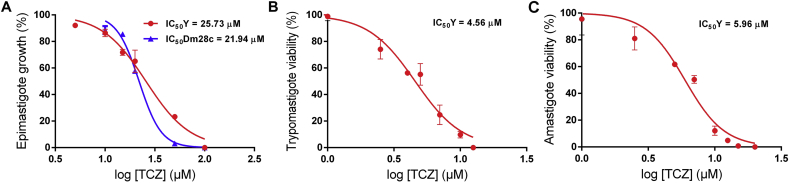
First, the effect of terconazole was evaluated on epimastigotes, the insect stage of T. cruzi, of the Y strain (DTU II). Epimastigotes were treated with the drug in concentrations between 0 and 100 μM and the trypanocidal effect was determined 48 h post treatment. The calculated IC50 (the concentration that kills 50% of the parasites) was 25.73 μM (±1.27). The reference drug, benznidazole, exhibited similar activity against this strain with an IC50 of 22.01 μM (±2.77). Considering that the DTU I is the most relevant DTU, in terms of geographical distribution, in South America, epimastigotes of the Dm28c strain were also tested. Similar results were obtained for terconazole with an IC50 value of 21.94 μM (±1.53), suggesting that terconazole is effective regardless of the DTU (Fig. 2A). On the contrary, the calculated IC50 for benznidazole was 1.8-fold higher for Dm28c epimastigotes, with a value of 38.55 μM (±1.76).
Fig. 2.
Effect of terconazole on epimastigotes, trypomastigotes and amastigotes of T. cruzi. Parasites were treated with terconazole in concentrations between 0 - 100 μM for 48 h (A) or 0–20 μM for 24 h (B and C). The concentrations required to inhibit 50% of parasite growth or parasite survival were calculated using epimastigotes of the Y (red line) and Dm28c (blue line) strains (A), trypomastigotes (B) and amastigotes (C) of the Y strain. The data is expressed as the mean ± standard deviation of three independent experiments.
3.2. Effect of terconazole on trypomastigotes and amastigotes
Terconazole activity was also evaluated against the mammalian life-stages of T. cruzi and the IC50 (concentration that decrease the viability of parasites in a 50%) were calculated 24 h post treatment. The drug presented trypanocidal activity against both parasitic forms, trypomastigotes and amastigotes, with calculated IC50 values of 4.56 μM (±0.32) and 5.96 μM (±0.35), respectively, as shown in Figs. 2B and 2C. Interestingly, these results demonstrate that trypomastigotes and amastigotes are 5.6 and 4.3-fold more susceptible to terconazole treatment than the epimastigote stage, respectively. Moreover, terconazole was found to be more effective than the control drug benznidazole against trypomastigotes and amastigotes. The calculated IC50 values of benznidazole were 12.71 μM (±1.53) for trypomastigotes and 11.4 μM (±0.5) for amastigotes.
The in vitro cytotoxic effect of terconazole was determined on mammalian cells (L929 line) and the IC50 value was obtained to calculate the selectivity index (SI) according to formula SI = IC50 against L929 cells/IC50 against tripomastigotes. Cells exposed to the drug for 24 h in a concentration range from 0 to 80 μM showed a SI of 5.1 (IC50 23.38μM ± 3.76).
3.3. Predicted mechanism of action of terconazole
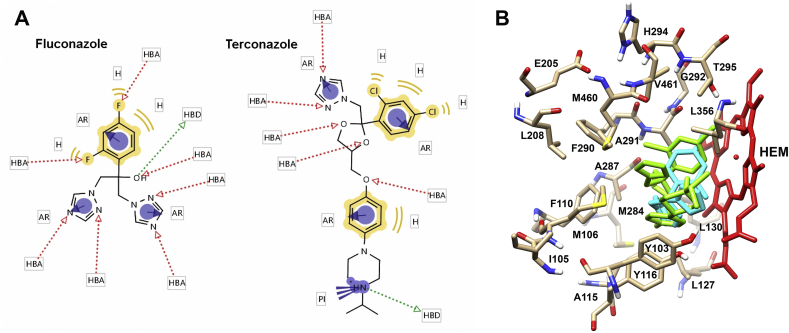
In order to predict if terconazole has similar properties to those experimentally determined for fluconazole, the common chemical reactive groups and the binding parameters to the protein CYP51 were calculated. To identify the common chemical features (groups that can participate in chemical interactions with a macromolecule) between terconazole and fluconazole the LigandScout algorithm was used. Feature-based structure alignments were performed and the similarities were calculated as the number of matched feature pairs (MFP; i.e. aromatic ring, hydrophobic area, hydrogen bond donor or acceptor, negative or positive ionizable atom and metal binding location). For these comparisons the 14 features of fluconazole were set as references and results showed that terconazole share all of them including 5 hydrogen bond acceptors, 1 hydrogen bond donor, 3 aromatic rings, 4 hydrophobic interactions and 1 positive ionizable group. Alignment results are schematized in Fig. 3A.
Fig. 3.
In silico predictions of the mechanism of action of terconazole. A) Reactive groups of terconazole and fluconazole. The LigandScout software was used to identify the common chemical features between fluconazole and terconazole. AR, aromatic ring (purple circles); H, hydrophobic area (yellow remarks); PI, positive ionizable atom (purple lines); HBA, hydrogen bond acceptor (red arrow); and HBD, hydrogen bond donor (green arrow). B) Molecular docking. Docking simulations were performed using the software Autodock 4.2.6 and the x-ray structure of the T. cruzi CYP51 (PDB ID: 2WX2). Residues and the heme group (HEM, red) corresponding to the fluconazole (green) and terconazole (light blue) binding sites in CYP51 are indicated.
Then, molecular docking simulations were performed in order to predict the interactions between terconazole and CYP51. In the reported x-ray structure of CYP51 from T. cruzi in complex with fluconazole (PDB ID: 2WX2), the ligand binds in the active site by coordination to the heme iron via the aromatic nitrogen atom of a triazole ring, by van der Waals and aromatic interactions. In addition, twenty residues were located within 7 A° of fluconazole (Y103, I105, M106, F110, A115, Y116, L127, L130, E205, L208, M284, A287, F290, A291, G292, H294, T295, L356, M460, and V461) (Chen et al., 2010). According to the molecular docking simulation, all of them, except I105 and E205 are in a similar position within CYP51 at the same distance from terconazole (Fig. 3B).
4. Discussion
Azoles were extensively tested as trypanocidal agents (Lepesheva et al., 2018). The first report for their use in T. cruzi included miconazole and econazole (Docampo et al., 1981). Some years later it was reported that ketoconazole has trypanocidal effect in a murine model of Chagas disease (McCabe et al., 1983). It was found that itraconazole decreases parasitemia to undetectable levels in murine models of T. cruzi infection (McCabe et al., 1986). Most recently, the results of the trial CHAGASAZOL, performed with chronic chagasic patients treated with posaconazole, were partially unsatisfactory because of the high rates of treatment failure (Molina et al., 2014). Additionally, the clinical trial performed using a prodrug of ravuconazole (E1224) resulted in a low success rate (Torrico et al., 2018). However, other studies revealed that the azole drugs called VNI, VFV and VT-1161 are highly efficient and selective to eradicate T. cruzi in murine models of Chagas disease (Guedes-da-Silva et al., 2017; Hoekstra et al., 2016; Lepesheva et al., 2015; Villalta et al., 2013). Similarly, two inhibitors of the last enzyme of the ergosterol synthesis, Δ24(25) sterol methyl transferase (24-SMT), 22,26-azasterol (AZA) and 24 (R,S),25-epiminolanosterol (EIL), inhibit the proliferation of T. cruzi epimastigotes and amastigotes and these effects are potentiated by CYP51 inhibitors. Synergic effects of AZA were also observed in a murine model of acute Chagas disease (Urbina et al., 1996). In addition, Braga et al. (2005) observed that other 24-SMT inhibitors (WSP488, WSP501 and WSP561) have antiproliferative effects on T. cruzi epimastigotes and induce marked ultrastructural changes in the cells.
Terconazole is an antifungal drug used to treat vaginal yeast infection, available as creams or suppositories. Terconazole was first described in the 1980s as a highly-active antifungal in dermatophytosis and candidiasis topical treatments and also possesses a moderate oral broad-spectrum activity tested in guinea pigs, rats, mice and turkeys (Van Cutsem et al., 1983). Here we demonstrate that terconazole has trypanocidal activity in epimastigotes from different DTUs and also in the clinically relevant life-stages of T. cruzi, trypomastigotes and amastigotes, with IC50 values similar to those calculated for other azoles such as posaconazole (IC50: 5.4 μM) (Botero et al., 2017). Furthermore, in this study we show that terconazole is more potent against trypomastigotes and amastigotes than the reference drug benznidazole not only according to the IC50 values obtained in this work but also to those previously reported by other authors (Moreno et al., 2010).
Related to the predicted mechanism of action, the molecular docking simulations suggest that terconazole inhibits the sterol biosynthesis by binding the T. cruzi cytochrome P450 14-α-demethylase in a very similar way that other active azoles such as fluconazole.
These results are relevant since terconazole began to be used almost 40 years ago, but unlike other fungicides, its trypanocidal activity was never studied in detail.
Because terconazole is used only as a vaginal cream (0.4% and 0.8%) or suppositories, there are very few reports on its oral toxicity. According to the DrugBank (https://www.drugbank.ca/) the oral lethal dose 50% (LD50) values were found to be up to 1700 mg/kg in rat studies (https://www.drugbank.ca/drugs/DB00251). This value is in accordance to that reported for posaconazole, with a LD50 up to 2700 mg/kg in rats (Cerilliant®, Sigma-Aldrich safety data sheet). During the clinical trials, some patients received posaconazole up to 1600 mg/day with similar adverse effects than the lower doses (https://www.drugbank.ca/drugs/DB01263). All these data allow us to suppose that, in case of being efficient as a trypanocidal agent in infected mice, terconazole could be administered orally for the treatment of Chagas disease.
Declarations
Author contribution statement
Chantal Reigada: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Melisa Sayé, Edward Valera-Vera, Mariana R. Miranda: Performed the experiments; Analyzed and interpreted the data.
Claudio A. Pereira: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT). Grants: FONCYT PICT 2013-2218 and 2015-0539. Global Challenges Research Fund (GCRF). Grant: MR/P027989/1. Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Researchers salaries and fellowships: Claudio A. Pereira, Mariana R. Miranda, Melisa Sayé and Edward Valera-Vera.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Special thanks to Lic. Fabio di Girolamo (IDIM-CONICET) for technical support.
References
- Andrews N.W., Colli W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J. Protozool. 1982;29:264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- Botero A., Keatley S., Peacock C., Thompson R.C. In vitro drug susceptibility of two strains of the wildlife trypanosome, Trypanosoma copemani: a comparison with Trypanosoma cruzi. Int. J. Parasitol Drugs Drug Resist. 2017;7:34–41. doi: 10.1016/j.ijpddr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga M.V., Magaraci F., Lorente S.O., Gilbert I., de Souza W. Effects of inhibitors of Delta24(25)-sterol methyl transferase on the ultrastructure of epimastigotes of Trypanosoma cruzi. Microsc. Microanal. 2005;11:506–515. doi: 10.1017/S143192760505035X. [DOI] [PubMed] [Google Scholar]
- Buckner F.S. Sterol 14-demethylase inhibitors for Trypanosoma cruzi infections. Adv. Exp. Med. Biol. 2008;625:61–80. doi: 10.1007/978-0-387-77570-8_6. [DOI] [PubMed] [Google Scholar]
- Chagas C. Nova Tripanosomiaze Humana: estudos sobre amorfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de uma nova entidade mórbida do homem. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Chen C.K., Leung S.S., Guilbert C., Jacobson M.P., McKerrow J.H., Podust L.M. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Neglected Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., Moreno S.N., Turrens J.F., Katzin A.M., Gonzalez-Cappa S.M., Stoppani A.O. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1981;3:169–180. doi: 10.1016/0166-6851(81)90047-5. [DOI] [PubMed] [Google Scholar]
- Ghannoum M.A., Rice L.B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes-da-Silva F.H., Batista D.G., Da Silva C.F., De Araujo J.S., Pavao B.P., Simoes-Silva M.R., Batista M.M., Demarque K.C., Moreira O.C., Britto C., Lepesheva G.I., Soeiro M.N. Antitrypanosomal activity of sterol 14alpha-demethylase (CYP51) inhibitors VNI and VFV in the Swiss mouse models of Chagas disease induced by the trypanosoma cruzi Y strain. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra W.J., Hargrove T.Y., Wawrzak Z., da Gama Jaen Batista D., da Silva C.F., Nefertiti A.S., Rachakonda G., Schotzinger R.J., Villalta F., Soeiro Mde N., Lepesheva G.I. Clinical candidate VT-1161's antiparasitic effect in vitro, activity in a murine model of Chagas disease, and structural characterization in complex with the target enzyme CYP51 from trypanosoma cruzi. Antimicrob. Agents Chemother. 2016;60:1058–1066. doi: 10.1128/AAC.02287-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva G.I., Friggeri L., Waterman M.R. CYP51 as drug targets for fungi and protozoan parasites: past, present and future. Parasitology. 2018;145:1820–1836. doi: 10.1017/S0031182018000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva G.I., Hargrove T.Y., Rachakonda G., Wawrzak Z., Pomel S., Cojean S., Nde P.N., Nes W.D., Locuson C.W., Calcutt M.W., Waterman M.R., Daniels J.S., Loiseau P.M., Villalta F. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. J. Infect. Dis. 2015;212:1439–1448. doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R.E., Araujo F.G., Remington J.S. Ketoconazole protects against infection with Trypanosoma cruzi in a murine model. Am. J. Trop. Med. Hyg. 1983;32:960–962. doi: 10.4269/ajtmh.1983.32.960. [DOI] [PubMed] [Google Scholar]
- McCabe R.E., Remington J.S., Araujo F.G. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 1986;35:280–284. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- Molina I., Gomez i Prat J., Salvador F., Trevino B., Sulleiro E., Serre N., Pou D., Roure S., Cabezos J., Valerio L., Blanco-Grau A., Sanchez-Montalva A., Vidal X., Pahissa A. Randomized trial of posaconazole and benznidazole for chronic Chagas' disease. N. Engl. J. Med. 2014;370:1899–1908. doi: 10.1056/NEJMoa1313122. [DOI] [PubMed] [Google Scholar]
- Moreno M., D'Avila D.,A., Silva M.N., Galvao L.M., Macedo A.M., Chiari E., Gontijo E.D., Zingales B. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Mem. Inst. Oswaldo Cruz. 2010;105:918–924. doi: 10.1590/s0074-02762010000700014. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Reigada C., Valera-Vera E.A., Saye M., Errasti A.E., Avila C.C., Miranda M.R., Pereira C.A. Trypanocidal effect of isotretinoin through the inhibition of polyamine and amino acid transporters in trypanosoma cruzi. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrico F., Gascon J., Ortiz L., Alonso-Vega C., Pinazo M.J., Schijman A., Almeida I.C., Alves F., Strub-Wourgaft N., Ribeiro I. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018;18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina J.A. Ergosterol biosynthesis and drug development for Chagas disease. Mem. Inst. Oswaldo Cruz. 2009;104(Suppl 1):311–318. doi: 10.1590/s0074-02762009000900041. [DOI] [PubMed] [Google Scholar]
- Urbina J.A., Vivas J., Lazardi K., Molina J., Payares G., Piras M.M., Piras R. Antiproliferative effects of delta 24(25) sterol methyl transferase inhibitors on Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Chemotherapy. 1996;42:294–307. doi: 10.1159/000239458. [DOI] [PubMed] [Google Scholar]
- Van Cutsem J., Van Gerven F., Zaman R., Janssen P.A. Terconazole - a new broad-spectrum antifungal. Chemotherapy. 1983;29:322–331. doi: 10.1159/000238215. [DOI] [PubMed] [Google Scholar]
- Villalta F., Dobish M.C., Nde P.N., Kleshchenko Y.Y., Hargrove T.Y., Johnson C.A., Waterman M.R., Johnston J.N., Lepesheva G.I. VNI cures acute and chronic experimental Chagas disease. J. Infect. Dis. 2013;208:504–511. doi: 10.1093/infdis/jit042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber G., Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]