Abstract
Prostate cancer is the second common etiology of cord compression after lung cancer. Its slow natural history justifies an aggressive treatment. The fact that the metastatic lesion precedes the primary tumor remains rare.
We report the case of a 86 year-old man who was admitted for heaviness of both lower limbs responsible for gait disorder. He had flaccid paraplegia. Spinal MRI showed an epidural lesion. Histology after surgery was compatible for a metastasis of prostatic adenocarcinoma.
Spinal cord compression due to prostate cancer is correlated with poor prognosis. The fact that the metastatic lesion precedes the primary tumor remains rare.
Keywords: Brain, Metastasis, Prostatic carcinoma
Introduction
Spinal cord compression is an important complication of metastatic tumours which is associated with gloomy prognosis. Prostate cancer is the second most common etiology of cord compression in men affecting l to 12% of all patients with between one-quarter and one-third of cases developing cord compression as a presenting feature. The slow natural history of prostate cancer justifies an extreme vigilance and an aggressive treatment in order to preserve neurologic abilities, to decrease pain, and to improve lifes’ quality. However little has changed by the time in term of neurologic recovery in spite of the improvement in imaging techniques.
Here we report the case of prostatic adenocarcinoma revealed by dorsal sipnal cord compression. The optimal method for the treatment of such metastasis will be discussed in this case report.
Case report
It is about a 86 year-old man with no particular medical or urological history who was admitted for rapid onset over 15–20 days of heaviness of both lower limbs responsible for gait disorder associated to intensive and embarrassing dorsal back pain leading to insomnia without sphincterian or sexual trouble. The whole symptomatology evolved in a context of apyrexia. On neurological examination, the patient had flaccid paraplegia with abolition of osteotendinous reflexes in both lower limbs associated to a left crural hypoesthesia and a sensory level at the 4th dorsal vertebra. An emergency spinal MRI was performed showing an epidural tissue process that engages and compresses the medullary cord and which is predominant on the postero-lateral side and extended over 50 mm. This process is accompagned by a medullary remplacement lesion of the body and te posterior arch of the 4th dorsal vertebra and without bone lysis, in hypo signal on T1 and T2 weighted images with moderately enhancement after Gadolinium injection (Fig. 1). The patient underwent an emergency laminectomy of the third and the 4th dorsal vertebra with total resection of the extra dural hemorragic lesion having a friable consistency with obvious healthy boundaries. The histopathological examination objectivized an invasive carcinomatous proliferation arranged in cribriform massifs and made of cubic carcinomatous cells with abundant cytoplasm and a strongly nucleated voluminous nucleus. The tumor dissociates mature bone spans. Mitoses are rare (Fig. 2). Immunohistochemistry was positive for prostatic spesifc antigen (PSA) and negative for keratine 7 and keratine 20 (Fig. 3). This aspect is compatible for a metastasis of prostatic adenocarcinoma.
Fig. 1.
Sagittal and axial spinal MRI showing an epidural tissue process (blue arrow) that engages and compresses the medullary cord and which is predominant on the postero-lateral side and extended over 50 mm. This process is accompagned by a medullary remplacement lesion of the body and te posterior arch of the 4th dorsal vertebra, in hypo signal on T1 and T2 weighted images with moderately enhancement after Gadolinium injection and without bone lysis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Histopathlogical section showing invasive carcinomatous proliferation arranged in cribriform massifs and made of cubic carcinomatous cells with abundant cytoplasm and a strongly nucleated voluminous nucleus. The tumor dissociates mature bone spans.
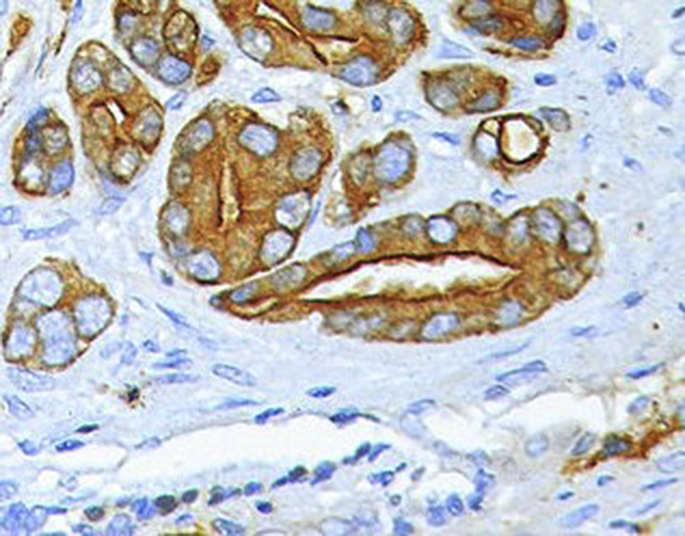
Fig. 3.
Immunohistochemistry section showing positivity for prostatic spesifc antigen (PSA).
Discussion
Spinal cord compression (SCC) is a common neurologic complication of advanced malignancies and must be considered as an oncologic emergency. Prostate cancer is the second cause of metastatic spinal cord compression in men. Its frequency is variable and 1–12% of prostate cancer patients will develop this SCC.1 The local extension of a vertebral metastasis leads to the spinal cord involvement. The time between the first neurological symptoms due to reversible edema and neurological troubles vary from days to months and representes a window of opportunity which should not be missed.2 Spinal back pain is the most common symptom occurring in 75–100% of the patients, usually progressive within days to weeks and even months, and it can be focal or radicular [2]. In prostate carcinoma patients, the lumbar spine is the most common site of initial metastasis unlike our case which was in the dorsal level. As a result, patients with lower back pain, but with a known history of prostate carcinoma. In our case we didn't have any history of prostatic illness despite the advanced age of our patient. Normal neurologic examination doesn't eliminate the possibility of spinal cord compression. Rodichok reported that 36% of patients who presented with back pain with normal neurologic findings, had epidural metastases on myelography.3 Most patients suffered from muscular weakness which is usually bilateral and touches both lower limbs. The degree of this disability is associated with the probability of success in preserving ambulation and represent so far a useful prognostic tool.2 Full spine standard radiography helps in the diagnostic of suspected spinal lesions. It may correctly predict the presence or absence of SCC in over 80% of symptomatic patients.3 Spine CT-scan provides good anatomy bony study and can't only determine the extent of bony destruction, but also evaluates the quality of bones above. Besides it explores a limited area in the spine so that imaging of the full spine is indicated in order to detect the compression at sites distant from the symptomatic lesion.2 MRI has the almost the same specificity and sensitivity for extradural masses as myelography.4 Moreover it has it has more increased sensitivity for diagnosing intramedullary lesions, paravertebral masses and even bone involvement. Pain relief and prevention of neurological deterioration are two important aspects to consider. Corticotherapy is usually instituted after the diagnosis. Greenberg noted that administration of high dose of dexamethasone had rapide and complete relief of pain before being irradiated. Immediate surgical decompression should be indicated in patients with an expected life span of at least 6 months who are worsening during radiation, who have had previous radiation in the involved site or who had spine compression of unknown histology.2 Radiotherapy should be the first mode of treatment, except in a rapidly progressive neurologic palsy secondary to bony compression from a collapsed vertebral body and severe mechanical pain secondary to bony destruction. Bisphosphonates have been used in patients with bone involvement. They may inhibit osteoclast activity, leading to a decrease in the formation of lytic bony lesions. Third generation biphosphonate like zoledronic acid, administered intravenously every three weeks reduced skeletal-related events compared to placebo according to a study conducted by Sâad.5
Conclusion
Spinal cord compression due to prostate cancer is correlated with destructive morbidity and poor prognosis. The fact that the metastatic lesion precedes the primary tumor remains rare. Combined laminectomy and radiotherapy are associated with improved neurological dysfunction and longer survival. Improvement in diagnostic and imaging techniques had little impact on functional outcome and neurologic recovery after treatment.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eucr.2019.100863.
Contributor Information
Mehdi Borni, Email: borni.mehdi13@gmail.com.
Brahim Kammoun, Email: kammoun.brahim28@gmail.com.
Fatma Kolsi, Email: kolsineifar.fatma@gmail.com.
Mohamed Zaher Boudawara, Email: zaher.boudawara@rns.tn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gilbert R.W., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 2.Flynn D.F., Shipley W.U. Management of spinal cord compression secondary to metastatic prostatic carcinoma. Urol Clin. 1991;18:145–152. [PubMed] [Google Scholar]
- 3.Rodichok L.D., Harper G.R., Ruckdeschel J.C. Early diagnosis of spinal epidural metastases. Am J Med. 1981;70:1181–1188. doi: 10.1016/0002-9343(81)90825-1. [DOI] [PubMed] [Google Scholar]
- 4.Carmody R.F., Yang P.J., Seeley G.W., Seeger J.F., Unger E.C., Johnson J.E. Spinal cord compression due to metastatic disease: diagnosis with MR imaging versus myelography. Radiology. 1989;173:225–229. doi: 10.1148/radiology.173.1.2675185. [DOI] [PubMed] [Google Scholar]
- 5.Saad F., Gleason D.M., Murray R. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.