Abstract
The recent discovery of minerals with metal-organic framework (MOF) structures has challenged the view of MOFs as purely synthetic materials. At the same time, the application of geo-inspired synthetic methodologies, such as accelerated ageing and pseudomorphic replication, has enabled a cleaner, more environmentally friendly synthesis of MOFs from mineral-like feedstocks, as well as the assembly of materials with structure controlled at both micro- and meso-scales. These almost concomitant developments have highlighted the previously unknown relationships between geology and MOF chemistry. Here, we outline examples of MOF structures found in minerals, and note geologically inspired approaches to MOF synthesis, as a means to highlight how the emergent geomimetic concepts in MOF chemistry can lead to advances in the design and synthesis of MOFs.
This article is part of the theme issue ‘Mineralomimesis: natural and synthetic frameworks in science and technology’.
Keywords: metal-organic frameworks, organic minerals, geomimetic, geochemistry, mechanochemistry, coordination polymers
1. Introduction
Metal-organic frameworks (MOFs) [1], advanced functional materials whose design is based on the regular and controllable assembly of structurally rigid molecular building blocks through coordination bonds, are the culmination of many decades of investigation on how to deploy metal–ligand bonds for the design and synthesis of extended solid-state architectures. After almost 30 years of developments [2], MOFs have now been demonstrated as unique designer materials, capable of combining and improving a number of functional properties, including record microporosity [3], efficient and selective catalysis [4], proton conductivity [5] and more [6–8]. As a result, MOFs have also recently entered commercialization [9], with reported commercial applications focusing on the storage, transport or delivery of small molecules [10]. The node-and-linker design of MOFs [11,12] and crystal engineering in general [13,14] have often taken inspiration from network structures found in minerals and other inorganic substances, as illustrated by mineralomimetic approaches [15,16] to design artificial metal cyanide frameworks. However, throughout their development MOFs have been considered as exclusively artificial materials, without direct naturally occurring metal-organic analogues. Very recently, the structural characterization of long-known [17] organic minerals [18] stepanovite and zhemchuzhnikovite [19,20], and the discovery of previously not known triazolite [21] and chanabayaite minerals [22], have revealed the natural appearance of two- and three-dimensional (2D and 3D) MOF structures [1,23,24]. Importantly, whereas some of the naturally occurring MOF minerals represent direct structural and chemical analogues of previously synthesized materials, others are structurally novel, suggesting intriguing opportunities for future materials design.
The discovery of MOFs as naturally occurring minerals has coincided with the recent emergence of synthetic approaches for MOFs that are inspired by geological processes and which seek to achieve cleaner, more efficient synthesis of MOFs starting from the simplest, and least expensive starting materials [25]. Specifically, such processes of mineral replacement [26], accelerated ageing [27] and mechanochemical synthesis [28] all focus on geologically relevant solvent-free processes and the use of mineral-like starting materials, notably metal oxides or carbonates, as safe, inexpensive MOF precursors [29].
We believe that the recent emergence of MOF structures in naturally occurring minerals, and growth of interest in geologically inspired syntheses of MOFs all highlight a strong and previously not noted connection between geology and MOF chemistry. Consequently, the aim of this brief review is to outline some of these newly discovered conceptual and practical links between MOF chemistry and geology, while highlighting the emergent opportunities for the design of new materials and for cleaner synthesis.
2. Metal-organic frameworks as minerals
(a). Metal oxalate frameworks: zhemchuzhnikovite and stepanovite
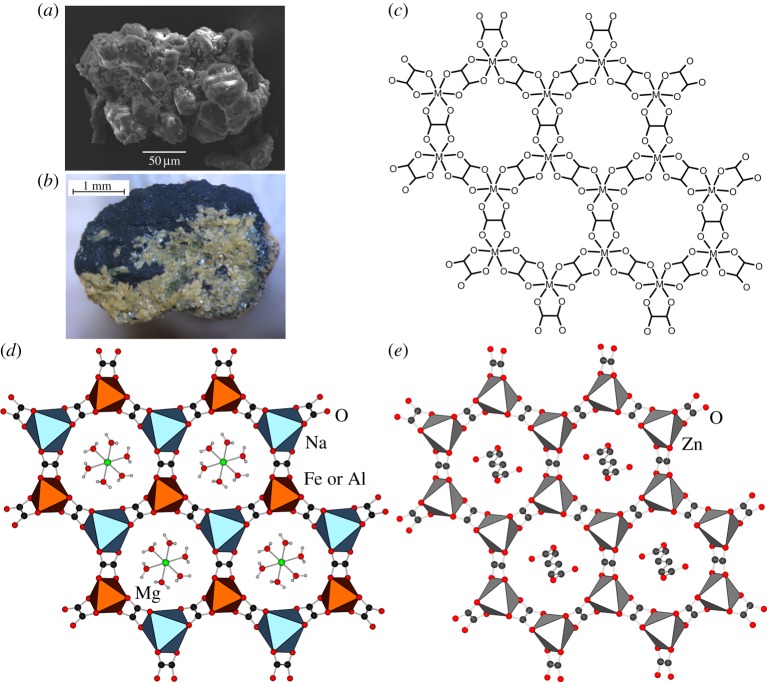
The existence of minerals with MOF structures was first described in 2016, driven by the analogy in chemical composition between a set of organic minerals first discovered in Siberia in the 1950s and 1960s (figure 1a,b), and layered MOF materials first developed for their magnetic properties by the Decurtins group in the 1990s [30]. In particular, the Decurtins group has demonstrated the formation of either 2D or 3D metal-organic architectures by combining oxalate anions (ox2−) with mono- (e.g. Li+, Na+) and trivalent cations (e.g. Cr3+). In the structures involving Na+ as the monovalent cation, the cations adopt an octahedral coordination geometry and assemble through bridging ox2− ligands into anionic open networks of (6,3)-topology (also knowns as honeycomb, or hcb, topology) (figure 1c) [30]. The negative charge of the hcb-networks is compensated through divalent counterions, resulting in an overall composition M1M2M3(ox)3, where M1, M2 and M3 represent mono-, di- and trivalent metal cations, respectively. Because an analogous composition was reported for the organic minerals stepanovite and zhemchuzhnikovite, in 2015 our team proposed [33] that these long known but never structurally characterized minerals could be based on open anionic MOF structures, a feature previously unreported in minerals. Specifically, Knipovich and co-workers have established that the composition of stepanovite was NaMgFe(ox)3 · nH2O (where n = 8 or 9), which would be consistent with the formation of a hcb-topology net composed on Na+, Fe3+ and ox2−, with Mg2+ as counterions. By analogy, we also speculated that zhemchuzhnikovite, reported with the composition NaMgFexAl1−x(ox)3 · nH2O (with n = 8 or 9), would be composed of anionic hcb-networks containing Na+ in combination with Fe3+ and Al3+ nodes. Indeed, these expectations were confirmed by single crystal X-ray diffraction experiments on synthetic samples of stepanovite and zhemchuzhnikovite synthesized in our laboratory [19,33], as well as on specimens of the two minerals obtained from the collection of Nefedov (figure 1a,b). Independently, the structure of synthetic zhemchuzhnikovite was also determined by Piro et al. [20]. Both minerals exhibit the anticipated open hcb-topology layers composed of Na+ and Fe3+ (and Al3+) nodes bridged by oxalate ions, with hydrated counterions situated in the cavities of each framework (figure 1d). The hcb-layers are separated by layers of ordered water molecules that are connected to neighbouring framework layers and ions through 1D (in zhemchuzhnikovite) or 3D (in stepanovite) network of hydrogen bonds.
Figure 1.
(a) Electron microscopy image of natural zhemchuzhnikovite [19]; (b) optical picture of natural stepanovite [19]; (c) schematic of a single hcb-topology layer in materials designed by Descurtins group [30]; (d) a single hcb-layer in structure of either zhemchuzhnikovite or stepanovite and (e) a single hcb-layer in a proton-conductive zinc oxalate MOF designed by the Kitagawa group [32,31]. (a,b) Adapted with permission from [19]. (Online version in colour.)
Whereas neither zhemchuzhnikovite nor stepanovite exhibit permanent porosity, which is often associated with MOFs, their structures nevertheless demonstrate the ability of forming open metal-organic structures with cavities of almost 1 nm diameter under geological conditions.
The major difference in the structures of the two minerals is in the mutual arrangement of neighbouring hcb-sheets. Whereas the sheets in the case of zhemchuzhnikovite arrange in the repeating AB fashion, the structure of stepanovite is more complex. In particular, the arrangement of hcb-layers of stepanovite can best be described as ABCA′B′C′-type stacking, where layers A′, B′ and C′ are related to their counterparts A, B and C, respectively, via crystallographic symmetry. Such complex arrangement leads to an unusually large crystallographic c-axis for stepanovite (ca 37 Å) compared with zhemchuzhnikovite (12.6 Å). Importantly, the stacking of hcb-layers in zhemchuzhnikovite leads to the formation of channels along the crystallographic c-axis, occupied by chains of hydrogen-bonded ions and water molecules. Such stacking of hcb-layers is analogous to the arrangement of sheets in analogous zinc-based oxalate MOFs developed by the Kitagawa group (figure 1e) [33,32], reported to exhibit very high proton conductivities in excess of 1 mS cm−1. It is, therefore, conceivable that both zhemchuzhnikovite as well as stepanovite could exhibit proton-conducting properties. Consequently, if structural analyses of the two minerals could have been accomplished at the time of their discovery, it is likely they could have provided early blueprints for MOFs and potentially even proton conductors.
(b). Carbon mineral challenge: inspiration for the discovery of metal-organic framework minerals
Stepanovite and zhemchuzhnikovite are scarce materials, belonging to a small group of organic minerals, i.e. mineral species that are based on carbon but are not carbonates of hydrogencarbonates. Interest in organic minerals has very recently been stimulated by the Carbon Mineral Challenge [34], an initiative directed towards the discovery of new carbon-containing minerals. The Carbon Mineral Challenge has been inspired by the need to improve the general understanding of the carbon cycle and the geological evolution of carbon minerals. Specifically, statistical models developed by Hazen et al. point to a significant gap in the number of carbon-containing minerals identified to date, and indicate that at least 135 carbon-containing minerals remain undiscovered as of 2015 [35]. Since that time, the Carbon Mineral Challenge has highlighted the discovery of at least 23 new carbon-containing minerals. While a majority of these are carbonates, they also include previously unknown transition metal oxalate or azolate species. Consequently, whereas the discovery of new organic minerals provides an important contribution to our understanding of the geological aspects of the planetary carbon cycle, it may also provide an inspiration for the development of new metal-organic materials.
(c). Triazolate-based metal-organic framework minerals: chanabayaite and triazolite
An excellent illustration of this possibility is provided by the pair of related minerals chanabayaite [22] and triazolite (figure 2a) [21], discovered in Pabellón de Pica, Chile in 2015 and 2016, respectively, with triazolite also included among the minerals associated with the Carbon Mineral Challenge. In both cases, the naturally occurring crystalline specimens were of sufficiently high quality to allow for structure determination using single crystal X-ray diffraction, which for both minerals revealed 3D metal-organic framework structures unprecedented in the context of geochemistry. Importantly, whereas both zhemchuzhnikovite and stepanovite are based on the very simple and frequently encountered oxalate ions, the chanabayaite and triazolite pair represents a so far unique occurrence of a more complex 1,2,4-triazolate ligand in a mineral. The structures of both minerals are best described in terms of rod-shaped secondary building units (SBUs) [36], i.e. linear coordination polymer chains composed of copper(II) ions bridged by chloride anions as µ-bridging ligands and nitrogen atoms in 1,2-positions of each 1,2,4-triazolate ion (figure 2b,c). Notably, such rod-like SBUs, composed of triazolate-bridged metal ions, with or without additional bridging halogen atoms, are a robust structural motif that has previously been observed in a large number of metal-organic structures of transition metals, containing iron, nickel, cobalt cadmium, zinc, vanadium, molybdenum and even silver [37–42].
Figure 2.
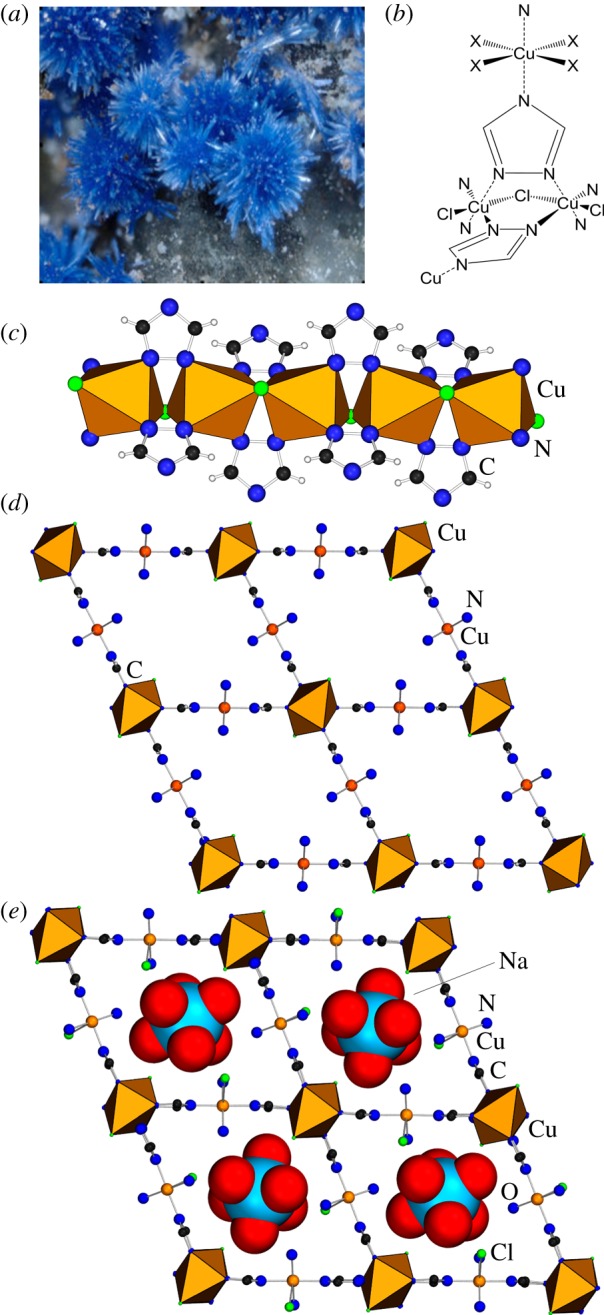
(a) Optical image of a cluster of triazolite crystals; (b) schematic view of the connectivity between metal ions and ligands in chanabayaite and triazolite; (c) fragment of the rod SBU found in the crystal structures of chanabayaite and triazolite; (d) view of the crystal structure of chanabayaite with guests omitted and (e) view of the crystal structure of triazolite, with hydrated sodium ion guests shown in space-filling. (a) Adapted with permission from [21]. (Online version in colour.)
In chanabayaite and triazolite, these chain-like SBUs are further crosslinked (figure 2d,e) into a 3D open framework through additional octahedrally coordinated copper(II) ions, with the axial coordination positions occupied by nitrogen atoms in the 4-position of triazolate ligands from different SBUs, and equatorial positions occupied by additional ammonia and/or chloride ligands. The overall result are framework structures with striking similarity to a family of MOFs based on divalent transition metal halides (e.g. CdCl2, CuBr2) and linear linkers based on 1,2,4-triazole-substituted ligands, such as 1,4-bis(1,2,4-triazol-4-yl)benzene (btzb), -ethylene (btze) etc. [43–45]. The structural similarity of triazolite and chanabayaite to the artificial MOFs is also evident in the comparison of crystallographic parameters, with chanabayaite exhibiting an identical space group and highly similar unit cell dimensions as several MOFs. Importantly, as synthetic MOFs have been demonstrated to exhibit high Langmuir surface areas, in the range of ca 500 m2 g−1, the striking structural similarity makes it likely that triazolite and chanabayaite could also exhibit microporous properties.
Comparison of the structures of the two triazolate-based minerals to analogous MOFs also reveals a potential opportunity for the future design of MOFs. Specifically, triazolite and chanabayaite represent inverted metal-organic framework (IMOF) analogues of the btzb- or btze-based synthetic materials [43–45], as described by Papaefstathiou & MacGillivray, i.e. framework materials in which the role of the linear organic linker has been replaced by a metal-based unit of linear connectivity [46]. In particular, the structures of two minerals exhibit two different types of copper(II) ions: one set of Cu2+ ions act as components of the rod-like SBU, while another set of Cu2+ ions act as linear linkers, connecting the SBUs into an open net. In such a structure the second set of Cu2+ ions effectively plays an identical structural role as 1,4-disubstituted phenyl rings in btzb-based MOFs. The ability to replace a complex organic linker with a combination of a simple organic heterocycle (i.e. 1,2,4-triazole) and a metal ion has not yet been explored in the context of triazolate-based MOFs and represents a potentially attractive, simple means for MOF design.
(d). Acetate-based minerals: hoganite and paceite
Although not MOFs, several other recently described organic minerals illustrate the potential of geochemical processes to generate building blocks and self-assembly motifs of interest in MOF design. Perhaps the simplest one among these is hoganite [47], a copper (II) acetate mineral reported in 2002 following discovery in the ferruginous gossan from the Potosi Pit in Broken Hill, Australia. Hoganite is hydrated copper(II) acetate, a well-known copper(II) reagent in most laboratories and is composed of discrete ‘paddlewheel’ units, in which pairs of Cu2+ ions are bridged by acetate ions acting as bis-monodentate ligands. Whereas hoganite is not a MOF, the paddlewheel unit is one of the central, ubiquitous SBUs in MOF design. Consequently, its appearance in a mineral structure opens up the possibility of finding other organic minerals that are bona fide MOFs based on paddlewheel units. Closely related to hoganite is paceite, a mixed calcium and copper(II) acetate mineral, found as thin crusts, up to 1 mm across, on surface of hoganite-bearing mineral specimens. Structurally, paceite is a linear coordination polymer of octahedrally coordinated Ca2+ and distorted square-planar Cu2+ ions bridged by acetate anions. The two types of metal ions alternate regularly in the coordination polymer, which is of considerable interest in the design of advanced materials seeking to combine metal ions with different electronic and catalytic properties [48].
(e). Chemistry informing mineralogy: deveroite as a lanthanide-based oxalate metal-organic framework
Zhemchuzhnikovite and stepanovite are most likely not the only cases of open oxalate MOF structures in mineralogy. This is strongly indicated by deveroite [49], a recently discovered cerium(III)-based mineral found in the Devero valley, Piedmont, Italy. Deveroite is characterized by the empirical formula Ce2(C2O4)3 × 10H2O and, whereas single crystal structure determination was not possible due to poor quality of specimens, it was determined to crystallize in the P21/c space group with unit cell parameters a = 11.240(8) Å, b = 9.635(11) Å and c = 10.339(12) Å, with β=114.41(10)° and V = 1019.6 Å3. Importantly, the synthetic form of deveroite was synthesized and structurally characterized by Ollendorff & Weigel [50], and was also established to be isostructural to analogous open-framework compounds of Nd, Pr, Gd, Ce, Eu, Ho, Tb, Dy, Er and Sm that have been known since the 1970s [50–56]. All these compounds display structures based on open, distorted hcb-frameworks. These lanthanide MOFs are structurally also very similar to analogous frameworks of Yb, Sc and Lu [57], which is not surprising given the similarity in cation sizes and chemical properties among the lanthanides (table 1).
Table 1.
Comparison of crystallographic parameters for deveroite and isostructural synthetic lanthanide oxalate frameworks first described by Ollendorff & Weigel [50].
| deveroite | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | |
|---|---|---|---|---|---|---|---|---|---|---|
| space group | P21/c | P21/c | P21/c | P21/c | P21/c | P21/c | P21/c | P21/a | P21/c | P21/c |
| a / Å | 11.240(8) | 11.370 | 11.347 | 11.254(9) | 11.678(2) | 11.5863(5) | 11.089 | 10.0549(4) | 10.997 | 11.433(2) |
| b / Å | 9.635(11) | 9.608 | 9.630 | 9.633(4) | 9.652(2) | 9.6080(2) | 9.635 | 9.6066(3) | 9.611 | 9.615(3) |
| c / Å | 10.339(12) | 10.490 | 10.392 | 10.331(6) | 10.277(2) | 10.1371(2) | 10.120 | 11.0306(6) | 10.020 | 9.988(3) |
| β /° | 114.41(10) | 114.57 | 114.52 | 114.52(5) | 118.92(2) | 118.906(2) | 114.25 | 114.097(4) | 114.11 | 118.76(2) |
| V / Å3 | 1019.6 | 1042.2 | 1033.2 | 1018.98 | 1013.93 | 987.882 | 985.839 | 972.63 | 966.648 | 962.522 |
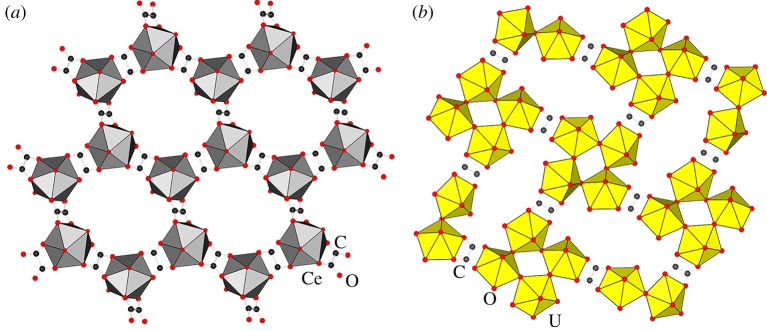
In all these isostructural or structurally similar lanthanide oxalates frameworks, the trivalent metal cation is coordinated by three oxalate anions, which also act as linkers that bridge neighbouring metal centres into a charge-neutral hcb-topology net. In these structures, each metal centre is also additionally coordinated with two or three molecules of water, depending on the metal (figure 3a).
Figure 3.
View of the metal-organic framework structures in lanthanide and actinide minerals: (a) single hcb-topology layer in deveroite [49] and (b) single sql-topology layer in the synthetic analogue of uroxite [58]. The lanthanide and actinide metal ions are represented as polyhedra. (Online version in colour.)
Besides the framework structure seen in deveroite and its synthetic analogues, other topologically distinct lanthanide oxalate MOFs have been obtained synthetically, for example square-grid topology (sql-topology) layered anionic frameworks that have been reported for several lanthanide ions, in the presence of alkaline metal, ammonium or hydronium ions as charge-balancing cations [59–62]. While such alternative framework structures could be likely candidates for not yet discovered carbon-based minerals their formation is expected to take place in at least mildly acidic environments. Consequently, the so far exclusive observation of only the hcb-structure may provide a hint of geological conditions under which deveroite and related minerals may have formed.
(f). Uroxite: an actinide-based metal-organic framework mineral
Uroxite is a recently discovered organic mineral, notably the first known uranyl oxalate which makes it also the first known organic mineral of uranium [58]. The mineral species was reportedly discovered on two separate locations in Colorado and Utah, USA by Kampf et al. [58]. Although the exact crystal structure of uroxite has not yet been published, the determined unit cell parameters and reported composition are nearly identical to three other previously published structures [63–65], all of which are identical to each other (table 2). Consequently, it is very likely that uroxite is a naturally occurring form of a previously synthetically prepared MOF.
Table 2.
Comparison of crystallographic parameters for uroxite and synthetic uranyl oxalates prepared by Duvieubourg et al. [63] Dalai et al. [64] and Gao et al. [65].
| uroxite | Duvieubourg | Dalai | Gao | |
|---|---|---|---|---|
| composition | [(UO2)2(C2O4)(OH)2H2O)2] · H2O | [(UO2)2(C2O4)(OH)2H2O)2] · H2O | [(UO2)2(C2O4)(OH)2H2O)2] · H2O | [(UO2)2(C2O4)(OH)2H2O)2] · H2O |
| space group | P21/c | P21/c | P21/c | P21/c |
| a / Å | 5.5698(2) | 5.5095(12) | 5.566(2) | 5.5561(4) |
| b / Å | 15.2877(6) | 15.195(3) | 15.263(3) | 15.2520(11) |
| c / Å | 13.3724(9) | 13.398(3) | 13.372(2) | 13.3506(9) |
| β /° | 94.015(7) | 93.927(3) | 93.99(2) | 93.9740(10) |
| V / Å3 | 1135.86 | 1119 | 1133.25 | 1128.63 |
The structures of these isostructural synthetic materials consist of 2D (4,4)-sql layers, comprising tetranuclear uranyl nodes interconnected into sheets by oxalate linkers, with additional water guest molecules occupying distorted square channels (figure 3b). Each of the tetranuclear nodes consists of four heptacoordinated uranyl cations with a pentagonal bipyramid coordination environment, arranged in a square. Each of the uranyl cations is coordinated by two uranyl oxygen atoms, two hydroxyl anions bridging between uranium cations within the tetranuclear node, one oxalate anion bridging two separate nodes, and one water molecule. The resulting layers stack in a slightly offset fashion, in that way creating channels that are occupied by guest water molecules.
3. Geomimetic synthesis of metal-organic frameworks and green chemistry
The outstanding interest of the research and chemical manufacturing communities, coupled with the recent announcement of first commercial applications of MOFs has highlighted the need to develop new, efficient and at the same time safer and more environmentally friendly approaches to the synthesis of such materials. In particular, the conventional solvothermal and solution-based approaches to MOFs, which are reliant on combining soluble metal salts with organic ligands at high temperatures and in high-boiling and often toxic solvents (e.g. N,N-dimethylformamide or DMF, N-methylpyrrolidine or NMP), are considered highly inadequate in the context of chemical manufacturing [66,67]. Notably, whereas such solvent-based techniques require soluble metal salts, such as nitrates or chlorides, the toxic, explosive and corrosive properties of such salts prevent their use in an industrial manufacturing context. Instead, safer and low-toxicity starting materials are preferred, such as sulfates, oxides, carbonates or hydroxides. Unfortunately, the poor solubility of such starting materials makes their use difficult. This problem has been tackled by both academia and by industry, for example by BASF reporting an electrolytic process for the synthesis of copper-based frameworks [68] such as copper(II) trimesate (also known as HKUST-1), in that way avoiding the need for soluble metal salts.
However, a more general solution to the challenges of accomplishing scalable, as well as safe and environmentally friendly synthesis of metal-organic materials is offered through techniques inspired by, or mimicking processes of mineral transformation. These techniques, which have emerged over the past 10 years provide an opportunity to conduct transformations of poorly soluble feedstocks, such as metal oxides or carbonates, by resorting to either elevated temperatures [69] (mineral replacement reactions) or solvent-free reaction environments (accelerated ageing [70], mechanochemistry [71]).
(a). Pseudomorphic replication: synthesis of mesostructured metal-organic framework materials by mineral replacement reactions
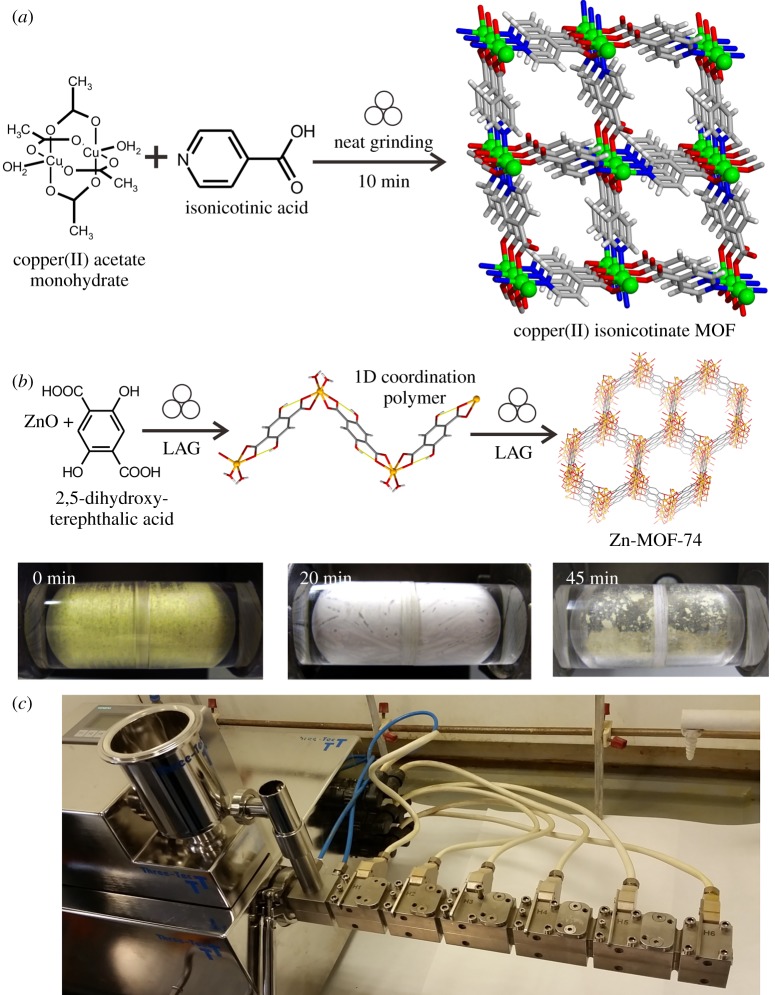
Mineral replacement processes are well-known geological transformations, whereupon one mineral species converts into another through a chemical reaction, typically upon exposure to a reactant solution [72,73]. Importantly, such reactions often proceed with the retention of overall mineral morphology, resulting in pseudomorphism. The ability to use mineral replacement reactions as a means to synthesize MOFs has been explored by Reboul and co-workers in a process termed pseudomorphic replication, wherein aluminium oxide starting material is exposed to a high-temperature (in the range 120–180°C) aqueous solution of 1,4-naphthalenedicarboxylic acid (H2ndc) [69]. Under such conditions, the reaction between Al2O3 and H2ndc proceeds rapidly, within seconds, to form the microporous metal-organic framework of composition Al(OH)ndc (figure 4). Importantly, if the reaction is conducted using micro-patterned alumina starting material the transformation proceeds with the retention of the porous pattern to provide a MOF material with an inverse opal microstructure.
Figure 4.
Time-resolved FESEM observation of the reaction of micropatterned alumina reactant with H2ndc at 140°C to form micropatterned Al(OH)ndc MOF with inverted opal structure: (a) starting material and after (b) 1 s, (c) 4 s, (d) 6 s, (e) 10 s, (f) 20 s, (g) 40 s and (h) 60 s. Adapted with permission from [68].
This type of transformation has also been used with success for the replacement reaction of a carbonate reactant [74], for example, in the high-temperature (120°C) microwave-heated reaction of biomineralized mesostructured calcium carbonate material from Baculogypsina sphaerulata with an aqueous solution of squaric acid (H2sq). After 12 h microwave irradiation, the sample underwent partial conversion to the open framework Casq·H2O with retention of the macropore morphology of the calcium carbonate substrate, with partial roughening [74].
(b). Accelerated ageing synthesis of metal-organic frameworks and coordination polymers
Among the major pathways for the formation of organic minerals is the interaction of inorganic minerals, such as oxides, sulfides, carbonates, with small organic molecules of biological origin. This pathway is of particular importance for the geological formation of metal oxalates, which represent the most abundant class of organic minerals, through the interaction of primary minerals with oxalic acid generated through lichen activity or present in, for example, guano [75,76]. Importantly, analogous processes have also been noted in the context of pharmaceutical materials, whereupon drug formulations of non-steroidal anti-inflammatory drug (NSAID) carboxylic acids such as ibuprofen were found to spontaneously react with magnesium oxide excipients [77]. Such reactivity was systematically studied by the Byrn group [78], who quantitatively monitored the spontaneous transformation of mixtures of MgO and flurbiprofen using X-ray powder diffraction.
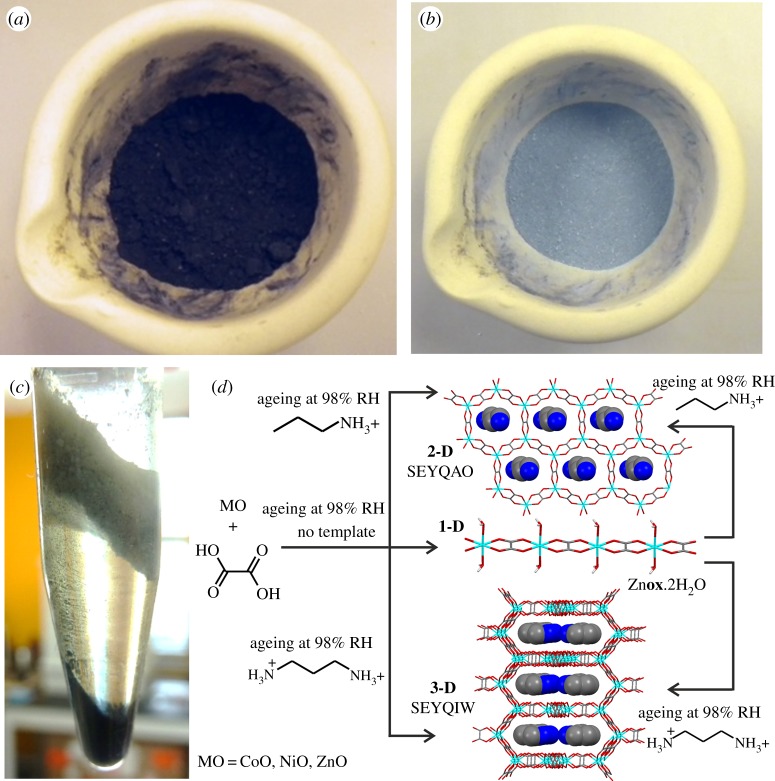
The methodology of ‘accelerated ageing’ synthesis of MOFs and coordination polymers is based on mimicking, accelerating and expanding such reactions for the processing of mineral-like feedstocks and their direct conversion into functional materials. A systematic exploration of the reactivity of oxalic acid with a range of main group and transition metal oxides was conducted by Qi et al. [79], who exposed stoichiometric mixtures of MgO, MnO, CoO, NiO, CuO (figure 5a,b), ZnO or PbO and H2ox to controlled conditions of temperature and humidity. In all cases, the mixtures spontaneously converted to metal oxalate coordination polymers, some of which are also known as organic minerals, e.g. the linear coordination polymers Mgox·2H2O (glushinskite), Mnox·2H2O (lindbergite) or Cuox·nH2O (mooloite) [76]. At room temperature and 98% relative humidity (RH), the transformations proceeded to quantitative conversion in most cases but only after a week and in some cases (e.g. PbO) over a month was needed. The reactions were significantly accelerated either by increasing the temperature to 45°C at 98% RH, or by mechanically activating the oxide starting materials by brief period (5 min) of pre-milling. In all cases, the transformations proceeded in the solid phase, demonstrating the ability to convert poorly soluble, high-melting mineral analogues into metal-organic compounds under mild, solvent-free conditions. The difference in reactivity of different metal oxides was also demonstrated [79] as a potential means to conduct recognition and separation of metal oxides in a mixture using density-based differences, without using solvents (figure 5c). Such solvent-free separation was demonstrated by exposing a mixture of ZnO, CuO and H2ox, in respective 1 : 1 : 1 stoichiometric ratio, to 98% RH at a temperature of 45°C. After several days ageing, the analysis of the reaction mixture revealed the selective transformation of zinc oxide into Znox·2H2O, with retention of copper(II) oxide. The significant difference in density of zinc oxalate (2.2 g cm−3) and CuO (6.3 g cm−3) enabled the gravity-based separation [80] of the two materials by flotation in the heavy liquid diiodomethane (CH2I2, with density 3.32 g cm−3) (figure 5c). The broader applicability of such solvent-free separation was also demonstrated on binary mixtures of CuO and PbO, as well as ZnO and PbO.
Figure 5.
Optical images of a mixture of copper(II) oxide and oxalic acid in 1 : 1 stoichiometric ratio: (a) before and (b) after ageing in air for 7 days, illustrating the formation of copper(II) oxalate. (c) Density-based separation of zinc and copper after exposing a mixture of oxides to a limited quantity of oxalic acid, followed by ageing [79]. The lower layer is CuO, while the upper layer is zinc oxalate formed through ageing. (d) Schematic illustration of the synthesis of open two- and three-dimensional oxalate frameworks upon ageing of cobalt(II), nickel(II) or zinc oxide in the presence of oxalic acid and suitable organoammonium templates, as shown in [79]. Photo credit for (a,b,c): T. Friščić. (Online version in colour.)
Whereas the accelerated ageing reactions of MgO, MnO and CuO demonstrated the formation of 1D coordination polymer structures found in naturally occurring minerals, as well as methods to accelerate such transformations, the ageing reactions of other metal oxides addressed by Qi et al. provided materials previously known in the scientific literature but not yet observed as minerals. This work also highlighted that accelerated ageing reactions, due to their very simple design and low technical requirements, can readily be scaled. Scalability was demonstrated by increasing the size of ZnO- and NiO-based reaction systems by two orders of magnitude, from hundreds of milligrams to tens of grams [79].
However, accelerated ageing reactions enables the synthesis of MOF structures with nanometre-sized cavities directly from metal oxides, without requiring solvents or elevated temperatures that are ubiquitous in the majority of MOF syntheses. This was demonstrated by Qi and co-workers [79] through the addition of stoichiometric amounts of alkylammonium salts, judiciously chosen based on earlier solution studies by Rao and Cheetham groups [81], to the ageing reactions involving ZnO, CoO or NiO and H2ox. The presence of alkylammonium templates induced the assembly of 2D open anionic frameworks with hcb-topology (figure 5d). Whereas this work demonstrated the ability to spontaneously assemble open MOF structures in reasonable amounts starting from simple metal oxide reactants, it also shows how ageing reactions can be templated for the formation of complex structures. This provides a potential hint on the formation of analogous hcb-frameworks found in the minerals stepanovite and zhemchuzhnikovite. As the alkylammonium cation templates in the MOFs obtained by accelerated ageing occupy the same positions as ions in the two minerals, it may be that the hydrated magnesium ions play a role in templating the formation of mineral MOFs.
How accelerated ageing reactivity can be used for the synthesis of popular MOF systems was investigated in 2012 by Cliffe et al. [82], who explored analogous reactivity between zinc oxide and differently substituted imidazoles. Notably, whereas Qi and co-workers have found that oxalic acid reacts with a range of metal oxides in humid air without deliquescence, in case of imidazoles the attempts of ageing reactions led to extensive deliquescence and little or no reactivity. However, accelerated ageing reactivity between ZnO and imidazoles could be accomplished through the addition of a protic catalyst, such as ammonium nitrate or sulfate salts. In the presence of such catalytic additives, the reactions proceeded without deliquescence, in a solid-to-solid fashion, providing non-porous 3D metal azolate frameworks [83] with zni- (for imidazole), dia- (for 2-methylimidazole, benzimidazole) and qtz-topology (for 2-ethylimidazole). Importantly, the formation of the dia-topology framework by reaction of ZnO with 2-methylimidazole (HMeIm) was found to take place via the intermediate appearance of the popular open Zn(MeIm)2 framework with sodalite (SOD) topology, known as ZIF-8 or MAF-4 [84,85].
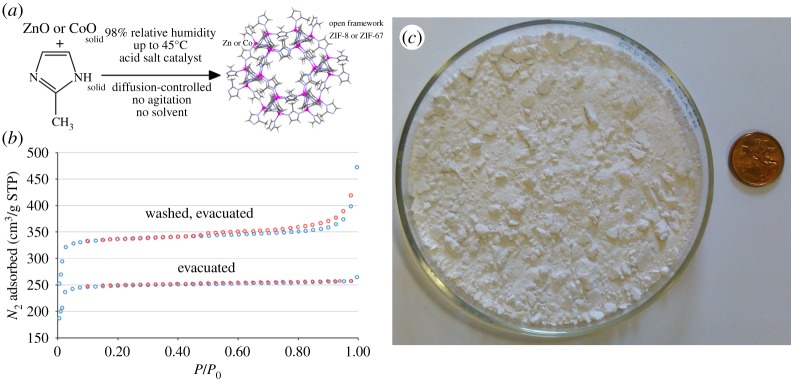
The synthesis of the ZIF-8 framework was accomplished through accelerated ageing by replacing simple ammonium salts as protic catalysts with more complex organoammonium salts, whose larger molecular structure enabled the stabilization of the open MOF structure. Specifically, Mottillo et al. [86] have found that either benzimidazolium sulfate or caffeinium sulfate can be used as catalysts to enable quantitative assembly of small-scale bulk amounts (5 g) of ZIF-8 directly from a 2 : 1 stoichiometric mixture of HMeIm and ZnO solids. The same approach was also readily applicable for the synthesis of the analogous material of cobalt(II), known as ZIF-67 (figure 6).
Figure 6.
Synthesis of zeolitic imidazolate frameworks by geomimetic accelerated ageing [82,86]: (a) schematic of the synthesis of selected zinc-and cobalt(II)-based MOFs; (b) nitrogen sorption isotherms for ZIF-8 framework samples obtained by accelerated ageing and (c) a 5 g sample of ZIF-8 obtained by accelerated ageing. Photo credit for (c): Dr C. Mottillo. (Online version in colour.)
The accelerated ageing approach was also applicable to the synthesis of other commercially relevant frameworks, such as copper(II) benzene-1,3,5-tricarboxylate (HKUST-1), synthesized by the Yuan group [87,88] in a variation of accelerated ageing technique involving ammonium salt catalysts and organic solvent vapours instead of conditions of high humidity. Importantly, accelerated ageing under humid conditions was also applicable for the activation of oxides of selected trivalent metals, notably La2O3, Nd2O3, Sm2O3 and Eu2O3 which were converted to 1D coordination polymers. Such reactivity again provides a possible route through which lanthanide- or actinide-based organic minerals, such as deveroite or uroxite, could form in a geological environment.
(c). Mechanochemical synthesis of metal-organic frameworks
Whereas accelerated ageing reactions focus on expanding the scope of mineral weathering reactivity, and accelerating it through variation of environmental conditions (e.g. temperature, atmosphere) or catalysts, another means to accelerate such transformations is via mechanochemistry [89], i.e. through ball milling or manual grinding. Indeed, mechanochemical transformations of selected metal oxides or carbonates with carboxylic acid or imidazole ligands have at this point been well studied [90], providing rapid (often within minutes), solvent-free and room-temperature approach to a wide range of MOFs. It was recently noted that most commercially relevant types of MOFs have now been made accessible through cleaner, safer mechanochemical methods, either in the form of batch synthesis by ball milling (figure 7a,b) [91,92], or continuous manufacture through twin screw extrusion (figure 7c) [93]. Whereas mechanochemical techniques are related to accelerated ageing, and in some cases have been inspired by ageing transformations observed in the context of pharmaceutical formulations, ball milling and screw extrusion are generally not considered as directly geomimetic techniques. This viewpoint, however, might be challenged as inorganic minerals are well known to undergo chemical transformations under high stress or impact in geological environments [94]. In that context, particularly interesting is the recent work of Hernández and co-workers [95], which explores the possibility that small organic building blocks important for the development of life on Earth, such as cyanide, might have been released from stable organic mineral species, such as ferrocyanides, through mechanochemical processes during, for example, meteoric impact.
Figure 7.
(a) The first reported mechanochemical synthesis of a MOF [91]; (b) synthesis of a MOF-74 type framework by mechanochemistry [92], with images of the milled sample for each reaction stage and (c) a twin-screw extruder used for continuous mechanochemical synthesis. (b) Adapted with permission from [92]. Photo credit for (c): Dr Deborah Crawford. (Online version in colour.)
4. Conclusion
Metal-organic frameworks are in many ways a unique class of materials. One unique aspect of their development over the past decades has been the lack of direct natural analogues. Here, we have highlighted how this paradigm is being challenged in the contexts of both materials design, as well as synthesis. On one hand, the emergent examples of naturally occurring minerals, such as zhemchuzhnikovite (discovered 1963), stepanovite (discovered 1953), deveroite (discovered 2013), chanabayaite (discovered 2015), triazolite (discovered 2016) and uroxite (discovered 2018) demonstrate the formation of metal-organic framework structures based on simple (oxalate) or relatively complex (1,2,4-triazole) organic ligands. On the other hand, geological processes of mineral weathering and mineral replacement have served as inspiration for the development of geomimetic synthesis approaches like accelerated ageing, which is aimed towards cleaner, safer and more efficient formation of MOFs, and pseudomorphic replication, which is aimed towards the deliberate and controlled assembly of microstructured material based on MOFs. We believe that the discovery and strengthening of connections between geochemistry and the chemistry of metal-organic frameworks strengthens both fields, by demonstrating the relevance of geological sciences in modern materials design and facilitating the design of new functional materials. In the latter context, we believe that an important driving force will be initiatives such as the Carbon Mineral Challenge, focused on the discovery of new organic minerals. Just in 2018, such initiatives encouraged the discovery of first zinc- (alterite) [96] and uranium-containing (uroxite) organic minerals. Considering the abundance of organic molecules already found in the composition of organic minerals, such as adenine, uric acid, mellitic acid and more, it is almost certain that other examples of MOFs exist in nature, such as BioMOFs—framework materials based on biologically relevant linkers such as adenine [97,98]. The discovery of organic minerals already demonstrates an accelerating trend and we expect that further mineral discoveries will be greatly facilitated by developing materials and structural analysis techniques, e.g. micro-electron diffraction on extremely small crystals [99], and improved abilities to investigate geological compositions of other planetary systems [100]. New organic minerals are likely to provide additional excitement to the diversity of structures and properties of geological materials—already the structures of zhemchuzhnikovite and stepanovite indicate the possibility of highly efficient geological proton conductors, while triazolite and chanabayaite suggest the appearance of highly microporous materials in nature.
Data accessibility
This article has no additional data.
Authors' contributions
Manuscript was prepared, written and proof-read by both authors.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge funding support by NSERC Discovery grant RGPIN-2017-06467, E. W. R. Steacie Memorial Fellowship grant SMFSU 507347-17 and McGill University.
References
- 1.Batten SR, Champness NR, Chen X-M, Garcia-Martinez J, Kitagawa S, Öhrström OM, Suh MP, Reedijk J. 2012. Coordination polymers, metal-organic frameworks and the need for terminology guidelines. CrystEngComm 14, 3001–3004. ( 10.1039/c2ce06488) [DOI] [Google Scholar]
- 2.Hoskins BF, Robson R. 1989. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 111, 5962–5964. ( 10.1021/ja00197a079) [DOI] [Google Scholar]
- 3.Farha OK, et al. 2012. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit? J. Am. Chem. Soc. 134, 15 016–15 021. ( 10.1021/ja3055639) [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen SBT, Hupp JT. 2009. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459 ( 10.1039/B807080F) [DOI] [PubMed] [Google Scholar]
- 5.Ramaswamy P, Wong NE, Shimizu GKH. 2014. MOFs as proton conductors – challenges and opportunities. Chem. Soc. Rev. 43, 5913–5932. ( 10.1039/c4cs00093e) [DOI] [PubMed] [Google Scholar]
- 6.de Lange MF, Verouden KJFM, Vlugt TJH, Gascon J, Kapteijn F. 2015. Adsorption-driven heat pumps: the potential of metal-organic frameworks. Chem. Rev. 115, 12 205–12 250. ( 10.1021/acs.chemrev.5b00059) [DOI] [PubMed] [Google Scholar]
- 7.Furukawa H, Cordova KE, O'Keeffe M, Yaghi OM. 2013. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 ( 10.1126/science.1230444) [DOI] [PubMed] [Google Scholar]
- 8.Bloch ED, Queen WL, Krishna R, Zadrozny JM, Brown CM, Long JR. 2012. Hydrocarbon separations in a metal-organic framework with open iron (II) coordination sites. Science 335, 1606–1610. ( 10.1126/science.1217544) [DOI] [PubMed] [Google Scholar]
- 9.Silva P, Vilela SMF, Tomé JPC, Almeida Paz FA. 2015. Multifunctional metal-organic frameworks: from academia to industrial applications. Chem. Soc. Rev. 44, 6774–6803. ( 10.1039/c5cs00307e) [DOI] [PubMed] [Google Scholar]
- 10.Mottillo C, Friščić T. 2018. From the benchtop to the pilot plant: making headway in the synthesis of metal-organic frameworks. Chimica Oggi – Chem. Today 35, 30–32. [Google Scholar]
- 11.Kitagawa S, Kitaura R, Noro S-i. 2004. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375. ( 10.1002/anie.200300610) [DOI] [PubMed] [Google Scholar]
- 12.Furukawa H, Kim J, Ockwig NW, O'Keeffe M, Yaghi OM. 2008. Control of vertex geometry, structure dimensionality, and pore metrics in the reticular synthesis of crystalline metal-organic frameworks and polyhedra. J. Am. Chem. Soc. 130, 11 650–11 661. ( 10.1021/ja803783c) [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS, Craig DC, Desiraju GR. 1995. Topological equivalences between organic and inorganic crystal structures: 1,3,5,7-tetrahydroxyadamantane and caesium chloride. J. Chem. Soc. Chem. Commun. 339–340. ( 10.1039/C39950000339) [DOI] [Google Scholar]
- 14.Reddy DS, Craig DC, Desiraju GR. 1994. Organic alloys: diamondoid networks in crystalline complexes of 1,3,5,7-tetrabromoadamantane, hexamethylenetetramine and carbon tetrachloride. J. Chem. Soc., Chem. Commun. 1457–1458. ( 10.1039/C39940001457) [DOI] [Google Scholar]
- 15.Iwamoto T, Nishikiori S-i, Kitazawa T, Yuge H. 1997. Mineralomimetic chemistry as a modern aspect of co-ordination chemistry. Dalton Trans. 4127–4136. ( 10.1039/A702539D) [DOI] [Google Scholar]
- 16.Kitazawa T. 1996. The structure of the mineralomimetic cadmium cyanide-dimethyl carbonate clathrate. J. Incl. Phen. Mol. Recog. Chem. 26, 153–159. ( 10.1007/BF01029939) [DOI] [Google Scholar]
- 17.Knipovich Y, Komkov AI, Nefedov EI. 1963. On stepanovite and the new mineral zhemchuzhnikovite. Trudy Vses. Nauchno-Issled. Geol. Inst. (V.S E.G.E.I.) 96, 131–135. (in Russian). [Google Scholar]
- 18.Echigo T, Kimata M. 2010. Crystal chemistry and genesis of organic minerals: a review of oxalate and polycyclic aromatic hydrocarbon minerals. Canad. Mineral. 48, 1329–1358. ( 10.3749/canmin.48.5.1329) [DOI] [Google Scholar]
- 19.Huskić I, Pekov IV, Krivovichev SV, Friščić T. 2016. Minerals with metal-organic framework structures. Sci. Adv. 2, e1600621 ( 10.1126/sciadv.1600621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piro OE, Echeverría GA, González-Barό AC, Baran EJ. 2016. Crystal and molecular structure and spectroscopic behavior of isotypic synthetic analogs of the oxalate minerals stepanovite and zhemchuzhnikovite. Phys. Chem. Mineral. 43, 287–300. ( 10.1007/s00269-015-0793-2) [DOI] [Google Scholar]
- 21.Chukanov NV, Zubkova NV, Möhn G, Pekov IV, Belakovsky DI, Van KV, Britvin SN, Puscharovsky DY. 2018. Triazolite, NaCu2(N3C2H2)2(NH3)2Cl3·4H2O, a new mineral species containing 1,2,4-triazolate anion, from a guano deposit at Pabellόn de Pica, Iquique Province, Chile. Mineral. Mag. 82, 1007–1014. ( 10.1180/minmag.2017.081.088) [DOI] [Google Scholar]
- 22.Chukanov NV, Zubkova NV, Möhn G, Pekov IV, Puscharovsky DYu, Zadov AE. 2015. Chanabayaite, Cu2(N3C2H2)Cl(NH3,Cl,H2O)4, a new mineral containing triazolate anion. Geol. Ore. Dep. 57, 712–720. ( 10.1134/S107570151508005X) [DOI] [Google Scholar]
- 23.Huskić I, Friščić T. 2018. Understanding geology through crystal engineering: coordination complexes, coordination polymers and metal-organic frameworks as minerals. Acta Crystallogr. B. 74, 539–559. ( 10.1107/S2052520618014762) [DOI] [Google Scholar]
- 24.Piro OE, Baran EJ. 2018. Crystal chemistry of organic minerals – salts of organic acids: the synthetic approach. Crystallogr. Rev. 24, 149–175. ( 10.1080/0889311X.2018.1445239) [DOI] [Google Scholar]
- 25.Julien PA, Mottillo C, Friščić T. 2017. Metal-organic frameworks meet scalable and sustainable synthesis. Green Chem. 19, 2729–2747. ( 10.1039/c7gc01078h) [DOI] [Google Scholar]
- 26.Putnis A. 2009. Mineral replacement reactions. Rev. Mineral. Geochem. 70, 87–124. ( 10.2138/rmg.2009.70.3) [DOI] [Google Scholar]
- 27.Mottillo C, Friščić T. 2017. Advances in solid-state transformations of coordination bonds: from the ball mill to the aging chamber. Molecules 22, 144 ( 10.3390/molecules22010144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friščić T. 2012. Supramolecular concepts and new techniques in mechanochemistry: cocrystals, cages, rotaxanes, open metal-organic frameworks. Chem. Soc. Rev. 41, 3493–3510. ( 10.1039/c2cs15332g) [DOI] [PubMed] [Google Scholar]
- 29.Zhan G, Zeng HC. 2017. Alternative synthetic approaches for metal-organic frameworks: transformation from solid matters. Chem. Commun. 53, 72–81. ( 10.1039/c6cc07094a) [DOI] [PubMed] [Google Scholar]
- 30.Descurtins S, Schmalle HW, Schneuwly P, Ensling J, Gütlich P. 1994. A concept for the synthesis of 3-dimensional homo- and bimetallic oxalate-bridged networks [M2(ox)3]n. Structural, Mössbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 116, 9521–9528. ( 10.1021/ja00100a016) [DOI] [Google Scholar]
- 33.Huskić I, Friščić T. 2015. Naturally occuring metal-organic frameworks. Acta Cryst. A71, s57–s58. ( 10.1107/S2053273315099131) [DOI] [Google Scholar]
- 32.Sadakiyo M, Yamada T, Kitagawa H. 2009. Rational designs for highly proton-conductive metal-organic frameworks. J. Am. Chem. Soc. 131, 9906–9907. ( 10.1021/ja9040016) [DOI] [PubMed] [Google Scholar]
- 31.Sadakiyo M, Yamada T, Honda K, Matsui H, Kitagawa H. 2014. Control of crystalline proton-conducting pathways by water-induced transformations of hydrogen-bonding networks in a metal-organic framework. J. Am. Chem. Soc. 136, 7701–7707. ( 10.1021/ja5022014) [DOI] [PubMed] [Google Scholar]
- 34.Deep Carbon Observatory. The Carbon Mineral Challenge. https://mineralchallenge.net/ Webpage (accessed on 21 January 2019).
- 35.Hazen RM, Hummer DR, Hystad G, Downs RT, Golden JJ. 2016. Carbon mineral ecology: predicting the undeiscovered minerals of carbon. Amer. Mineral. 101, 889–906. ( 10.2138/am-2016-5546) [DOI] [Google Scholar]
- 36.Rosi NL, Kim J, Eddaoudi M, Chen B, O'Keeffe M, Yaghi OM. 2005. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. Acc. Chem. Res. 127, 1504–1518. ( 10.1021/ja045123o) [DOI] [PubMed] [Google Scholar]
- 37.Habib HA, Hoffmann A, Höppe HA, Steinfeld G, Janiak C. 2009. Crystal structure solid-state cross polarization magic angle spinning 13C NMR correlation in luminescent d10 metal-organic frameworks constructed with the 1,2-bis(1,2,4-triazol-4-yl)ethane ligand. Inorg. Chem. 48, 2166–2180. ( 10.1021/ic802069k) [DOI] [PubMed] [Google Scholar]
- 38.Sharga OV, Lysenko AB, Krautscheid H, Domasevitch KV. 2010. A three-dimensional heterometallic CuI/VIV 1,2-bis(1,2,4-triazol-4-yl)- ethane framework: a new insight into the structure of vanadium oxyfluoride coordination hybrids. Acta Crystallogr. C. 66, 269–272. ( 10.1107/S0108270110034323) [DOI] [PubMed] [Google Scholar]
- 39.Lysenko AB, Senchyk GA, Lincke J, Lässig D, Fokin AA, Butova ED, Schreiner PR, Krautscheid H, Domasevitch KV. 2010. Metal oxide-organic frameworks (MOOFs), a new series of coordination hybrids constructed from molybdenum(VI) oxide and bitopic 1,2,4-triazole linkers. Dalton Trans. 39, 4223–4231. ( 10.1039/b922732f) [DOI] [PubMed] [Google Scholar]
- 40.Liang N, Wang J, Yuan D, Li B, Li H. 2010. A novel three-dimensional network silver coordination polymer with flexible bis(1,2,4-triazol-4-yl)ethane. Inorg. Chem. Commun. 13, 844–846. ( 10.1016/j.inoche.2010.04.009) [DOI] [Google Scholar]
- 41.Savard D, Cook C, Enright GD, Korobkov I, Burchell TJ, Murugesu M. 2011. Gradual spin crossover behaviour in a linear trinuclear FeII complex. CrystEngComm 13, 5190–5197. ( 10.1039/C1CE05275F) [DOI] [Google Scholar]
- 42.Cui YF, Qian X, Chen Q, Li BL, Li HY. 2012. A polythreading coordination array formed from a 3D microporous cation network and 1D anion ladders. CrystEngComm 14, 1201–1204. ( 10.1039/C2CE06397B) [DOI] [Google Scholar]
- 43.Wang L, Ye Y, Li Z, Lin Q, Ouyang J, Liu L, Zhang Z, Xiang S. 2017. Highly selective adsorption of C2/C1 mixtures and solvent-dependent thermochromic properties in metal–organic frameworks containing infinite copper-halogen chains. Cryst. Growth Des. 17, 2081–2089. ( 10.1021/acs.cgd.7b00060) [DOI] [Google Scholar]
- 44.Bondar O, Lukashuk LV, Lysenko AB, Krautscheid H, Rusanov EB, Chernegac AN, Domasevitch KV. 2008. New microporous copper(II) coordination polymers based upon bifunctional 1,2,4-triazole/tetrazolate bridges. CrystEngComm 10, 1216–1226. ( 10.1039/B806671J) [DOI] [Google Scholar]
- 45.Ding B, Yang P, Liu YY, Wang Y, Du GX. 2013. Hydrothermal syntheses and characterization of a series of luminescent Cd(II) frameworks with pyridine-based and benzene-based bis-triazole ligands. CrystEngComm 15, 2490–2503. ( 10.1039/C3CE26998A) [DOI] [Google Scholar]
- 46.Papaefstathiou GS, MacGillivray LR. 2002. An inverted metal-organic framework with compartmentalized cavities constructed by using an organic bridging unit derived from the solid state. Angew. Chem. Int. Ed. 41, 2070–2073. () [DOI] [PubMed] [Google Scholar]
- 47.Hibbs DE, Kolitsch U, Leverett P, Sharpe JL, Williams PA. 2002. Hoganite and paceite, two new acetate minerals from the Potosi mine, Broken Hill. Australia. Min. Mag. 66, 459–464. ( 10.1180/0026461026630042) [DOI] [Google Scholar]
- 48.Marti RM, Howe JD, Morelock CR, Conradi MS, Walton KS, Sholl DS, Hayes SE. 2017. CO2 Dynamics in pure and mixed-metal MOFs with open metal sites. J. Phys. Chem. C. 121, 25 778–25 787. ( 10.1021/acs.jpcc.7b07179) [DOI] [Google Scholar]
- 49.Guastoni A, Nestola F, Gentile P, Zorzi F, Alvaro M, Lanza A, Perutto L, Shiazza M, Casati NM. 2013. Deveroite-(Ce): a new REE-oxalate from Mount Cervandone, Devero Valley, Western-Central Alps, Italy. Min. Mag. 77, 3019–3026. ( 10.1180/minmag.2013.077.7.11) [DOI] [Google Scholar]
- 50.Ollendorff W, Weigel F. 1969. The crystal structure of some lanthanide oxalate decahydrates, 0020–1650Ln2(C2O4)3·10H2O, with Ln = La, Ce, Pr and Nd. Inorg. Nucl. Chem. Lett. 5, 263–269. (doi:10.1016/ (69)80196-0) [Google Scholar]
- 51.Hansson E. 1970. Structural studies on the rare earth carboxylates. 5. Crystal and molecular structure of neodymium(III) oxalate 10.5-Hydrate. Acta Chem. Scand. 24, 2969–2982. ( 10.3891/acta.chem.scand.24-2969) [DOI] [Google Scholar]
- 52.Chi H. 1970. Location of gadolinium ions in hydrated oxalate by ESR. Bull. Chem. Soc. Jpn. 43, 1703–1707. ( 10.1246/bcsj.43.1703) [DOI] [Google Scholar]
- 53.Wang P, et al. 2013. Two-/three-dimensional open lanthanide–organic frameworks containing rigid/flexible dicarboxylate ligands: synthesis, crystal structure and photoluminescent properties. CrystEngComm 15, 1931–1949. ( 10.1039/C3CE26684B) [DOI] [Google Scholar]
- 54.Liu TF, Zhang W, Sun WH, Cao R. 2011. Conjugated ligands modulated sandwich structures and luminescence properties of lanthanide metal–organic frameworks. Inorg. Chem. 50, 5242–5248. ( 10.1021/ic200579j) [DOI] [PubMed] [Google Scholar]
- 55.Rong-Hua Z, Han-Guo L. 2007. Poly[[triaquasesqui-(2-oxalato-terbium(III)] methanol solvate]. Acta Crystallogr. E. 63, m2925 ( 10.1107/S1600536807055043) [DOI] [Google Scholar]
- 56.Zhang Y, Bhadbhade M, Scales N, Karatchevtseva I, Price JR, Lu K, Lumpkin GR. 2014. Dysprosium complexes with mono-/di-carboxylate ligands—from simple dimers to 2D and 3D frameworks. J. Solid State Chem. 219, 1–8. ( 10.1016/j.jssc.2014.07.007) [DOI] [Google Scholar]
- 57.Hansson E. 1973. Structural studies of rare earth carboxylates 16. The crystal and molecular structure of tetra-aquo tris-oxalato diytterbium(III) dihydrate. Acta Chem. Scand. 27, 823–834. ( 10.3891/acta.chem.scand.27-0823) [DOI] [Google Scholar]
- 58.Kampf AR, Plášil J, Nash BP, Němec I, Marty J. 2018. Uroxite, IMA 2018-100. Eur. J. Min. 30, 1181–1189. ( 10.1127/ejm/2018/0030-2819) [DOI] [Google Scholar]
- 59.Bataille T, Louër D. 1999. Yttrium sodium oxalate tetrahydrate, [Y(H2O)]Na(C2O4)2·3H2O. Acta Crystallogr. C. 55, 1760–1762. ( 10.1107/S0108270199009683) [DOI] [Google Scholar]
- 60.McDonald TRR, Spink JM. 1967. The crystal structure of a double oxalate of yttrium and ammonium, NH4Y(C2O4)2·H2O. Acta Crystallogr. 23, 944–949. ( 10.1107/S0365110X67004104) [DOI] [Google Scholar]
- 61.Steinfink H, Brunton GD. 1970. Crystal structure of erbium oxalate trihydrate. Inorg. Chem. 9, 2112–2115. ( 10.1021/ic50091a030) [DOI] [Google Scholar]
- 62.Chapelet-Arab B, Duvieubourg L, Nowogrocki G, Abraham F, Grandjean S. 2006. U(IV)/Ln(III) mixed site in polymetallic oxalato complexes. Part III: Structure of Na[Yb(C2O4)2(H2O)]·3H2O and the derived quadratic series (NH4+)1−x[Ln1−xUx(C2O4)2(H2O)]·(3 + x) H2O, Ln = Y, Pr–Sm, Gd, Tb. J. Solid State Chem. 179, 4029–4036. ( 10.1016/j.jssc.2006.09.007) [DOI] [Google Scholar]
- 63.Duvieubourg L, Nowogrocki G, Abraham F, Grandjean S. 2005. Hydrothermal synthesis and crystal structures of new uranyl oxalate hydroxides: α- and β-[(UO2)2(C2O4)(OH)2(H2O)2] and [(UO2)2 (C2O4)(OH)2 (H2O)2]·H2O. J. Solid State Chem. 178, 3437–3444. ( 10.1016/j.jssc.2005.08.018) [DOI] [Google Scholar]
- 64.Dalai S, Bera M, Rana A, Chowdhuri DS, Zangrando E. 2010. Combination of covalent and hydrogen bonding in the formation of 3D uranyl-carboxylate networks. Inorg. Chim. Acta. 363, 3407–3412. ( 10.1016/j.ica.2010.06.043) [DOI] [Google Scholar]
- 65.Gao X, Song J, Sun LX, Xing YH, Bai FY, Shi Z. 2016. A family of uranium–carboxylic acid hybrid materials: synthesis, structure and mixed-dye selective adsorption. New J. Chem. 40, 6077–6085. ( 10.1039/C6NJ00109B) [DOI] [Google Scholar]
- 66.Czaja AU, Trukhan N, Müller U. 2009. Industrial applications of metal-organic frameworks. Chem. Soc. Rev. 38, 1284–1293. ( 10.1039/b804680h) [DOI] [PubMed] [Google Scholar]
- 67.Gaab M, Trukhan N, Maurer S, Gummaraju R, Müller U. 2012. The progression of Al-based metal-organic frameworks – from academic research to industrial production and applications. Micropor. Mesopor. Mater. 157, 131–136. ( 10.1016/j.micromeso.2011.08.016) [DOI] [Google Scholar]
- 68.Mueller U, Schubert M, Teich F, Puetter H, Schierle-Arndt K, Pastré J. 2006. Metal-organic frameworks – prospective industrial applications. J. Mater. Chem. 16, 626–636. ( 10.1039/b511962f) [DOI] [Google Scholar]
- 69.Reboul J, et al. 2012. Mesoscopic architectures of porous coordination polymers fabricated by pseudmorphic replication. Nature Mater. 11, 717–723. ( 10.1038/nmat3359) [DOI] [PubMed] [Google Scholar]
- 70.Jia C, Wang J, Feng X, Lin Q, Yuan W. 2014. Efficient vapour-assisted aging and liquid-assisted grinding synthesis of a microporous copper-adeninate framework. CrystEngComm 16, 6552–6555. ( 10.1039/C4CE00533C) [DOI] [Google Scholar]
- 71.James SL, et al. 2012. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 41, 413–447. ( 10.1039/c1cs15171a) [DOI] [PubMed] [Google Scholar]
- 72.Putnis A. 2002. Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Min. Mag. 66, 689–708. ( 10.1180/0026461026650056) [DOI] [Google Scholar]
- 73.Banfield JF, Barker WW, Welch SA, Taunton A. 1999. Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl Acad. Sci. USA 96, 3404–3411. ( 10.1073/pnas.96.7.3404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sumida K, Hu M, Furukawa S, Kitagawa S. 2016. Structuralization of Ca2+-based metal-organic frameworks prepared via coordination replication fo calcium carbonate. Inorg. Chem. 55, 3700–3705. ( 10.1021/acs.inorgchem.6b00397) [DOI] [PubMed] [Google Scholar]
- 75.Gadd GM. 2010. Metals, minerals, and microbes: geomicrobiology and bioremediation. Microbiol. 156, 609–643. ( 10.1099/mic.0.037143-0) [DOI] [PubMed] [Google Scholar]
- 76.Clarke RM, Williams IR. 1986. Moolooite, a naturally occurring hydrated copper oxalate from Western Australia. Min. Mag. 50, 295–298. ( 10.1180/minmag.1986.050.356.15) [DOI] [Google Scholar]
- 77.Chow EHH, Strobridge FC, Friščić T. 2010. Mechanochemistry of magnesium oxide revisited: facile derivatisation of pharmaceuticals using coordination and supramolecular chemistry. Chem. Commun. 46, 6368–6370. ( 10.1039/c0cc01337d) [DOI] [PubMed] [Google Scholar]
- 78.Byrn SR, Xu W, Newman AW. 2001. Chemical reactivity in solid-state pharmaceuticals: formulation implications. Adv. Drug Deliv. Rev. 48, 115–136. ( 10.1016/S0169-409X(01)00102-8) [DOI] [PubMed] [Google Scholar]
- 79.Qi F, Stein RS, Friščić T. 2014. Mimicking mineral neogenesis for the clean synthesis of metal-organic materials from mineral feedstocks: coordination polymers, MOFs and metal oxide separation. Green Chem. 16, 121–132. ( 10.1039/c3gc41370e) [DOI] [Google Scholar]
- 80.Atwood JL. 2015. Separation of active pharmaceutical ingredients (APIs) from excipients in pharmaceutical formulations. Cryst. Growth Des. 15, 2874–2877. ( 10.1021/acs.cgd.5b00317) [DOI] [Google Scholar]
- 81.Vaidhyanathan R, Natarajan S, Cheetham AK, Rao CNR. 1999. New open-framework zinc oxalates synthesized in the presence of structure-directing organic amines. Chem. Mater. 11, 3636–3642. ( 10.1021/cm990434x) [DOI] [Google Scholar]
- 82.Cliffe MJ, Mottillo C, Stein RS, Bučar DK, Friščić T. 2012. Accelerated aging: a low energy, solvent-free alternative to solvothermal and mechanochemical synthesis of metal-organic materials. Chem. Sci. 3, 2495–2500. ( 10.1039/c2sc20344h) [DOI] [Google Scholar]
- 83.Zhang J-P, Zhang Y-B, Lin J-B, Chen X-M. 2012. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 112, 1001–1033. ( 10.1021/cr200139g) [DOI] [PubMed] [Google Scholar]
- 84.Park KS, Ni Z, Côte AP, Choi JY, Huang R, Uribe-Romo FJ, Chae HK, O'Keeffe M, Yaghi OM. 2006. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl Acad. Sci. USA 103, 10 186–10 191. ( 10.1073/pnas.0602439103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang X-C, Lin Y-Y, Zhang J-P, Chen X-M. 2006. Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 45, 1557–1559. ( 10.1002/anie.200503778) [DOI] [PubMed] [Google Scholar]
- 86.Mottillo C, Lu Y, Pham M-H, Cliffe MJ, Do T-O, Friščić T. 2013. Mineral neogenesis as an inspiration for mild, solvent-free synthesis of bulk microporous metal-organic frameworks from metal (Zn, Co) oxides. Green Chem. 15, 2121–2131. ( 10.1039/c3gc40520f) [DOI] [Google Scholar]
- 87.Feng X, Jia C, Wang J, Cao X, Tang P, Yuan W. 2015. Efficient vapor-assisted aging synthesis of functional and highly crystalline MOFs from CuO and rare earth sesquioxides/carbonates. Green Chem. 17, 3740–3745. ( 10.1039/c5gc00378d) [DOI] [Google Scholar]
- 88.Tang P, Jia C, Jiang Y, Gong W, Cao X, Yang J, Yuan W. 2016. Reactivity studies of metal-organic frameworks under vapor-assisted aging: structural interconversions and transformations. Eur. J. Inorg. Chem. 2016, 5617–5622. ( 10.1002/ejic.201600907) [DOI] [Google Scholar]
- 89.Do JL, Friščić T. 2017. Mechanochemistry: a force of synthesis. ACS Cent. Sci. 3, 13–19. ( 10.1021/acscentsci.6b00277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beldon PJ, Fábián L, Stein RS, Thirumurugan A, Cheetham AK, Friščić T. 2010. Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry. Angew. Chem. Int. Ed. 49, 9640–9643. ( 10.1002/anie.201005547) [DOI] [PubMed] [Google Scholar]
- 91.Pichon A, Lazuen-Garay A, James SL. 2006. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm 8, 211–214. ( 10.1039/b513750k) [DOI] [Google Scholar]
- 92.Julien PA, et al. 2016. In situ monitoring and mechanism of the mechanochemical formation of a microporous MOF-74 framework. J. Am. Chem. Soc. 138, 2929–2932. ( 10.1021/jacs.5b13038) [DOI] [PubMed] [Google Scholar]
- 93.Crawford D, Casaban J, Haydon R, Giri N, McNally T, James SL. 2015. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 6, 1645–1649. ( 10.1039/c4sc03217a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nermoen A, Korsnes RI, Aursjø O, Madland MV, Kjørslevik TAC, Østensen G. 2016. How stress and temperature conditions affect rock-fluid chemistry and mechanical deformation. Front. Phys. 4, 2 ( 10.3389/fphy.2016.00002) [DOI] [Google Scholar]
- 95.Bolm C, Mocci R, Schumacher C, Turberg M, Puccetti F, Hernández JG. 2018. Mechanochemical activation of iron cyano complexes: a prebiotic impact scenario for the synthesis of α-amino acid derivatives. Angew. Chem. Int. Ed. 57, 2423–2426. ( 10.1002/anie.201713109) [DOI] [PubMed] [Google Scholar]
- 96.Yang H, Gibbs RB, Evans SH, Downs RT, Jabrin Z. 2018. Alterite, IMA 2018-070. CNMNC Newsletter No. 45, October 2018, p. 1043. Eur. J. Min. 30, 1037–1043. ( 10.1127/ejm/2018/0030-2810) [DOI] [Google Scholar]
- 97.An J, Geib SJ, Rosi NJ. 2009. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 131, 8376–8377. ( 10.1021/ja902972w) [DOI] [PubMed] [Google Scholar]
- 98.McKinlay AC, Morris RE, Horcajada P, Férey G, Gref R, Couvreur P, Serre C. 2010. BioMOFs: metal-organic frameworks for biological and medical applications. Angew. Chem Int. Ed. 49, 6260–6266. ( 10.1002/anie.201000048) [DOI] [PubMed] [Google Scholar]
- 99.Gruene T, et al. 2018. Rapid structure determination of microcrystalline molecular compounds using electron diffraction. Angew. Chem. Int. Ed. 57, 16 313–16 317. ( 10.1002/anie.201811318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michalski JR, Kraft MD, Diedrich T, Sharp TG, Christensen PR. 2003. Thermal emission spectroscopy of the silica polymorphs and considerations for remote sensing of Mars. Geophys. Res. Lett. 30, 1–4. ( 10.1029/2003GL018354) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.