Abstract
Electroactive and conducting framework materials, encompassing coordination polymers and metal–organic frameworks, have captured the imagination of the scientific community owing to their highly designable nanoporous structures and their potential applications in electrochromic devices, electrocatalysts, porous conductors, batteries and solar energy harvesting systems, among many others. While they are now considered integral members of the broader field of inorganic materials, it is timely to reflect upon their strengths and challenges compared with ‘traditional’ solid-state materials such as minerals, pigments and zeolites. Indeed, the latter have been known since ancient times and have been prized for centuries in fields as diverse as art, archaeology and industrial catalysis. This opinion piece considers a brief historical perspective of traditional electroactive and conducting inorganic materials, with a view towards very recent experimental progress and new directions for future progress in the burgeoning area of coordination polymers and metal–organic frameworks. Overall, this article bears testament to the rich history of electroactive solids and looks at the challenges inspiring a new generation of scientists.
This article is part of the theme issue ‘Mineralomimesis: natural and synthetic frameworks in science and technology’.
Keywords: coordination polymers, metal–organic frameworks, electroactive materials
1. Introduction
(a). Historical perspectives on traditional electroactive and conducting inorganic materials
Inorganic materials can be defined broadly as those derived from non-living sources that contain no organically produced carbon, such as rocks, minerals, glass, ceramics and metals; indeed most of the Earth is inorganic, comprising mainly oxide- and sulfide-based minerals. These materials can be natural or synthetic in origin, as in the case of pigments which are among the most historically significant members of the class. In the context of archaeology and art history, colour is one of the most important properties as exemplified by Egyptian blue, a pigment with early ancient Egyptian and Roman origins, which has been identified as calcium copper silicate (CaCuSi4O10) and is believed to be the first synthetically derived pigment of its colour at the time of its development [1]. The ability to manufacture pigments exhibiting a wide spectrum was historically significant as certain natural minerals were rare and difficult to work with.
In the modern age, the first synthetic inorganic pigment is widely documented as Prussian blue, Fe7(CN)18, which was synthesized accidentally in the seventeenth century by a colour-maker, Diesbach, from the reaction of iron sulfate and potash [2]. The intense blue coloration of this compound arises from its absorption of light in the red region of the electromagnetic spectrum at around 680 nm via a phenomenon known as ‘mixed valency’, which results in an intervalence charge transfer (IVCT) band [3]. Here, electron transfer occurs between alternate Fe(II) and Fe(III) centres linked by the bridging cyanide (CN−) ligands within the infinite three-dimensional cubic array of . An important property of Prussian blue that has been exploited in electrochromic devices [4] is its electroactivity which enables the in situ generation, using electrochemical methods, of the fully oxidized analogue (Berlin green), or the fully reduced state (Everitt's salt) [5]. The physical changes in the redox states of Prussian blue are further illustrated by the electronic and conducting properties: Prussian blue itself is a semiconductor, Berlin green exhibits a slight increase in conductivity from Prussian blue due to improved energy matching between high- and low-spin Fe(III) ions, and Everitt's salt containing purely Fe(II) is an insulator [6,7]. While the conductivities of the Prussian blues do not rival those of traditional inorganic materials such as metals, structure–function relationships in this general class of materials have been the subject of significant attention over the past five decades through variation of the metal ions, their redox states, and the presence of vacancies and guest species. For example, substitution of iron for heavier transition metals with more diffuse orbitals (e.g. Ru, Os) yields more delocalized systems [2,8]. Experimental studies have revealed that the compound K1.2Ru3.6[Ru(CN)6]3·16H2O shows a considerably higher electrical conductivity (5.69 × 10−3 S cm−1) than that of the parent compound due to increased framework delocalization [9]. However, the closed-shell nature of the cyanido ligand and the localized valence electrons of the metals prohibit full electron delocalization, as charge transfer is governed by hopping between sites that are relatively charge localized.
In addition to its prominence as a pigment in artworks as eminent as those of van Gogh, and as a dye-stuff for fabrics including those used in the manufacture of uniforms for the Prussian Army (from which it derives its name), Prussian blue analogues have gained commercial interests in electrochromic devices [4], ion exchange and host–guest chemistry [10,11], as well as battery materials for reversible lithium intercalation, among other applications [12]. Indeed, orally administered Prussian blue is listed with the World Health Organisation as one if its most important therapies for the sequestration of poisonous heavy metals [13]. Prussian blue and its analogues are considered members of inorganic materials known as ‘open framework compounds’ owing to the three-dimensional nature of the crystalline lattice that generates porosity in the material—a property that underlies its utility in these wide-ranging applications.
Zeolites represent arguably the most historically important open framework inorganic materials of both natural and synthetic origin [14]. Although the field is dominated by aluminosilicates owing to their stability and their wide industrial utility in catalysis, separations and ion exchange, zeolitic structures based on over 25 elements of the periodic table are known [15]. The periodic porous nature of these systems has been crucial in enabling structure elucidation in concert with their physico-chemical properties. In the context of their electroactive properties, the systematically variable sizes and charge selectivities of zeolites have been exploited to produce modified electrodes for electroanalysis of ions; however, the reproducibility of these electroanalytical platforms has generally been considered low due to the challenge of obtaining reproducible surfaces [16]. More recent approaches to producing electroactive and conducting zeolitic materials have exploited the host properties of the materials to confine organic species such as conducting polymers [17]. These inorganic–organic hybrid materials are known to aid the protection of the polymer from degradation and ageing, while retaining its fast reversible electrical conductivity. Meanwhile, the chemical resistance and high thermal stability of zeolite hosts are considered advantageous and enable the materials to be used in sensing applications, particularly for different cations [17].
A driving force for developments in zeolites has been the modulation of the pores for selective adsorption and other applications. Herein lies a challenge for these systems, as the limited sizes of the polyhedral centres and the means for their connection pose restrictions on the structures that can be achieved [15]. Nevertheless, in the late 1970s the work of Gravereau, Garnier and Hardy on zeolitic materials with ion-exchange properties demonstrated important progress [18].
(b). Towards infinitely tunable open framework coordination compounds
In the context of tuning the porosities of open framework compounds, a closely related strategy with rich historical origins over the past century has been the development of crystalline coordination polymers (CPs). Here, rigid organic moieties with preselected coordination modes coordinate to metal ions or metal clusters, creating a virtually limitless number of potential frameworks. This field gained significant momentum in the 1980s owing to the pioneering work by Robson and Hoskins who reported a series of ‘three-dimensional infinite polymeric frameworks’ (see [19]), followed by Kitagawa and co-workers' reports of ‘novel coordination polymers’ in the early 1990s. By the mid-1990s, Yaghi and co-workers determined a method for ‘guest binding and removal’ from the pores of CPs, which launched an explosion of research into metal–organic frameworks (MOFs) [20,21] for use in gas storage and separation. CPs and MOFs consist of metal ions or clusters coordinated by organic linkers to form one-, two- or three-dimensional networks [22]. They are self-assembled into highly crystalline compounds in which the building blocks are connected in a periodic, orderly fashion, and are infinitely extended.
The enormous possibilities in terms of the different combinations of organic and inorganic constituents give rise to countless structures with wide-ranging potential properties. Thus, frameworks with a variety of targeted chemical and physical functionalities can be achieved by designing metal and organic building units using molecular chemistry. ‘Crystal engineering’ is a promising way to tune the properties of frameworks either by the use of carefully selected starting materials in synthesis, or by post-synthesis modification involving either the framework backbone itself, or molecules and ions intercalated within the pores as guest species [23]. In the latter case, guest exchange can result in a framework with either a mixture of properties reminiscent of both guest and as-synthesized framework, or completely new physical and electronic characteristics. Structural modifications can also be induced through post-synthesis modification, through interactions between exchanged guest and framework, or through the displacement of framework metal and/or organic components using techniques such as solvent assisted linker exchange (SALE) [24].
Owing to their highly tunable surface areas and pore volumes, frameworks have been successfully implicated in an expanding number of applications including selective gas sorption, separations, sensing and drug delivery [25,26]. A recent trend that has emerged over the past decade is the incorporation of redox-active components into frameworks. The combination of redox modularity with porosity fulfils important prerequisites that underlie applications such as electrocatalysis and capacitance. Frameworks with electroactive or conductive properties, which can be introduced via de novo synthesis or post-synthesis modification, are also promising candidates as the basis of electronics applications including sensors and electrochromic devices, owing to their rich electronic and optical properties.
(c). Scope
In view of the rapidly paced nature of outputs in the field of CPs and MOFs, this perspective endeavours to highlight important general trends and very recent developments in experimental studies of electroactive and conducting frameworks following other recent reviews in the area [27–30]. In this regard, our discussion does not provide an exhaustive review of work in the area, for which the reader is referred to other excellent articles [12,31,32]. It is also important to distinguish the electroactive and conductive properties discussed herein as those intrinsic to the backbone of the framework material itself, rather than ionic conductivity through the pores of the material; although the combination of both properties has been shown to generate interesting new phenomena that could be exploited in applications such as supercapacitors [33].
2. Electroactive and conducting metal–organic frameworks
(a). Approaches to achieving electroactivity and conductivity in framework materials
Owing to their composition of one or more transition-metal-based ions and a combination of different organic ligands, a unique advantage of electroactive frameworks compared with many traditional inorganic materials is the potential access to a multitude of different redox states. If harnessed, the varied functions of these states as a result of their distinct structural, electronic, optical, magnetic, fluorescent and host–guest properties underpin a wide range of potential applications in which electroactivity and nanoporosity within a solid material is a significant asset.
Approaches to achieve redox activity in frameworks include integrating redox-active metal ions or organic ligands, by the post-synthetic introduction of redox-active molecules through covalent bonding to the metals or ligands, or as guests located in the pores [29]. The various redox states in these materials can be accessed by in situ electrochemical modulation or ex situ chemical oxidation and reduction. Important considerations here include the stability of the redox states and the diffusion of counterions into the pores for charge balance. In this regard, the coordination geometry of the metal ions and the structures of the organic components must remain robust to redox modulation. Moreover, guest species must be carefully selected for optimal diffusion through the pores without causing structural degradation.
In general, two synthetic approaches are taken to generate electroactive frameworks, the most common being the use of redox-active ligands and/or metals. Organic ligands based on tetrathiafulvalene (TTF) [34], tetracyanoquinodimethane (TCNQ) [35], viologen [36] and 2,5-dihydroxy-1,4-benzoquinone (H2dhbq) [37,38], among others, have been shown to impart their redox-active properties into framework materials. Alternatively, the use of redox-active d-block metals has also been used as an approach to form electroactive frameworks, de novo [39,40]. For example, the Cr(III) analogue of HKUST-1, [Cr3(btc)2] (btc = 1,3,5-benzenetricarboxylate), was shown to reversibly electrochemically cycle between the Cr(III)/(IV) and Cr(III)/(V) redox states [41]. In contrast to the parent HKUST-1 [42], redox modulation of [Cr3(btc)2] was found to promote CO2 sorption due to the stronger affinity towards higher oxidized states of chromium.
Post-synthetic intercalation of redox-active components is another common approach towards electroactive frameworks. An advantage of this method is that potential instability of frameworks can be avoided by choosing a robust parent system such that virtually any framework can be rendered electroactive. A notable example of this approach is the SALE method proposed by Farha & Hupp [43]. The zirconium framework, [Zr6(μ3-OH)8(OH)8(TBzPy)2] (NU-1000) (TBzPy = 1,3,6,8-tetrakis(p-benzoic acid)pyrene) was found to exhibit bias-switchable permselectivity upon SALE with ferrocene carboxylic acid [44], and the cation permeability was found to depend on the ferrocene/ferrocenium redox state. Ion permselectivity of this form has potential uses in framework-based sensors [45] and electrocatalysis [46,47]. Post-synthetic metathesis of metal ions has also been widely explored, such as the case of metathesis of [Zn4O(bdc)] (MOF-5) (bdc = 1,4-benzenedicarboxylate) with a series of redox-active first-row transition metal salts [48]. The Cr(II) exchanged MOF-5 analogue demonstrated the ability to oxidize to Cr(III) upon treatment with NOBF4 as confirmed by UV/Vis/NIR spectroscopy, while the Fe(II) exchanged framework displayed NO activation properties.
In addition to the potential applications of electroactive frameworks, at the fundamental level, these materials provide a unique opportunity to study deeply fundamental aspects of charge transfer in three-dimensional coordination space. Two key structural features enabling these fundamental insights include (i) the ability to study the precise atomic arrangement via crystallography, and (ii) the ease of modularity of components allowing for structure–property relationships to be investigated. While electroactivity in frameworks can be isolated to particular components, this property is recognized as an important prerequisite to imbue useful and interesting physical phenomena such as mixed valency and long-range electronic delocalization leading to conductivity [28].
In the context of fundamental charge transfer interactions in frameworks, ‘through-bond’ and ‘through-space’ mechanisms have been identified (figure 1) [32], with an increasing number of recent examples of dual experimental and computational studies aiding in the elucidation of mechanisms [27,30]. A major driving force for experimentalists has been the quest to achieve long-range conductivity, whereby charge transfer interactions are delocalized by exploiting redox matching of the components (this could be achieved through donor–acceptor or mixed-valence mechanisms) or π-interactions (such as π-conjugation or stacking). As the field progresses, attention is turning towards the computational and theoretical community to achieve new understandings of the underlying mechanisms in order to guide experimental design.
Figure 1.
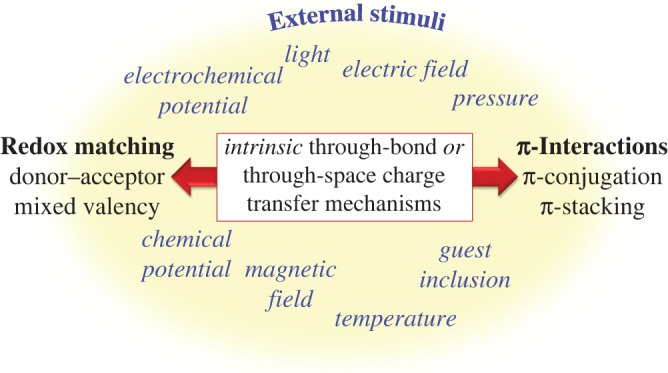
Mechanisms for charge transfer in framework materials including ‘through-bond’ and ‘through-space’ interactions such as redox matching and π-interactions. External stimuli can also be used to induce changes in the charge transfer properties. (Online version in colour.)
Through-bond mechanisms to achieve charge transfer leading to conductivity in frameworks have exploited conjugated organic ligands and metal ions containing relatively ‘loosely’ bound electrons. One of the first conductive frameworks, [Cu[Cu(pdt)2]] (pdt = 2,3-pyrazinedithiolate), was reported in 2009 by Takaishi and co-workers [49], in which Cu(II) ions were linked by pdt ligands forming two-dimensional sheets. The sheets were subsequently connected by copper bis(dithiolene) units to form the three-dimensional framework. The high-energy unpaired electrons of Cu(II) increased the charge density, contributing to the relatively high conductivity of the material which occurred through the [Cu(pdt)] sheets via the copper bis(dithiolene) units.
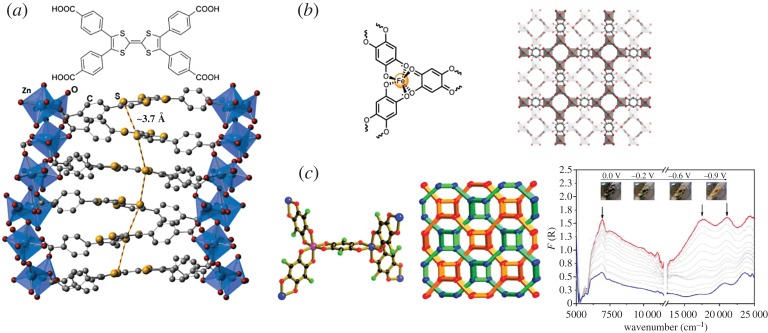
Charge transport through space has been realized by non-covalent interactions (π-stacking) between adjacent ligands which are close packed and provide sufficient orbital overlap, enabling a pathway for band-like charge transfer. A series of TTF-based MOFs, [M2(TTFTB)] (M = Mn, Co, Zn, Cd; TTFTB = tetrathiafulvalene tetrabenzoate), was reported by Dincă and co-workers in which TTF moieties were π–π stacked and the S···S distances between neighbouring TTFs were relatively short (similar to those in TTF–TCNQ and other conductive charge-transfer complexes), which were found to possess cation-dependent conductivity [50] (figure 2a). The smaller TTFTB separation found in frameworks containing the larger metal ions resulted in a higher conductivity, which was attributed to the greater charge mobility. Partial oxidation of the TTF cores was also found in the as-synthesized framework in the case of [Zn2(TTFTB)], from which the resulting radicals improved the charge density [51]. Donor–acceptor frameworks such as the tetrathiafulvalene–naphthalene diimide (TTF–NDI) material [(Zn(DMF))2(TTFTC)(DPNDI)]n (H4TTFTC represents tetrathiafulvalenetetracarboxylic acid and DPNDI represents di(4-pyridyl)-naphthalenediimide) have been strategically designed, where alternating TTF and NDI redox units are stacked at a distance of 3.6 Å [52]. This is strongly reminiscent of donor–acceptor charge transfer salts [53], and indeed the as-synthesized framework displayed radical and charge delocalization characteristics as evidenced electrochemically, spectroscopically and by spectroelectrochemistry.
Figure 2.
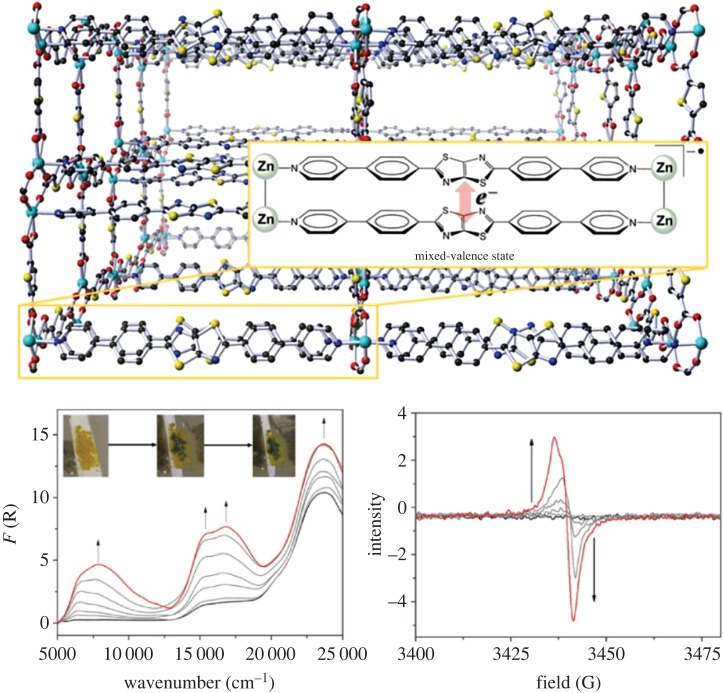
Examples of frameworks displaying higher electronic delocalization. (a) Ligand-based delocalization through proximal S···S contacts in the [Zn2(TTFTB)]n framework where TTFTB = tetrathiafulvalenetetrabenzoate as shown at the top. (b) Mixed ligand–metal redox activity giving rise to charge delocalization throughout the backbone of where dhbq represents dhbq2− (2,5-dioxidobenzoquinone) and dhbq3− (1,2-dioxido-4,5-semiquinone) in a mixed-valence state. (c) The H2fan (3,6-difluoro-2,5-dihydroxy-1,4-benzoquinone) analogue [(NBu4)2[Fe2(fan)3]]n demonstrating electrochemical modulation of mixed-valence behaviour, probed by UV/Vis/NIR spectroelectrochemistry (bottom right). Reproduced with permission from the American Chemical Society [66–68]. (Online version in colour.)
The first study to systematically probe localized through-space mixed-valence characteristics was conducted on a cofacial thiazolo[5,4-d]thiazole (TzTz) framework [Zn2(DPPTzTz)2(TDC)2]n where DPPTzTz represents 2,5-bis(4-(4-pyridynyl)phenyl)thiazolo[5,4-d]thiazole and H2TDC represents thiophene-2,5-dicarboxylic acid [54] (figure 3). Electrochemical or chemical reduction of the cofacial TzTz units to the mixed-valence state resulted in an intra-dimer mixed-valence interaction, which was evidenced by a characteristic near-infrared (NIR) band in the solid-state UV/Vis/NIR spectroelectrochemistry. Application of Marcus–Hush analysis revealed a class II Robin and Day IVCT interaction in the material [55,56]. Computational studies also revealed an important distance dependence for IVCT, opening up possibilities for tuning charge transfer through careful modulation of cofacial stacking geometries. Subsequently, by replacing the thiophene based co-ligand with the selenophene analogue, the incorporation of transition metal nodes other than Zn(II) was achieved [57], allowing structure–activity relationships to be elucidated.
Figure 3.
Localized through-space intervalence charge transfer (IVCT) in the cofacial framework [Zn2(DPPTzTz)2(TDC)2]n where DPPTzTz represents 2,5-bis(4-(4-pyridynyl)phenyl)thiazolo[5,4-d]thiazole and H2TDC represents thiophene-2,5-dicarboxylic acid. Upon electrochemical reduction to the radical mixed-valence state (according to the EPR spectroelectrochemistry shown on bottom right), an IVCT interaction within TzTz dimers was evidenced by a characteristic NIR band in UV/Vis/NIR spectroelectrochemistry (bottom left). Reproduced with permission from the American Chemical Society [54]. (Online version in colour.)
(b). Electronic delocalization in frameworks: towards conducting frameworks
While the majority of framework materials are insulators due to the use of diamagnetic metals and redox-innocent/closed-shell ligands, the aforementioned approaches have been investigated as a basis for engendering long-range electronic delocalization of the framework [27,30,32]. Redox-active components have been found to play an integral role in mediating charge transfer.
Electroactive framework materials have also attracted much attention for their potential uses as cathode or anode materials in batteries owing to their tailored design and permanent porosity [58–60]. [FeIII(OH)0.8F0.2(bdc)]·H2O (MIL-53(Fe)·H2O) (bdc2− = 1,4-benzenedicarboxylate) was the first example that exploited redox activity to mediate ion insertion [61]. In this framework, the reversible insertion of lithium ions generated the reduced form of the framework, allowing this material to act as cathode material. Mössbauer spectroscopy on the lithiated species revealed that almost half of the Fe(III) ions were reduced to Fe(II). The capacitance of MIL-53(Fe)·H2O only reached 70 mA h g−1 with the uptake of 0.6 lithium ions per formula unit, and further charging was limited due to framework decomposition. Although the capacitance of MIL-53(Fe)·H2O was low in contrast to proven battery materials, this work provided an impetus for frameworks to be investigated as electrochemical capacitors. An interesting example of a framework that exploits redox activity, porosity and conductivity to achieve supercapacitance has been demonstrated. Nanosheets of [Ni3(HITP)2] (HITP = 2,3,6,7,10,11-hexaaminotriphenylene) were found to exhibit high conductivity (2 S cm−1) and a NIR transition in the absorption spectrum, but also microporosity due to the extended linker [62]. Electrochemical studies revealed a purely capacitive response upon cycling cathodically between 0.02 and −0.6 V, creating a window of opportunity for this system to be investigated towards energy storage [33]. Moreover, the excellent electronic properties of [Ni3(HITP)2] allowed for the pelletized compound to act solely as the active electrode material in an anode half-cell without the need for conductive or emulsion additives. The gravimetric capacitance of this material was 111 F g−1 with only 10% loss of this capacitance over 10 000 cycles.
Changes in the redox states of materials are often accompanied by colour changes and a number of studies have aimed to exploit this phenomenon in frameworks for electrochromic devices [63]. The NDI-based framework [Ni2(NDISA)(H2O)2] (NDISA = N,N′-bis(3-carboxy-4-hydroxyphenyl)-1,4,5,8-naphthalenetetradicarboximide) was found to possess two stable redox states [63]. The deposition of the framework onto fluorine-doped tin oxide (FTO) and the subsequent spectroelectrochemical measurements revealed that the material underwent reversible electrochromic switching over a short (7 s) time scale. In a similar study, a series of two-dimensional nanosheets synthesized using bis(terpyridine)-based ligands exhibited the properties of a layered electrochromic device [64]. The preparation of the frameworks using a liquid–liquid interface method yielded nanometre thin films with relatively robust mechanical properties. The integration of two variants of this framework (cobalt and iron analogues) yielded a twofold electrochromic device. Commercially, electrochromic switching is already being implemented in applications such as transparent-to-dark windows and photo-switches [65]. The multifaceted nature of electroactive framework materials has great potential to enhance and expand on current technologies.
To date, the most successful strategy for the design of conducting frameworks has been the incorporation of a mixture of both metal- and ligand-based redox activity [28]. This is demonstrated by a number of examples, such as where dhbq represents dhbq2− (2,5-dioxidobenzoquinone) and dhbq3− (1,2-dioxido-4,5-semiquinone), where the ligands exist in a mixed-valence state (figure 2b) [67], as well as [(NBu4)2[Fe2(fan)3]]n where H2fan is 3,6-difluoro-2,5-dihydroxy-1,4-benzoquinone (figure 2c) [68]. In both examples, mediation of mixed-valence ligand states across the iron centres results in conductivities of 0.16 and 0.0055 S cm−1, respectively. Further improvements in conductivity have been achieved in two-dimensional CP sheets (termed CONASHs, coordination nanosheets, by Nishihara and co-workers [69]) constructed from benzenehexathiol (BHT), triphenylenehexathiol (THT) and their amine-substituted derivatives, which are coordinated in a bis-chelating fashion to redox-active transition metals such as Co(II), Ni(II) and Fe(II) to form planes of metal bis(dithiolene) components linked by benzene or triphenylene moieties [62,70–77]. The metal bis(dithiolene) cores within these CPs feature excellent mixing of thiol and metal coordination orbital energies, resulting in record-breaking room-temperature conductivities such as 1580 S cm−1 reported for the CuBHT analogue by Xu and co-workers [73]. Metallic conductivity at lower temperatures has also been reported for the CoTHT analogue by Marinescu and co-workers [76].
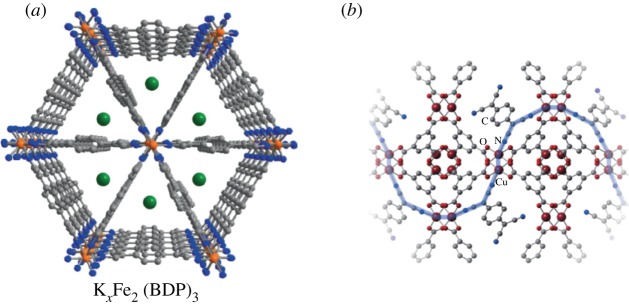
Post-synthetic doping with reductant or oxidant guests has also been shown to be a powerful strategy to control the redox characteristics of the ensuing framework, thereby directly influencing the conductivities. This is exemplified by a recent study from Long and co-workers on the framework [Fe2(BDP)3]n where BDP represents 1,4-benzenedipyrazolate (figure 4a) [40]. Here, reduction to form [KxFe2(BDP)3]n where 0 < x ≤ 2 resulted in a 10 000-fold anisotropic enhancement in conductivity. Less predictably so, post-synthetic modification has also been applied to introduce new mixed metal–guest electron delocalization pathways, as demonstrated by Talin, Allendorf and co-workers for [Cu3(BTC)2]n (H3BTC = 1,3,5-benzenetricarboxylic acid) [78]. Here, introduction of TCNQ leads to new electronic pathways between redox-active Cu nodes resulting in a dramatic conductivity enhancement (figure 4b).
Figure 4.
Examples of post-synthetic modification of frameworks to improve the electronic conductivity. (a) [Fe2(BDP)3]n where BDP represents 1,4-benzenedipyrazolate, where chemical reduction improves the conductivity and (b) the introduction of new pathways for electronic delocalization (blue) in the framework [Cu3(BTC)2]n by intercalation with TCNQ guests. Reproduced with permission from Macmillan Publishers Limited [40,78].
(c). Techniques for probing electroactive and conducting frameworks
For discrete redox-active molecules, probing localized charge transfer interactions can be routinely achieved using electrochemical, spectroscopic and spectroelectrochemical (SEC) techniques [79,80]. A common issue faced in the characterization of electroactive framework materials arises from their solid-state nature which often makes quantification of key optical and electrochemical parameters difficult. However, recent developments in characterization methods for electroactive frameworks have addressed a number of these challenges [57,81].
Solid-state DC electrochemistry is often employed as the primary method of assessing potential redox reactions in framework materials. Commonly used methods such as cyclic, square-wave and differential pulse voltammetry can yield qualitative data on the redox processes. In contrast to its solution-state counterpart, however, quantitative electrochemical measurements in the solid state are sometimes challenging to obtain due to the high capacitance versus Faradaic currents observed. While diffusion plays an integral role in solution-state electrochemistry, frameworks can often hinder the diffusion of counterions such that the observed redox processes may only be reflective of surface-confined processes [82]. However, diffusion of counterions can occur if an appropriately sized electrolyte is chosen. More recently, methods such as slow-scan voltammetry have been used to promote intercalation of ions into frameworks rather than on the crystallite surface [67]. Redox state modulation is often coupled with structural changes and a number of frameworks are susceptible to degradation as a result. Thus, additional characterization such as powder X-ray diffraction or vibrational spectroscopy should be undertaken to confirm a given framework's stability towards electrochemical cycling. While mechanical immobilization of frameworks onto electroactive surfaces such as glassy carbon is often adequate for laboratory-scale analyses, this is insufficient for applications where the interface with an electroactive substrate is important (e.g. electrocatalysis, electrochromic or ‘smart’ windows). Here, robust surface attachment of frameworks is crucial to ensure the efficacy of a device; methods include electrophoretic deposition [83], oriented epitaxial growth [84], and bottom-up assembly onto self-assembled monolayers [85], among others. A challenge here lies in the optimization of a given method for each framework—a non-trivial issue that requires significant future attention for commercialization of these materials [86].
While DC voltammetry is often used as the first step in the analysis of electroactive frameworks, the lack of quantitative data and the aforementioned drawbacks are such that determining more complex information, such as redox mechanisms and reaction kinetics, is non-trivial. An alternative method is the use of Fourier-transform AC voltammetry, which is a technique generalized by Bond and co-workers enabling Faradaic currents to be analysed without interference from high capacitive effects in materials [87]. In frameworks, the use of AC voltammetry has enabled mechanistic insights into the redox chemistry of zeolitic imidazolate frameworks, and the technique promises further utility for assessing electroactive mechanisms in a wide range of other materials [81,88].
In concert with electrochemistry, spectroelectrochemistry (SEC) is an invaluable tool for the in situ assessment of electrogenerated species [89]. This technique has played an integral role in the characterization of mixed-valence complexes, metal oxides, polymers and many other materials. SEC has also been coupled with commonly employed spectroscopic methods such as electron paramagnetic resonance (EPR) [90], IR/Raman [91,92] and UV/Vis [93]. The in situ nature of this experiment allows for the observation of unstable redox species at varying environmental (temperature, pressure and atmosphere) conditions and at variable ranges of spectroscopic time scales. Spectroelectrochemical cells are often custom-built for a given spectroscopic method and a number of designs are available in the literature. The most common variant, SEC Vis/NIR, has been widely employed in framework chemistry and has yielded important insights into optical and vibrational characteristics as a function of redox state [29,52]. For example, the combination of SEC Vis/NIR and EPR methods was essential to the characterization of the through-space IVCT interaction in the three-dimensional pillared framework [Zn2(BPPTzTz)2(tdc)2] shown in figure 3 [54].
Assessing the redox-state-dependent conductive properties of frameworks has relied, to date, on ex situ methods, often involving an appropriate oxidant (e.g. halides) or reductant (e.g. nitrosonium salts) via the solution or vapour phases. Careful consideration of the redox potential and stoichiometric quantity of oxidant/reductant enables the generation of frameworks with a systematic gradation of redox states from which conductivities can be measured (see for example [40] and [94]). In practice, control over the redox state can be difficult to achieve via these methods which require careful scrutiny to ensure that the structural integrity of materials is maintained. Traditionally, two- or four-point probe techniques along with electrical impedance spectroscopy have been used to assess conductivities; however, it is well acknowledged that grain boundary and contact resistance effects can lead to multiple order-of-magnitude inaccuracies. Anisotropic single-crystal measurements are thus considered crucial for more accurate conductivity assessments [50], with conductivity-based atomic force microscopy and scanning electron microscopy having been used when the crystal dimensions become intractable [77]. Nanojunction growth has provided another means of assessing conductivities in the move towards device fabrication of frameworks [78].
In combination with these ‘contact’ methods for assessing conductivities, ‘contactless’ techniques that negate issues such as contact resistances have been of significant interest. Here, charge mobilities can be assessed using microwave conductivity measurements [66], while the aforementioned SEC techniques yield information on the optical features of framework materials. In the latter case, a significant avenue for future work is the quantitative association between spectral signatures for charge transfer such as IVCT bands, with the solid-state conductivities. Indeed, this connection has already been considered for Prussian blue [95], but remains an open question for framework materials.
3. Future outlook: challenges and opportunities
The aforementioned examples demonstrate that electroactive and conductive frameworks have yielded important fundamental insights into charge transfer phenomena in three-dimensional coordination space due to the ability to interrogate structure–function relationships; however, more studies of this type are needed. By virtue of the tunability of physico-chemical phenomena in response to redox-state changes, an electroactive material is endowed with multifunctional properties which, if harnessed, could be exploited for practical applications. These range from exploiting the gas separation and storage properties such as electroswing adsorption [96], through to their implementation in unique electronic devices wherein coexisting electronic, optical and magnetic properties, for example, could be used [86]. The ability to modulate the electroactive and conductive properties with stimuli such as in situ electrochemical or ex situ chemical methods are now relatively well trialled; however, there is excellent potential to exploit other external stimuli such as pressure, light, magnetic and electric fields to modulate the electroactive and conductive properties. The ‘flexibility’ of some frameworks also offers the prospect of linking mechanical function (e.g. ‘piezo’ effects) with the electroactivity and conductivity. These lesser-used and in some cases ‘untapped’ stimuli offer interesting prospects for the future as they are known to modulate the electroactive and conducting properties of solids such as conducting and superconducting charge transfer salts [27]. Indeed, the tantalizing prospect of discovering a new class of superconductors in frameworks is also apparent, that may or may not arise upon variation of temperature or pressure, for example.
A significant issue hampering the future application of frameworks has been their lack of robustness and their instability under ambient conditions where zeolites, for example, possess superior structural properties. In this regard, very recent efforts have sought to integrate electroactive properties into framework scaffolds that are recognized as being more thermally and chemically robust, including those based on Zr(IV) [97].
For applications of electroactive frameworks in photo-, electro- and photoelectrocatalysis, Morris and co-workers have recently shown that there is no evidence to suggest an enhancement of capabilities brought about by strong long-range electronic coupling [98]. For these applications, localized charge transfer interactions have been demonstrated to be beneficial for electrocatalytic activity by providing avenues for stabilizing excited transition states.
Where conductivity is, however, desired for applications in electronic devices, the question of defects within the structures and their effects on electronic properties must be addressed; recently, the understanding and, indeed, optimization of defects within frameworks have been shown to have important implications for gas separation and adsorption [99]. A further critical issue identified by Dincă and co-workers is the variability in measurement methods for conductive frameworks that render comparisons between them challenging, sometimes presenting 2–3 orders of magnitude differences in the results [32]. The development of ‘contactless methods’ is a major advance here, but more systematic comparisons are needed on given frameworks. The development of in situ spectroelectrochemical methods that link the optical and electronic properties also offers prospects if quantitative connections can be established between, for example, the optical IVCT bands reminiscent of charge delocalization in frameworks and the conductivities. Systematic studies that probe the transition from localized to delocalized charge transfer would be particularly fruitful here, as they have been over the decades for discrete dinuclear complexes [79]. In the case of electroactive frameworks, interfacing the solids with appropriate electroactive surfaces has represented a non-trivial exercise, with optimization required for each framework. This presents an important avenue of endeavour given that their applications will be dependent on robust, reproducible devices.
Finally, an aspect not discussed herein, but which is critical within the area of electroactive and conducting frameworks, is the importance of computational calculations in concert with experimental studies. For electroactive materials, standard density functional theory (DFT) approaches have been used and provide a favourable description of localized charge transfer interactions where a ‘fragment’ of the framework structure can be used to approximate the three-dimensional solid (see for example [54]). For more highly conductive materials, band structure methods have been employed; however, questions remain as to the feasibility of these methods beyond the semiconducting regime given that correlation effects become increasingly important as the degree of electronic delocalization increases. This challenge offers fertile ground for experimentalists, theoreticians and computational chemists to unite. Equally important is the collaboration of chemists, traditionally occupied in fundamental analyses of frameworks, with engineers, mathematicians and physicists who bring new insights to bridge the disciplines. These challenges are difficult, albeit ultimately rewarding, ones for the emerging field of electroactive and conducting frameworks.
Acknowledgements
The Australian Research Council is gratefully acknowledged for financial support.
Data accessibility
This article has no additional data.
Authors' contributions
This article was jointly written by all authors.
Competing interests
We have no competing interests.
Funding
This research was supported by the Australian Research Council.
References
- 1.Coccato A, Moens L, Vandenabeele P. 2017. On the stability of mediaeval inorganic pigments: a literature review of the effect of climate, material selection, biological activity, analysis and conservation treatments. Herit. Sci. 5, 1–25. ( 10.1186/s40494-017-0125-6) [DOI] [Google Scholar]
- 2.Dunbar KR, Heintz RA. 1996. Chemistry of transition metal cyanide compounds: modern perspectives. Prog. Inorg. Chem. 45, 283–391. ( 10.1002/9780470166468.ch4) [DOI] [Google Scholar]
- 3.Ellis D, Eckhoff M, Neff VD. 1981. Electrochromism in the mixed-valence hexacyanides. 1. Voltammetric and spectral studies of the oxidation and reduction of thin films of Prussian blue. J. Phys. Chem. 85, 1225–1231. ( 10.1021/j150609a026) [DOI] [Google Scholar]
- 4.Itaya K, Shibayama K, Akahoshi H, Toshima S. 1982. Prussian-blue-modified electrodes: an application for a stable electrochromic display device. J. Appl. Phys. 53, 804–805. ( 10.1063/1.329997) [DOI] [Google Scholar]
- 5.Itaya K, Uchida I, Neff VD. 2002. Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc. Chem. Res. 19, 162–168. ( 10.1021/ar00126a001) [DOI] [Google Scholar]
- 6.Rosseinsky DR, Tonge JS, Berthelot J, Cassidy JF. 1987. Site-transfer conductivity in solid iron hexacyanoferrates by dielectric relaxometry, voltammetry and spectroscopy. Prussian Blue, congeners and mixtures. J. Chem. Soc., Faraday Trans. 1 83, 231–243. ( 10.1039/f19878300231) [DOI] [Google Scholar]
- 7.Pajerowski DM, Watanabe T, Yamamoto T, Einaga Y. 2011. Electronic conductivity in Berlin green and Prussian blue. Phys. Rev. B 83, 153202 ( 10.1103/PhysRevB.83.153202) [DOI] [Google Scholar]
- 8.Robin MB. 1962. Colour and electronic configurations of Prussian blue. Inorg. Chem. 1, 337–342. ( 10.1021/ic50002a028) [DOI] [Google Scholar]
- 9.Behera JN, D'Alessandro DM, Soheilnia N, Long JR. 2009. Synthesis and characterization of ruthenium and iron–ruthenium Prussian blue analogues. Chem. Mater. 21, 1922–1926. ( 10.1021/cm900230p) [DOI] [Google Scholar]
- 10.Kaye SS, Long JR. 2005. Hydrogen storage in the dehydrated Prussian blue analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 127, 6506–6507. ( 10.1021/ja051168t) [DOI] [PubMed] [Google Scholar]
- 11.Chapman KW, Southon PD, Weeks CL, Kepert CJ. 2005. Reversible hydrogen gas uptake in nanoporous Prussian Blue analogues. Chem. Commun. 2005 (26), 3322–3324. ( 10.1039/b502850g) [DOI] [PubMed] [Google Scholar]
- 12.Maspoch D, Ruiz-Molina D, Veciana J. 2007. Old materials with new tricks: multifunctional open-framework materials. Chem. Soc. Rev. 36, 770–818. ( 10.1039/b501600m) [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation 2017. WHO model lists of essential medicines. Geneva, Switzerland: World Health Organisation. See https://www.who.int/medicines/publications/essentialmedicines/en/. [Google Scholar]
- 14.Liu J, Yu J.. 2016. Towards greener and designed synthesis of zeolite materials. In Zeolites and zeolite-like materials (eds Sels BF, Kustov LM), ch. 1, pp. 1–32. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 15.Cheetham AK, Ferey G, Loiseau T. 1999. Open-framework inorganic materials. Angew. Chem. Int. Edn. 38, 3268–3292. () [DOI] [PubMed] [Google Scholar]
- 16.Walcarius A. 1996. Zeolite-modified electrodes: analytical applications and prospects. Electroanalysis 8, 971–986. ( 10.1002/elan.1140081102) [DOI] [Google Scholar]
- 17.Jaymand M. 2014. Conductive polymers/zeolite (nano-)composites: under-exploited materials. RSC Adv. 4, 33 935–33 954. ( 10.1039/C4RA03067B) [DOI] [Google Scholar]
- 18.Gravereau P, Garnier E, Hardy A. 1979. Les hexacyanoferrates zeolithiques: structure cristalline de K2Zn3[Fe(CN)6]2.xH2O. Acta Crystallogr. B 35, 2843–2848. ( 10.1107/S0567740879010797) [DOI] [Google Scholar]
- 19.Robson R. 2008. Design and its limitations in the construction of bi- and poly-nuclear coordination complexes and coordination polymers (aka MOFs): a personal view. Dalton Trans. 2008 (38), 5113–5131. ( 10.1039/B805617J) [DOI] [PubMed] [Google Scholar]
- 20.Eddaoudi M, Moler DB, Li H, Chen B, Reineke TM, O'Keeffe M, Yaghi OM. 2001. Modular chemistry: secondary building units as a basis for the design of highly porous and robust metal–organic carboxylate frameworks. Acc. Chem. Res. 34, 319–330. ( 10.1021/ar000034b) [DOI] [PubMed] [Google Scholar]
- 21.Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O'Keeffe M, Yaghi OM. 2003. Hydrogen storage in microporous metal–organic frameworks. Science 300, 1127–1129. ( 10.1126/science.1083440) [DOI] [PubMed] [Google Scholar]
- 22.Batten SR, Champness NR, Chen X-M, Garcia-Martinez J, Kitagawa S, Öhrström L, O'Keeffe M, Suh MP, Reedijk J. 2012. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 14, 3001–3004. ( 10.1039/C2CE06488J) [DOI] [Google Scholar]
- 23.Cohen SM. 2012. Postsynthetic methods for the functionalization of metal–organic frameworks. Chem. Rev. 112, 970–1000. ( 10.1021/cr200179u) [DOI] [PubMed] [Google Scholar]
- 24.Lalonde MB, Mondloch JE, Deria P, Sarjeant AA, Al-Juaid SS, Osman OI, Farha OK, Hupp JT. 2015. Selective solvent-assisted linker exchange (SALE) in a series of zeolitic imidazolate frameworks. Inorg. Chem. 54, 7142–7144. ( 10.1021/acs.inorgchem.5b01231) [DOI] [PubMed] [Google Scholar]
- 25.Lazaro IA, Forgan RS. 2019. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 380, 230–259. ( 10.1016/j.ccr.2018.09.009) [DOI] [Google Scholar]
- 26.Kirchon A, Feng L, Drake HF, Joseph EA, Zhou HC. 2018. From fundamentals to applications: a toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 47, 8611–8638. ( 10.1039/c8cs00688a) [DOI] [PubMed] [Google Scholar]
- 27.Usov PM, Leong CF, D'Alessandro DM. 2017. Conducting framework materials. In Functional supramolecular materials: from surfaces to MOFs (ed. R Banerjee), ch. 7, pp. 247–280. London, UK: The Royal Society of Chemistry. [Google Scholar]
- 28.Murase R, Leong CF, D'Alessandro DM. 2017. Mixed valency as a strategy for achieving charge delocalization in semiconducting and conducting framework materials. Inorg. Chem. 56, 14 373–14 382. ( 10.1021/acs.inorgchem.7b02090) [DOI] [PubMed] [Google Scholar]
- 29.D'Alessandro DM. 2016. Exploiting redox activity in metal–organic frameworks: concepts, trends and perspectives. Chem. Commun. 52, 8957–8971. ( 10.1039/C6CC00805D) [DOI] [PubMed] [Google Scholar]
- 30.Leong CF, Usov PM, D'Alessandro DM. 2016. Intrinsically conducting metal–organic frameworks. MRS Bull. 41, 858–864. ( 10.1557/mrs.2016.241) [DOI] [Google Scholar]
- 31.Givaja G, Amo-Ochoa P, Gomez-Garcia CJ, Zamora F. 2012. Electrical conductive coordination polymers. Chem. Soc. Rev. 41, 115–147. ( 10.1039/c1cs15092h) [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Campbell MG, Dincă M. 2016. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Edn. 55, 3566–3579. ( 10.1002/anie.201506219) [DOI] [PubMed] [Google Scholar]
- 33.Sheberla D, Bachman JC, Elias JS, Sun CJ, Shao-Horn Y, Dinca M. 2017. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–224. ( 10.1038/nmat4766) [DOI] [PubMed] [Google Scholar]
- 34.Wang H-Y, Cui L, Xie J-Z, Leong CF, D'Alessandro DM, Zuo J-L. 2017. Functional coordination polymers based on redox-active tetrathiafulvalene and its derivatives. Coord. Chem. Rev. 345, 342–361. ( 10.1016/j.ccr.2016.10.011) [DOI] [Google Scholar]
- 35.Shimomura S, Kitagawa S. 2011. Soft porous crystal meets TCNQ: charge transfer-type porous coordination polymers. J. Mater. Chem. 21, 5537–5546. ( 10.1039/c1jm10208g) [DOI] [Google Scholar]
- 36.Aulakh D, Varghese JR, Wriedt M. 2015. A new design strategy to access zwitterionic metal–organic frameworks from anionic viologen derivates. Inorg. Chem. 54, 1756–1764. ( 10.1021/ic5026813) [DOI] [PubMed] [Google Scholar]
- 37.Kitagawa S. 2002. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 224, 11–34. ( 10.1016/s0010-8545(01)00369-1) [DOI] [Google Scholar]
- 38.Mercuri ML, Congiu F, Concas G, Sahadevan SA. 2017. Recent advances on anilato-based molecular materials with magnetic and/or conducting properties. Magnetochemistry 3, 17 ( 10.3390/magnetochemistry3020017) [DOI] [Google Scholar]
- 39.Park JG, Aubrey ML, Oktawiec J, Chakarawet K, Darago LE, Grandjean F, Long GJ, Long JR. 2018. Charge delocalization and bulk electronic conductivity in the mixed-valence metal–organic framework Fe(1,2,3-triazolate)2(BF4)x. J. Am. Chem. Soc. 140, 8526–8534. ( 10.1021/jacs.8b03696) [DOI] [PubMed] [Google Scholar]
- 40.Aubrey ML, et al. 2018. Electron delocalization and charge mobility as a function of reduction in a metal–organic framework. Nat. Mater. 17, 625–632. ( 10.1038/s41563-018-0098-1) [DOI] [PubMed] [Google Scholar]
- 41.Leszczynski MK, Kornowicz A, Prochowicz D, Justyniak I, Noworyta K, Lewinski J. 2018. Straightforward synthesis of single-crystalline and redox-active Cr(II)-carboxylate MOFs. Inorg. Chem. 57, 4803–4806. ( 10.1021/acs.inorgchem.8b00395) [DOI] [PubMed] [Google Scholar]
- 42.Chui SS. 1999. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 283, 1148–1150. ( 10.1126/science.283.5405.1148) [DOI] [PubMed] [Google Scholar]
- 43.Deria P, Bury W, Hupp JT, Farha OK. 2014. Versatile functionalization of the NU-1000 platform by solvent-assisted ligand incorporation. Chem. Commun. 50, 1965–1968. ( 10.1039/c3cc48562e) [DOI] [PubMed] [Google Scholar]
- 44.Hod I, Bury W, Gardner DM, Deria P, Roznyatovskiy V, Wasielewski MR, Farha OK, Hupp JT. 2015. Bias-switchable permselectivity and redox catalytic activity of a ferrocene-functionalized, thin-film metal–organic framework compound. J. Phys. Chem. Lett. 6, 586–591. ( 10.1021/acs.jpclett.5b00019) [DOI] [PubMed] [Google Scholar]
- 45.Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. 2012. Metal–organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125. ( 10.1021/cr200324t) [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Zhu D, Guo C, Vasileff A, Qiao S-Z. 2017. Design strategies toward advanced MOF-derived electrocatalysts for energy-conversion reactions. Adv. Energy Mater. 7, 1700518 ( 10.1002/aenm.201700518) [DOI] [Google Scholar]
- 47.Solomon MB, Church TL, D'Alessandro DM. 2017. Perspectives on metal–organic frameworks with intrinsic electrocatalytic activity. CrystEngComm 19, 4049–4065. ( 10.1039/c7ce00215g) [DOI] [Google Scholar]
- 48.Brozek CK, Dinca M. 2013. Ti3+-, V2+/3+-, Cr2+/3+-, Mn2+-, and Fe2+-substituted MOF-5 and redox reactivity in Cr- and Fe-MOF-5. J. Am. Chem. Soc. 135, 12 886–12 891. ( 10.1021/ja4064475) [DOI] [PubMed] [Google Scholar]
- 49.Takaishi S, et al. 2009. Electroconductive porous coordination polymer Cu[Cu(pdt)2] composed of donor and acceptor building units. Inorg. Chem. 48, 9048–9050. ( 10.1021/ic802117q) [DOI] [PubMed] [Google Scholar]
- 50.Park SS, Hontz ER, Sun L, Hendon CH, Walsh A, Van Voorhis T, Dinca M. 2015. Cation-dependent intrinsic electrical conductivity in isostructural tetrathiafulvalene-based microporous metal–organic frameworks. J. Am. Chem. Soc. 137, 1774–1777. ( 10.1021/ja512437u) [DOI] [PubMed] [Google Scholar]
- 66.Narayan TC, Miyakai T, Seki S, Dinca M. 2012. High charge mobility in a tetrathiafulvalene-based microporous metal–organic framework. J. Am. Chem. Soc. 134, 12 932–12 935. ( 10.1021/ja3059827) [DOI] [PubMed] [Google Scholar]
- 67.Darago LE, Aubrey ML, Yu CJ, Gonzalez MI, Long JR. 2015. Electronic conductivity, ferrimagnetic ordering, and reductive insertion mediated by organic mixed-valence in a ferric semiquinoid metal–organic framework. J. Am. Chem. Soc. 137, 15 703–15 711. ( 10.1021/jacs.5b10385) [DOI] [PubMed] [Google Scholar]
- 68.Murase R, et al. 2017. Mixed valency in a 3D semiconducting iron–fluoranilate coordination polymer. Inorg. Chem. 56, 9025–9035. ( 10.1021/acs.inorgchem.7b01038) [DOI] [PubMed] [Google Scholar]
- 51.Leong CF, Wang C-H, Ling CD, D'Alessandro DM. 2018. A spectroscopic and electrochemical investigation of a tetrathiafulvalene series of metal–organic frameworks. Polyhedron 154, 334–342. ( 10.1016/j.poly.2018.07.023) [DOI] [Google Scholar]
- 52.Leong CF, Chan B, Faust TB, D'Alessandro DM. 2014. Controlling charge separation in a novel donor–acceptor metal–organic framework via redox modulation. Chem. Sci. 5, 4724–4728. ( 10.1039/c4sc01551g) [DOI] [Google Scholar]
- 53.Jiang H, Yang X, Cui Z, Liu Y, Li H, Hu W, Kloc C. 2014. Adjusting tetrathiafulvalene (TTF) functionality through molecular design for organic field-effect transistors. CrystEngComm 16, 5968–5983. ( 10.1039/C3CE41849A) [DOI] [Google Scholar]
- 54.Hua C, Doheny PW, Ding B, Chan B, Yu M, Kepert CJ, D'Alessandro DM. 2018. Through-space intervalence charge transfer as a mechanism for charge delocalization in metal–organic frameworks. J. Am. Chem. Soc. 140, 6622–6630. ( 10.1021/jacs.8b02638) [DOI] [PubMed] [Google Scholar]
- 55.Robin MB, Day P. 1968. Mixed valence chemistry—a survey and classification. In Advances in inorganic chemistry and radiochemistry (eds Emeléus HJ, Sharpe AG), vol. 10, pp. 247–422. Cambridge, MA: Academic Press. [Google Scholar]
- 56.Hush NS. 1968. Homogeneous and heterogeneous optical and thermal electron transfer. Electrochim. Acta 13, 1005–1023. ( 10.1016/0013-4686(68)80032-5) [DOI] [Google Scholar]
- 57.Ding B, Hua C, Kepert CJ, D'Alessandro DM. 2019. Influence of structure–activity relationships on through-space intervalence charge transfer in metal–organic frameworks with cofacial redox-active units. Chem. Sci. 10, 1392–1400. ( 10.1039/c8sc01128a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patwardhan S, Schatz GC. 2015. Theoretical investigation of charge transfer in metal organic frameworks for electrochemical device applications. J. Phys. Chem. C 119, 24 238–24 247. ( 10.1021/acs.jpcc.5b06065) [DOI] [Google Scholar]
- 59.Wang L, Han Y, Feng X, Zhou J, Qi P, Wang B. 2016. Metal–organic frameworks for energy storage: batteries and supercapacitors. Coord. Chem. Rev. 307, 361–381. ( 10.1016/j.ccr.2015.09.002) [DOI] [Google Scholar]
- 60.Zhang Z, Awaga K. 2016. Redox-active metal–organic frameworks as electrode materials for batteries. MRS Bull. 41, 883–889. ( 10.1557/mrs.2016.245) [DOI] [Google Scholar]
- 61.Ferey G, Millange F, Morcrette M, Serre C, Doublet ML, Greneche JM, Tarascon J-M. 2007. Mixed-valence Li/Fe-based metal–organic frameworks with both reversible redox and sorption properties. Angew. Chem. Int. Edn. 46, 3259–3263. ( 10.1002/anie.200605163) [DOI] [PubMed] [Google Scholar]
- 62.Sheberla D, Sun L, Blood-Forsythe MA, Er S, Wade CR, Brozek CK, Aspuru-Guzik A, Dinca M. 2014. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a semiconducting metal–organic graphene analogue. J. Am. Chem. Soc. 136, 8859–8862. ( 10.1021/ja502765n) [DOI] [PubMed] [Google Scholar]
- 63.AlKaabi K, Wade CR, Dincă M. 2016. Transparent-to-dark electrochromic behavior in naphthalene-diimide-based mesoporous MOF-74 analogs. Chem 1, 264–272. ( 10.1016/j.chempr.2016.06.013) [DOI] [Google Scholar]
- 64.Takada K, Sakamoto R, Yi ST, Katagiri S, Kambe T, Nishihara H. 2015. Electrochromic bis(terpyridine)metal complex nanosheets. J. Am. Chem. Soc. 137, 4681–4689. ( 10.1021/ja510788b) [DOI] [PubMed] [Google Scholar]
- 65.Dolgopolova EA, Shustova NB. 2016. Metal–organic framework photophysics: optoelectronic devices, photoswitches, sensors, and photocatalysts. MRS Bull. 41, 890–896. ( 10.1557/mrs.2016.246) [DOI] [Google Scholar]
- 69.Maeda H, Sakamoto R, Nishihara H. 2016. Coordination programming of two-dimensional metal complex frameworks. Langmuir 32, 2527–2538. ( 10.1021/acs.langmuir.6b00156) [DOI] [PubMed] [Google Scholar]
- 70.Kambe T, et al. 2013. π-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 135, 2462–2465. ( 10.1021/ja312380b) [DOI] [PubMed] [Google Scholar]
- 71.Cui J, Xu Z. 2014. An electroactive porous network from covalent metal–dithiolene links. Chem. Commun. 50, 3986–3988. ( 10.1039/c4cc00408f) [DOI] [PubMed] [Google Scholar]
- 72.Campbell MG, Sheberla D, Liu SF, Swager TM, Dinca M. 2015. Cu3(hexaiminotriphenylene)2: an electrically conductive 2D metal–organic framework for chemiresistive sensing. Angew. Chem. Int. Edn. 54, 4349–4352. ( 10.1002/anie.201411854) [DOI] [PubMed] [Google Scholar]
- 73.Huang X, et al. 2015. A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 6, 7408 ( 10.1038/ncomms8408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang P, Hou X, Liu L, Mi J, Dong M. 2015. Two-dimensional π-conjugated metal bis(dithiolene) complex nanosheets as selective catalysts for oxygen reduction reaction. J. Phys. Chem. C 119, 28 028–28 037. ( 10.1021/acs.jpcc.5b09148) [DOI] [Google Scholar]
- 75.Liu H, Li X, Chen L, Wang X, Pan H, Zhang X, Zhao M. 2016. Gas adsorption effects on the electronic properties of two-dimensional nickel bis(dithiolene) complex. J. Phys. Chem. C 120, 3846–3852. ( 10.1021/acs.jpcc.5b11699) [DOI] [Google Scholar]
- 76.Clough AJ, Skelton JM, Downes CA, de la Rosa AA, Yoo JW, Walsh A, Melot BC, Marinescu SC. 2017. Metallic conductivity in a two-dimensional cobalt dithiolene metal–organic framework. J. Am. Chem. Soc. 139, 10 863–10 867. ( 10.1021/jacs.7b05742) [DOI] [PubMed] [Google Scholar]
- 77.Sun X, Wu K-H, Sakamoto R, Kusamoto T, Maeda H, Nishihara H. 2017. Conducting π-conjugated bis(iminothiolato)nickel nanosheet. Chem. Lett. 46, 1072–1075. ( 10.1246/cl.170382) [DOI] [Google Scholar]
- 78.Talin AA, et al. 2014. Tunable electrical conductivity in metal–organic framework thin-film devices. Science 343, 66–69. ( 10.1126/science.1246738) [DOI] [PubMed] [Google Scholar]
- 79.D'Alessandro DM, Keene FR. 2006. Current trends and future challenges in the experimental, theoretical and computational analysis of intervalence charge transfer (IVCT) transitions. Chem. Soc. Rev. 35, 424–440. ( 10.1039/b514590m) [DOI] [PubMed] [Google Scholar]
- 80.Hua C, Rizzuto FJ, Zhang X, Tuna F, Collison D, D'Alessandro DM. 2017. Spectroelectrochemical properties of a Ru(II) complex with a thiazolo[5,4-d]thiazole triarylamine ligand. New J. Chem. 41, 108–114. ( 10.1039/C6NJ02802K) [DOI] [Google Scholar]
- 81.Usov PM, Simonov AN, Bond AM, Murphy MJ, D'Alessandro DM. 2017. Untangling complex redox chemistry in zeolitic imidazolate frameworks using Fourier transformed alternating current voltammetry. Anal. Chem. 89, 10 181–10 187. ( 10.1021/acs.analchem.7b01224) [DOI] [PubMed] [Google Scholar]
- 82.Carbó AD. 2009. Electrochemistry of porous materials. Boca Raton, FL: CRC Press. [Google Scholar]
- 83.Hod I, et al. 2014. Directed growth of electroactive metal–organic framework thin films using electrophoretic deposition. Adv. Mater. 26, 6295–6300. ( 10.1002/adma.201401940) [DOI] [PubMed] [Google Scholar]
- 84.Gu ZG, Zhang J. 2019. Epitaxial growth and applications of oriented metal–organic framework thin films. Coord. Chem. Rev. 378, 513–532. ( 10.1016/j.ccr.2017.09.028) [DOI] [Google Scholar]
- 85.Rubio-Gimenez V, et al. 2018. Bottom-up fabrication of semiconductive metal–organic framework ultrathin films. Adv. Mater. 30, 1704291 ( 10.1002/adma.201704291) [DOI] [PubMed] [Google Scholar]
- 86.Stassen I, Burtch N, Talin A, Falcaro P, Allendorf M, Ameloot R. 2017. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 46, 3185–3241. ( 10.1039/c7cs00122c) [DOI] [PubMed] [Google Scholar]
- 87.Bond AM, Duffy NW, Guo SX, Zhang J, Elton D. 2005. Changing the look of voltammetry. Can FT revolutionize voltammetric techniques as it did for NMR? Anal. Chem. 77, 186A–195A. ( 10.1021/ac053370k) [DOI] [PubMed] [Google Scholar]
- 88.Usov PM, McDonnell-Worth C, Zhou F, MacFarlane DR, D'Alessandro DM. 2015. The electrochemical transformation of the zeolitic imidazolate framework ZIF-67 in aqueous electrolytes. Electrochim. Acta 153, 433–438. ( 10.1016/j.electacta.2014.11.150) [DOI] [Google Scholar]
- 89.Kaim W, Fiedler J. 2009. Spectroelectrochemistry: the best of two worlds. Chem. Soc. Rev. 38, 3373–3382. ( 10.1039/b504286k) [DOI] [PubMed] [Google Scholar]
- 90.Harder SR, Feinberg BA, Ragsdale SW. 1989. A spectroelectrochemical cell designed for low temperature electron paramagnetic resonance titration of oxygen-sensitive proteins. Anal. Biochem. 181, 283–287. ( 10.1016/0003-2697(89)90244-3) [DOI] [PubMed] [Google Scholar]
- 91.Niaura G, Gaigalas AK, Vilker VL. 1997. Moving spectroelectrochemical cell for surface Raman spectroscopy. J. Raman Spectrosc. 28, 1009–1011. () [DOI] [Google Scholar]
- 92.Krejčik M, Daněk M, Hartl F. 1991. Simple construction of an infrared optically transparent thin-layer electrochemical cell: applications to the redox reactions of ferrocene, Mn2(CO)10 and Mn(CO)3(3,5-di-t-butyl-catecholate). J. Electroanal. Chem. Interfacial Electrochem. 317, 179–187. ( 10.1016/0022-0728(91)85012-E) [DOI] [Google Scholar]
- 93.Usov PM, Fabian C, D'Alessandro DM. 2012. Rapid determination of the optical and redox properties of a metal–organic framework via in situ solid state spectroelectrochemistry. Chem. Commun. 48, 3945–3947. ( 10.1039/c2cc30568b) [DOI] [PubMed] [Google Scholar]
- 94.Schneider C, et al. 2018. High electrical conductivity and high porosity in a Guest@MOF material: evidence of TCNQ ordering within Cu3BTC2 micropores. Chem. Sci. 9, 7405–7412. ( 10.1039/c8sc02471e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.England SJ, Kathirgamanathan P, Rosseinsky DR. 1980. Perturbation calculation from the charge-transfer spectrum data of intervalence site-transfer D.C. conductivity in Prussian Blue. J. Chem. Soc., Chem. Commun. 1980 (17), 840–841. ( 10.1039/c39800000840) [DOI] [Google Scholar]
- 96.Leong CF, Faust TB, Turner P, Usov PM, Kepert CJ, Babarao R, Thornton AW, D'Alessandro DM. 2013. Enhancing selective CO2 adsorption via chemical reduction of a redox-active metal–organic framework. Dalton Trans. 42, 9831–9839. ( 10.1039/c3dt00083d) [DOI] [PubMed] [Google Scholar]
- 97.Johnson BA, Bhunia A, Fei H, Cohen SM, Ott S. 2018. Development of a UiO-type thin film electrocatalysis platform with redox-active linkers. J. Am. Chem. Soc. 140, 2985–2994. ( 10.1021/jacs.7b13077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin S, Usov PM, Morris AJ. 2018. The role of redox hopping in metal–organic framework electrocatalysis. Chem. Commun. 54, 6965–6974. ( 10.1039/C8CC01664J) [DOI] [PubMed] [Google Scholar]
- 99.Ren JW, Ledwaba M, Musyoka NM, Langan HW, Mathe M, Liao SJ, Pang W. 2017. Structural defects in metal–organic frameworks (MOFs): formation, detection and control towards practices of interests. Coord. Chem. Rev. 349, 169–197. ( 10.1016/j.ccr.2017.08.017) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.