Abstract
Heterogeneity within the Alzheimer's disease (AD) syndromic spectrum is typically classified in a domain-specific manner (e.g., language vs. visual cognitive function). The central aim of this study was to investigate whether impairment in visual cognitive tasks thought to be subserved by posterior cortical dysfunction in non-amnestic AD presentations is associated with tau, amyloid, or neurodegeneration in those regions using 18F-AV-1451 and 11C-PiB positron emission tomography (PET) and magnetic resonance imaging (MRI). Sixteen amyloid-positive patients who met criteria for either Posterior Cortical Atrophy (PCA; n = 10) or logopenic variant Primary Progressive Aphasia (lvPPA; n = 6) were studied. All participants underwent a structured clinical assessment, neuropsychological battery, structural MRI, amyloid PET, and tau PET. The neuropsychological battery included two visual cognitive tests: VOSP Number Location and Benton Facial Recognition. Surface-based whole-cortical general linear models were used to first explore the similarities and differences between these biomarkers in the two patient groups, and then to assess their regional associations with visual cognitive test performance. The results show that these two variants of AD have both dissociable and overlapping areas of tau and atrophy, but amyloid is distributed with a stereotyped localization in both variants. Performance on both visual cognitive tests were associated with tau and atrophy in the right lateral and medial occipital association cortex, superior parietal cortex, and posterior ventral occipitotemporal cortex. No cortical associations were observed with amyloid PET. We further demonstrate that cortical atrophy has a partially mediating effect on the association between tau pathology and visual cognitive task performance. Our findings show that non-amnestic variants of AD have partially dissociable spatial patterns of tau and atrophy that localize as expected based on symptoms, but similar patterns of amyloid. Further, we demonstrate that impairments of visual cognitive dysfunction are strongly associated with tau in visual cortical regions and mediated in part by atrophy.
Keywords: Alzheimer's disease, Amyloid imaging, Tau imaging, Brain atrophy, Visual function
Highlights
-
•
The Visual-cognitive Impairment Rating (VIR) scale is designed to rate visual cognitive and functional impairment.
-
•
Performance on two visual cognitive tasks was associated with tau and atrophy in posterior cortical regions.
-
•
Cortical atrophy is a partial mediator of the association between tau pathology and visual cognitive task performance.
1. Introduction
Alzheimer's disease (AD) may present as a diverse spectrum of clinical syndromes arising from a common underlying pathology. Apart from the typical amnestic presentations, other clinical variants arising from AD pathology are posterior cortical atrophy (PCA) and logopenic variant primary progressive aphasia (lvPPA) (Koedam et al., 2010; Snowden et al., 2007) among other presentations. PCA, commonly thought of as a “visual variant” of the AD syndrome (Benson et al., 1988), is defined clinically by a progressive decline in higher-order visual processing and other posterior cortical functions and radiologically associated with prominent tau pathology in the occipital, posterior parietal, and posterior temporal lobes (Crutch, et al., 2017; Mendez et al., 2002; Tang-Wai et al., 2004). In contrast, lvPPA is characterized primarily by variably non-fluent speech, difficulties with word retrieval and sentence repetition, and phonological speech errors (Gorno-Tempini et al., 2011). In addition to predominant language deficits, lvPPA patients also exhibit impairments in visual construction, visual attention, and spatial working memory (Foxe et al., 2013; Watson et al., 2018), which have been attributed to dysfunction of the superior parietal lobule, a region implicated in spatial perception, attention, and working memory (Bueicheku et al., 2015; Cabeza, 2008). Though both PCA and lvPPA demonstrate visual cognitive impairments, the nature and extent of these specific impairments, along with the understanding of how pathology drives these impairments mechanistically, is still not well understood. Additionally, the neuroanatomy of atrophy in PCA and lvPPA, while dissociable, seems to share the overlapping involvement of posterior cortical regions extending into the occipital lobe (Migliaccio et al., 2009; Warren et al., 2012). Here we aimed to bring these lines of observation together in a study of the relationship between amyloid, tau, and neurodegeneration (Jack Jr. et al., 2018) and visual cognitive impairment across non-amnestic AD syndromes of PCA and lvPPA.
Visual cognition is supported by two major cortical pathways: the ventral and dorsal pathways. The ventral pathway, which extends from the ventral visual association cortex through the occipitotemporal cortex to the anterior temporal lobe and is responsible for conscious visual perception of objects, faces, and colors (Mishikin et al., 1983). The dorsal pathway, which extends from dorsal visual association cortex to posterior parietal and frontal cortical regions, is thought to differentiate into multiple pathways subserving visual attention, space perception, and visually guided action (Kravitz et al., 2011). Although deficits in higher-order visual information processing are observable across the AD spectrum, it is unclear whether the deficits are dissociable into those associated with impairments in dorsal or ventral cortical visual pathways (Galton et al., 2000; Ross et al., 1996; Tsai et al., 2011). A recent expert consensus classification proposes that the majority of PCA patients have dorsal stream deficits and a minority have ventral stream deficits (Crutch, et al., 2017). Although one study of early symptom clusters in PCA supports this view (Tsai et al., 2011), it has not yet been investigated rigorously using psychometric tests and tau PET imaging.
The focus of this study was to determine if visual cognitive task performance supported by these posterior visual processing streams are specifically associated with tau pathology in those regions measured by tau (18F-AV-1451) positron emission tomography (PET) and cortical atrophy across two atypical phenotypes of AD. We aim to build on several previously published studies that have demonstrated syndrome-specific patterns of tau deposition using this PET tracer across the AD syndromic spectrum (Day et al., 2017; Ossenkoppele et al., 2015a; Ossenkoppele et al., 2015b; Ossenkoppele et al., 2016; Xia et al., 2017), and would build on that work to clarify our understanding of how tau pathology and neurodegeneration in atypical variants of the AD syndrome impact visuospatial cognition. In relation to this primary aim, we also endeavored to improve clinical characterization of visual cognitive deficits across the AD syndromic spectrum by creating a visual cognitive rating scale to serve as an adjust to the standard Clinical Dementia Rating (CDR) scale utilized frequently in clinical research. We also performed an exploratory analysis of whether amyloid, tau, and atrophy have both dissociable localization and partially overlapping localization in these two non-amnestic presentations of AD. In light of previous work suggesting that tau and atrophy appear in parallel (Jack Jr. et al., 2002), and that the impact of tau pathology on cognition may be mediated by grey matter loss (Bejanin et al., 2017), a secondary aim was to compare associations between visual cognition and tau pathology to associations between visual cognition and cortical atrophy, and investigate to what extent cortical atrophy mediates the relationship between tau pathology and visual cognitive task performance. Building on work that has linked visual dysfunction in both PCA and lvPPA to posterior parietal dysfunction, these non-amnestic AD patients were investigated together in pooled analyses to leverage a wide range of performance on the visual cognitive tasks and range of posterior cortical dysfunction. We hypothesized that a traditional neuropsychological task designed to measure space perception (VOSP Number Location) would be related to tau and atrophy in the right hemisphere occipitoparietal cortex, while a neuropsychological task designed to measure face perception (Benton Facial Recognition) would be related to tau and atrophy in occipitotemporal cortex, including the fusiform face area. We also performed a preliminary analysis of mediation to test the hypothesis from the pathology literature that neurofibrillary tangles drive neurodegeneration (at least in part), which causes cognitive impairment (Giannakopoulos et al., 2003; Gomez-Isla et al., 1997; Terry et al., 1991).
2. Methods
2.1. Participants
Sixteen individuals were recruited from the Massachusetts General Hospital (MGH) Frontotemporal Disorders Unit PPA and PCA Programs (Putcha et al., 2018). See Table 1 for full demographic data. All patients received a standard clinical evaluation comprising a comprehensive neurological and psychiatric history and exam, structured informant interviews, and a neuropsychological battery including the National Alzheimer's Coordinating Center (NACC) Uniform Data Set (UDS) version 3.0 battery (See Table 2.), Frontotemporal Lobar Degeneration (FTLD) module, and a battery focused on visuospatial cognition. Clinical formulation was performed as we have described—through consensus discussions in our multidisciplinary team—with each patient being classified based on all clinical information as having mild cognitive impairment or dementia (global clinical status), and then each patient's cognitive-behavioral syndrome being diagnosed (Dickerson et al., 2017). Ten patients met diagnostic criteria for PCA (Crutch, et al., 2017; Mendez et al., 2002; Tang-Wai et al., 2004), and 6 patients met criteria for lvPPA (Gorno-Tempini et al., 2011). Participants also underwent neuroimaging sessions which included a high-resolution 3 Tesla MRI, 18F-AV-1451 positron emission tomography (tau PET), and Pittsburgh Compound B (PiB) positron emission tomography (amyloid PET) imaging. We only included patients in this study who had a positive amyloid PET scan, as assessed by visual read according to previously published procedures (Rabinovici et al., 2010), and met biomarker criteria for probable Alzheimer's disease (distribution volume ratio (DVR) > 1.2; Villeneuve et al., 2015a). We chose to analyze a combined sample of PCA and lvPPA in order to leverage the natural heterogeneity and range in behavioral performance and tau signal in posterior cortical regions across patients, which allows us to investigate these brain-behavior relationships.
Table 1.
Clinical and demographic characteristics. The mean (SD) is presented for each continuous demographic factor. * indicates a statistically significant difference (p < 0.05) between PCA and lvPPA groups. M = Male; F = Female, R = Right Handed; L = Left Handed; FLR DVR = Frontal-Lateral-Retrosplenial Distribution Volume Ratio. CDR = Clinical Dementia Rating. VIR = Visual Impairment Rating.
| Demographic | ALL (N = 16) | PCA (N = 10) | lvPPA (N = 6) |
|---|---|---|---|
| Age (years) | 66.6 (7.8) | 66.1 (9.4) | 67.3 (4.7) |
| Sex (Male: Female) | 5 M: 11F | 3 M: 7F | 2 M: 4F |
| Education (years) | 16.7 (2.3) | 17.2 (1.4) | 15.8 (3.4) |
| Handedness (R:L) | 14R: 2 L | 9R: 1 L | 5R: 1 L |
| Symptom duration (years) | 4.0 (1.1) | 4.0 (0.8) | 4.0 (1.5) |
| Amyloid PET FLR DVR | 1.6 (0.2) | 1.6 (0.2) | 1.7 (0.2) |
| CDR Global | 0 (N = 1); 0.5 (N = 10); 1 (N = 5) |
0.5 (N = 6); 1 (N = 4) |
0 (N = 1) 0.5 (N = 4); 1 (N = 1) |
| CDR sum of boxes | 3.6 (1.9) | 4.3 (1.9) | 2.5 (1.5) |
| CDR supplemental language | 0 (N = 6) 0.5 (N = 6) 1 (N = 3) 2 (N = 1) |
0 (N = 6) 0.5 (N = 3) 1 (N = 1) |
0.5 (N = 3) 1 (N = 2) 2 (N = 1) |
| VIR (Visual-cognitive impairment rating) | 0 (N = 5) 0.5 (N = 1) 1 (N = 10) |
1 (N = 10) | 0 (N = 5) 0.5 (N = 1) |
Table 2.
Neuropsychological Test Performance. All tests presented in this table are part of the NACC UDS3 Battery and supplement FTLD module. The mean (SD) is presented for each continuous demographic factor. * indicates a statistically significant difference (p < 0.05) between PCA and lvPPA groups. MOCA = Montreal Cognitive Assessment. NACC=National Alzheimer's Coordinating Center. UDS = Unified Data Set. FTLD = Frontotemporal Lobar Degeneration.
| Test | ALL (N = 16) | PCA (N = 10) | lvPPA (N = 6) |
|---|---|---|---|
| MOCA (out of 30) | 17.1 (5.8) | 18.3 (6.8) | 15.2 (3.2) |
| Longest digit span forward | 5.1 (2.1)* | 6.0 (1.6) | 4.0 (2.08) |
| Longest digit span backward | 3.3 (1.5) | 3.5 (1.4) | 2.9 (1.6) |
| Longest spatial span forward | 3.2 (1.1)* | 2.6 (0.5) | 4.3 (1.4) |
| Longest spatial span backward | 2.9 (1.0)* | 2.4 (0.5) | 3.7 (1.3) |
| Craft story learning (/44) | 9.3 (5.8) | 11.6 (6.1) | 6.0 (3.7) |
| Craft story delayed recall (/44) | 7.4 (6.5) | 8.8 (7.5) | 5.3 (4.4) |
| Craft story % retention | 73.5 (33.6) | 67.7 (37.2) | 82.1 (28.2) |
| Benson figure copy (/17) | 10.8 (6.4)* | 3.5 (1.3) | 15.6 (1.4) |
| Benson figure delayed recall (/17) | 4.5 (4.3)* | 1.3 (1.5) | 6.7 (4.2) |
| Letter fluency (F/L/A/S) | 13*/14/12/16 | 17/16/14/18 | 9/11/6/10 |
| Category fluency (Animals/Vegetables/Fruits) | 12/7/8 | 13/7/10 | 10/8/6 |
| Multilingual naming Test (/32) | 19.8 (7.8) | 18.4 (9.4) | 22.0 (4.4) |
| Multilingual naming test with semantic and phonemic cues (/32) | 25.3 (5.8) | 25 (7.4) | 26 (2.8) |
| Word picture matching (/20) | 19.8 (0.4) | 19.7 (0.5) | 20.0 (0) |
| Semantic associates (/16) | 15.9 (0.3) | 15.8 (0.4) | 16.0 (0) |
MRI, tau PET, and amyloid PET data from twenty-six cognitively normal older adults (mean age = 67.8 ± 4.5 years, 13 M/13F) was used to determine the magnitude of imaging abnormalities in the patients. These individuals were evaluated rigorously using a structured evaluation including the NACC UDS 3.0 as part of their participation in the Massachusetts Alzheimer's Disease Center Longitudinal Cohort. Clinical status was deemed cognitively normal for all control participants (CDR = 0) and neuropsychological performance was within normal limits. They were also determined to have normal brain structure based on MRI and low cerebral amyloid based on a visual read and quantitative analysis of amyloid PET (DVR < 1.1).
Individuals were excluded from this cohort if they had a primary psychiatric or other neurologic disorder. This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All participants and their informants/caregivers provided informed consent in accordance with the protocol approved by the Partners HealthCare Human Research Committee Institutional Review Board in Boston, Massachusetts.
2.2. Visual-cognitive Impairment Rating (VIR)
We developed the Visual-cognitive Impairment Rating (VIR) scale to characterize the clinical severity of patients in the visual cognitive domain. The format of the rating scale is similar to a Clinical Dementia Rating (CDR) scale (Morris, 1993), and is meant to be used as an adjunct to the existing CDR scale. The VIR is designed to capture the overall level of visual-cognitive impairment as it relates to cerebral dysfunction, such as visual object agnosia, prosopagnosia, visuoperceptual impairment, simultanagnosia, hemispatial neglect, hemianopsia, or cortical blindness, that are not attributed to primary ocular or extra-ocular movement dysfunction or cognitive impairment in non-visual domains. The VIR was rated by integrating information about visual-cognitive symptoms in daily life and visuoperceptual and visual-cognitive abnormalities on examination, without knowledge of neuropsychological test performance. VIR ratings of all participants in this study, as well as CDR ratings, were conducted by a neurologist (S.M.M, B.C.D, or M.C.E.), and were based on a semi-structured patient and informant interview and a neurologic examination of the patient that specifically includes evaluation of cortical visual dysfunction. VIR ratings were as follows: 0 = clinically normal, 0.5 = questionable/very mild impairment, 1 = mild impairment, 2 = moderate impairment, 3 = severe impairment, and reported in Table 1 along with CDR scores. VIR scoring guidelines are detailed in Supplementary Material Table 1.
2.3. Visual cognitive tasks
Within a maximum of four months from the PET and MR scans, all participants underwent a neuropsychological task battery assessing visual-cognitive skills. We chose to include two tasks in the current analysis – one that has been associated with dorsal visual stream processing: Visual Object Space Processing battery (VOSP): Number Location Test (Warrington and James, 1991), and one that has been associated with ventral visual stream processing: the Benton Facial Recognition Test (Benton and Van Allen, 1968). In the VOSP Number Location test, ten arrays are presented and each array is comprised of two squares arranged one above the other. The top square contains numbers arranged randomly. The bottom square contains only a black dot. The patient is asked to verbally identify which number corresponds to the same spatial location as the black dot. Each correct identification earns one point (maximum 10). In the Benton Facial Recognition test, patients are shown arrays of black and white faces, and asked to match a sample face to either one out of six sample faces, or three out of six sample faces shown in different lighting and orientation conditions. Each correctly identified face earns one point (maximum 27). Percent correct and normative data for each test based on age- and education-based published norms (Herrera-Guzman et al., 2004; Spreen and Strauss, 1991) are reported. Performance differences between PCA and lvPPA subgroups were investigated using independent samples t-tests. The association between neuropsychological performance and measures of tau and cortical thickness were investigated using Pearson's bivariate correlations. For these analyses, as well as routine group comparisons on demographic factors, p values <.05 were considered statistically significant. Primary hypothesis-driven analyses were conducted on just two tests of interest with no corrections for multiple comparisons applied. Statistical analyses were conducted in IBM SPSS Version 24.0 (Armonk, NY).
2.4. Neuroimaging data acquisition and analysis
All sixteen patients underwent 18F-AV-1451 (tau) and 11C- Pittsburgh Compound B (amyloid) PET scans. The 18F-AV-1451 radiotracer was prepared at MGH with a radiochemical yield of 14% ± 3% and specific activity of 216 ± 60 GBq/μmol (5837 ± 1621 mCi/μmol) at the end of synthesis (60 min) and validated for human use (Shoup et al., 2013). Scans were acquired from 80 to 100 min after a 10.0 ± 1.0 mCi bolus injection in 4 × 5 min frames. The 11C-PiB PET radiotracer was acquired with an 8.5 to 15 mCi bolus injection followed immediately by a 60-min dynamic acquisition in 69 frames (12 × 15 seconds, 57 × 60 seconds). All PET data were acquired using a Siemens/CTI (Knoxville, TN) ECAT HR+ scanner (3D mode; 63 image planes; 15.2 cm axial field of view; 5.6 mm transaxial resolution and 2.4 mm slice interval). Data were reconstructed and attenuation corrected; each frame was evaluated to verify adequate count statistics; interframe head motion was corrected prior to further processing.
All patients also underwent a high-resolution MRI scan (Siemens TIM Trio 3.0 Tesla) with an average time delay of 23 ± 21 days from PET scans, and included acquisition of T1-weighted multi-echo magnetization prepared rapid acquisition gradient echo (MPRAGE) structural images. The MRI analysis methods employed here have been previously described in detail (Dale et al., 1999; Fischl et al., 2004), including cortical thickness processing and spherical registration to align subjects' cortical surfaces. Visual inspection confirmed accurate registration between anatomical and PET volumes. To evaluate the anatomy of PET binding, each individual's PET data set was rigidly co-registered to the subject's MPRAGE data using SPM8 (Wellcome Department of Cognitive Neurology, Function Imaging Laboratory, London). Similar to a previous report, 18F-AV-1451 specific binding was expressed as the standardized uptake value ratio (SUVR) using the whole cerebellar grey matter as a reference (Johnson et al., 2016). 11C-PiB PET data were expressed as the distribution volume ratio (DVR) with the cerebellar grey matter as a reference (Becker et al., 2011), where regional time-activity curves (TAC) were used to compute regional DVRs for each ROI using the Logan graphical method applied to data from 40 to 60 min after injection. PET data were not partial volume corrected and were performed using geometric transform matrix as implemented in FreeSurfer stable release version 6.0.
Using methods we have previously published (Makaretz et al., 2018; Xia et al., 2017), whole cortex general linear models (GLM) were created to determine where tau, amyloid, and cortical atrophy were present in PCA and lvPPA patients separately, compared to a control group using FreeSurfer version 6.0. We also generated group overlap maps in each imaging modality. Effect size maps from the GLMs were created, and thresholds were adjusted based on which modality was examined for illustrative purposes (gamma >0.8 for tau PET, > 0.3 for cortical atrophy, and > 0.4 for amyloid PET). Then, to determine if visual task performance was related to tau, amyloid or cortical atrophy in hypothesized regions, statistical surface maps were generated by computing a GLM for the effects of the task performance on each imaging variable at each vertex point on the cortical surface, controlling for age. This analysis was implemented using mri_glmfit with task scores as the independent variables of interest and tau PET SUVR, amyloid PET DVR, or cortical thickness as the dependent variable, controlling for age. Given our relatively small sample size and specific a priori hypotheses, an uncorrected statistical threshold of p < 0.01 was set.
As mentioned above, we also aimed to determine if cortical thickness mediates the relationship between tau and visual task performance (Bejanin et al., 2017). To do this, we created overlap maps that included only vertices that showed a significant association between cognitive performance and both tau PET and cortical thickness (at p < 0.01). The mean tau PET SUVR and cortical thickness measures were then extracted from the overlapping region of interest and used to perform mediation analysis, conducted separately for each task using multiple regression in SPSS version 24. In Step 1, task performance was regressed on tau SUVR to examine the total effect of tau on task performance. In Step 2, cortical atrophy was regressed on tau SUVR. In Step 3, we performed multiple regression analysis where we regressed task performance on both tau SUVR and cortical atrophy. In Step 4, we compared the standardized regression coefficients (beta) of the tau SUVR predictor computed at Step 1 (total effect) and Step 3 (direct effect) to determine the amount of mediation by cortical atrophy. As age, sex, and education did not appear to be confounds based on non-significant bivariate correlations with either the independent or dependent variables, these demographic factors were not included in the mediation analysis.
3. Results
3.1. Clinical characteristics and cognitive performance in non-amnestic AD
Table 1 shows sample characteristics. With regard to global clinical status, the patients included in this study were either classified as having mild cognitive impairment (CDR = 0.5) or mild dementia (CDR = 1). One exception was a very mild lvPPA patient who had a global CDR of 0, though this patient was rated with focal language deficits (CDR = 1 on the supplemental language box) impacting the CDR community affairs box score (0.5). There were no differences between PCA and lvPPA groups on any clinical or demographic variable, except for the expected differences on domain-specific ratings of impairment in the language domain (CDR supplemental Language box score), where lvPPA patients were rated as more impaired compared to PCA, and in the visual-cognitive domain score (VIR), where PCA patients were more impaired than lvPPA.
Performance on the VOSP Number Location and Benton Facial Recognition tests (Fig. 1) revealed that the PCA patients performed worse than the lvPPA patients: on the VOSP Number Location Test, mean % correct was 33.7 ± 11.9 in the PCA group, and 81.7 ± 18.3 in the lvPPA group (t = 5.9, df = 12, p = 0.00007), and on the Benton Facial Recognition Test, mean % correct was 67.7 ± 10.4 in the PCA group, and 84.6 ± 8.6 in the lvPPA group (t = 3.2, df = 12, p = 0.009). Normatively, the PCA group was severely impaired (z-score = −4.3 ± 0.91) and the lvPPA group was intact (z-score = −0.70 ± 1.4) on the VOSP Number Location test. Nevertheless, there was a range of performance within each group as shown in scatterplots (Fig. 3 and Fig. 4). At the individual level, all PCA patients and two lvPPA patients performed under the cutoff for clinical impairment (z < −1.5). On the Benton Facial Recognition Test, the PCA group was moderately impaired (z-score = −2.6 ± 1.5), and the lvPPA group was intact (z-score = −0.20 ± 1.0) compared to normative data. Individually, 7 out of 8 PCA patients and no lvPPA patients performed under the cutoff for clinical impairment (z < −1.5). However, 2 out of 6 lvPPA patients had z-scores less than −1.2, indicating vulnerability on this test. Symptom duration was not related to performance on either task (all p > 0.5). Age, education, and sex were not related to performance on either task (all p > .10). Performance on the two tasks was highly correlated in the whole group (r = 0.76, p = .003), but not correlated when examined within each syndromic subgroup separately (PCA: r = 0.52, p = .23; lvPPA: r = 0.41, p = .42).
Fig. 1.

Visual cognitive task performance in non-amnestic AD. The PCA group performed worse than the lvPPA group on both tasks: one thought to target the dorsal visual stream (VOSP Number Location), and one thought to target the ventral visual stream (Benton Facial Recognition). * indicates group differences at p < 0.05.
Fig. 3.
Impaired performance on the VOSP Number Location test is associated with elevated tau and atrophy in dorsal and ventral visual cortical regions. Whole cortex general linear models demonstrate that (A) tau PET signal and (B) cortical thickness were associated with performance in topographically similar regions in predominantly right hemisphere posterior parietal and ventral occipitotemporal cortex, after controlling for age. Results show maps of statistical significance values, thresholded at p < 0.01. Scatterplots illustrate the effects depicted in the maps, with values derived from the entire correlated region shown for each modality. These examples show robust relationships between impaired VOSP Number Location performance and (C) elevated tau signal (r = −0.89, p = 0 00002) as well as (D) cortical atrophy (r = 0.92, p = 0.000003).
Fig. 4.
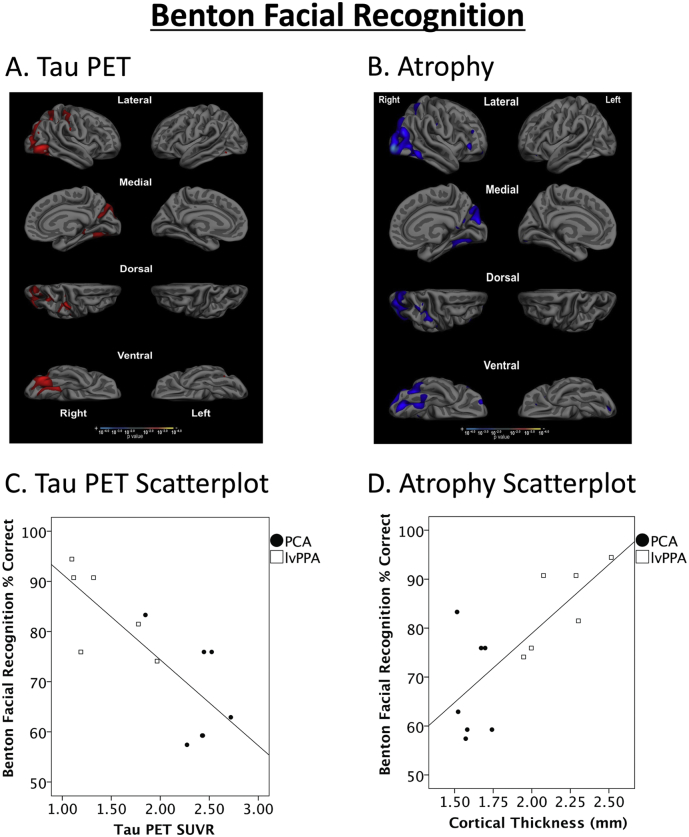
Impaired performance on the Benton Facial Recognition test is associated with elevated tau and atrophy in ventral and dorsal visual cortical regions. Whole cortex general linear models demonstrate that (A) tau PET signal and (B) cortical thickness were associated with performance in occipitotemporal cortex, as well as right hemisphere posterior parietal lobule, after controlling for age. Results show maps of statistical significance values, thresholded at p < 0.01. Scatterplots illustrate the effects depicted in the maps, with values derived from the entire correlated region shown for each modality. These examples show robust relationships between impaired Benton Facial recognition performance and (C) elevated tau signal (r = −0.79, p = 0 001) as well as (D) cortical atrophy (r = 0.74, p = 0.004).
On the remainder of the testing battery (See Table 2), PCA patients demonstrated better performance compared to lvPPA patients on an auditory attention task (Digit Span Forward; t = 2.5, p = 0.02) and letter fluency (F words; t = 3.8, p = 0.003), but performed more poorly compared to the lvPPA group on spatial attention (Spatial Span Forward; t = −2.8, p = 0.01) and spatial working memory (Spatial Span Backward, t = −2.5, p = 0.03) tasks. PCA patients also performed worse than lvPPA on the Benson Figure Copy (t = −14.08, p = 0.000006) and Delayed Recall (t = −2.4, p = 0.04), and 6 out of 10 of the PCA patients were so impaired that they could not attempt the task. PCA patients performed comparably (p > 0.05) to the lvPPA group on an auditory-verbal working memory task (Digit Span Backward), and on the Craft story memory learning, recall, and recognition. No individual in either PCA or lvPPA group demonstrated impairment on two basic visual recognition tests (Word Picture Matching and Semantic Associates), demonstrating intact basic visual function necessary for object recognition and semantic memory.
3.2. Tau PET, cortical atrophy, and amyloid PET signal are distinct but overlapping in PCA and lvPPA
We explored the localization of tau, amyloid, and atrophy in the cerebral cortex of the two groups, as well as the overlap between both groups. This exploration was meant to illustrate areas of common and distinct pathology between these groups, as well as the relationships of the imaging measures to each other. Compared to age-matched control participants, maps of cortical tau in PCA (Fig. 2A, left) revealed occipital, ventral and posterolateral temporal, lateral parietal, precuneus, and posterior cingulate cortex tau pathology with a slight rightward predominance. In contrast, maps of cortical tau in lvPPA (Fig. 2A, right) revealed left greater than right tau pathology in lateral temporal, lateral parietal, precuneus, and posterior cingulate cortices. Tau pathology common to both PCA and lvPPA groups (Fig. 2A, central) is in bilateral posterolateral temporal, inferior parietal, precuneus, and posterior cingulate cortex. Compared to age-matched control participants, maps of cortical thickness in PCA patients (Fig. 2B, left) revealed a pattern very similar to the localization of tau pathology in this group. Cortical thickness maps in lvPPA patients (Fig. 2B, right) revealed atrophy that again was localized in a manner that closely corresponded to tau pathology but extended more anteriorly in the temporal cortex. The localization of cortical atrophy common to both PCA and lvPPA groups is small (Fig. 2B, central), and observed primarily in posterolateral temporal, inferior parietal, and posterior cingulate cortices. Lastly, compared to control participants, maps of amyloid PET revealed a stereotyped pattern in both PCA (Fig. 2C, left) and lvPPA groups (Fig. 2C, right), adhering to the typical spatial localization of amyloid and including large regions of the lateral temporal, medial parietal, and medial and lateral frontal lobes. Areas of overlap common to PCA and lvPPA comprised medial and lateral frontal lobes and medial parietal cortex.
Fig. 2.
Tau (18F-AV-1451) PET, amyloid (11C-PiB) PET, and Cortical Atrophy in non-amnestic AD. A. Maps of cortical tau PET in PCA (left), lvPPA (right), and areas of overlap common to the two groups (center), compared to age-matched control participants. Effect size, calculated by FreeSurfer as gamma, is displayed, with minimum threshold set to 0.8. B. Maps of cortical thickness in PCA (left), lvPPA (right), and areas of overlap atrophy common to the two groups (center), compared to age-matched control participants. Effect size (gamma) is displayed, with minimum threshold set to 0.3. C. Maps of cortical amyloid PET in PCA (left), lvPPA groups (right), and areas of overlap amyloid PET signal common to the two groups (center), compared to age-matched control participants. Effect size (gamma) is displayed, with minimum threshold set to 0.4. Thresholds were selected for illustrative purposes.
3.3. Visual cognitive task performance associated with tau and atrophy in posterior visual processing regions
Performance on the VOSP Number Location, a test hypothesized to measure spatial attention, was correlated with tau PET in bilateral visual association areas, right hemisphere superior parietal cortex, and predominantly right hemisphere lateral and medial occipitotemporal cortex, such that increased tau signal in these regions was associated with decreased task performance after controlling for age (Fig. 3A). No associations between task performance and tau signal was observed in anterior temporal or frontal lobe regions. Task performance was also strongly correlated with cortical thickness in largely the same regions (Fig. 3B). No regions showed significant associations between task performance and amyloid PET after controlling for age (Supplementary Material Fig. 1, left). Performance on the Benton Facial Recognition test, hypothesized to measure face perception, was negatively associated with tau PET in bilateral visual association areas and right hemisphere fusiform gyrus, but also in predominantly right hemisphere superior parietal cortex and intraparietal sulcus, such that increased tau in these regions was associated with decreased task performance after controlling for age (Fig. 4A). Task performance was also positively associated with cortical thickness in largely the same regions (Fig. 4B). No regions showed significant associations between task performance and amyloid PET after controlling for age (Supplementary Material Fig. 1, right). As neither sex nor education was related to performance on either visual cognitive task, inclusion of these factors as covariates in these regression analyses did not change the results.
3.4. Cortical atrophy partially mediates the relationship between tau pathology and VOSP number location performance
We conducted a preliminary mediation analysis with a strong a priori hypothesis that cortical atrophy would partially mediate the relationship between tau PET and visual cognitive performance. We created overlap maps that included vertices where both tau and cortical atrophy were associated with VOSP Number Location performance (Fig. 5A, center). The mean tau SUVR and cortical thickness measures were then extracted from this overlapping region of interest and used to perform mediation analysis (Fig. 5B). As shown in Supplementary Material Fig. 2, SUVR was strongly correlated with cortical thickness measures from this overlap region of interest (r = −0.79, p = 0.0002). Both tau PET and cortical atrophy demonstrated strong independent relationships with task performance as discussed above. Partial correlation analysis showed that the association between tau PET and performance on the VOSP Number Location test remained significant, but to a lesser degree, when cortical thickness was taken into account (ß = −0.47, p = 0.02; Supplementary Material Fig. 3). In sum, our mediation analysis revealed both direct and atrophy-mediated relationships between tau pathology and VOSP Number Location performance (Fig. 5B). We repeated the same analysis for the tau PET and atrophy correlates of Benton Facial Recognition task performance. The associations between Benton Facial Recognition performance and tau pathology (ß = -0.79, p = 0.001) as well as between Benton Facial Recognition performance and cortical thickness (ß =0.74, p = 0.004) were very similar, and the tau PET SUVR was very strongly negatively correlated with cortical thickness (r = −0.82, p = 0.00009). Because of this very high degree of correspondence, when we examined the partial correlations between tau PET SUVR and performance on the Benton Facial Recognition test when cortical thickness was taken into account, this relationship was no longer significant (ß = −0.57, p = 0.14). In fact, the relationship between cortical thickness and Benton Facial Recognition performance was also no longer significant (ß = −0.26, p = 0.47), despite the overall regression model explaining 65% of the task variance with both predictors entered (F = 9.1, p = 0.006). This suggests that our measures of tau pathology and cortical atrophy in this analysis shared too much variance to disentangle the unique variance of each contributor to predicting task performance. As such, our mediation analysis on the Benton Facial Recognition task revealed only direct effects of tau PET on task performance, as well as cortical atrophy on task performance, but not a mediating effect of atrophy. Inclusion of age, sex, and education as covariates did not change the outcome of the model.
Fig. 5.
Cortical atrophy partially mediates the relationship between tau pathology and visual cognitive task performance on VOSP Number Location. Tau PET and cortical atrophy measurements from the overlap region (A, center) were included in the mediation model to predict visual cognitive task performance. Filled arrows represent significant effects of the mediation analysis (B), indicating both direct and indirect effects of tau pathology on VOSP task performance.
4. Discussion
PCA and lvPPA syndromes represent non-amnestic presentations of AD that are associated with partially overlapping clinical, neuroanatomical, and pathological profiles (Warren et al., 2012). Although deficits in higher-order visual processing are observable across the AD syndromic spectrum, it has so far been unclear whether the deficits are clearly attributable to dysfunction of dorsal or ventral visual pathways, or both (Galton et al., 2000; Ross et al., 1996; Tsai et al., 2011). Here we hypothesized specific regional associations between performance on visual cognitive tasks and imaging markers of tau PET and cortical atrophy, but not amyloid PET. We further hypothesized that cortical atrophy would partially mediate the relationship between tau and task performance, based on previous work suggesting the parallel processes of tau deposition and grey matter volume loss in AD (Gomez-Isla et al., 1997; Jack Jr. et al., 2002; Whitwell et al., 2008).
We first explored amyloid, tau, and neurodegeneration in these two clinical phenotypes of AD to examine whether we could observe both dissociable and overlapping regions of pathology when these two variants were compared. In PCA, we found that tau and cortical atrophy were closely co-localized in bilateral dorsal occipital and superior parietal cortex and precuneus, as well as in lateral and medial ventral occipital and posterior ventral temporal cortex, with a slight right hemisphere predominance. In lvPPA, we found that tau pathology and cortical atrophy were closely co-localized with each other in lateral temporal, inferior parietal, and precuneus cortical regions with a left hemisphere predominance. The subgroups showed some overlap in regions affected by tau pathology and atrophy, primarily in posterolateral temporal, inferior parietal, and medial parietal regions, expanding on prior reports in these AD variants using similar imaging markers (Ossenkoppele et al., 2015b; Ossenkoppele et al., 2016; Xia et al., 2017). In contrast, amyloid was found in widespread regions of frontal, lateral temporal, lateral parietal, and precuneus cortices in both groups, with a high degree of overlap between PCA and lvPPA amyloid PET maps, consistent with many previously published studies demonstrating that amyloid PET does not distinguish patients with distinct clinical syndromes at the symptomatic stage of the disease (Day et al., 2017; de Souza et al., 2011; Ossenkoppele et al., 2016; Rosenbloom et al., 2011; Xia et al., 2017).
As expected, our PCA patients were rated as functionally impaired on our novel Visual-cognitive Impairment Rating (VIR) scale. Their impairments on visual cognitive task performance—obtained independently of the VIR—provide convergent validity for the VIR. Regarding the visual cognitive tasks, our PCA patients demonstrated impairment on both visuospatial cognition tasks of spatial attention and facial recognition, and these task impairments were correlated. That is, our sample does not demonstrate very dissociable performance on these tests. These results do not support previous reports on individuals with PCA that have suggested a greater dorsal compared to ventral visual stream dysfunction (Tsai et al., 2011). We did not have a sample size sufficient to directly address the question of increasing ventral stream dysfunction with more progressed stages of the disease, though this remains an important topic of future investigation. Importantly, none of our patients demonstrated impairment on basic tests of visual object matching (word picture matching, semantic associates), indicating that they did not have early visual perceptual or associative processing impairments that might have had global effects on these higher-order visual cognitive tests.
We demonstrated that visual cognitive impairment was related to both tau burden and cortical atrophy, such that worse task performance was related to increased tau burden and cortical atrophy in predominantly right hemisphere occipital, superior parietal, and ventral occipitotemporal regions for both the “dorsal” space perception task as well as the “ventral” facial recognition task. Though we did not observe the regional specificity we hypothesized, we did identify right hemisphere predominance as would be expected of the neuroanatomical substrates supporting visual cognitive task performance. One previous study which found regional relationships between cortical atrophy and space perception deficits compared to object perception deficits in PCA (Lehmann et al., 2011) also observed a large amount of common cortical atrophy across both dorsal and ventral deficit groups when compared to age-matched control participants, such that the group with predominantly ventral dysfunction also demonstrated atrophy in dorsal regions and vice versa. This is consistent with our findings in the current study, as we observed some associations between our “dorsal” task and ventral pathology, and some associations between our “ventral” task and dorsal pathology. We interpret this finding to reflect the fact that visual cognitive tasks used in clinical settings often do not purely target the function they are designed to isolate. Specifically, the VOSP Number Location test is a test of spatial attention, but also requires an individual to accurately recognize numbers, which is associated with bilateral fusiform and lingual regions of the ventral object recognition stream (Knops, 2017). Similarly, though the Benton Facial Recognition test is designed to measure face recognition, it also requires an individual to scan through an array of faces and scan between target and foils in order to answer correctly, functions that rely heavily on dorsal attention systems. There are well documented connections between the two streams in the macaque (Borra et al., 2008; Borra et al., 2010), purported to integrate disparate information from each visual stream together to enable meaningful interactions with the environment (Milner, 2017). We conclude that although the VOSP Number Location test was designed to measure space perception, it may also recruit functions supported by the ventral visual stream. Similarly, though the Benton Facial Recognition test is a measure of face perception, it may also relies on intact functioning of the dorsal attention system. Another likely possibility is that our sample may not be representative of the full spectrum of visual cognitive deficits. That is, given the breadth of impairment observed on dorsal stream tasks with relative preservation of ventral stream tasks (visual object recognition on tests of semantic associates, word-picture matching), the majority of our PCA sample may be predominantly impaired in the dorsal regions without enough ventrally-impaired individuals to fully test this dorsal vs. ventral dissociation.
We did not observe any significant regional associations between amyloid PET and visual cognitive task performance, further supporting the neuropathological observations that the localization of amyloid plaques does not contribute strongly to cognitive impairments (Nelson et al., 2012). In contrast, the associations between task performance and tau burden were quite strong and regionally specific, similar to cortical atrophy correlates, consistent with previous studies showing the close correspondence between tau and neurodegeneration as the likely drivers of cognitive impairments (Bejanin et al., 2017; Ossenkoppele et al., 2015b; Xia et al., 2017). These results support a large body of previous neuropathological and in vivo work demonstrating that neurofibrillary tangle pathology is closely associated with neuronal loss, and likely a main driver of cognitive changes in AD (Giannakopoulos et al., 2003; Gomez-Isla et al., 1997; Terry et al., 1991). Our mediation analysis confirmed that cortical atrophy is a partial mediator of the relationship between tau pathology and performance on the VOSP Number Location test, such that tau pathology has an independent association with visuospatial cognition, but also has an indirect effect on cognition via cortical atrophy. However, we did not observe a mediation effect when examining predictors of performance on the Benton Facial Recognition test, as our measures of tau deposition and cortical atrophy were much more tightly correlated in relation to this task than with the VOSP Number Location analysis, making it impossible to tease apart the relative contributions of each biomarker. This finding of the partially mediating role of cortical atrophy is generally consistent with previous work showing a similar mediating role of grey matter loss in the relationship between tau and performance on broad cognitive domains (Bejanin et al., 2017) as well as the mediating role of glucose metabolism (18F-FDG PET) in the relationship between tau and global cognition in AD (Saint-Aubert et al., 2016). Taken together, these studies all emphasize the role of neurofibrillary tangle pathology as the main driving factor of AD-related neurodegeneration, which in turn has been shown to more proximally impact cognitive performance (Terry et al., 1991). The only previous study to our knowledge to have examined the relative contributions of grey matter volume and tau deposition specifically in relation to visuospatial cognition, Bejanin et al., 2017, observed a full mediation of grey matter in explaining the relationship between tau and task performance. The difference in strength of mediation observed (partial vs. full) may be attributed in part to the difference in how visuospatial cognition was defined, as their outcome measure was a composite score including both the VOSP Number Location test and a test of visuoconstruction (Benson Figure Copy), both purportedly relying predominantly on the dorsal stream. Alternatively, as Bejanin et al., 2017 also included a cohort of individuals with the amnestic syndrome of AD in addition to PCA and lvPPA patients, this difference could also be attributed to varying co-pathologies that may be more common in older age and may vary across AD syndromic groups (e.g., microvascular disease (Villeneuve et al., 2015b). Of note, Bejanin et al., found the same regions (predominantly right hemisphere middle occipital gyrus, lateral and medial parietal cortex, and posterior temporal cortex) of tau deposition and grey matter volume loss associated with poorer performance on those visuospatial tasks, when they examined the amnestic group alone. These observations together emphasize the importance of these posterior cortical regions to visuospatial functions across the syndromic spectrum of AD, as well as driving role of tau deposition and its relation to neurodegeneration and visual cognitive decline.
Our study has some limitations. First, our study sample of 16 individuals was relatively small, and as such may have been underpowered to detect the mediation effects that were hypothesized. Reassuringly, the associations between task performance and markers of tau and atrophy demonstrated a robust association and full range with the patients included, suggesting that these effects are strong but require replication in a larger sample. Second, as mentioned above, the majority of our PCA patients were likely to be more impaired in dorsal compared to ventral stream functions, and as such, our sample may have lacked the full spectrum of visual cognitive impairments necessary to tease apart the relative contributions of dorsal versus ventral visual stream involvement in the tasks studied here. This too can be addressed with a larger sample size in replication, although our anecdotal experience leads us to believe that isolated ventral variants of PCA are relatively rare. Alternatively, perhaps a demanding task of visual object matching which produces a full range of performance in PCA patients may further assist in teasing apart dorsal versus ventral stream impairment. A related consideration is that studying patients at even earlier stages of their disease course (i.e., all CDR 0.5) may yield more distinct regional localization of pathology that may separate dorsal and ventral visual stream dysfunction. Another possibility is that we are observing a compensatory upregulation of both visual streams in patients who are experiencing progressive neurodegeneration in regions affecting visuospatial function, similar to what has previously been shown with hippocampal hyperactivation in amnestic presentations of AD (e.g., Putcha et al., 2011); future incorporation of functional neuroimaging into mediational models tested in this study may help distinguish the relative contributions of the dorsal and ventral visual streams to visuospatial cognitive decline. Finally, the associations between tau pathology, cortical atrophy, and visual cognitive task performance reported in this study were cross-sectional and correlational in nature. Causal relationships between these biomarkers of AD pathology themselves and with visual cognitive decline requires longitudinal investigation.
In summary, we provide further evidence that PCA and lvPPA are part of a continuous spectrum of heterogeneity in non-amnestic AD, with the common feature of amyloid pathology in highly stereotyped locations but tau and neurodegeneration in some specific overlapping regions (posterolateral temporal, inferior parietal, posterior cingulate/precuneus) but sparing medial temporal cortex, and thus, episodic memory retention. The distinctive localization of tau and neurodegeneration to language or visual systems drives differences in the clinical phenotypes. While tau and atrophy in visual cortical regions are clearly two important co-localized contributors to visual cognitive dysfunction in AD variants, cortical atrophy was found to have a partially mediating effect on the association between tau pathology and visuospatial task performance, while amyloid does not have a regionally specific association with visual cognitive task performance.
Funding
This research was supported by NIH grants R21 AG051987, R01 DC014296, P01AG005134, and P50 AG005134 and by the David Mooney Family Fund for PCA Research. This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the MGH, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant number(s) S10RR021110, S10RR023043, S10RR023401. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Competing interests
Dr. Dickerson has been a consultant for Lilly, Inc.
Acknowledgements
The authors would like to thank the patients and families who participated in this research, without whose partnership this research would not have been possible. We would also like to thank Joseph Locascio, PhD of Massachusetts General Hospital and Harvard Medical Schools for biostatistics consultation on this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101889.
Appendix A. Supplementary data
Supplementary material
References
- Becker J.A., Hedden T., Carmasin J., Maye J., Rentz D.M., Putcha D., Fischl B., Greve D.N., Marshall G.A., Salloway S., Marks D., Buckner R.L., Sperling R.A., Johnson K.A. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann. Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejanin A., Schonhaut D.R., La Joie R., Kramer J.H., Baker S.L., Sosa N., Ayakta N., Cantwell A., Janabi M., Lauriola M., O'Neil J.P., Gorno-Tempini M.L., Miller Z.A., Rosen H.J., Miller B.L., Jagust W.J., Rabinovici G.D. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain. 2017;140:3286–3300. doi: 10.1093/brain/awx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D.F., Davis R.J., Snyder B.D. Posterior cortical atrophy. Arch. Neurol. 1988;45:789–793. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Van Allen M.W. Impairment in facial recognition in patients with cerebral disease. Trans. Am. Neurol. Assoc. 1968;93:38–42. [PubMed] [Google Scholar]
- Borra E., Belmalih A., Calzavara R., Gerbella M., Murata A., Rozzi S., Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Borra E., Ichinohe N., Sato T., Tanifuji M., Rockland K.S. Cortical connections to area TE in monkey: hybrid modular and distributed organization. Cereb. Cortex. 2010;20:257–270. doi: 10.1093/cercor/bhp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueicheku E., Ventura-Campos N., Palomar-Garcia M.A., Miro-Padilla A., Parcet M.A., Avila C. Functional connectivity between superior parietal lobule and primary visual cortex "at rest" predicts visual search efficiency. Brain Connect. 2015;5:517–526. doi: 10.1089/brain.2015.0352. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutch S.J., Schott J.M., Rabinovici G.D., Murray M., Snowden J.S., van der Flier W.M., Dickerson B.C., Vandenberghe R., Ahmed S., Bak T.H., Boeve B.F., Butler C., Cappa S.F., Ceccaldi M., de Souza L.C., Dubois B., Felician O., Galasko D., Graff-Radford J., Graff-Radford N.R., Hof P.R., Krolak-Salmon P., Lehmann M., Magnin E., Mendez M.F., Nestor P.J., Onyike C.U., Pelak V.S., Pijnenburg Y., Primativo S., Rossor M.N., Ryan N.S., Scheltens P., Shakespeare T.J., Suarez Gonzalez A., Tang-Wai D.F., Yong K.X.X., Carrillo M., Fox N.C., on behalf of the Alzheimer's Association, I. A. A. s. D. a. A. S. P. I. A Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13:870–884. doi: 10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Day G.S., Gordon B.A., Jackson K., Christensen J.J., Rosana Ponisio M., Su Y., Ances B.M., Benzinger T.L.S., Morris J.C. Tau-PET binding distinguishes patients with early-stage posterior cortical atrophy from amnestic Alzheimer disease dementia. Alzheimer Dis. Assoc. Disord. 2017;31:87–93. doi: 10.1097/WAD.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L.C., Corlier F., Habert M.O., Uspenskaya O., Maroy R., Lamari F., Chupin M., Lehericy S., Colliot O., Hahn-Barma V., Samri D., Dubois B., Bottlaender M., Sarazin M. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer's disease. Brain. 2011;134:2036–2043. doi: 10.1093/brain/awr130. [DOI] [PubMed] [Google Scholar]
- Dickerson B.C., McGinnis S.M., Xia C., Price B.H., Atri A., Murray M.E., Mendez M.F., Wolk D.A. Approach to atypical Alzheimer's disease and case studies of the major subtypes. CNS Spectr. 2017;22:439–449. doi: 10.1017/S109285291600047X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Segonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Foxe D.G., Irish M., Hodges J.R., Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. J. Int. Neuropsychol. Soc. 2013;19:247–253. doi: 10.1017/S1355617712001269. [DOI] [PubMed] [Google Scholar]
- Galton C.J., Patterson K., Xuereb J.H., Hodges J.R. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P., Herrmann F.R., Bussiere T., Bouras C., Kovari E., Perl D.P., Morrison J.H., Gold G., Hof P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T., Hollister R., West H., Mui S., Growdon J.H., Petersen R.C., Parisi J.E., Hyman B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann. Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Guzman I., Pena-Casanova J., Lara J.P., Gudayol-Ferre E., Bohm P. Influence of age, sex, and education on the visual object and space perception battery (VOSP) in a healthy normal elderly population. Clin. Neuropsychol. 2004;18:385–394. doi: 10.1080/1385404049052421. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Jr., Dickson D.W., Parisi J.E., Xu Y.C., Cha R.H., O'Brien P.C., Edland S.D., Smith G.E., Boeve B.F., Tangalos E.G., Kokmen E., Petersen R.C. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J.L., Montine T., Phelps C., Rankin K.P., Rowe C.C., Scheltens P., Siemers E., Snyder H.M., Sperling R., Contributors NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Schultz A., Betensky R.A., Becker J.A., Sepulcre J., Rentz D., Mormino E., Chhatwal J., Amariglio R., Papp K., Marshall G., Albers M., Mauro S., Pepin L., Alverio J., Judge K., Philiossaint M., Shoup T., Yokell D., Dickerson B., Gomez-Isla T., Hyman B., Vasdev N., Sperling R. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops A. Probing the neural correlates of number processing. Neuroscientist. 2017;23:264–274. doi: 10.1177/1073858416650153. [DOI] [PubMed] [Google Scholar]
- Koedam E.L., Lauffer V., van der Vlies A.E., van der Flier W.M., Scheltens P., Pijnenburg Y.A. Early-versus late-onset Alzheimer's disease: more than age alone. J. Alzheimers Dis. 2010;19:1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- Kravitz D.J., Saleem K.S., Baker C.I., Mishkin M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Barnes J., Ridgway G.R., Wattam-Bell J., Warrington E.K., Fox N.C., Crutch S.J. Basic visual function and cortical thickness patterns in posterior cortical atrophy. Cereb. Cortex. 2011;21:2122–2132. doi: 10.1093/cercor/bhq287. [DOI] [PubMed] [Google Scholar]
- Makaretz S.J., Quimby M., Collins J., Makris N., McGinnis S., Schultz A., Vasdev N., Johnson K.A., Dickerson B.C. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J. Neurol. Neurosurg. Psychiatry. 2018;89:1024–1031. doi: 10.1136/jnnp-2017-316409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez M.F., Ghajarania M., Perryman K.M. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2002;14:33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- Migliaccio R., Agosta F., Rascovsky K., Karydas A., Bonasera S., Rabinovici G., Miller B., Gorno-Tempini M.L. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner A.D. How do the two visual streams interact with each other? Exp. Brain Res. 2017;235:1297–1308. doi: 10.1007/s00221-017-4917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishikin M., Ungerleider L.G., Macko K. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- Morris J.C. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Del Tredici K., Duyckaerts C., Frosch M.P., Haroutunian V., Hof P.R., Hulette C.M., Hyman B.T., Iwatsubo T., Jellinger K.A., Jicha G.A., Kovari E., Kukull W.A., Leverenz J.B., Love S., Mackenzie I.R., Mann D.M., Masliah E., McKee A.C., Montine T.J., Morris J.C., Schneider J.A., Sonnen J.A., Thal D.R., Trojanowski J.Q., Troncoso J.C., Wisniewski T., Woltjer R.L., Beach T.G. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Cohn-Sheehy B.I., La Joie R., Vogel A.C., Moller C., Lehmann M., van Berckel B., Seeley W.W., Pijnenburg Y., Gorno-Tempini M.L., Kramer J.H., Barkhof F., Rosen H., van der Flier W.M., Jagust W., Miller B., Scheltens P., Rabinovici G. Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer's disease. Hum. Brain Mapp. 2015;36:4421–4437. doi: 10.1002/hbm.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Schonhaut D.R., Baker S.L., O'Neil J.P., Janabi M., Ghosh P.M., Santos M., Miller Z.A., Bettcher B.M., Gorno-Tempini M.L., Miller B.L., Jagust W.J., Rabinovici G.D. Tau, amyloid, and hypometabolism in a patient with posterior cortical atrophy. Ann. Neurol. 2015;77:338–342. doi: 10.1002/ana.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Schonhaut D.R., Scholl M., Lockhart S.N., Ayakta N., Baker S.L., O'Neil J.P., Janabi M., Lazaris A., Cantwell A., Vogel J., Santos M., Miller Z.A., Bettcher B.M., Vossel K.A., Kramer J.H., Gorno-Tempini M.L., Miller B.L., Jagust W.J., Rabinovici G.D. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D., Brickhouse M., O'Keefe K., Sullivan C., Rentz D., Marshall G., Dickerson B., Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J. Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha D., McGinnis S.M., Brickhouse M., Wong B., Sherman J.C., Dickerson B.C. Executive dysfunction contributes to verbal encoding and retrieval deficits in posterior cortical atrophy. Cortex. 2018;106:36–46. doi: 10.1016/j.cortex.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici G.D., Furst A.J., Alkalay A., Racine C.A., O'Neil J.P., Janabi M., Baker S.L., Agarwal N., Bonasera S.J., Mormino E.C., Weiner M.W., Gorno-Tempini M.L., Rosen H.J., Miller B.L., Jagust W.J. Increased metabolic vulnerability in early-onset Alzheimer's disease is not related to amyloid burden. Brain. 2010;133:512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom M.H., Alkalay A., Agarwal N., Baker S.L., O'Neil J.P., Janabi M., Yen I.V., Growdon M., Jang J., Madison C., Mormino E.C., Rosen H.J., Gorno-Tempini M.L., Weiner M.W., Miller B.L., Jagust W.J., Rabinovici G.D. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76:1789–1796. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.J., Graham N., Stuart-Green L., Prins M., Xuereb J., Patterson K., Hodges J.R. Progressive biparietal atrophy: an atypical presentation of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 1996;61:388–395. doi: 10.1136/jnnp.61.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Aubert L., Almkvist O., Chiotis K., Almeida R., Wall A., Nordberg A. Regional tau deposition measured by [(18)F]THK5317 positron emission tomography is associated to cognition via glucose metabolism in Alzheimer's disease. Alzheimers Res. Ther. 2016;8:38. doi: 10.1186/s13195-016-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup T.M., Yokell D.L., Rice P.A., Jackson R.N., Livni E., Johnson K.A., Brady T.J., Vasdev N. A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J. Label. Compd. Radiopharm. 2013;56:736–740. doi: 10.1002/jlcr.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J.S., Stopford C.L., Julien C.L., Thompson J.C., Davidson Y., Gibbons L., Pritchard A., Lendon C.L., Richardson A.M., Varma A., Neary D., Mann D.M.A. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43:835–845. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
- Spreen O., Strauss E. Oxford University Press; New York: 1991. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- Tang-Wai D.F., Graff-Radford N.R., Boeve B.F., Dickson D.W., Parisi J.E., Crook R., Caselli R.J., Knopman D.S., Petersen R.C. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tsai P.H., Teng E., Liu C., Mendez M.F. Posterior cortical atrophy: evidence for discrete syndromes of early-onset Alzheimer's disease. Am. J. Alzheimers Dis. Other Dement. 2011;26:413–418. doi: 10.1177/1533317511418955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I., Madison C., Ayakta N., Ghosh P.M., La Joie R., Arthur-Bentil S.K., Vogel J.W., Marks S.M., Lehmann M., Rosen H.J., Reed B., Olichney J., Boxer A.L., Miller B.L., Borys E., Jin L.W., Huang E.J., Grinberg L.T., DeCarli C., Seeley W.W., Jagust W. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S., Wirth M., La Joie R. Are AD-typical regions the convergence point of multiple pathologies? Front. Aging Neurosci. 2015;7:42. doi: 10.3389/fnagi.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J.D., Fletcher P.D., Golden H.L. The paradox of syndromic diversity in Alzheimer disease. Nat. Rev. Neurol. 2012;8:451–464. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- Warrington E., James M. Bury St Edmunds. Thames Valley Test Company; England: 1991. The visual object and space perception battery. [Google Scholar]
- Watson C.L., Possin K., Allen I.E., Hubbard H.I., Meyer M., Welch A.E., Rabinovici G.D., Rosen H., Rankin K.P., Miller Z., Santos-Santos M.A., Kramer J.H., Miller B.L., Gorno-Tempini M.L. Visuospatial functioning in the primary progressive aphasias. J. Int. Neuropsychol. Soc. 2018;24:259–268. doi: 10.1017/S1355617717000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell J.L., Josephs K.A., Murray M.E., Kantarci K., Przybelski S.A., Weigand S.D., Vemuri P., Senjem M.L., Parisi J.E., Knopman D.S., Boeve B.F., Petersen R.C., Dickson D.W., Jack C.R., Jr. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C., Makaretz S.J., Caso C., McGinnis S., Gomperts S.N., Sepulcre J., Gomez-Isla T., Hyman B.T., Schultz A., Vasdev N., Johnson K.A., Dickerson B.C. Association of in Vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017;74:427–436. doi: 10.1001/jamaneurol.2016.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material