Summary
Stem cells provide a sensitive model to study exposure to toxicants, such as cigarette smoke. Electronic cigarettes (ECs) are popular nicotine delivery devices, often targeted to youth and pregnant mothers. However, little is known about how chemicals in ECs might affect neural stem cells, and in particular their mitochondria, organelles that maintain cell functionality and health. Here we show that the mechanism underlying EC-induced stem cell toxicity is stress-induced mitochondrial hyperfusion (SIMH), a transient survival response accompanied by increased mitochondrial oxidative stress. We identify SIMH as a survival response to nicotine, now widely available in EC refill fluids and in pure form for do-it-yourself EC products. These observed mitochondrial alterations combined with autophagy dysfunction to clear damaged mitochondria could lead to faulty stem cell populations, accelerate cellular aging, and lead to acquired mitochondriopathies. Any nicotine-containing product may likewise stress stem cells with long-term repercussions for users and passively exposed individuals.
Video Abstract
Subject Areas: Medicine, Smoking, Cell Biology, Stem Cells Research, Biological Sciences Research Methodologies
Graphical Abstract
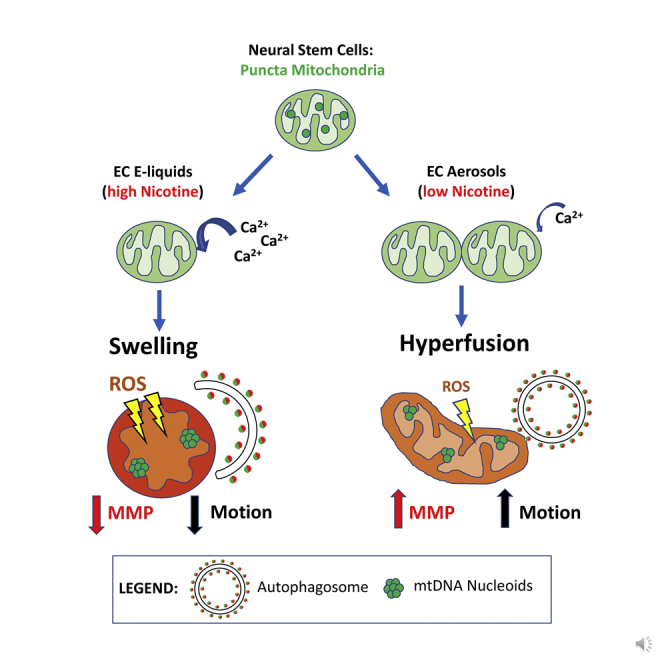
Highlights
-
•
E-cigarettes cause stress-induced mitochondrial hyperfusion (SIMH) in stem cells
-
•
SIMH was accompanied by alterations in mitochondrial morphology and dynamics
-
•
The SIMH survival response was caused by nicotine
-
•
Damaged mitochondria in stem cells could accelerate aging and lead to diseases
Medicine; Smoking; Cell Biology; Stem Cells Research; Biological Sciences Research Methodologies
Introduction
Throughout life, stem cells are critical in organ development, maintenance of homeostasis, and tissue renewal or repair. The damage to stem cells that accumulates normally or following exposure to toxicants alters their ability to maintain cell functioning and can lead to disease or aging in adults (Behrens et al., 2014, Oh et al., 2014, Pazhanisamy, 2009, Schultz and Sinclair, 2016). During development, stem cells of the nervous system are particularly vulnerable to toxicants (Rodier, 1995). Because stem cells are present throughout life, their risk of accumulating damage is high. Stem cells are also often more sensitive to stress than differentiated cells (Bahl et al., 2012, Behar et al., 2014), making them excellent models for evaluating safe limits of exposure to potential toxicants (Betts, 2010, Liu et al., 2017, Talbot et al., 2014, Talbot and Lin, 2011, Yin et al., 2015).
Toxicants, such as tobacco smoke, have detrimental effects on the health of stem cells (Kee et al., 2010, Lin et al., 2010, Lin et al., 2009, Lin et al., 2015, Talbot, 2008, Talbot and Lin, 2011). Stem cells exposed to tobacco products or nicotine have diminished regenerative potential because they are less proliferative, migratory, and able to differentiate (Greenberg et al., 2017, Ng et al., 2015, Shaito et al., 2017). Electronic cigarettes (EC) are popular new tobacco products that aerosolize nicotine and flavor chemicals through heating (Kim et al., 2016). Although originally introduced as safer or smoking cessation replacements, recent studies have shown that ECs indeed cause various forms of toxicity (National Academies of Sciences, Engineering, and Medicine, 2018). Embryonic stem cells and neural stem cells (NSCs) were highly sensitive to some EC refill fluids in vitro (Bahl et al., 2012, Behar et al., 2014). However, little is known about the mechanisms underlying stem cell toxicity and the toxicants present in ECs.
Mitochondria are excellent models for toxicological studies with stem cells because they are sensitive indicators of stress (Attene-Ramos et al., 2013, Belyaeva et al., 2008, Meyer et al., 2013). Furthermore, mitochondria control stemness (Berger et al., 2016, Margineantu and Hockenbery, 2016, Wanet et al., 2015), and their decline may underlie age-related changes in stem cell functioning (Norddahl et al., 2011, Ross et al., 2013, Tilly and Sinclair, 2013, Zhang et al., 2018). Stem cells have evolved pro-survival mechanisms centered around mitochondria, such as autophagic turnover (mitophagy) (Green et al., 2011), asymmetric segregation of mitochondria and damaged proteins during cell division (Bufalino et al., 2013, Katajisto et al., 2015, Rujano et al., 2006), and stress-induced mitochondrial hyperfusion (SIMH) (Bahl et al., 2016, Nunnari and Suomalainen, 2012, Tondera et al., 2009). These studies support the idea that mitochondria are critical in regulating stem cell health. However, it is not fully understood which stress responses stem cells activate when exposed to ECs and which chemicals are responsible for inducing stress.
To date, the effects of ECs on mitochondrial dysfunction are relatively unexplored (Lerner et al., 2016). The purpose of this study was to characterize the effects of EC refill fluids and their aerosols on stem cell mitochondria and to identify the ingredient in EC products that activates SIMH. NSCs were chosen for study as their mitochondria are well-defined and amenable to analysis using video bioinformatics tools (Bahl et al., 2016, Bahl et al., 2012, Bhanu and Talbot, 2015). Equally important, NSCs are potential targets of EC aerosol as inhaled chemicals travel efficiently to the brain via the olfactory tracks (Kozlovskaya et al., 2014). In this study, we show that the effects of ECs on the mitochondria are mediated by nicotine, and not by the transfer of volatile organic chemicals or solvents (propylene glycol and vegetable glycerin) (Figures S1–S3). Details on the procedure that were used in this study are given in the Transparent Methods in the Supplemental Information.
Results
EC Liquids and Aerosols Inhibit Autophagic Flux in Stem Cells
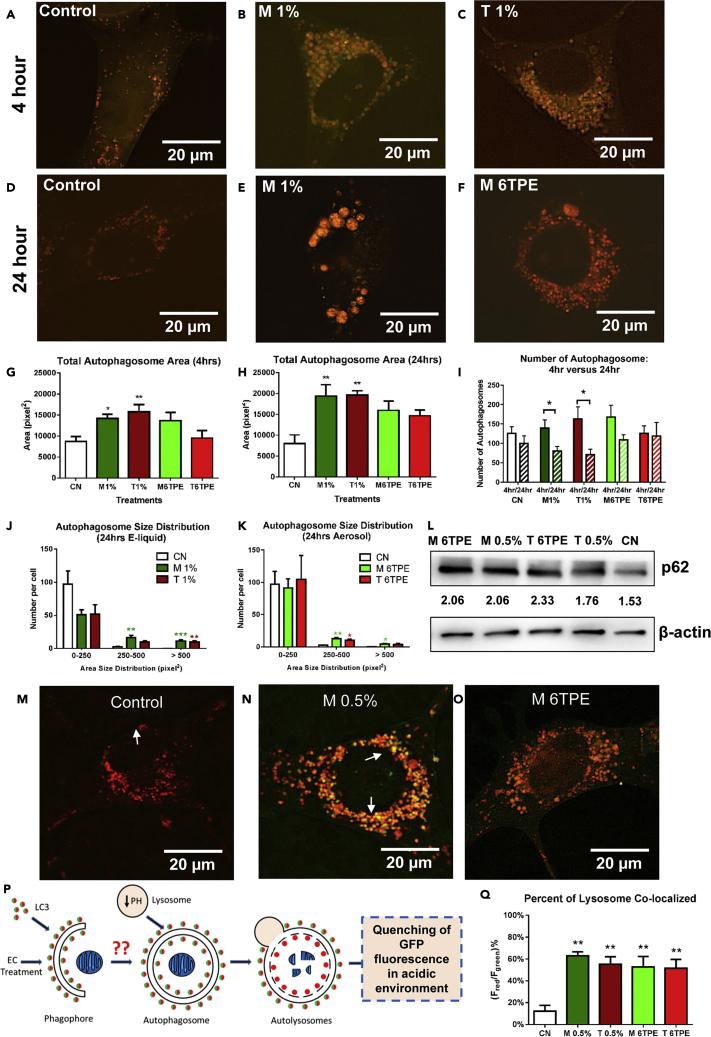
To assess the effect of EC fluids and aerosols on autophagy, NSCs were transfected with a tandem-tagged microtubule-associated protein 1 light chain 3 beta reporter (ptfLC3 plasmid), which labels autophagosomes. The reporter is tagged to both monomeric red fluorescent protein (mRFP ), which is photostable, and monomeric green fluorescent protein (mGFP), which quenches in the acidic environment of autophagosomes and autolysosomes (Kimura et al., 2007). Figures 1A–1F show mGFP and mRFP fluorescence in control and EC-treated NSCs. E-liquid concentrations are given in percent, and aerosol concentrations are given in TPE (total puff equivalents). One TPE = 0.22% of e-liquid or 6TPE = 1.3% of e-liquid. More details on this conversion are given in the Transparent Methods, as previously reported (Behar et al., 2018a). By 24 h of treatment, the autophagosomes in the treated group were larger than in the control and had more GFP fluorescence (resulting in a yellow colocalized image in Figure 1B), indicating an increase in autophagosome pH.
Figure 1.
EC Liquid or Aerosol Exposure Impairs Autophagy Clearance
(A–C) Autophagy mRFP-EGFP-LC3 reporter images of control, menthol 1% e-liquid, and tobacco 1% liquid NSCs after 4 h. Scale bar, 20 μm.
(D–F) Autophagy reporter images of control, menthol 1% e-liquid, and menthol 6TPE aerosol-treated NSCs after 24 h. Scale bar, 20 μm.
(G and H) Quantification of total autophagosome area after 4 and 24 h of treatment.
(I) Comparison of the number of autophagosomes between 4 and 24 h of treatment. Significance of autophagosome number was determined by comparing the two time points.
(J and K) Size distribution plot revealing an increase in medium and large autophagosomes in 24-h e-liquid- and aerosol-treated NSCs.
(L) Western blot analysis of p62, an autophagy marker revealing increased expression in e-liquid and aerosol treatments.
(M–O) Autolysosome (mApple-pHluorin-LAMP1) reporter images of control, menthol 0.5% e-liquid, and menthol aerosol-treated NSCs after 24 h. Arrows point to individual autolysosomes. Scale bar, 20 μm.
(P) A schematic diagram showing the autophagosome to autolysosome maturation and quenching of pHluorin (green) fluorescence intensity upon lysosome acidification.
(Q) Red-to-green fluorescence ratio of the autolysosome reporter showing a loss of the acidity of the autolysosome.
Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, **p < 0.01, ***p < 0.001). Data are represented as mean ± SEM.
CellProfiler image processing software was used to segment and quantify the total area of autophagosomes in cells treated for 4 h (Figures 1A–1C and 1G) and 24 h (Figures 1D–1F and 1H) with e-liquid or aerosol. There was a time-dependent increase in the total area of the autophagosomes with e-liquids inducing a greater increase than aerosols. There was also a slight increase in the number of autophagosomes by 4 h, but by 24 h the number of autophagosomes in e-liquid-exposed cells decreased, likely due to fusion of autophagosomes into large autolysosomes (Figure 1I). The enlarged autophagosomes also had higher GFP fluorescence, indicative of a pH increase. The data were plotted as size distribution graphs, separating the autophagosomes into three bin sizes (Figures 1J and 1K). This analysis showed an increase in the medium and large-sized autophagosomes in all treatment groups. After 24 h of treatment, p62 (sequestosome-1), which targets toxic cellular waste for autophagy, was also increased in all conditions (Figure 1L).
Autophagosomes normally merge with lysosomes, forming autolysosomes in which damaged cargo is degraded. An autolysosome-targeted pHluorein-LAMP1 reporter was used to determine if an increase in the pH of autolysosomes diminished proteolysis (Figures 1M–1O). This reporter is tagged with both mApple, which is photostable, and pHluorin (a pH-sensitive GFP), which quenches in the acidic environment of autolysosomes (Figure 1P). In contrast to controls, which showed predominantly red fluorescence (Figure 1M), merged images of liquid- and aerosol-treated cells showed an increase in yellow or orange signal, indicating the pH in autolysosomes was not acidic (Figures 1N and 1O). The percent of co-localization of the mApple and pHluorin channels increased in all treatment groups, indicating a higher-than-normal pH in autolysosomes (Figure 1Q), which could decrease degradation and contribute to increased autophagic load and backup of flux.
Mitochondria Exposed to E-Liquids and Aerosols Are Mostly Protected against Mitophagy
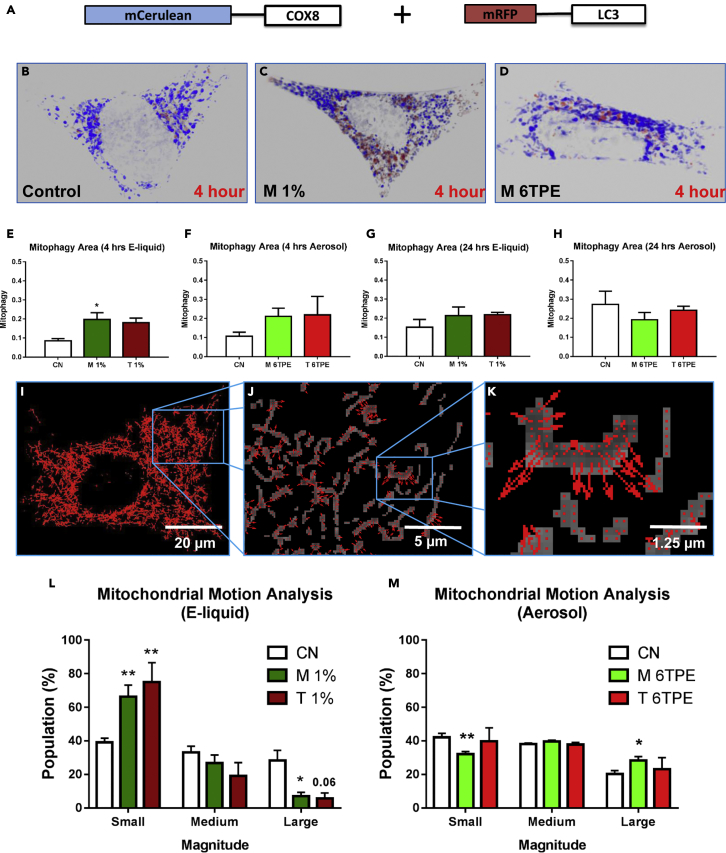
Mitophagy (mitochondrial autophagy) protects cells by eliminating damaged or dysfunctional mitochondria thereby preventing propagation of pro-apoptotic signaling (National Center for Chronic Disease Prevention and Health Promotion (U.S.) and Office on Smoking and Health (U.S.), 2016). To quantity mitophagy, NSCs were co-transfected with the ptfLC3 plasmid, which targets autophagosomes (only mRFP channel shown) and an mCerulean-tagged mitochondria-targeted plasmid (Figure 2A). Cells were treated for 4 and 24 h, and images were taken using Nikon Ti Eclipse microscope and a Zeiss Airyscan super-resolution microscope. Similar to untreated controls (Figure 2B), cells treated with e-liquid (Figure 2C) or aerosol (Figure 2D) and their corresponding 3D Zeiss Airyscan super-resolution videos (Videos S1, S2, S3, S4, S5, and S6) showed little overlap of the mitochondria and autophagosomes. CellProfiler software was used to quantify the percentage of overlap (co-localization) between the mCerulean and mRFP channels. After 4 and 24 h of treatment, mitophagy had not increased significantly, except in the high-dose 1% menthol group (Figures 2E–2H). These data show that mitochondria, for the most part, are protected from mitophagy in cells treated with e-liquids and aerosols.
Figure 2.
Mitochondria are Protected Against Mitophagy and Display Altered Motion
(A) Dual transfection with mCerulean mitochondrial-targeted (blue) and mRFP-tagged autophagosome (red) reporters.
(B–D) Control and 4-h e-liquid and aerosol-treated NSC super-resolution images were 3D reconstructed to visualize mitophagy (blue mitochondria engulfed by red autophagosomes).
(E–H) Ratio of co-localized area divided by total mitochondrial area after 4- and 24-h treatments showed primarily no significant change in mitophagy except in the 4-h menthol e-liquid treatment.
(I–K) Motion images at increasing magnifications of an NSC treated with a low dose of e-liquids. Arrows indicate direction and magnitude of motion for each pixel. Scale bar, 20 μm (I); 5μm (J); 1.25 μm (K).
(L) Motion analysis of 24-h e-liquid treatments showed a significant increase in small-magnitude vectors and a decrease in large-magnitude vectors, indicating an overall decrease in motion.
(M) Motion analysis of 24-h aerosol treatments showed a significant decrease in small-magnitude vectors and an increase in large-magnitude vectors for the menthol group, indicating an overall increase in motion.
Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, **p < 0.01). Data are represented as mean ± SEM.
This video shows a rotating 3D reconstruction of a control NSC. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of a control NSC. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 4 h of VM 6TPE treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of Video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 24 h of VM 6TPE treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of NSC after 4 h of VM 1% Treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 24 h of VM 1% treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of Video = 10 s (5 frames/second).
EC Liquids and Aerosols Altered Mitochondrial Dynamics
Mitochondrial motion analysis was done on time-lapse images of living cells using MitoMo (http://vislab.ucr.edu/SOFTWARE/software.php), a motion-magnification algorithm that quantifies and sums the magnitude of mitochondrial motion over all pixels. Motion analysis at the pixel level allows quantification in cases where tracking individual organelles is not possible. Examples of the motion vectors obtained with MitoMo are depicted in Figures 2I–2K. The total motion (net sum of all motion vectors) was classified as small, medium, or large. Treatment with e-liquids decreased large motion and increased small motion relative to controls (Figure 2L). In contrast, menthol aerosol-treated NSCs exhibited a decrease in small motion and an increase in large motion (Figure 2M). The tobacco-aerosol-treated group showed a similar increase in large motion but was not statistically significant (Figure 2M). The hyperfused mitochondria (aerosol) had greater motion than the control, whereas the swollen mitochondria (high e-liquid concentrations) had less motion than the control.
ECs Caused Mitochondrial Swelling and Stress-Induced Mitochondrial Hyperfusion
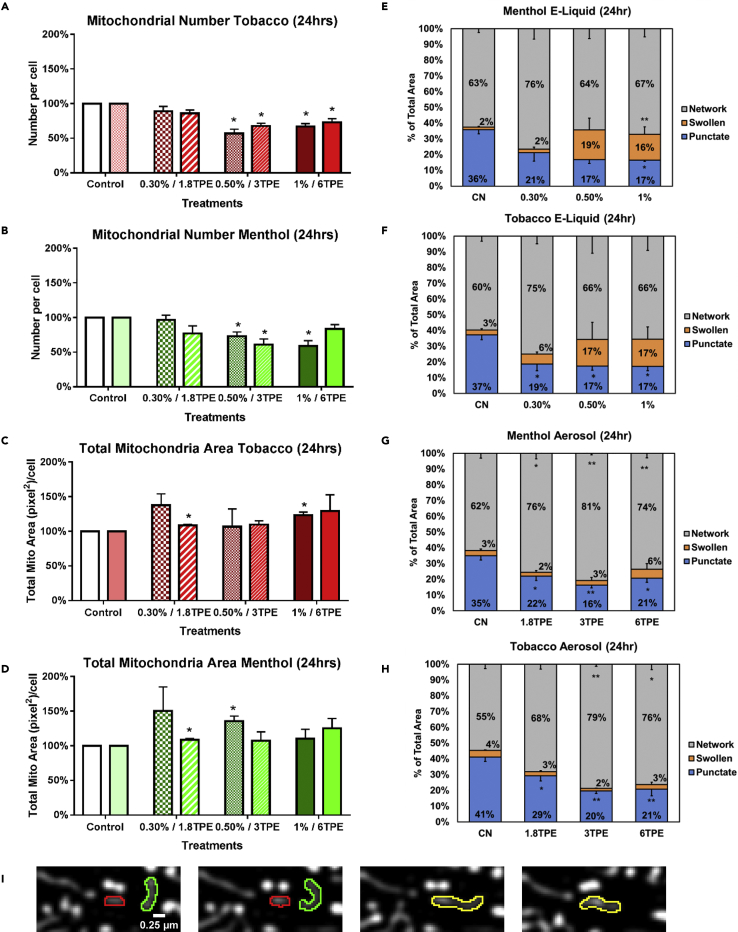
The effect of e-liquid and aerosol on mitochondrial number and morphology was investigated using a stably transfected MitoTimer-NSC line, which provided a strong fluorescent signal suitable for segmenting and classifying mitochondrial morphology. Cells were incubated for 24 h with menthol- or tobacco-flavored e-liquids or aerosols and compared against untreated controls by segmenting and quantifying mitochondrial number and area using CellProfiler software. The number of mitochondria in e-liquid and aerosol-treated cells decreased dose dependently (Figures 3A and 3B), whereas the total mitochondrial area increased in some treatments (Figures 3C and 3D). These data support the hypothesis that mitochondria are undergoing hyperfusion, which results in a decrease in the number of mitochondria. The increase in total mitochondrial area in some concentrations supports mitochondrial biogenesis.
Figure 3.
EC Liquid or Aerosol Exposure Results in Swollen and Hyperfused Mitochondria
(A and B) Mitochondrial number was quantified and normalized to the control group for 24-h tobacco and menthol e-liquid and aerosol treatments, showing a general decrease in mitochondria number.
(C and D) Total mitochondrial area was quantified and normalized to the control group for 24-h tobacco and menthol e-liquid and aerosol treatments, revealing an increase in some concentrations.
(E and F) Morphological classification of dotted, networked, and swollen mitochondria treated for 24 h with menthol or tobacco e-liquid, showing mitochondrial hyperfusion in the low concentrations and swelling in the higher concentrations.
(G and H) Morphological classification of dotted, networked, and swollen mitochondria after 24 h of treatment with menthol or tobacco aerosol, showing mitochondrial hyperfusion in all concentrations.
(I) A representative image of two mitochondria fusing to form the networked phenotype. Scale bar, 0.25 μm.
Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, **p < 0.01). Data are represented as mean ± SEM.
To further investigate EC-induced hyperfusion, mitochondria in treated cells were classified morphologically as punctate, swollen, or networked using MitoMo software. Transition from the punctate to networked morphology occurs during hyperfusion (Bahl et al., 2016, Tondera et al., 2009). The percentage of each morphological type was calculated relative to total mitochondrial area (Figures 3E–3H). In the e-liquid-treated cells, mitochondrial hyperfusion increased at 0.3% concentration and swelling increased in the 0.5% and 1% treatment groups (Figures 3E and 3F). Menthol and tobacco aerosols caused a decrease in the number of punctate mitochondria and a corresponding increase in the networked morphology (Figures 3G and 3H). These changes further support the idea that EC aerosol caused SIMH, which is a pro-survival protective response (Bahl et al., 2016, Tondera et al., 2009). Time-lapse imaging of living cells showed examples of punctate mitochondria fusing to form larger networked mitochondria (Figure 3I).
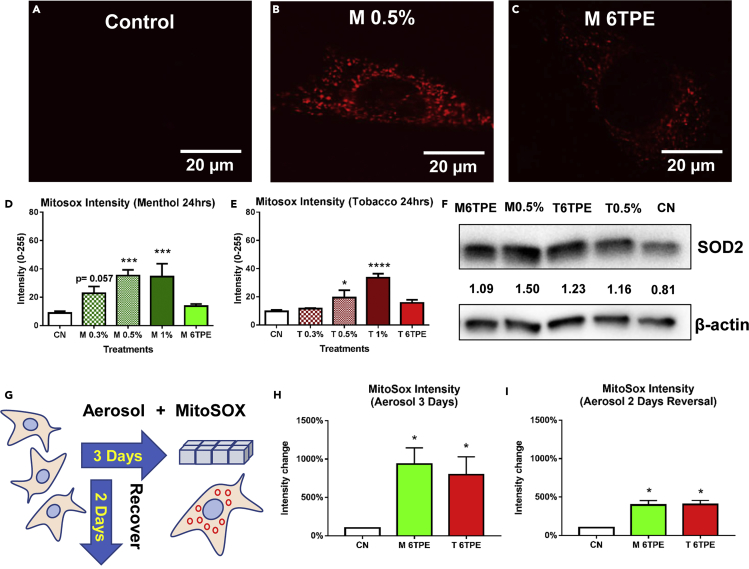
EC Liquids and Aerosols Increased Mitochondrial Superoxide Levels
As hyperfusion can increase superoxide production (Bahl et al., 2016), we next loaded cells with MitoSOX Red, a mitochondrial-targeted dye that produces red fluorescence in the presence of superoxide anion. Loaded cells were incubated for 24 h with menthol and tobacco e-liquids or aerosols, and MitoSOX fluorescence was analyzed using CL-Quant software. There was a dose-dependent increase in MitoSOX intensity in the e-liquid-treated cells, and a modest increase in the aerosol treatments after 24 h (Figures 4A–4E). To determine if the increase in mitochondrial reactive oxygen species (ROS) induced an antioxidant response, superoxide dismutase 2 (SOD2), a mitochondrial enzyme that converts superoxide into hydrogen peroxide and diatomic oxygen, was quantified using western blots. Cells treated for 24 h with either e-liquid or aerosol had significantly elevated SOD2 levels when compared with controls (Figure 4F).
Figure 4.
Exposure to EC Liquid or Aerosol Causes Superoxide Production and an Antioxidant Response
(A–C) Control and 24-h e-liquid- and aerosol-treated NSCs labeled with MitoSOX Red showing increased levels of superoxide in the mitochondria. Scale bar, 20 μm.
(D and E) MitoSOX intensity levels were quantified for all menthol and tobacco treatments and showed a concentration response increase in all groups with the e-liquid groups being significant.
(F) Western blot of superoxide dismutase 2 (SOD2), a superoxide scavenger, showing increased expression in most treatment groups.
(G) A schematic diagram representing prolonged (3 days treated) aerosol treatments and reversal (2 days treated, 2 days reversal) experiments, followed by MitoSOX quantification.
(H) 3-day aerosol treatment caused an increase in MitoSOX intensity relative to control cells.
(I) 2-day aerosol treatment followed by 2 days of recovery decreased intensity relative to (H) but did not produce a full recovery.
Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, ***p < 0.001, ****p < 0.0001). Data are represented as mean ± SEM.
To determine if longer aerosol treatment would elevate mitochondrial ROS levels, NSCs were treated for 3 days and then loaded with MitoSOX dye (Figure 4G). There was a statistically significant increase in MitoSOX fluorescence in the menthol and tobacco 6TPE aerosol-treated cells (Figure 4H). Furthermore, treatment for 2 days followed by 2 days of recovery did not completely reverse the MitoSOX fluorescence (Figure 4I), suggesting that the increase in mitochondrial ROS was not readily reversible.
EC Exposure Induces Mitochondrial Protein Oxidation
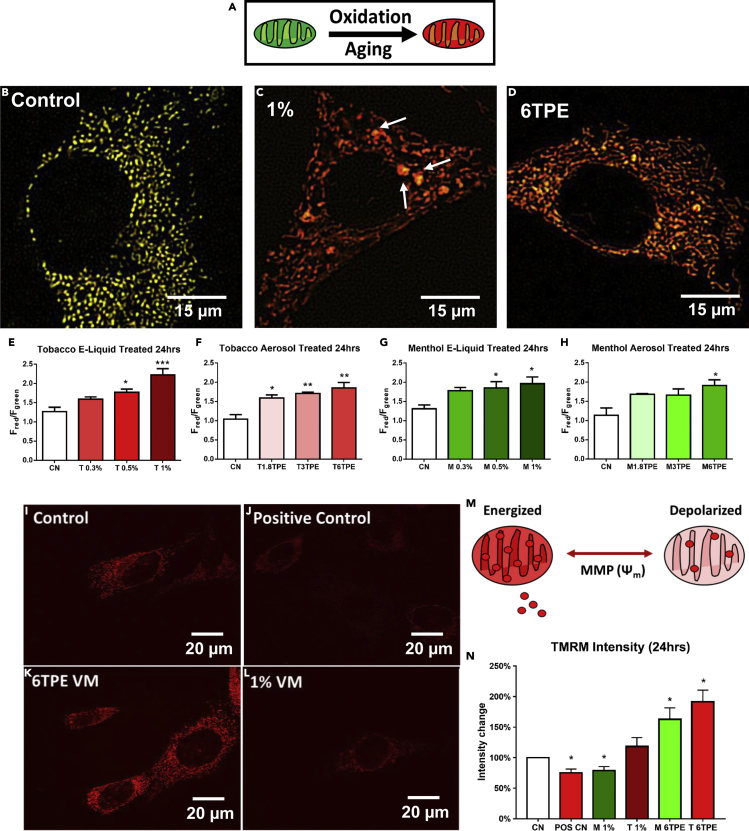
The MitoTimer reporter cell line was used to assess the effects of EC liquid and aerosol on mitochondrial protein oxidation. MitoTimer is composed of the colorimeter Timer protein tagged to the cytochrome c oxidase subunit VIII gene, which targets it to the mitochondria (Laker et al., 2014). This reporter detects an increase in mitochondrial protein oxidation or aging by an increase in the red/green fluorescence ratio (Figure 5A). MitoTimer-NSCs were treated for 24 h with EC liquids or aerosols. Control cells (Figure 5B) expressed more green fluorescence than menthol treated cells which showed an increase in red fluorescence, as demonstrated in the merged images of the red and green channels (Figures 5C and 5D). The red/green fluorescence ratio was quantified using CL-Quant image processing software and showed a concentration-dependent increase in all e-liquid and aerosol treatments (Figures 5E–5H). The swollen mitochondria also had a high red-to-green MitoTimer ratio, indicative of high levels of ROS (white arrows in Figure 5C). These increases in the red/green ratio correspond to a significant elevation in oxidation of mitochondrial proteins in e-liquid- and aerosol-treated cells compared with untreated controls.
Figure 5.
EC Liquids and Aerosols Increase Mitochondrial Protein Oxidation and Membrane Potential
(A) A schematic of the mitochondrial-targeted reporter MitoTimer, which shifts from green to red fluorescence upon protein oxidation and aging. Scale bar, 15 μm.
(B–D) Control and 24-h e-liquid- and aerosol-treated MitoTimer-NSCs. Arrows point to swollen mitochondria.
(E–H) MitoTimer red to green ratio indicative of mitochondrial protein oxidation levels were quantified, revealing a dose-response increase in all treatments.
(I–L) Control and 24-h menthol e-liquid, menthol aerosol, and KCN/oligomycin-treated NSCs labeled with the mitochondrial membrane potential dye TMRM. Scale bar, 20 μm.
(M) A schematic of TMRM dye sequestering by normal mitochondria, versus depolarized mitochondria, which are leaky and have weaker TMRM fluorescence.
(N) TMRM fluorescence intensity was quantified and normalized to the control group, showing a loss of membrane potential in the menthol 1% high concentration and increased membrane potential in both aerosol treatments. Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, **p < 0.01, ***p < 0.001). Data are represented as mean ± SEM.
EC Liquids and Aerosols Alter the Mitochondrial Membrane Potential
To examine mitochondrial membrane potential (MMP), cells were labeled with tetramethylrhodamine, methyl ester (TMRM) dye and then treated with e-liquid or aerosol for 24 h (Figures 5I–5L). This cell-permeant, cationic, red dye is sequestered by active mitochondria in live cells but leaks out of depolarized mitochondria (Figure 5M). As a positive control, cells were de-energized with 2.5 mM potassium cyanide (KCN), a respiratory inhibitor, and 1 μg/mL of oligomycin, a mitochondrial ATPase inhibitor (Figure 5J). Treatment with menthol aerosols increased TMRM accumulation in the mitochondria (Figure 5K), which is consistent with the observed mitochondrial hyperfusion and subsequent metabolic shift; however, the TMRM signal was decreased in the 1% menthol e-liquid group (Figure 5L), suggesting this concentration damages the mitochondria and causes membrane leakage. The TMRM fluorescence intensity was quantified using CL-Quant software and normalized to the control (Figure 5N). In agreement with the fluorescent images, the positive control and 1% menthol e-liquid decreased the MMP, whereas the aerosols significantly increased the potential. TMRM measurements were then done after 30 min, 1 h, 2 h, 4 h, and 24 h for the menthol e-liquid group. Initially the membrane potential rises (likely due to an increase in ROS), but by 24 h, it dropped due to membrane damage (Figure S4).
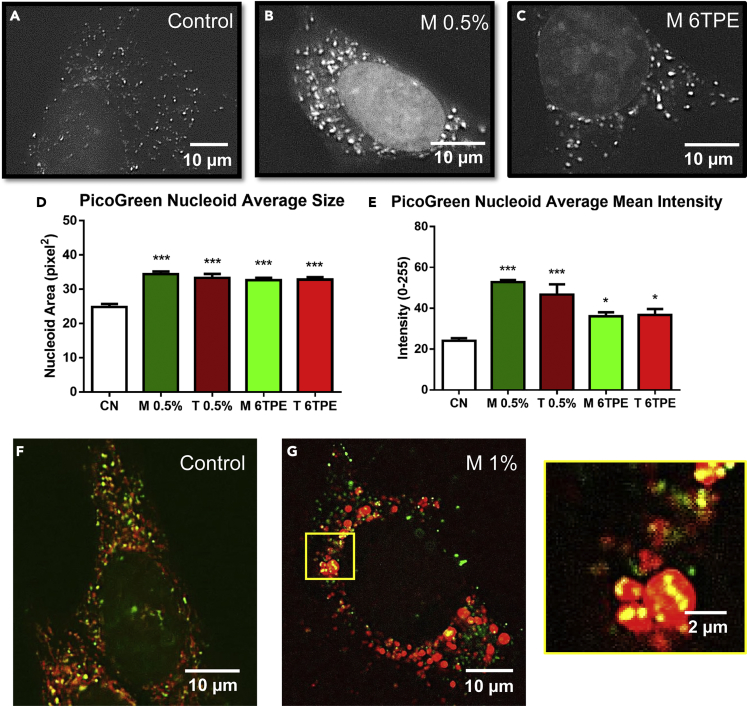
EC Treatment Induced Aggregation of Mitochondrial Nucleoids and mtDNA Damage
Elevation of ROS in mitochondria could affect their DNA (mtDNA). To investigate this possibility, cells were treated with e-liquids (0.5%) and aerosols (6TPE) for 24 h then incubated with Quant-iT PicoGreen dye, which labels dsDNA including mitochondrial nucleoids. Control cells exhibited multiple small fluorescent green nucleoids (Figure 6A), whereas the treated cells had larger brighter nucleoids (Figures 6B and 6C), indicative of mtDNA nucleoid aggregation. Quantification using CL-Quant demonstrated an increase in both the average mtDNA nucleoid area/cell and nucleoid intensity in all treated groups relative to controls (Figures 6D and 6E). There was also an increase in the nuclear PicoGreen signal, especially in the e-liquid-treated group (Figure 6B), perhaps due to damage of the nuclear envelope. PicoGreen signal was also co-labeled with MitoTracker dye (Figures 6F and 6G), revealing aggregated PicoGreen signals within the swollen mitochondria treated with 1% menthol e-liquid.
Figure 6.
EC Liquids and Aerosols Cause Aggregation of mtDNA Nucleoids
(A–C) Control and 24-h menthol e-liquid- and aerosol-treated NSCs imaged lived with PicoGreen dye, which labels mtDNA. Scale bar, 10 μm.
(D and E) PicoGreen fluorescence puncta were quantified to show an increase in average mtDNA nucleoid size and average mean intensity.
(F and G) Control and 24-hr menthol e-liquid-treated NSCs labeled with PicoGreen (mtDNA) and MitoTracker-Red (mitochondria), showing aggregated mtDNA in the menthol 1% e-liquid-treated condition. Scale bar, 20 μm (F, G); 2μm (G). Data are represented as mean ± SEM.
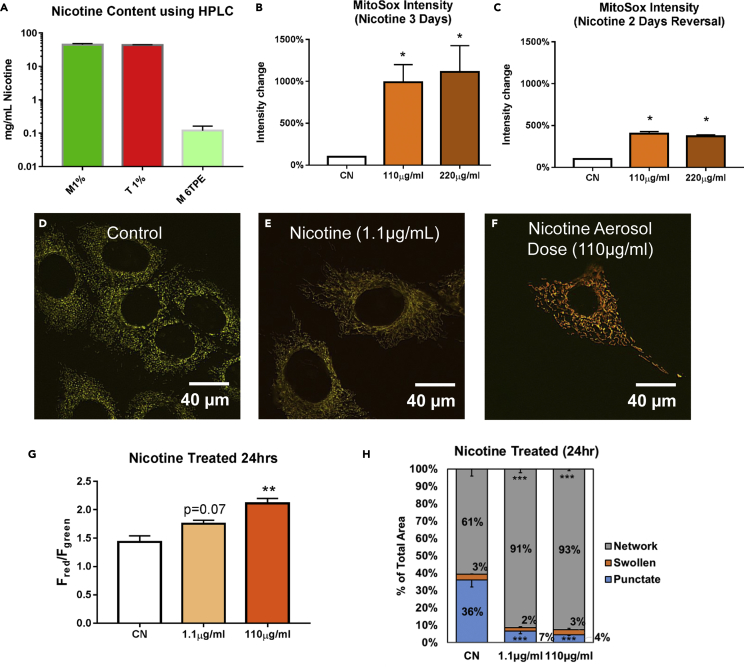
Nicotine Caused Increased Protein Oxidation and Mitochondrial Hyperfusion
Nicotine concentrations in 1% EC liquids and 6TPE aerosols from tobacco and menthol-flavored disposable cartomizers were analyzed using high-performance liquid chromatography, as described previously (Bahl et al., 2012). E-liquids had an average of 44 mg nicotine/mL, whereas aerosols had lower concentrations of 110 μg/mL (Figure 7A). To determine if nicotine was contributing to oxidative stress in mitochondria, cells were treated for 3 days with 110 or 220 μg of nicotine/mL (corresponding to concentrations found in aerosol), and labeled with MitoSOX Red. Fluorescent images were captured, and average intensity/cell was quantified using CL-Quant software. There was a statistically significant increase in the average MitoSOX fluorescent intensity, relative to untreated controls, in the nicotine-treated groups (Figure 7B). Superoxide production was lower in cells that were allowed to recover for 2 days following 2 days of nicotine treatment; nevertheless, superoxide remained significantly elevated compared with the untreated control (Figure 7C).
Figure 7.
Nicotine-Induced Mitochondrial Protein Oxidation and Hyperfusion
(A) Nicotine concentrations in the 1% e-liquid and 6TPE aerosols were quantified using high-performance liquid chromatography.
(B) 3-day nicotine treatments (110 μg/mL and 220 μg/mL, corresponding to amount of nicotine found in aerosols) increased MitoSOX intensity relative to control cells.
(C) 2-day nicotine treatment followed by 2 days of recovery decreased ROS production, but did not produce a full recovery.
(D–F) Control and 24-h nicotine treatment (110 μg/mL, and 1.1 μg/mL corresponding to a 100-fold lower concentration). Scale bar, 40 μm.
(G) Both concentrations of nicotine increased the MitoTimer red/green fluorescent ratio indicative of mitochondrial protein oxidation.
(H) Both concentrations of nicotine caused a significant increase in mitochondrial hyperfusion (increase of networked mitochondria relative to punctate and swollen morphologies).
Asterisks on top of each bar indicate the statistical significance. (*p < 0.05, **p < 0.01, ***p < 0.001). See also Figure S2. Data are represented as mean ± SEM.
To determine if nicotine was contributing to mitochondrial protein oxidation and SIMH, the MitoTimer-NSCs were treated with nicotine at 110 μg/mL (corresponding to 6TPE of aerosol) and 1.1 μg/mL (100-fold lower concentration than found in aerosol) (Figures 7E and 7F). Live images were collected after 24 h, and the fluorescent ratios of the red to green channels were quantified using CL-Quant software. There was a concentration-dependent effect on mitochondrial protein oxidation in both nicotine-treated groups, with a statistically significant increase in the 110 μg/mL dose (Figure 7G). The mitochondrial morphologies showed both a statistically significant increase in the networked mitochondria and a decrease in the punctate mitochondria in both treatment groups (Figure 7H). The induction by nicotine of mitochondrial hyperfusion and an increase in superoxide production is consistent with the changes observed in the aerosol-treated cells (Figures 3G, 3H, 4H, 4I, 5F, and 5H).
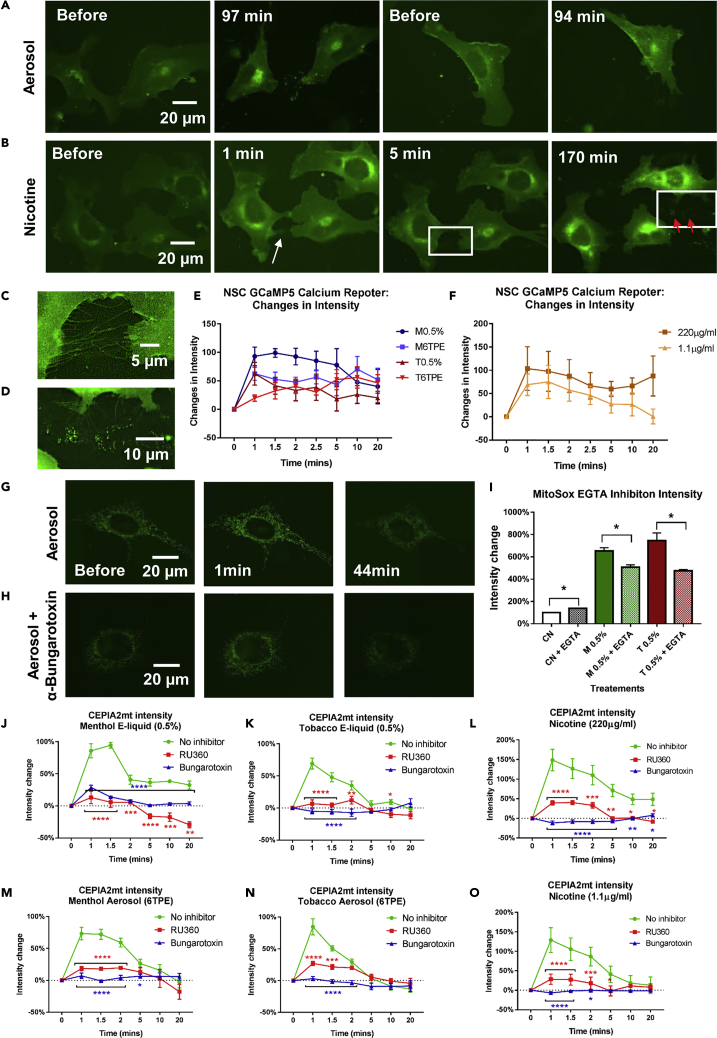
EC and Nicotine Exposure Induce Ca2+ Influx Leading to Plasma Membrane Retraction and Intracellular Calcium Overload
Nicotine can activate nicotinic acetylcholine receptors (nAChRs) on cell membranes in the central nervous system and other non-neuronal cells, resulting in an influx of cations, including Ca2+ (Sharma and Vijayaraghavan, 2002). To evaluate the effect of nicotine and EC exposure on nAChR-calcium signaling, NSCs were transfected with a calcium reporter (GCaMP5) consisting of a circularly permuted green fluorescent protein (cpGFP), calmodulin (CaM), and the Ca2+/CaM-binding “M13” peptide (Akerboom et al., 2012). Transfected cells were imaged live before and after the addition of e-liquids, aerosols, and various concentrations of nicotine. A rapid increase in fluorescence was visible within 1 min of adding of 6TPE aerosol (Figure 8A) and 1.1 μg/mL of nicotine (Figure 8B). The fluorescence signal accumulated in the perinuclear region, presumably due to sequestering of calcium by the endoplasmic reticulum (ER). In addition, the plasma membrane began to retract, particularly at cell-to-cell contacts (white arrow in Figure 8B, higher magnification shown in Figure 8C). After approximately 170 min, some of these retractions pinched off forming small extracellular fragments with elevated fluorescence indicative of high levels of calcium (red arrows in Figure 8B and higher magnification shown in Figure 8D). The GCaMP5 fluorescence intensity was quantified for all concentrations over time (Figures 8E and 8F), and statistical analysis was done by comparing each time to time = 0 min (Table S1). These data show that Ca2+ levels did not return to control levels in the higher doses, resulting in Ca2+ overload.
Figure 8.
EC Liquids and Aerosols Elevate Mitochondrial Calcium
(A and B) Treatment of GCaMP5-transfected NSC with (A) Aerosol T 6TPE and (B) 110 μμg/mL of nicotine caused a rapid influx of calcium, which remained elevated in perinuclear regions. White arrow points to plasma membrane retraction at cell contacts, and red arrows point to extracellular fragments with high levels of calcium. Scale bar, 20 μm.
(C) Nicotine treatment resulted in a rapid retraction of cell membranes and dissociation of cell-to-cell adhesion. Scale bar, 5 μm.
(D) Nicotine treatment resulted in pinching off of small extracellular fragments with elevated fluorescence indicative of high levels of calcium. Scale bar, 10 μm.
(E and F) GCaMP5 fluorescence intensity before and after addition of e-liquids, aerosols, and nicotine.
(G) Treatment of CEPIA2mt (mitochondrial-targeted calcium reporter)-transfected NSCs with aerosol caused a rapid increase in fluorescence intensity indicative of mitochondrial calcium influx. Scale bar, 20 μm.
(H) The increase in CEPIA2mt fluorescence was attenuated in the presence of α-bungarotoxin, an α7 nAChR blocker. Scale bar, 20 μm.
(I) Increased MitoSOX Red fluorescence intensity was partially inhibited by the calcium chelator EGTA.
(J–O) Mitochondrial calcium levels were assessed using CEPIA2mt fluorescence intensity prior and after addition of e-liquids, aerosols, and nicotine doses (green lines). Red lines represent calcium levels in the presence of RU360, and blue lines, in the presence of α-bungarotoxin. Statistical analysis was conducted comparing inhibitor curves against the control curves.
Asterisks on top of each bar and points indicate the statistical significance. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Data are represented as mean ± SEM.
EC-Induced Mitochondrial Calcium Influx Can Be Blocked to Prevent Mitochondrial Damage
Intracellular calcium can accumulate in mitochondria, where it regulates various cellular functions (Santo-Domingo and Demaurex, 2010). Unlike the ER, mitochondria are a passive Ca2+ buffer, and calcium accumulation in the mitochondria triggers ROS production (Rizzuto et al., 2012). To examine the effect of nicotine and EC exposure on mitochondrial calcium levels, NSCs were transfected with a genetically encoded Ca2+ indicator, calcium-measuring organelle-entrapped protein indicator (CEPIA) (Suzuki et al., 2014). Transfected cells were imaged live before and after the addition of e-liquids, aerosols, and various concentrations of nicotine. All treatments resulted in an increase in fluorescence signal within 1 min of treatment followed by a decrease in fluorescence by 44 min (Figure 8G). The fluorescence intensity was quantified using CL-Quant software and the normalized change in intensity relative to the “before” (0 min) time point was plotted (green lines on Figures 8J–8O). Most treatments were significantly different from the 0 min control, and fluorescent intensity returned to basal levels in some groups by 20 min (Figures 8K and 8M–8O). However, in the case of 0.5% menthol and the 220 μg/mL nicotine, the calcium levels did not return to resting states (Figures 8J and 8L), indicating a persistent elevation in mitochondrial calcium.
Mitochondria have Ca2+ transporters, such as the mitochondrial Ca2+ uniporter (MCU) (Santo-Domingo and Demaurex, 2010), that allow import of a high Ca2+ load against a concentration gradient. To block Ca2+ uptake through MCU, NSCs were pre-incubated with Ru360, a cell-permeable dinuclear ruthenium amine complex that binds to MCU with high affinity. Similar to previous CEPIA2mt experiments, cells were treated with e-liquids, aerosols, and various concentrations of nicotine, this time following 10 min of pre-treatment with 10 μM Ru360 (red lines on Figures 8J–8O). The changes in normalized fluorescent intensity were plotted over 20 min, revealing a significant dampening of the intracellular calcium level.
Functional α7 nAChRs are found in the cell plasma membrane, and recently they have been found in the mitochondrial membrane (Gergalova et al., 2012, Skok et al., 2016). To block the α7 nAChRs on the cell membrane and the mitochondria, CEPIA2mt-transfected NSCs were pretreated for 10 min with 100 nM antagonist α-bungarotoxin, and then treated with various concentrations of e-liquids, aerosols, and nicotine (Figure 8H, blue lines on Figures 8J–8N). The changes in normalized fluorescence intensity over 20 min revealed a significant inhibition of the mitochondrial calcium influx, likely due to dual blockage of the nicotinic receptors at the cell and mitochondrial membranes.
Lastly, to test whether calcium blockage could inhibit mitochondrial effects, NSCs were incubated with 1 μM calcium chelator EGTA and treated with 0.5% e-liquid doses for 4 h. The MitoSOX fluorescence intensity was quantified revealing a statistically significant decrease in mitochondrial superoxide production compared with the treatments without EGTA (Figure 8H). These data indicate that calcium overload is a contributing factor to the EC-induced mitochondrial defects.
Discussion
This is the first demonstration that e-liquids and aerosols from a leading brand of ECs induce a stress response in stem cells that includes interruption of autophagic flux without mitophagy, mitochondrial hyperfusion accompanied by oxidative stress, and mtDNA aggregation. Moreover, this is the first demonstration that these cellular responses can be attributed to nicotine, which by itself mimicked the EC products. Nicotine concentrations that caused cellular stress responses were lower than or similar to the concentrations found in the aerosols that we tested. The hyperfusion stress response appears to be a protective mechanism to guard against mitophagy. We further showed that nicotine caused increased mitochondrial superoxide production, which could be reduced by inhibiting calcium influx. Calcium influx, due to EC or nicotine exposure, could be reversed using an α7 nAChR blocker, chelating extracellular calcium, or blocking the MCU channel. Taken together, these data support the conclusion that tobacco products containing nicotine can stress stem cells and induce a transient survival response.
Autophagy promotes quality control and cell health by sequestering and degrading damaged cellular proteins and organelles, including mitochondria (Glick et al., 2010, Green et al., 2011). Degradation within autolysosomes depends on acidity and the functioning of acid hydrolases and vacuolar-type proton ATPase (Kimura et al., 2007). Factors that elevate the pH in autolysosomes prevent degradation and turnover of cellular components. Treatment of NSCs with EC products resulted in an increase in autophagosomes and autolysosomes, in agreement with a recent study showing an increase in LC3, an autophagy marker, in airway epithelial cells exposed to EC aerosols (Shivalingappa et al., 2015). Our study shows that backup of autophagic flux is due to loss of the acidic environment within autolysosomes. The increase in lysosomal pH is likely caused by nicotine, a weak base, that can diffuse across the cell membrane and accumulate in lysosomes by proton trapping (Govind et al., 2009, Thyberg et al., 1983).
Mitophagy normally occurs when damaged mitochondria fragment into small punctate mitochondria that can readily be engulfed by autophagosomes (Ashrafi and Schwarz, 2012, Ni et al., 2015). Treatment with EC liquids and aerosols triggered autophagy, but paradoxically, not mitophagy. Treated mitochondria formed interconnected tubular networks (hyperfusion), which likely protected them from mitophagy (Figures 2E–2H). The small but significant increase in mitophagy seen in the 1% menthol e-liquid is likely due to clearance of the swollen mitochondria, which were elevated in this group (Figure 2E). In agreement with this idea, swelling was accompanied by loss of the MMP, thereby putting the leaky mitochondria at risk for targeted degradation (Figure 5L). Thus, while SIMH has several consequences for the mitochondria, one of them appears to be to protect mitochondria from mitophagy.
High concentrations of e-liquids caused mitochondrial swelling, likely due to an excess of mitochondrial calcium, which did not protect against mitophagy. Calcium overload can trigger mitochondrial impairment, especially in cases where calcium uptake is accompanied by oxidative stress (Rizzuto et al., 2012). It is now known that nAChRs are also present on the mitochondrial membrane (Gergalova et al., 2012, Skok et al., 2016), and that nicotine can enter cells. As shown in our study, nicotine treatment (binding to nAChRs) leads to a large influx of calcium, both into the cytoplasm and into the mitochondria. The e-liquids had much higher concentrations of nicotine than the aerosol, which likely accounts for the production of swollen mitochondria due to calcium overload. An excess of mitochondrial calcium may cause opening of the mitochondrial permeability transition pore, which in turn can lead to swelling of the mitochondrial matrix as water, small molecules, and protons enter, resulting in outer membrane rupture (Lemasters et al., 2009). The nicotine concentration difference between the e-liquid and aerosol probably comes about because of the relatively poor transfer of nicotine to the aerosol in the cig-a-like product used in this study. This may explain why the toxicity of 6TPE aerosols is lower than that of the 1% e-liquids. Processes such as heating and aerosolization and the design of the EC could affect the efficiency of transfer of nicotine and other fluid chemicals to the aerosols. For cartomizer-style ECs, the heating temperatures are relatively low, which likely contributed to the low nicotine transfer. Our laboratory has previously shown that heating can significantly affect chemical transfer to EC aerosol (Behar et al., 2018b).
SIMH, which was first described by Tondera et al. in response to treatment with stress stimuli (e.g., UV irradiation, actinomycin D, cycloheximide), is now recognized as a transient survival response in cells exposed to toxic substances (Shutt and McBride, 2013, Tondera et al., 2009). Our data show that cells responded to low doses of EC liquids and aerosol and nicotine by undergoing SIMH accompanied by increased ROS production, which in turn increased oxidation of mitochondrial proteins. Increased protein oxidation can irreversibly damage mitochondria and thereby cause changes in the integrity of mitochondrial membranes and their membrane potential (Handy and Loscalzo, 2012, Lagouge and Larsson, 2013). Changes in the permeability of the mitochondrial membrane to proteins, such as cytochrome c, is a critical initiation factor for apoptosis and other signaling cascades (Gergalova et al., 2012, Lenaz, 1998), suggesting that high doses or prolonged exposure to EC aerosols eventually result in cell death.
Changes similar to those observed in our study have also been reported in NSCs treated with third-hand smoke (THS) extracts (Bahl et al., 2016). THS is a rich source of nicotine, which likely caused SIMH and the accompanying changes. During chronic treatment of NSC with THS for 15 days, mitochondria maintained an elevated membrane potential (MMP) and proliferated at a faster rate than untreated controls. However, by 30 days of exposure, both the MMP and proliferation rate had decreased, demonstrating that by this time the SIMH survival response was collapsing. This is likely due to the accumulated oxidative stress and suggests that cell death would follow. These data support the idea that SIMH is a transient survival response that enables mitochondria and cells to combat stress, but that chronic exposure may eventually overwhelm the protection provided by SIMH. It is probable that respiratory cells in the users of these products would respond to aerosolized nicotine with SIMH rather than mitochondrial swelling.
We further showed for the first time that SIMH is accompanied by aggregation of mitochondrial nucleoids. mtDNA aggregation has also been observed in cells in which Drp1, a protein required for mitochondrial fission, has been knocked out (Ban-Ishihara et al., 2013, Ishihara et al., 2015). Other studies show that anti-cancer drugs, such as doxorubicin, intercalate into mtDNA, causing their aggregation accompanied by mitochondrial hyperfusion (Ashley and Poulton, 2009, Tondera et al., 2009). This study suggests that remodeled nucleoids were able to exclude doxorubicin and maintain mtDNA synthesis. These studies show that mitochondrial nucleoids are dynamic structures that aggregate when cells are stressed, or genes are knocked out in a manner, leading to mitochondrial fusion. Nucleoid aggregation may occur as part of the SIMH scenario to protect mtDNA from the high levels of DNA-damage-inducing superoxide that form during hyperfusion (Lenaz, 1998, Shokolenko et al., 2009). Alternatively, EC chemical(s) capable of intercalating into DNA may cause nucleoid aggregation.
In addition to initiating a cascade of responses in mitochondria, calcium influx led to retraction of cell-to-cell contacts and calcium accumulation inside the cell and mitochondria. The massive calcium influx that occurred during treatment with e-liquids, aerosols, and nicotine could be inhibited by ruthenium RU360 and α-bungarotoxin, a blocker of α7 nicotinic receptors. Calcium overload can pose serious cellular effects (Bagur and Hajnóczky, 2017, Celsi et al., 2009, Zhivotovsky and Orrenius, 2011). Fragments encapsulating Ca2+ (shown in Figure 8D) may provide a protective mechanism for sequestering and expelling excessive amounts of intracellular calcium. This cellular retraction may have significant implications in epithelial cells that depend on tight cell junctions to maintain selectively permeable barriers (Brune et al., 2015). Intracellular calcium levels can return to resting levels by efflux through the plasma membrane Ca2+ transport ATPase and the Na+/Ca2+ exchanger (NCX), as well as uptake into the ER/sarcoplasmic reticulum via the sarco/endoplasmic reticulum Ca2+-ATPase (Bagur and Hajnóczky, 2017). Although it appears that in the short term (by 20 min, Figures 8E and 8F) calcium is lowered, prolonged incubation (170 min, Figure 8B) resulted in an accumulation of intracellular calcium. This increase in perinuclear calcium after 3 h of treatment, which is likely accumulation in the ER, could potentially lead to ER stress. Eventually, calcium overload can cause cell death via several mechanisms including Ca2+-mediated mitochondrial permeabilization (Bagur and Hajnóczky, 2017).
Mitochondrial dysfunction and oxidative damage are hallmarks of aging and disease (Bogenhagen, 2010). Tobacco smoke and nicotine cause premature aging (Spano et al., 2012) and other disorders such as cancer (Grando, 2014, Macleod et al., 2013, Tan et al., 2008), neurodegenerative disorders (Martin, 2012, Nunnari and Suomalainen, 2012), and cardiovascular disease (Centers for Disease Control and Prevention (U.S.) et al., 2010) due to their effects on the mitochondria. Our studies suggest that chronic exposure to EC aerosols could contribute to aging via several pathways including (1) impaired autophagy and the inability to clear damaged cellular components, (2) increased oxidative stress and protein oxidation, and (3) mtDNA aggregation leading to impaired segregation of nucleoids. The elevation of mitochondrial ROS can induce mtDNA damage, which in turn can accelerate aging by interfering with the antioxidant response and the mitochondrial life cycle (Oh et al., 2014, Lagouge and Larsson, 2013). Aggregated mitochondrial nucleoids, which were observed in the hyperfused mitochondria, may further impair a cell's ability to segregate mutated nucleoids, which would contribute to mitochondrial dysfunction and aging (Bogenhagen, 2010).
ECs are often considered harm reduction products (Kim et al., 2016, McNeill et al., 2015). However, our data show that nicotine, which is found in most ECs, elicits cellular responses similar to those observed in EC aerosol-treated NSCs and those reported previously in THS-treated cells (Bahl et al., 2016). The concentrations of nicotine that we tested are likely relevant to what an EC user would receive. In our study, the EC aerosol had nicotine concentrations of 110 μg/mL on average. Nicotine concentrations of 1.1 μg/mL (100-fold less than what was found in our EC aerosols) induced the SIMH and increased mitochondrial superoxide. One study reported that when participants used an EC containing 36 mg/mL nicotine, their plasma nicotine levels received a “boost” of 12.5 ng/mL (Lopez et al., 2016). This is comparable to what conventional cigarette users achieve (nicotine boost of ∼16 ng/mL). When delivered through the lung, the concentration in brain nicotine “boost” after smoking one conventional cigarette is 0.7 μg/mL (434 nM) on average (Rose et al., 2010). The 1.1 μg/mL nicotine concentration tested in this study is in the range that NSCs can be exposed to in vivo.
SIMH occurred in response to EC- and nicotine-induced stress and represents a transient survival response that may enable stem cells to live until the stress is removed. However, removal of aerosol or nicotine treatment did not fully restore superoxide and MMP to basal levels. During the exposure period, oxidative damage accumulates in mitochondrial proteins and probably also in mtDNA (Shokolenko et al., 2009), so that even if cells recover, they may be genetically and physiologically abnormal owing to increased oxidative damage. These observations are critical as they suggest that EC aerosols are not without harm and that long-term exposures could elevate the possibility of premature aging and disease. By extension, any nicotine delivery device could have the same effects and may not be safe for long-term use. This situation is confounded by several observations. First, nicotine has been reported in counterfeit ECs and in some refill fluids labeled to contain no nicotine (Davis et al., 2015, Omaiye et al., 2017), making it difficult for EC users to avoid nicotine even if they wish to do so. Second, when ECs deliver nicotine poorly, EC users show compensatory puffing to achieve adequate nicotine (Behar et al., 2015, Schroeder and Hoffman, 2014), making it likely that EC users will adjust their topography to receive sufficient nicotine to stress stem cells.
Recent studies suggest that teens who use ECs are up to seven times more likely to start smoking conventional cigarettes (National Academies of Sciences, Engineering, and Medicine, 2018), and tobacco is ranked extremely high on the addiction scale (Nutt et al., 2007). Past studies have shown that adolescents are not likely to refrain from smoking tobacco cigarettes when told that smoking may shorten their life expectancy because death is so remote from their young age. However, endpoints that are proximal to their age, such as smoking induces facial wrinkles by age 25, have had greater impact on smoking initiation and cessation in adolescents (Morita, 2007, Seitz et al., 2012). Our data, which show oxidative damage to stem cells, when expanded in future studies may likewise be helpful in providing information that could prevent the use of nicotine-containing products that may diminish the quality of life and life expectancy.
Damage to stem cells could have significant impact on a developing embryo or infant (Kee et al., 2010). Cigarette smoking during pregnancy can induce physical impairments and cognitive defects in the progeny (Clifford et al., 2012, Mund et al., 2013). Prenatal exposure to nicotine also alters pathways in the brain that are critical for motor and cognitive functions and behavioral responses (Dwyer et al., 2009, Wickstrom, 2007). This is a concern given the fact that nicotine is still present in devices, such as nicotine patches and ECs, which are often recommended as cigarette substitutes for pregnant women who smoke. Our data support re-evaluation of recommending any nicotine products to women during pregnancy.
In conclusion, our data show that exposure of stem cells to e-liquids, aerosols, or nicotine produces a stress response that leads to SIMH, which itself increases oxidative stress in cells. Of particular importance is the finding that nicotine alone can induce the changes observed with EC aerosols. This supports the idea that ECs are not as harmless as often claimed and that even short-term exposure can stress cells in a manner that may lead, with chronic use, to morbidity or disease. These observations are likely to pertain to any product containing nicotine.
Limitations of the Study
It would be appropriate in the future to expand work on ECs to other cell types, such as human respiratory epithelium, a target of EC aerosols (National Academies of Sciences, Engineering, and Medicine, 2018). It remains to be shown that similar changes occur in cells in humans using EC products and compare sub-chronic and chronic exposures. Also, we used only one brand of ECs. Other brands may produce stronger or weaker effects. Finally, our aerosols were produced using one protocol. Humans using ECs use various puffing topographies (Behar et al., 2015), which may affect the results.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The mNSCs were a generous gift from Evan Snyder, San Diego CA. We thank Madeline Vega for her help in reviewing the manuscript. We also thank David Carter from the UCR Microscopy Core for his help with the AiryScan microscope. Research reported in this publication was supported by NIDA, NIEHS, and the FDA Center for Tobacco Products (CTP) grant #s R01DA036493, R21DA037365, and R01ES029741. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Author Contributions
Project administration and funding acquisition, P.T.; Conceptualization, A.Z. and P.T.; Investigation, A.Z. and E.O.; Software and formal analysis, A.Z., R.P., A.C., and S.L.; Writing – Original Draft, A.Z. and P.T.; Writing – Review & Editing, all authors.
Declaration of Interests
The authors have no competing interests to declare.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.034.
A video abstract is available at https://doi.org/10.1016/j.isci.2019.05.034#mmc8.
Supplemental Information
References
- Akerboom J., Chen T.-W., Wardill T.J., Tian L., Marvin J.S., Mutlu S., Calderon N.C., Esposti F., Borghuis B.G., Sun X.R. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Akerboom, J., Chen, T.-W., Wardill, T.J., Tian, L., Marvin, J.S., Mutlu, S., Calderon, N.C., Esposti, F., Borghuis, B.G., Sun, X.R., et al. (2012). Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci.. 32, 13819-13840. [DOI] [PMC free article] [PubMed]
- Ashley N., Poulton J. Anticancer DNA intercalators cause p53 dependent mitochondrial DNA nucleoid re-modelling. Oncogene. 2009;28:3880–3891. doi: 10.1038/onc.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ashley, N., and Poulton, J.. (2009). Anticancer DNA intercalators cause p53 dependent mitochondrial DNA nucleoid re-modelling. Oncogene 28, 3880-3891. [DOI] [PMC free article] [PubMed]
- Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2012;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ashrafi, G., and Schwarz, T.L.. (2012). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ.. 20, 31-42. [DOI] [PMC free article] [PubMed]
- Attene-Ramos M.S., Huang R., Sakamuru S., Witt K.L., Beeson G.C., Shou L., Schnellmann R.G., Beeson C.C., Tice R.R., Austin C.P. Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem. Res. Toxicol. 2013;26:1323–1332. doi: 10.1021/tx4001754. [DOI] [PMC free article] [PubMed] [Google Scholar]; Attene-Ramos, M.S., Huang, R., Sakamuru, S., Witt, K.L., Beeson, G.C., Shou, L., Schnellmann, R.G., Beeson, C.C., Tice, R.R., Austin, C.P., et al. (2013). Systematic study of mitochondrial toxicity of environmental chemicals using quantitative high throughput screening. Chem. Res. Toxicol.. 26, 1323-1332. [DOI] [PMC free article] [PubMed]
- Bagur R., Hajnóczky G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bagur, R., and Hajnoczky, G.. (2017). Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol. Cell 66, 780-788. [DOI] [PMC free article] [PubMed]
- Bahl V., Lin S., Xu N., Davis B., Wang Y.H., Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012;34:529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]; Bahl, V., Lin, S., Xu, N., Davis, B., Wang, Y.H., and Talbot, P.. (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol.. 34, 529-537. [DOI] [PubMed]
- Bahl V., Johnson K., Phandthong R., Zahedi A., Schick S.F., Talbot P. Thirdhand cigarette smoke causes stress-induced mitochondrial hyperfusion and alters the transcriptional profile of stem cells. Toxicol. Sci. 2016;153:55–69. doi: 10.1093/toxsci/kfw102. [DOI] [PubMed] [Google Scholar]; Bahl, V., Johnson, K., Phandthong, R., Zahedi, A., Schick, S.F., and Talbot, P.. (2016). Thirdhand cigarette smoke causes stress-induced mitochondrial hyperfusion and alters the transcriptional profile of stem cells. Toxicol. Sci. 153, 55-69. [DOI] [PubMed]
- Ban-Ishihara R., Ishihara T., Sasaki N., Mihara K., Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl. Acad. Sci. U S A. 2013;110:11863–11868. doi: 10.1073/pnas.1301951110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ban-Ishihara, R., Ishihara, T., Sasaki, N., Mihara, K., and Ishihara, N.. (2013). Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc. Natl. Acad. Sci. U S A 110, 11863-11868. [DOI] [PMC free article] [PubMed]
- Behar R.Z., Davis B., Wang Y., Bahl V., Lin S., Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. In Vitro. 2014;28:198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]; Behar, R.Z., Davis, B., Wang, Y., Bahl, V., Lin, S., and Talbot, P.. (2014). Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. In Vitro. 28, 198-208. [DOI] [PubMed]
- Behar R.Z., Hua M., Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]; Behar, R.Z., Hua, M., and Talbot, P.. (2015). Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10, 1-18. [DOI] [PMC free article] [PubMed]
- Behar R.Z., Wang Y., Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control. 2018;27:325–333. doi: 10.1136/tobaccocontrol-2016-053472. [DOI] [PMC free article] [PubMed] [Google Scholar]; Behar, R.Z., Wang, Y., and Talbot, P.. (2018a). Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control 27, 325-333. [DOI] [PMC free article] [PubMed]
- Behar R.Z., Luo W., Mcwhirter K.J., Pankow J.F., Talbot P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-25575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Behar, R.Z., Luo, W., Mcwhirter, K.J., Pankow, J.F., and Talbot, P.. (2018b). Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep.. 8, 1-11. [DOI] [PMC free article] [PubMed]
- Behrens A., van Deursen J.M., Rudolph K.L., Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 2014;16:201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]; Behrens, A., van Deursen, J.M., Rudolph, K.L., and Schumacher, B.. (2014). Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 16, 201-207. [DOI] [PMC free article] [PubMed]
- Belyaeva E.A., Dymkowska D., Wieckowski M.R., Wojtczak L. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol. 2008;231:34–42. doi: 10.1016/j.taap.2008.03.017. [DOI] [PubMed] [Google Scholar]; Belyaeva, E.A., Dymkowska, D., Wieckowski, M.R., and Wojtczak, L.. (2008). Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol.. 231, 34-42. [DOI] [PubMed]
- Berger E., Rath E., Yuan D., Waldschmitt N., Khaloian S., Allgauer M., Staszewski O., Lobner E.M., Schottl T., Giesbertz P. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 2016;7:13171. doi: 10.1038/ncomms13171. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berger, E., Rath, E., Yuan, D., Waldschmitt, N., Khaloian, S., Allgauer, M., Staszewski, O., Lobner, E.M., Schottl, T., Giesbertz, P., et al. (2016). Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun.. 7, 13171. [DOI] [PMC free article] [PubMed]
- Betts K.S. Growing knowledge: using stem cells to study developmental neurotoxicity. Environ. Health Perspect. 2010;118:a432–a437. doi: 10.1289/ehp.118-a432. [DOI] [PMC free article] [PubMed] [Google Scholar]; Betts, K.S.. (2010). Growing knowledge: using stem cells to study developmental neurotoxicity. Environ. Health Perspect.. 118, a432-a437. [DOI] [PMC free article] [PubMed]
- Bhanu B., Talbot P. Springer International Publishing; 2015. Video Bioinformatics: From Live Imaging to Knowledge. [Google Scholar]; Bhanu, B., and Talbot, P.. (2015). Video Bioinformatics: From Live Imaging to Knowledge (Switzerland: Springer International Publishing).
- Bogenhagen D.F. Does mtDNA nucleoid organization impact aging? Exp. Gerontol. 2010;45:473–477. doi: 10.1016/j.exger.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bogenhagen, D.F.. (2010). Does mtDNA nucleoid organization impact aging? Exp. Gerontol.. 45, 473-477. [DOI] [PMC free article] [PubMed]
- Brune K., Frank J., Schwingshackl A., Finigan J., Sidhaye V.K. Pulmonary epithelial barrier function: some new players and mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L731–L745. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brune, K., Frank, J., Schwingshackl, A., Finigan, J., and Sidhaye, V.K.. (2015). Pulmonary epithelial barrier function: some new players and mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L731-L745. [DOI] [PMC free article] [PubMed]
- Bufalino M.R., DeVeale B., van der Kooy D. The asymmetric segregation of damaged proteins is stem cell-type dependent. J. Cell Biol. 2013;201:523–530. doi: 10.1083/jcb.201207052. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bufalino, M.R., DeVeale, B., and van der Kooy, D.. (2013). The asymmetric segregation of damaged proteins is stem cell-type dependent. J. Cell Biol. 201, 523-530. [DOI] [PMC free article] [PubMed]
- Celsi F., Pizzo P., Brini M., Leo S., Fotino C., Pinton P., Rizzuto R. Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim. Biophys. Acta. 2009;1787:335–344. doi: 10.1016/j.bbabio.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Celsi, F., Pizzo, P., Brini, M., Leo, S., Fotino, C., Pinton, P., and Rizzuto, R.. (2009). Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Biochim. Biophys. Acta. 1787, 335-344. [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention (U.S.) National Center for Chronic Disease Prevention and Health Promotion (U.S.) Office on Smoking and Health (U.S.) Centers for Disease Control and Prevention; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [PubMed] [Google Scholar]; Centers for Disease Control and Prevention (U.S.); National Center for Chronic Disease Prevention and Health Promotion (U.S.); Office on Smoking and Health (U.S.) (2010). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. (Atlanta: Centers for Disease Control and Prevention). [PubMed]
- Clifford A., Lang L., Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol. Teratol. 2012;34:560–570. doi: 10.1016/j.ntt.2012.09.004. [DOI] [PubMed] [Google Scholar]; Clifford, A., Lang, L., and Chen, R.. (2012). Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol. Teratol.. 34, 560-570. [DOI] [PubMed]
- Davis B., Dang M., Kim J., Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob. Res. 2015;17:134–141. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis, B., Dang, M., Kim, J., and Talbot, P.. (2015). Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob. Res. 17, 134-141. [DOI] [PMC free article] [PubMed]
- Dwyer J.B., McQuown S.C., Leslie F.M. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dwyer, J.B., McQuown, S.C., and Leslie, F.M.. (2009). The dynamic effects of nicotine on the developing brain. Pharmacol. Ther.. 122, 125-139. [DOI] [PMC free article] [PubMed]
- Gergalova G., Lykhmus O., Kalashnyk O., Koval L., Chernyshov V., Kryukova E., Tsetlin V., Komisarenko S., Skok M. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS One. 2012;7:e31361. doi: 10.1371/journal.pone.0031361. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gergalova, G., Lykhmus, O., Kalashnyk, O., Koval, L., Chernyshov, V., Kryukova, E., Tsetlin, V., Komisarenko, S., and Skok, M.. (2012). Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS One 7, e31361. [DOI] [PMC free article] [PubMed]
- Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]; Glick, D., Barth, S., and Macleod, K.F.. (2010). Autophagy: cellular and molecular mechanisms. J. Pathol.. 221, 3-12. [DOI] [PMC free article] [PubMed]
- Govind A.P., Vezina P., Green W.N. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Govind, A.P., Vezina, P., and Green, W.N.. (2009). Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol.. 78, 756-765. [DOI] [PMC free article] [PubMed]
- Grando S.A. Connections of nicotine to cancer. Nat. Rev. Cancer. 2014;14:419–429. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]; Grando, S.A.. (2014). Connections of nicotine to cancer. Nat. Rev. Cancer 14, 419-429. [DOI] [PubMed]
- Green D.R., Galluzzi L., Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]; Green, D.R., Galluzzi, L., and Kroemer, G.. (2011). Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333, 1109-1112. [DOI] [PMC free article] [PubMed]
- Greenberg J.M., Carballosa C.M., Cheung H.S. Concise review: the deleterious effects of cigarette smoking and nicotine usage and mesenchymal stem cell function and implications for cell-based therapies. Stem Cells Transl. Med. 2017;6:1815–1821. doi: 10.1002/sctm.17-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]; Greenberg, J.M., Carballosa, C.M., and Cheung, H.S.. (2017). Concise review: the deleterious effects of cigarette smoking and nicotine usage and mesenchymal stem cell function and implications for cell-based therapies. Stem Cells Transl. Med.. 6, 1815-1821. [DOI] [PMC free article] [PubMed]
- Handy D.E., Loscalzo J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]; Handy, D.E., and Loscalzo, J.. (2012). Redox regulation of mitochondrial function. Antioxid. Redox Signal.. 16, 1323-1367. [DOI] [PMC free article] [PubMed]
- Ishihara T., Ban-Ishihara R., Maeda M., Matsunaga Y., Ichimura A., Kyogoku S., Aoki H., Katada S., Nakada K., Nomura M. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission Is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol. Cell. Biol. 2015;35:211–223. doi: 10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishihara, T., Ban-Ishihara, R., Maeda, M., Matsunaga, Y., Ichimura, A., Kyogoku, S., Aoki, H., Katada, S., Nakada, K., Nomura, M., et al. (2015). Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission Is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol. Cell. Biol. 35, 211-223. [DOI] [PMC free article] [PubMed]
- Katajisto P., Dohla J., Chaffer C.L., Pentinmikko N., Marjanovic N., Iqbal S., Zoncu R., Chen W., Weinberg R.A., Sabatini D.M. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–343. doi: 10.1126/science.1260384. [DOI] [PMC free article] [PubMed] [Google Scholar]; Katajisto, P., Dohla, J., Chaffer, C.L., Pentinmikko, N., Marjanovic, N., Iqbal, S., Zoncu, R., Chen, W., Weinberg, R.A., and Sabatini, D.M.. (2015). Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 348, 340-343. [DOI] [PMC free article] [PubMed]
- Kee K., Flores M., Cedars M.I., Reijo Pera R.A. Human primordial germ cell formation is diminished by exposure to environmental toxicants acting through the AHR signaling pathway. Toxicol. Sci. 2010;117:218–224. doi: 10.1093/toxsci/kfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kee, K., Flores, M., Cedars, M.I., and Reijo Pera, R.A.. (2010). Human primordial germ cell formation is diminished by exposure to environmental toxicants acting through the AHR signaling pathway. Toxicol. Sci. 117, 218-224. [DOI] [PMC free article] [PubMed]
- Kim K.-H., Kabir E., Jahan S.A. Review of electronic cigarettes as tobacco cigarette substitutes: their potential human health impact. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016;34:262–275. doi: 10.1080/10590501.2016.1236604. [DOI] [PubMed] [Google Scholar]; Kim, K.-H., Kabir, E., and Jahan, S.A.. (2016). Review of electronic cigarettes as tobacco cigarette substitutes: their potential human health impact. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 34, 262-275. [DOI] [PubMed]
- Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]; Kimura, S., Noda, T., and Yoshimori, T.. (2007). Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452-460. [DOI] [PubMed]
- Kozlovskaya L., Abou-Kaoud M., Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control Release. 2014;189:133–140. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]; Kozlovskaya, L., Abou-Kaoud, M., and Stepensky, D.. (2014). Quantitative analysis of drug delivery to the brain via nasal route. J. Control Release 189, 133-140. [DOI] [PubMed]
- Lagouge M., Larsson N.G. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013;273:529–543. doi: 10.1111/joim.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lagouge, M., and Larsson, N.G.. (2013). The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med.. 273, 529-543. [DOI] [PMC free article] [PubMed]
- Laker R.C., Xu P., Ryall K.A., Sujkowski A., Kenwood B.M., Chain K.H., Zhang M., Royal M.A., Hoehn K.L., Driscoll M. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. 2014;289:12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]; Laker, R.C., Xu, P., Ryall, K.A., Sujkowski, A., Kenwood, B.M., Chain, K.H., Zhang, M., Royal, M.A., Hoehn, K.L., Driscoll, M., et al. (2014). A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. 289, 12005-12015. [DOI] [PMC free article] [PubMed]
- Lemasters J., Theruvath T., Zhong Z., Nieminen A. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lemasters, J., Theruvath, T., Zhong, Z., and Nieminen, A.. (2009). Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 1787, 1395-1401. [DOI] [PMC free article] [PubMed]
- Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]; Lenaz, G.. (1998). Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta. 1366, 53-67. [DOI] [PubMed]
- Lerner C.A., Rutagarama P., Ahmad T., Sundar I.K., Elder A., Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016;477:620–625. doi: 10.1016/j.bbrc.2016.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lerner, C.A., Rutagarama, P., Ahmad, T., Sundar, I.K., Elder, A., and Rahman, I.. (2016). Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun.. 477, 620-625. [DOI] [PMC free article] [PubMed]
- Lin S., Tran V., Talbot P. Comparison of toxicity of smoke from traditional and harm-reduction cigarettes using mouse embryonic stem cells as a novel model for preimplantation development. Hum. Reprod. 2009;24:386–397. doi: 10.1093/humrep/den419. [DOI] [PubMed] [Google Scholar]; Lin, S., Tran, V., and Talbot, P.. (2009). Comparison of toxicity of smoke from traditional and harm-reduction cigarettes using mouse embryonic stem cells as a novel model for preimplantation development. Hum. Reprod.. 24, 386-397. [DOI] [PubMed]
- Lin S., Fonteno S., Weng J.H., Talbot P. Comparison of the toxicity of smoke from conventional and harm reduction cigarettes using human embryonic stem cells. Toxicol. Sci. 2010;118:202–212. doi: 10.1093/toxsci/kfq241. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lin, S., Fonteno, S., Weng, J.H., and Talbot, P.. (2010). Comparison of the toxicity of smoke from conventional and harm reduction cigarettes using human embryonic stem cells. Toxicol. Sci. 118, 202-212. [DOI] [PMC free article] [PubMed]
- Lin S.C., Yip H., Phandthong R., Davis B., Talbot P. Evaluation of dynamic cell processes and behavior using video bioinformatics tools. In: Bhanu B., Talbot P., editors. Video Bioinformatics. Springer International Publishing; 2015. pp. 167–186. [Google Scholar]; Lin, S.C., Yip, H., Phandthong, R., Davis, B., and Talbot, P.. (2015). Evaluation of dynamic cell processes and behavior using video bioinformatics tools. In Video Bioinformatics, B. Bhanu and P. Talbot, eds. (Switzerland: Springer International Publishing), pp. 167-186.
- Liu S., Yin N., Faiola F. Prospects and frontiers of stem cell toxicology. Stem Cells Dev. 2017;26:1528–1539. doi: 10.1089/scd.2017.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu, S., Yin, N., and Faiola, F.. (2017). Prospects and frontiers of stem cell toxicology. Stem Cells Dev. 26, 1528-1539. [DOI] [PMC free article] [PubMed]
- Lopez A.A., Hiler M.M., Soule E.K., Ramôa C.P., Karaoghlanian N.V., Lipato T., Breland A.B., Shihadeh A.L., Eissenberg T. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob. Res. 2016;18:720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lopez, A.A., Hiler, M.M., Soule, E.K., Ramoa, C.P., Karaoghlanian, N.V., Lipato, T., Breland, A.B., Shihadeh, A.L., and Eissenberg, T.. (2016). Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob. Res. 18, 720-723. [DOI] [PMC free article] [PubMed]
- Macleod K.F., Boland M.L., Chourasia A.H., Macleod K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013;3:1–28. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]; Macleod, K.F., Boland, M.L., Chourasia, A.H., and Macleod, K.F.. (2013). Mitochondrial dysfunction in cancer. Front. Oncol.. 3, 1-28. [DOI] [PMC free article] [PubMed]
- Margineantu D.H., Hockenbery D.M. Mitochondrial functions in stem cells. Curr. Opin. Genet. Dev. 2016;38:110–117. doi: 10.1016/j.gde.2016.05.004. [DOI] [PubMed] [Google Scholar]; Margineantu, D.H., and Hockenbery, D.M.. (2016). Mitochondrial functions in stem cells. Curr. Opin. Genet. Dev. 38, 110-117. [DOI] [PubMed]
- Martin L.J. Biology of mitochondria in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Martin, L.J.. (2012). Biology of mitochondria in neurodegenerative diseases, Prog. Mol. Biol. Transl. Sci. 107, 355-415. [DOI] [PMC free article] [PubMed]
- McNeill A., Brose L.S., Calder R., Hitchman S.C., Hajek P., McRobbie H. Public Health England; 2015. E-cigarettes: an Evidence Update: a Report Commissioned by Public Health England. [Google Scholar]; McNeill, A., Brose, L.S., Calder, R., Hitchman, S.C., Hajek, P., and McRobbie, H.. (2015). E-cigarettes: an Evidence Update: a Report Commissioned by Public Health England, Public Health England.
- Meyer J.N., Leung M.C.K., Rooney J.P., Sendoel A., Hengartner M.O., Kisby G.E., Bess A.S. Mitochondria as a target of environmental toxicants. Toxicol. Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meyer, J.N., Leung, M.C.K., Rooney, J.P., Sendoel, A., Hengartner, M.O., Kisby, G.E., and Bess, A.S.. (2013). Mitochondria as a target of environmental toxicants. Toxicol. Sci. 134, 1-17. [DOI] [PMC free article] [PubMed]
- Morita A. Tobacco smoke causes premature skin aging. J. Dermatol. Sci. 2007;48:169–175. doi: 10.1016/j.jdermsci.2007.06.015. [DOI] [PubMed] [Google Scholar]; Morita, A.. (2007). Tobacco smoke causes premature skin aging. J. Dermatol. Sci. 48, 169-175. [DOI] [PubMed]
- Mund M., Louwen F., Klingelhoefer D., Gerber A. Smoking and pregnancy - a review on the first major environmental risk factor of the unborn. Int. J. Environ. Res. Public Health. 2013;10:6485–6499. doi: 10.3390/ijerph10126485. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mund, M., Louwen, F., Klingelhoefer, D., and Gerber, A.. (2013). Smoking and pregnancy - a review on the first major environmental risk factor of the unborn. Int. J. Environ. Res. Public Health 10, 6485-6499. [DOI] [PMC free article] [PubMed]
- National Academies of Sciences, Engineering, and Medicine . The National Academies Press; 2018. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]; National Academies of Sciences, Engineering, and Medicine. (2018). Public Health Consequences of E-Cigarettes. (Washington, DC: The National Academies Press). [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (U.S.) Office on Smoking and Health (U.S.) Centers for Disease Control and Prevention; 2016. E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. [PubMed] [Google Scholar]; National Center for Chronic Disease Prevention and Health Promotion (U.S.); Office on Smoking and Health (U.S.) (2016). E-cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. (Atlanta: Centers for Disease Control and Prevention). [PubMed]
- Ng T.K., Huang L., Cao D., Yip Y.W.-Y., Tsang W.M., Yam G.H.-F., Pang C.P., Cheung H.S. Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci. Rep. 2015;5:7828. doi: 10.1038/srep07828. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ng, T.K., Huang, L., Cao, D., Yip, Y.W.-Y., Tsang, W.M., Yam, G.H.-F., Pang, C.P., and Cheung, H.S.. (2015). Cigarette smoking hinders human periodontal ligament-derived stem cell proliferation, migration and differentiation potentials. Sci. Rep.. 5, 7828. [DOI] [PMC free article] [PubMed]
- Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ni, H.M., Williams, J.A., and Ding, W.X.. (2015). Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6-13. [DOI] [PMC free article] [PubMed]
- Norddahl G.L., Pronk C.J., Wahlestedt M., Sten G., Nygren J.M., Ugale A., Sigvardsson M., Bryder D. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]; Norddahl, G.L., Pronk, C.J., Wahlestedt, M., Sten, G., Nygren, J.M., Ugale, A., Sigvardsson, M., and Bryder, D.. (2011). Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8, 499-510. [DOI] [PubMed]
- Nunnari J., Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nunnari, J., and Suomalainen, A.. (2012). Mitochondria: in sickness and in health. Cell 148, 1145-1159. [DOI] [PMC free article] [PubMed]
- Nutt D., King L.A., Saulsbury W., Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]; Nutt, D., King, L.A., Saulsbury, W., and Blakemore, C.. (2007). Development of a rational scale to assess the harm of drugs of potential misuse. Lancet 369, 1047-1053. [DOI] [PubMed]
- Oh J., Lee Y.D., Wagers A.J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oh, J., Lee, Y.D., and Wagers, A.J.. (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med.. 20, 870-880. [DOI] [PMC free article] [PubMed]
- Omaiye E.E., Cordova I., Davis B., Talbot P. Counterfeit electronic cigarette products with mislabeled nicotine concentrations. Tob. Regul. Sci. 2017;3:347–357. doi: 10.18001/TRS.3.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Omaiye, E.E., Cordova, I., Davis, B., and Talbot, P.. (2017). Counterfeit electronic cigarette products with mislabeled nicotine concentrations. Tob. Regul. Sci. 3, 347-357. [DOI] [PMC free article] [PubMed]
- Pazhanisamy S.K. Stem cells, DNA damage, ageing and cancer. Hematol. Oncol. Stem Cell Ther. 2009;2:375–384. doi: 10.1016/s1658-3876(09)50005-2. [DOI] [PubMed] [Google Scholar]; Pazhanisamy, S.K.. (2009). Stem cells, DNA damage, ageing and cancer. Hematol. Oncol. Stem Cell Ther.. 2, 375-384. [DOI] [PubMed]
- Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]; Rizzuto, R., De Stefani, D., Raffaello, A., and Mammucari, C.. (2012). Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566-578. [DOI] [PubMed]
- Rodier P.M. Developing brain as a target of toxicity. Environ. Health Perspect. 1995;103:73–76. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rodier, P.M.. (1995). Developing brain as a target of toxicity. Environ. Health Perspect.. 103, 73-76. [DOI] [PMC free article] [PubMed]
- Rose J.E., Mukhin A.G., Lokitz S.J., Turkington T.G., Herskovic J., Behm F.M., Garg S., Garg P.K. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc. Natl. Acad. Sci. U S A. 2010;107:5190–5195. doi: 10.1073/pnas.0909184107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rose, J.E., Mukhin, A.G., Lokitz, S.J., Turkington, T.G., Herskovic, J., Behm, F.M., Garg, S., and Garg, P.K.. (2010). Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc. Natl. Acad. Sci. U S A 107, 5190-5195. [DOI] [PMC free article] [PubMed]
- Ross J.M., Stewart J.B., Hagström E., Brené S., Mourier A., Coppotelli G., Freyer C., Lagouge M., Hoffer B.J., Olson L. Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature. 2013;501:412–415. doi: 10.1038/nature12474. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ross, J.M., Stewart, J.B., Hagstrom, E., Brene, S., Mourier, A., Coppotelli, G., Freyer, C., Lagouge, M., Hoffer, B.J., Olson, L., et al. (2013). Germline mitochondrial DNA mutations aggravate ageing and can impair brain development. Nature 501, 412-415. [DOI] [PMC free article] [PubMed]
- Rujano M.A., Bosveld F., Salomons F.A., Dijk F., van Waarde M.A.W.H., van der Want J.J.L., de Vos R.A.I., Brunt E.R., Sibon O.C.M., Kampinga H.H. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 2006;4:e417. doi: 10.1371/journal.pbio.0040417. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rujano, M.A., Bosveld, F., Salomons, F.A., Dijk, F., van Waarde, M.A.W.H., van der Want, J.J.L., de Vos, R.A.I., Brunt, E.R., Sibon, O.C.M., and Kampinga, H.H.. (2006). Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, e417. [DOI] [PMC free article] [PubMed]
- Santo-Domingo J., Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]; Santo-Domingo, J., and Demaurex, N.. (2010). Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta. 1797, 907-912. [DOI] [PubMed]
- Schroeder M.J., Hoffman A.C. Electronic cigarettes and nicotine clinical pharmacology. Tob. Control. 2014;23:ii30–ii35. doi: 10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schroeder, M.J., and Hoffman, A.C.. (2014). Electronic cigarettes and nicotine clinical pharmacology. Tob. Control 23, ii30-ii35. [DOI] [PMC free article] [PubMed]
- Schultz M.B., Sinclair D.A. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143:3–14. doi: 10.1242/dev.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schultz, M.B., and Sinclair, D.A.. (2016). When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development 143, 3-14. [DOI] [PMC free article] [PubMed]
- Seitz C.M., Strack R.W., Wyrick D.L. Cigarette smoking and facial wrinkles: a review of the literature. J. Smok. Cessat. 2012;7:18–24. [Google Scholar]; Seitz, C.M., Strack, R.W., and Wyrick, D.L.. (2012). Cigarette smoking and facial wrinkles: a review of the literature. J. Smok. Cessat.. 7, 18-24.
- Shaito A., Saliba J., Husari A., El-Harakeh M., Chhouri H., Hashem Y., Shihadeh A., El-Sabban M. Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Sci. Rep. 2017;7:14281. doi: 10.1038/s41598-017-14634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shaito, A., Saliba, J., Husari, A., El-Harakeh, M., Chhouri, H., Hashem, Y., Shihadeh, A., and El-Sabban, M.. (2017). Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Sci. Rep.. 7, 14281. [DOI] [PMC free article] [PubMed]
- Sharma G., Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]; Sharma, G., and Vijayaraghavan, S.. (2002). Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 53, 524-534. [DOI] [PubMed]
- Shivalingappa P.C., Hole R., Westphal C.V., Vij N. Airway exposure to e-cigarette vapors impairs autophagy and induces aggresome formation. Antioxid. Redox Signal. 2015;24:186–204. doi: 10.1089/ars.2015.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shivalingappa, P.C., Hole, R., Westphal, C.V., and Vij, N.. (2015). Airway exposure to e-cigarette vapors impairs autophagy and induces aggresome formation. Antioxid. Redox Signal.. 24, 186-204. [DOI] [PMC free article] [PubMed]
- Shokolenko I., Venediktova N., Bochkareva A., Wilson G.L., Alexeyev M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009;37:2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shokolenko, I., Venediktova, N., Bochkareva, A., Wilson, G.L., and Alexeyev, M.F.. (2009). Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 37, 2539-2548. [DOI] [PMC free article] [PubMed]
- Shutt T.E., McBride H.M. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim. Biophys. Acta. 2013;1833:417–424. doi: 10.1016/j.bbamcr.2012.05.024. [DOI] [PubMed] [Google Scholar]; Shutt, T.E., and McBride, H.M.. (2013). Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim. Biophys. Acta 1833, 417-424. [DOI] [PubMed]
- Skok M., Gergalova G., Lykhmus O., Kalashnyk O., Koval L., Uspenska K. Nicotinic acetylcholine receptors in mitochondria: subunit composition, function and signaling. Neurotransmitter. 2016;3:e1290. [Google Scholar]; Skok, M., Gergalova, G., Lykhmus, O., Kalashnyk, O., Koval, L., and Uspenska, K.. (2016). Nicotinic acetylcholine receptors in mitochondria: subunit composition, function and signaling. Neurotransmitter 3, e1290.
- Spano D., Heck C., Antonellis P., De Christofori G., Zollo M. Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 2012;22:234–249. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]; Spano, D., Heck, C., Antonellis, P. De Christofori, G., and Zollo, M.. (2012). Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 22, 234-249. [DOI] [PubMed]
- Suzuki J., Kanemaru K., Ishii K., Ohkura M., Okubo Y., Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]; Suzuki, J., Kanemaru, K., Ishii, K., Ohkura, M., Okubo, Y., and Iino, M.. (2014). Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun.. 5, 4153. [DOI] [PMC free article] [PubMed]
- Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res. C Embryo Today. 2008;84:61–72. doi: 10.1002/bdrc.20120. [DOI] [PubMed] [Google Scholar]; Talbot, P.. (2008). In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res. C Embryo Today 84, 61-72. [DOI] [PubMed]
- Talbot P., Lin S. Mouse and human embryonic stem cells: can they improve human health by preventing disease? Curr. Top. Med. Chem. 2011;11:1638–1652. doi: 10.2174/156802611796117621. [DOI] [PubMed] [Google Scholar]; Talbot, P., and Lin, S.. (2011). Mouse and human embryonic stem cells: can they improve human health by preventing disease? Curr. Top. Med. Chem. 11, 1638-1652. [DOI] [PubMed]
- Talbot P., Nieden N.I., Lin S., Martinez I., Guan B., Bhanu B. Use of video bioinformatics tools in stem cell toxicology. In: Sahu S.C., Casciano D.A., editors. Handbook of Nanotoxicology, Nanomedicine and Stem Cell Use in Toxicology. John Wiley & Sons Ltd.; 2014. pp. 379–402. [Google Scholar]; Talbot, P., Nieden, N.I., Lin, S., Martinez, I., Guan, B., and Bhanu, B.. (2014). Use of video bioinformatics tools in stem cell toxicology. In Handbook of Nanotoxicology, Nanomedicine and Stem Cell Use in Toxicology, S.C. Sahu and D.A. Casciano, eds. (West Sussex, United Kingdom: John Wiley & Sons Ltd.), pp. 379-402.
- Tan D., Goerlitz D.S., Dumitrescu R.G., Han D., Seillier-Moiseiwitsch F., Spernak S.M., Orden R.A., Chen J., Goldman R., Shields P.G. Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis. 2008;29:1170–1177. doi: 10.1093/carcin/bgn034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tan, D., Goerlitz, D.S., Dumitrescu, R.G., Han, D., Seillier-Moiseiwitsch, F., Spernak, S.M., Orden, R.A., Chen, J., Goldman, R., and Shields, P.G.. (2008). Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis 29, 1170-1177. [DOI] [PMC free article] [PubMed]
- Thyberg J., Hedin U., Stenseth K., Nilsson J. Effects of nicotine on the fine structure of cultivated mouse peritoneal macrophages. Acta Pathol. Microbiol. Immunol. Scand. A. 1983;91:23–30. doi: 10.1111/j.1699-0463.1983.tb02722.x. [DOI] [PubMed] [Google Scholar]; Thyberg, J., Hedin, U., Stenseth, K., and Nilsson, J.. (1983). Effects of nicotine on the fine structure of cultivated mouse peritoneal macrophages. Acta Pathol. Microbiol. Immunol. Scand. A 91, 23-30. [DOI] [PubMed]
- Tilly J.L., Sinclair D.A. Germline energetics, aging, and female infertility. Cell Metab. 2013;17:838–850. doi: 10.1016/j.cmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tilly, J.L., and Sinclair, D.A.. (2013). Germline energetics, aging, and female infertility. Cell Metab.. 17, 838-850. [DOI] [PMC free article] [PubMed]
- Tondera D., Grandemange S., Jourdain A., Karbowski M., Mattenberger Y., Herzig S., Da Cruz S., Clerc P., Raschke I., Merkwirth C. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tondera, D., Grandemange, S., Jourdain, A., Karbowski, M., Mattenberger, Y., Herzig, S., Da Cruz, S., Clerc, P., Raschke, I., Merkwirth, C., et al. (2009). SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J.. 28, 1589-1600. [DOI] [PMC free article] [PubMed]
- Wanet A., Arnould T., Najimi M., Renard P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015;24:1957–1971. doi: 10.1089/scd.2015.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wanet, A., Arnould, T., Najimi, M., and Renard, P.. (2015). Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 24, 1957-1971. [DOI] [PMC free article] [PubMed]
- Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr. Neuropharmacol. 2007;5:213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wickstrom, R.. (2007). Effects of nicotine during pregnancy: human and experimental evidence. Curr. Neuropharmacol.. 5, 213-222. [DOI] [PMC free article] [PubMed]
- Yin N., Yao X., Qin Z., Wang Y.L., Faiola F. Assessment of Bisphenol A (BPA) neurotoxicity in vitro with mouse embryonic stem cells. J. Environ. Sci. (China) 2015;36:181–187. doi: 10.1016/j.jes.2015.06.004. [DOI] [PubMed] [Google Scholar]; Yin, N., Yao, X., Qin, Z., Wang, Y.L., and Faiola, F.. (2015). Assessment of Bisphenol A (BPA) neurotoxicity in vitro with mouse embryonic stem cells. J. Environ. Sci. (China) 36, 181-187. [DOI] [PubMed]
- Zhang H., Menzies K.J., Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145 doi: 10.1242/dev.143420. dev143420. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang, H., Menzies, K.J., and Auwerx, J.. (2018). The role of mitochondria in stem cell fate and aging. Development 145, dev143420. [DOI] [PMC free article] [PubMed]
- Zhivotovsky B., Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]; Zhivotovsky, B., and Orrenius, S.. (2011). Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 50, 211-221. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows a rotating 3D reconstruction of a control NSC. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of a control NSC. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 4 h of VM 6TPE treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of Video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 24 h of VM 6TPE treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosomes (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of NSC after 4 h of VM 1% Treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of video = 10 s (5 frames/second).
This video shows a rotating 3D reconstruction of an NSC after 24 h of VM 1% treatment. mCerulean-tagged mitochondria (COX8) are labeled blue, and mRFP-tagged autophagosome (LC3) are labeled red. Length of Video = 10 s (5 frames/second).