Abstract
Seven previously unreported cyclonerane derivatives, namely, 3,7,11-trihydroxycycloneran-10-one, cycloneran-3,7,10,11-tetraol, cycloneran-3,7,11-triol, 11,12,15-trinorcycloneran-3,7,10-triol, 7,10S-epoxycycloneran-3,15-diol, 7,10R-epoxycycloneran-3,15-diol, and (10Z)-15-acetoxy-10-cycloneren-3,7-diol, were isolated in addition to the known (10Z)-cyclonerotriol, (10E)-cyclonerotriol, catenioblin C, and chokol E from the culture of Trichoderma asperellum A-YMD-9-2, an endophytic fungus obtained from the marine red alga Gracilaria verrucosa. The structures of previously unreported compounds were established by spectroscopic techniques, including 1D/2D NMR, MS, and IR. The isolation of these new cyclonerane derivatives greatly adds to the structural diversity of unusual cyclonerane sesquiterpenes, and several isolates exhibit potent inhibition against some marine phytoplankton species.
Keywords: Trichoderma asperellum, sesquiterpene, cyclonerane
1. Introduction
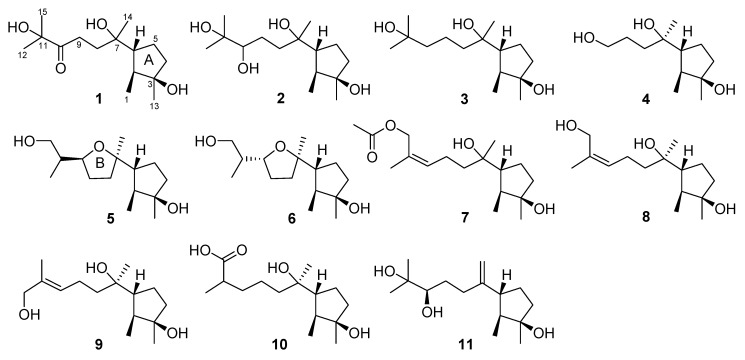
Trichoderma species have proven to be prolific sources of diterpenes and sesquiterpenes, especially some unusual terpene skeletons, such as harziane, proharziane, wickerane, citrinovirin, trichaspin, and cyclonerane [1,2,3,4,5,6,7,8]. Of the cyclonerane-type sesquiterpenes, cyclonerodiol and its analogs have been isolated from several species of Trichoderma [4,5,6,9,10,11,12,13,14]. To date, around 30 cyclonerane sesquiterpenes have been characterized, and almost half of them occurred in Trichoderma species. In our ongoing search for new and bioactive metabolites from marine algicolous fungi [15], an endophytic strain (A-YMD-9-2) of Trichoderma asperellum, isolated from the marine red alga Gracilaria verrucosa, was chemically examined. As a result, seven new cyclonerane derivatives, namely, 3,7,11-trihydroxycycloneran-10-one (1), cycloneran-3,7,10,11-tetraol (2), cycloneran-3,7,11-triol (3), 11,12,15-trinorcycloneran-3,7,10-triol (4), 7,10S-epoxycycloneran-3,15-diol (5), 7,10R-epoxycycloneran-3,15-diol (6), and (10Z)-15-acetoxy-10-cycloneren-3,7-diol (7), together with four known compounds, namely, (10Z)-cyclonerotriol (8) [16], (10E)-cyclonerotriol (9) [16,17], catenioblin C (10) [18], and chokol E (11) [19], were isolated and identified (Figure 1). Herein, the isolation, structure elucidation, and bioactivity of these compounds are described in detail.
Figure 1.
Structures of 1–11 (the stereochemistry in 1–8 and 10 only represents relative configuration).
2. Results and Discussion
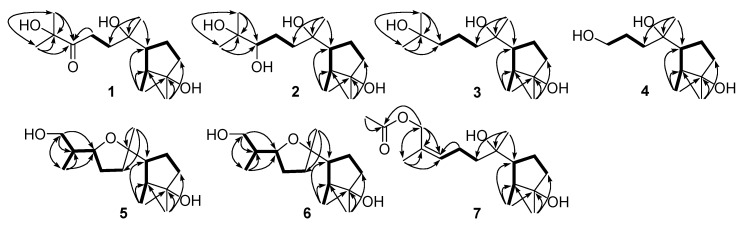
Compound 1 was obtained as a colorless oil. The molecular formula C15H28O4 was determined by HREIMS m/z 272.1991 [M]+ (calculated for C15H28O4, 272.1988), requiring two degrees of unsaturation. Its IR absorption bands at 3440 and 1713 cm−1 suggested the presence of hydroxy and carbonyl groups. In combination with HSQC data, the 1H NMR spectrum (Table 1) showed one methyl doublet, four methyl singlets, and an array of signals at δH 1.5–2.6 for four methylenes and two methines. Aided by the 90° and 135° DEPT experiments, 15 resonances in the 13C NMR spectrum (Table 2) were assigned to five methyls, four methylene sp3, two methine sp3, three quaternary sp3 and one quaternary sp2 carbons. The 13C NMR spectrum of 1 exhibited the signals at δC 79.4 and 215.1, attributed to an oxygenated quaternary sp3 carbon and a ketone carbonyl group, respectively, instead of the signals for the vinyl group in cyclonerodiol [20]. HMBC correlations from H3-12 (δH 1.30) to C-10 (δC 215.1), C-11 (δC 79.4), and C-15 (δC 28.0) and from H3-15 (δH 1.29) to C-10, C-11, and C-12 (δC 27.8) established the connectivity between C-10 and C-11, which then extended to C-8 (δC 31.5) as corroborated by the HMBC correlation from H2-9 (δH 2.51) to C-10 as well as by the COSY correlation from H2-8 (δH 2.12 and 1.89) to H2-9. The connectivity around ring A was deduced to be the same as those of cyclonerodiol based on their identical NMR data, and this was confirmed by the COSY correlations from H3-1 (δH 1.03)/H-2 (δH 1.56)/H-6 (δH 1.97)/H2-5 (δH 1.88 and 1.63)/H2-4 (δH 1.67 and 1.56) and HMBC correlations from H3-1 to C-2 (δC 44.9), C-3 (δC 81.4), and C-6 (δC 55.1), from H3-13 (δH 1.25) to C-2, C-3, and C-4 (δC 40.4), and from H3-14 (δH 1.19) to C-6, C-7 (δC 76.0), and C-8 (δC 31.5) (Figure 2). The NOESY correlation from H3-1 to H-6 further verified that Me-1 was syn to H-6, while the NOESY correlations from H3-1 to H-5a (δH 1.88) and from H3-13 to H-5b (δH 1.63) suggested Me-1 to be opposite to Me-13. The above information evidenced 1 to be 3,7,11-trihydroxycycloneran-10-one.
Table 1.
1H NMR Data for 1–7 (500 MHz, in CDCl3, δ in ppm, J in Hz).
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 (β) | 1.03, d (6.8) | 1.03, d (6.8) | 1.03, d (6.8) | 1.05, d (6.8) | 1.02, d (6.8) | 1.02, d (6.8) | 1.04, d (6.8) |
| 2 (α) | 1.56, m | 1.55, m | 1.59, m | 1.60, m | 1.50, m | 1.54, m | 1.60, m |
| 4a | 1.67, m | 1.69, m | 1.67, m | 1.69, m | 1.68, m | 1.68, m | 1.68, m |
| 4b | 1.56, m | 1.56, m | 1.55, m | 1.56, m | 1.59, m | 1.58, m | 1.55, m |
| 5a | 1.88, m | 1.88, m | 1.85, m | 1.88, m | 1.89, m | 1.89, m | 1.85, m |
| 5b | 1.63, m | 1.56, m | 1.54, m | 1.55, m | 1.49, m | 1.40, m | 1.55, m |
| 6 (β) | 1.97, m | 1.89, m | 1.85, m | 1.87, m | 1.95, m | 2.00, m | 1.84, m |
| 8a | 2.12, m | 1.73, m | 1.44, m | 1.57, m | 1.75, m | 1.86, m | 1.50, t (8.3) |
| 8b | 1.89, m | 1.59, m | 1.64, m | 1.69, m | |||
| 9a | 2.51, m | 1.61, m | 1.44, m | 1.68, m | 1.92, m | 1.83, m | 2.16, m |
| 9b | 1.40, m | 1.71, m | 1.79, m | ||||
| 10 | 3.38, br d (10.3) a | 1.45, m | 3.68, m | 4.07, ddd (7.6, 6.9, 4.1) | 4.04, ddd (9.8, 5.6, 4.3) | 5.41, t (7.3) | |
| 11 | 1.93, m | 2.00, m | |||||
| 12 | 1.30, s | 1.16, s | 1.22, s | 0.93, d (7.0) | 0.90, d (7.1) | 1.74, br s | |
| 13 (α) | 1.25, s | 1.25, s | 1.25, s | 1.26, s | 1.24, s | 1.25, s | 1.25, s |
| 14 | 1.19, s | 1.15, s | 1.16, s | 1.17, s | 1.14, s | 1.17, s | 1.16, s |
| 15a | 1.29, s | 1.21, s | 1.22, s | 3.65, d (10.8, 6.1) | 3.69, dd (10.8, 6.9) | 4.62, d (11.9) | |
| 15b | 3.61, d (10.8, 4.1) | 3.58, dd (10.8, 3.7) | 4.57, d (11.9) | ||||
| CH3CO | 2.06, s |
a H-10 is coupled with H-9a and H-9b, but one of the couplings only results in broad resonance.
Table 2.
13C NMR data for 1–7 (125 MHz, in CDCl3, δ in ppm).
| Position | δC, Type | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 14.4, CH3 | 14.5, CH3 | 14.7, CH3 | 14.6, CH3 | 14.0, CH3 | 13.8, CH3 | 14.7, CH3 |
| 2 | 44.9, CH | 44.7, CH | 44.4, CH | 44.5, CH | 45.4, CH | 45.3, CH | 44.4, CH |
| 3 | 81.4, C | 81.5, C | 81.5, C | 81.4, C | 81.3, C | 81.4, C | 81.4, C |
| 4 | 40.4, CH2 | 40.4, CH2 | 40.5, CH2 | 40.5, CH2 | 40.5, CH2 | 40.4, CH2 | 40.5, CH2 |
| 5 | 24.8, CH2 | 24.5, CH2 | 24.4, CH2 | 24.6, CH2 | 25.1, CH2 | 25.4, CH2 | 24.5, CH2 |
| 6 | 55.1, CH | 54.5, CH | 54.3, CH | 54.9, CH | 54.2, CH | 54.1, CH | 54.6, CH |
| 7 | 76.0, C | 75.1, C | 75.0, C | 74.8, C | 85.7, C | 86.0, C | 74.8, C |
| 8 | 31.5, CH2 | 37.0, CH2 | 41.1, CH2 | 36.8, CH2 | 35.7, CH2 | 34.8, CH2 | 40.5, CH2 |
| 9 | 32.8, CH2 | 25.8, CH2 | 18.7, CH2 | 27.2, CH2 | 28.1, CH2 | 27.4, CH2 | 22.5, CH2 |
| 10 | 215.1, C | 79.0, CH | 44.5, CH2 | 63.6, CH2 | 80.3, CH | 84.2, CH | 131.1, CH |
| 11 | 79.4, C | 73.4, C | 71.2, C | 38.2, CH | 37.4, CH | 130.0, C | |
| 12 | 27.8, CH3 | 23.5, CH3 | 29.4, CH3 | 11.8, CH3 | 12.0, CH3 | 21.6, CH3 | |
| 13 | 26.3, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 |
| 14 | 23.5, CH3 | 25.0, CH3 | 25.2, CH3 | 25.2, CH3 | 23.1, CH3 | 26.4, CH3 | 25.0, CH3 |
| 15 | 28.0, CH3 | 26.7, CH3 | 29.5, CH3 | 66.9, CH2 | 66.7, CH2 | 63.3, CH2 | |
| CH3CO | 171.3, C | ||||||
| CH3CO | 21.1, CH3 | ||||||
Figure 2.
Key COSY (bold lines) and HMBC (arrows) correlations of 1–7.
Compound 2 was isolated as a colorless oil, and its molecular formula was established as C15H30O4, which has two more hydrogen atoms than that of 1, based on the (+)-HREIMS m/z 297.2037 [M + Na]+ (calculated for C15H30O4Na, 297.2042). A detailed comparison of its NMR signals (Table 1 and Table 2) with those of 1 revealed the presence of ring A and its affiliated groups in 2. In addition, this compound exhibited a deshielded broad doublet (J = 10.3 Hz) at δH 3.38 in the 1H NMR spectrum. Combined with the HSQC correlation of this proton signal to the carbon signal at δC 79.0, this signal was attributed to the oxymethine proton on C-10. This was supported by the HMBC correlations from oxymethine sp3 carbon at δC 79.0 (C-10) to H3-12 (δH 1.16) and H3-15 (δH 1.21) as well as a COSY correlation from H-10 to H2-9 (δH 1.61 and 1.40) (Figure 2). Thus, 2 was deduced to be cycloneran-3,7,10,11-tetraol. Previously, cyclonerodiol B from the mangrove-derived endophytic fungus Trichoderma sp. Xy24 was assigned the same structure as 2, but it should be revised to epicyclonerodiol oxide in view of their identical NMR data [9,13]. To confirm the absolute configuration at C-10, Mosher’s reactions were performed under the conditions described previously [21]. Unfortunately, no esterified products were obtained after the purification via preparative thin-layer chromatography (TLC).
Compound 3 was purified as a colorless oil, and its HREIMS m/z 258.2198 [M]+ (calculated for C15H30O3, 258.2195) produced the molecular formula C15H30O3, which has one oxygen atom less than that of 2. Its 13C NMR spectrum (Table 2) showed a close similarity to that of 2, except for the lack of the signal for an oxymethine group and the presence of the signal for a methylene group of C-10 (δC 44.5), which was supported by its HMBC correlations to H3-12 (δH 1.22) and/or H3-15 (δH 1.22). Other HMBC and COSY correlations, as shown in Figure 2, further confirmed 3 to be cycloneran-3,7,11-triol, a 10-deoxy derivative of 2.
Compound 4 was also isolated as a colorless oil. A molecular formula C12H24O3 was assigned by HREIMS m/z 216.1724 [M]+ (calculated for C12H24O3, 216.1725), implying one degree of unsaturation. Its 1H and 13C NMR data (Table 1 and Table 2) resembled those of 2, except for the lack of signals for a 2-hydroxy-2-propyl group (δH 1.16 and 1.21, δC 23.5, 26.7, and 73.4) and the presence of signals for a hydroxymethylene group (δH 3.68 and δC 63.6). This group was deduced to be situated at the side chain terminus on the basis of HMBC correlations from H3-14 (δH 1.17) to C-6 (δC 54.9), C-7 (δC 74.8), and C-8 (δC 36.8) and COSY correlations from H2-10 (δH 3.68) to H2-9 (δH 1.68) and from H2-9 to H2-8 (δH 1.57). HMBC correlations (Figure 2) from H3-1 (δH 1.05) to C-2 (δC 44.5), C-3 (δC 81.4), and C-6, and from H3-13 (δH 1.26) to C-2, C-3, and C-4 (δC 40.5), and COSY correlations from H3-1 to H2-4 (δH 1.69 and 1.56) via H-2 (δH 1.60), H-6 (δH 1.87), and H2-5 (δH 1.88 and 1.55), further verified the connectivity of ring A. Thus, 4 was proposed to be 11,12,15-trinorcycloneran-3,7,10-triol, possibly a degradation product of 1 or 2. To ascertain the relative configuration at C-7, the energy-minimized conformers, regardless of the rotation of the methyl and hydroxy groups as well as the side chain, of 7α- and 7β-methyl isomers optimized at the B3LYP/6-31G(d) level in chloroform, were subjected to the 13C NMR calculations using the gauge-independent atomic orbital (GIAO) method at the B3LYP/6-31+G(d,p) level via Gaussian 09 software [22]. Both experimental and calculated shifts were input into Sarotti’s DP4+ sheet (see https://sarotti-nmr.weebly.com) [23], and the calculated data for 7α- and 7β-methyl isomers displayed 73.74% and 26.26% DP4+ probabilities, respectively. Thus, the methyl group at C-7 was tentatively assigned as α orientation.
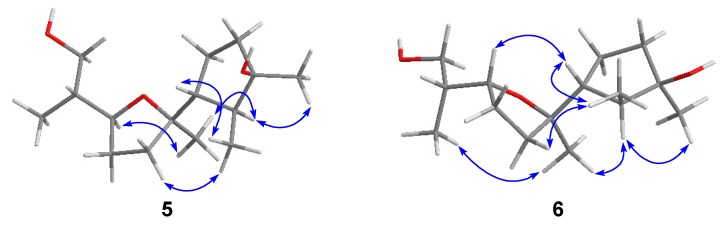
Compound 5 was obtained as a colorless oil, and the HREIMS spectrum afforded the molecular formula C15H28O3, suggesting two degrees of unsaturation. However, examination of the 1H and 13C NMR data (Table 1 and Table 2) indicated the absence of any unsaturated bond. Thus, this compound was suggested to possess a bicyclic skeleton. As the NMR data of 5 resembled those of 7,10-epoxycycloneran-3,11,12-triol, the presence of a 7,10-epoxycyclonerane framework was proposed [5]. However, the signal for a methine group appeared in the 13C NMR spectrum of 5 instead of that for an oxygenated quaternary carbon in the 13C NMR spectrum of 7,10-epoxycycloneran-3,11,12-triol. The fact that the methine group was located at C-11 (δC 38.2) of 5 was corroborated by its HMBC correlations with H3-12 (δH 0.93) and H2-15 (δH 3.65 and 3.61). HMBC correlations (Figure 2) from H3-12 and H2-15 to C-10 (δC 80.3), from H3-1 (δH 1.02) to C-2 (δC 45.4), C-3 (δC 81.3), and C-6 (δC 54.2), from H3-13 (δH 1.24) to C-2, C-3, and C-4 (δC 40.5), and from H3-14 (δH 1.14) to C-6, C-7 (δC 85.7), and C-8 (δC 35.7) further confirmed the structure of 5 as 11-deoxy derivative of 7,10-epoxycycloneran-3,11,12-triol. Additionally, H-10 (δH 4.07) and Me-14 (δH 1.14) were on the same face of ring B by their NOESY correlation (Figure 3). The NOESY correlations from H3-1 to H-5a (δH 1.89) and H-6 (δH 1.95) and from H3-13 to H-5b (δH 1.49) validated the relative configurations around ring A. However, the relative configuration at C-11 remains unsolved.
Figure 3.
Key NOESY correlations of 5 and 6.
Compound 6 was isolated as a colorless oil and has the same molecular formula as that of 5 by HREIMS m/z 256.2028 [M]+ (calcd for C15H28O3, 256.2038). Its 1H and 13C NMR data (Table 1 and Table 2) also resembled those of 5, except for the deshielded signal for C-10 (δC 84.2). Thus, 6 was deduced to be a C-10 epimer of 5, as supported by the NOESY correlation from H3-12 (δH 0.90) to H3-14 (δH 1.17). The similar chemical shifts of C-7 (δC 86.0) and C-10 (δC 84.2) to those of epicyclonerodiol oxide further evidenced the opposite orientation of H-10 and C-14 [9]. The relative configurations around ring A and at C-7 were deduced to be the same as those of 5 based on their identical 1H and 13C NMR data. Moreover, because both C-6 and C-7 are chiral centers, the rotation of the C-6–C-7 single bond is restricted to some extent. Thus, the relative configuration at C-7 could be locked by the NOESY correlations from H3-14 to H-2 (δH 1.54) and H-5b (δH 1.40) and from H-8a (δH 1.86) to H3-1 (δH 1.02) and H-2 (Figure 3). As is the case for 5, the relative configuration at C-11 (δC 37.4) is not determined.
Compound 7 was obtained as a colorless oil. Its molecular formula was determined to be C17H30O4 by HREIMS (m/z 298.2138), corresponding to three degrees of unsaturation. The carbonyl signal at δC 171.3 and the methyl signals at δH 2.06 and δC 21.1 as well as their HMBC correlation indicated the presence of an acetyl group. The remaining NMR data (Table 1 and Table 2) showed a close similarity to those of (10Z)-cyclonerotriol (8) [16]. Thus, 7 was speculated to be an acetylated derivative of 8, and the acetoxy group was attached to C-15, as evidenced from the HMBC correlation from H2-15 (δH 4.62 and 4.57) to the carbonyl carbon (δC 171.3). Other HMBC and COSY correlations (Figure 2) further validated the structure of 7, and the Z geometry was corroborated by the NOESY correlations between H2-15 and H2-9 (δH 2.16) and between H3-12 (δH 1.74) and H-10 (δH 5.41).
To develop new inhibitors against harmful microalgae that seriously threaten marine aquaculture, 1–11 were evaluated for their growth inhibition against four marine phytoplankton species (Chattonella marina, Heterosigma akashiwo, Karlodinium veneficum, and Prorocentrum donghaiense) [24]. The results (Table 3) show that 10, with IC50 values ranging from 1.6 to 2.0 μg/mL, is the most potent to inhibit the four phytoplankton species tested. An analysis of the structure–activity relationship suggested that the carboxyl group at C-15 may greatly contribute to the inhibitory ability of cyclonerane sesquiterpenes. Moreover, the toxicity of 10 to the marine zooplankton Artemia salina was also assayed [7], but it does not exhibit any lethal effect at the concentration of 100 μg/mL.
Table 3.
Inhibition against four marine phytoplankton species by 1–11.
| Compound | IC50 (μg/mL) | |||
|---|---|---|---|---|
| Chattonella marina | Heterosigma akashiwo | Karlodinium veneficum | Prorocentrum donghaiense | |
| 1 | 5.2 | 8.0 | 10 | 9.9 |
| 2 | 8.8 | 21 | 76 | 6.5 |
| 3 | 61 | 73 | 71 | 40 |
| 4 | 13 | 73 | 6.3 | 34 |
| 5 | 2.4 | 26 | 3.9 | 20 |
| 6 | 5.8 | 37 | 5.5 | 15 |
| 7 | 59 | 14 | 35 | 7.3 |
| 8 | 42 | 50 | 12 | 3.4 |
| 9 | 15 | 53 | 43 | 1.1 |
| 10 | 1.6 | 1.8 | 1.6 | 2.0 |
| 11 | 62 | 9.4 | 13 | 6.0 |
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations and ECD curves were determined on a Chirascan CD spectrometer (Applied Photophysics Ltd., Surrey, UK). IR spectra were obtained on a Nicolet iS10 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). NMR spectra were recorded on a Bruker Avance III 500 NMR spectrometer (Bruker Corp., Billerica, MA, USA). Low- and high-resolution EI and ESI+ mass spectra were measured on an Autospec Premier P776 mass spectrometer (Waters Corp., Milford, MA, USA) and an Agilent G6230 TOF mass spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA), respectively. Column chromatography (CC) was carried out with silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), RP-18 (AAG12S50, YMC Co., Ltd., Kyoto, Japan), and Sephadex LH-20 (GE Healthcare, Uppsala, Sweden). Thin-layer chromatography (TLC) was performed with precoated silica gel plates (GF-254, Qingdao Haiyang Chemical Co., Qingdao, China). Quantum chemical calculations were run with Gaussian 09 software (Gaussian, Inc., Wallingford, CT, USA).
3.2. Fungal Material and Fermentation
The fungal strain Trichoderma asperellum A-YMD-9-2 (Moniliaceae) was obtained from the inner tissue of the marine red alga Gracilaria verrucosa collected from Yangma Island, Yantai, China in August 2016. The fungus was identified by morphological observation and by analysis of the ITS regions of its rDNA, whose sequence data were deposited at GenBank with the accession number MH819724. The fermentation was performed statically at room temperature for 40 days in 200 × 1 L Erlenmeyer flasks, each containing 50 g rice, 0.6 g peptone, 50 mL pure water, and 50 mL natural seawater from the coast of Yantai, China.
3.3. Extraction and Isolation
The mycelia and broth were separated by filtration at the end of the fermentation, and the former were dried in the shade and exhaustively extracted with CH2Cl2 and MeOH (1:1, v/v). After removing organic solvents under a vacuum, the residue was partitioned between EtOAc and H2O to afford an EtOAc-soluble extract (202.1 g). The broth was directly extracted with EtOAc and then concentrated to give an extract (10.3 g). Based on the similar TLC profiles, these two parts were combined and subjected to silica gel CC with step-gradient solvent systems consisting of petroleum ether (PE)/EtOAc (50:1 to 0:1) and then CH2Cl2/MeOH (10:1 to 0:1) to give eight fractions (Frs. 1–8). Fr. 3 eluted with PE/EtOAc (2:1) and was further purified by CC on RP-18 (MeOH/H2O, 3:2) and silica gel (PE/EtOAc, 6:1) to obtain 1 (11.3 mg), 5 (5.2 mg), and 6 (5.9 mg). Fr. 4 eluted with PE/EtOAc (1:1) and was further purified by CC on RP-18 (MeOH/H2O, 1:1 to 3:2) and Sephadex LH-20 (MeOH) and preparative TLC (CH2Cl2/MeOH, 15:1) to yield 7 (8.1 mg), 8 (33.8 mg), 10 (10.1 mg), and 11 (3.6 mg). Fr. 5 eluted with EtOAc and was further purified by CC on RP-18 (MeOH/H2O, 2:3 to 3:2) and Sephadex LH-20 (MeOH) and preparative TLC (CH2Cl2/MeOH, 10:1) to give 2 (26.0 mg), 4 (1.7 mg), and 9 (10.0 mg). Fr. 6 eluted with CH2Cl2/MeOH (10:1) and was further purified by CC on RP-18 (MeOH/H2O, 1:1) and Sephadex LH-20 (MeOH) and preparative TLC (CH2Cl2/MeOH, 7:1) to produce 3 (12.0 mg).
3,7,11-Trihydroxycycloneran-10-one (1): Colorless oil; [α]20D − 24 (c 0.44, MeOH); IR (KBr) vmax 3440, 2960, 2932, 1713, 1460, 1663, 1374, 1239, 1125, 1013, 921 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S1 and S2); 2D NMR data, Figures S3–S6; EIMS m/z (%) 272 [M]+ (<1), 196 (12), 178 (45), 141 (95), 136 (44), 121 (39), 95 (39), 71 (40), 56 (40), 44(100); HREIMS m/z 272.1991 [M]+ (calcd for C15H28O4, 272.1988) (Figure S7).
Cycloneran-3,7,10,11-tetraol (2): Colorless oil; [α]20D − 30 (c 1.0, MeOH); IR (KBr) vmax 3416, 2967, 1654, 1460, 1378, 1266, 1162, 1074, 1032, 921, 885 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S8 and S9); 2D NMR data, Figures S10–S13; ESI+MS m/z 297 [M + Na]+; HRESI+MS m/z 297.2037 [M + Na]+ (calcd for C15H30O4Na, 297.2042) (Figure S14).
Cycloneran-3,7,11-triol (3): Colorless oil; [α]20D − 18 (c 0.62, MeOH); IR (KBr) vmax 3406, 2965, 1654, 1460, 1377, 1164, 920, 885 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S15 and S16); 2D NMR data, Figures S17–S20; EIMS m/z (%) 258 [M]+ (<1), 207 (25), 157 (28), 139 (81), 127 (70), 109 (81), 95 (38), 81(45), 44(100); HREIMS m/z 258.2198 [M]+ (calcd for C15H30O3, 258.2195) (Figure S21).
11,12,15-Trinorcycloneran-3,7,10-triol (4): Colorless oil; [α]20D − 39 (c 0.062, MeOH); IR (KBr) vmax 3426, 2928, 1720, 1635, 1460, 1383, 1054, 920 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S22 and S23); 2D NMR data, Figures S24–S27; EIMS m/z (%) 216 [M]+ (<1), 165 (11), 157 (11), 139 (79), 103 (31), 96 (40), 85 (98), 81(58), 44(100); HREIMS m/z 216.1724 [M]+ (calcd for C12H24O3, 216.1725) (Figure S28).
7,10S-epoxycycloneran-3,15-diol (5): Colorless oil; [α]20D +11 (c 0.20, MeOH); IR (KBr) vmax 3426, 2965, 2931, 2876, 1654, 1460, 1376, 1205, 1092, 1024, 919 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S29 and S30); 2D NMR data, Figures S31–S34; EIMS m/z (%) 256 [M]+ (<1), 183 (20), 143 (100), 139 (32), 125 (35), 113 (30), 59 (42), 44(100); HREIMS m/z 256.2034 [M]+ (calcd for C15H28O3, 256.2038) (Figure S35).
7,10R-epoxycycloneran-3,15-diol (6): Colorless oil; [α]20D +6.5 (c 0.31, MeOH); IR (KBr) vmax 3426, 2965, 2931, 2868, 1635, 1460, 1376, 1206, 1152, 1093, 1024, 919 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S36 and S37); 2D NMR data, Figures S38–S41; EIMS m/z (%) 256 [M]+ (<1), 237 (8), 179 (10), 143 (90), 125 (32), 95 (28), 56 (41), 44(100); HREIMS m/z 256.2028 [M]+ (calcd for C15H28O3, 256.2038) (Figure S42).
(10Z)-15-Acetoxy-10-cycloneren-3,7-diol (7): Colorless oil; [α]20D − 11 (c 0.31, MeOH); IR (KBr) vmax 3452, 2963, 2932, 1735, 1654, 1460, 1370, 1240, 1025, 920, 885 cm−1; 1H and 13C NMR data, Table 1 and Table 2 (Figures S43 and S44); 2D NMR data, Figures S45–S48; EIMS m/z (%) 256 [M]+ (<1), 139 (33), 125 (98), 107 (28), 81 (27), 44(100); HREIMS m/z 298.2138 [M]+ (calcd for C17H30O4, 298.2144) (Figure S49).
4. Conclusions
Chemical examination of the marine-alga-endophytic fungus Trichoderma asperellum A-YMD-9-2 led to the isolation and identification of seven new (1–7) and four known (8–11) cyclonerane derivatives, including an unprecedented trinorcyclonerane (4). The discovery of these new isolates greatly diversifies the unusual cyclonerane sesquiterpenes. Among the isolates, 10 displays significant inhibition of the four phytoplankton species and features no toxicity to any of the zooplankton tested.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/5/252/s1, Figures S1–S49: 1D/2D NMR and HRMS spectra of compounds 1–7.
Author Contributions
Conceptualization, N.-Y.J.; methodology, Y.-P.S., F.-P.M., and X.-L.Y.; software, Y.-P.S. and X.-L.Y.; validation, N.-Y.J.; formal analysis, Y.-P.S. and N.-Y.J.; investigation, Y.-P.S. and X.-H.L.; resources, X.-H.L.; data curation, Y.-P.S. and N.-Y.J.; writing—original draft preparation, Y.-P.S.; writing—review and editing, N.-Y.J.; visualization, N.-Y.J.; supervision, N.-Y.J.; project administration, N.-Y.J. and F.-P.M.; funding acquisition, N.-Y.J. and F.-P.M.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31670355; the Natural Science Foundation for Distinguished Young Scholars of Shandong Province, grant number JQ201712; Key Cutting-Edge Research Program of the CAS, grant number QYZDB-SSW-DQC013; the Science and Technology Service Network Initiative of the CAS, grant number KFJ-STS-ZDTP-023; and the Youth Innovation Promotion Association of the CAS, grant number 2013138.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Su D., Ding L., He S. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini-Rev. Med. Chem. 2018;18:1702–1713. doi: 10.2174/1389557518666180727130826. [DOI] [PubMed] [Google Scholar]
- 2.Reino J.L., Guerrero R.F., Hernández-Galán R., Collado I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008;7:89–123. doi: 10.1007/s11101-006-9032-2. [DOI] [Google Scholar]
- 3.Shi Z.Z., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018;81:319–325. doi: 10.1016/j.bioorg.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Song Y.P., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018;81:2553–2559. doi: 10.1021/acs.jnatprod.8b00714. [DOI] [PubMed] [Google Scholar]
- 5.Song Y.P., Liu X.H., Shi Z.Z., Miao F.P., Fang S.T., Ji N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry. 2018;152:45–52. doi: 10.1016/j.phytochem.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Liang X.R., Miao F.P., Song Y.P., Liu X.H., Ji N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016;26:5029–5031. doi: 10.1016/j.bmcl.2016.08.093. [DOI] [PubMed] [Google Scholar]
- 7.Miao F.P., Liang X.R., Yin X.L., Wang G., Ji N.Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012;14:3815–3817. doi: 10.1021/ol3014717. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T., Izumi N., Ui H., Sueki A., Masuma R., Nonaka K., Hirose T., Sunazuka T., Nagai T., Yamada H., et al. Wickerols A and B: novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron. 2012;68:9267–9271. doi: 10.1016/j.tet.2012.08.066. [DOI] [Google Scholar]
- 9.Fujita T., Takaishi Y., Takeda Y., Fujiyama T., Nishi T. Fungal metabolites. II. Structural elucidation of minor metabolites, valinotricin, cyclonerodiol oxide, and epicyclonerodiol oxide, from Trichoderma polysporum. Chem. Pharm. Bull. 1984;32:4419–4425. doi: 10.1248/cpb.32.4419. [DOI] [Google Scholar]
- 10.Macías F.F., Varela R.M., Simonet A.M., Cutler H.G., Cutler S.J., Eden M.A., Hill R.A. Bioactive carotanes from Trichoderma virens. J. Nat. Prod. 2000;63:1197–1200. doi: 10.1021/np000121c. [DOI] [PubMed] [Google Scholar]
- 11.Zheng C.J., Sun P.X., Jin G.L., Qin L.P. Sesquiterpenoids from Trichoderma atroviride, an endophytic fungus in Cephalotaxus fortunei. Fitoterapia. 2011;82:1035–1038. doi: 10.1016/j.fitote.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Wu S.H., Zhao L.X., Chen Y.W., Huang R., Miao C.P., Wang J. Sesquiterpenoids from the endophytic fungus Trichoderma sp. PR-35 of Paeonia delavayi. Chem. Biodivers. 2011;8:1717–1723. doi: 10.1002/cbdv.201000236. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M., Zhao J.L., Liu J.M., Chen R.D., Xie K.B., Chen D.W., Feng K.P., Zhang D., Dai J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017;19:651–658. doi: 10.1080/10286020.2016.1251908. [DOI] [PubMed] [Google Scholar]
- 14.Pang X., Lin X., Tian Y., Liang R., Wang J., Yang B., Zhou X., Kaliyaperumal K., Luo X., Tu Z., Liu Y. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat. Prod. Res. 2018;32:105–111. doi: 10.1080/14786419.2017.1338286. [DOI] [PubMed] [Google Scholar]
- 15.Ji N.Y., Wang B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016;80:301–342. doi: 10.1007/s13225-016-0358-9. [DOI] [Google Scholar]
- 16.Li X., Kim Y.H., Jung J.H., Kang J.S., Kim D.K., Choi H.D., Son B.W. Microbial transformation of the bioactive sesquiterpene, cyclonerodiol, by the ascomycete Penicillium sp. and the actinomycete Streptomyces sp. Enzyme Microb. Technol. 2007;40:1188–1192. doi: 10.1016/j.enzmictec.2006.09.002. [DOI] [Google Scholar]
- 17.Hanson J.R., Hitchcock P.B., Nyfeler R. Cyclonerotriol [6-(3-hydroxy-2,3-dimethylcyclopentyl)-2- methylhept-2-ene-1,6-diol], a new sesquiterpenoid metabolite of Fusariurn culmorum. J. Chem. Soc. Perkin Trans. 1. 1975:1586–1590. doi: 10.1039/p19750001586. [DOI] [Google Scholar]
- 18.Wu H.Y., Wang Y.L., Tan J.L., Zhu C.Y., Li D.X., Huang R., Zhang K.Q., Niu X.M. Regulation of the growth of cotton bollworms by metabolites from an entomopathogenic fungus Paecilomyces cateniobliquus. J. Agric. Food Chem. 2012;60:5604–5608. doi: 10.1021/jf302054b. [DOI] [PubMed] [Google Scholar]
- 19.Koshino H., Togiya S., Terada S., Yoshihara T., Sakamura S., Shimanuki T., Sato T., Tajimi A. New fungitoxic sesquiterpenoids, chokols A-G, from stromata of Epichloe typhina and the absolute configuration of chokol E. Agric. Biol. Chem. 1989;53:789–796. doi: 10.1271/bbb1961.53.789. [DOI] [Google Scholar]
- 20.Laurent D., Goasdoue N., Kohler F., Pellegrin F., Platzer N. Characterization of cyclonerodiol isolated from corn infested by Fusarium Moniliforme sheld.: one- and two-dimensional 1H and 13C NMR study. Magn. Reson. Chem. 1990;28:662–664. doi: 10.1002/mrc.1260280721. [DOI] [Google Scholar]
- 21.Su B.N., Park E.J., Mbwambo Z.H., Santarsiero B.D., Mesecar A.D., Fong H.H.S., Pezzuto J.M., Kinghorn A.D. New chemical constituents of euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. J. Nat. Prod. 2002;65:1278–1282. doi: 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- 22.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09, Revision C.01. Gaussian, Inc.; Wallingford, CT, USA: 2010. [Google Scholar]
- 23.Grimblat N., Zanardi M.M., Sarotti A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015;80:12526–12534. doi: 10.1021/acs.joc.5b02396. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z.Z., Miao F.P., Fang S.T., Yin X.L., Ji N.Y. Sulfurated diketopiperazines from an algicolous isolate of Trichoderma virens. Phytochem. Lett. 2018;27:101–104. doi: 10.1016/j.phytol.2018.07.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.