Abstract
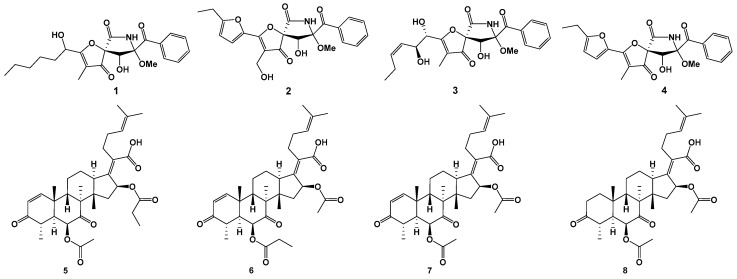
Two new spiro-heterocyclic γ-lactam derivatives, cephalimysins M (1) and N (2), were isolated from the fermentation cultures of the marine-derived fungus Aspergillus fumigatus CUGBMF17018. Two known analogues, pseurotin A (3) and FD-838 (4), as well as four previously reported helvolic acid derivatives, 16-O-propionyl-16-O-deacetylhelvolic acid (5), 6-O-propionyl-6-O-deacetylhelvolic acid (6), helvolic acid (7), and 1,2-dihydrohelvolic acid (8) were also identified. One-dimensional (1D), two-dimensional (2D) NMR, HRMS, and circular dichroism spectral analysis characterized the structures of the isolated compounds.
Keywords: marine-derived Aspergillus fumigatus, spiro-heterocyclic γ-lactam, cephalimysins
1. Introduction
Marine-derived fungi are important resources of structurally and biologically diverse secondary metabolites in drug discovery [1,2,3,4,5,6]. A series of novel marine natural compounds have been isolated from marine-derived fungi of Aspergillus fumigatus strains, such as E-β-trans-5,8,11-trihydroxybergamot-9-ene and β-trans-2β,5,15-trihydroxybergamot-10-ene [7], diketopiperazines [8,9], indole alkaloids [10], fumigaclavine C [11], fumiquinazoline K [12], and gliotoxin analogues [13]. During our ongoing efforts to search for new bioactive metabolites from marine-derived fungi, an Aspergillus fumigatus strain CUGBMF170049 was isolated from a sediment sample that was collected from the Bohai Sea, China. Chemical investigations on an EtOAc-MeOH extracted fraction of its solid fermentation cultures resulted in the characterization of two new spiro-heterocyclic γ-lactam derivatives, cephalimysins M (1) and N (2), along with two known analogues, pseurotin A (3) [14], and FD-838 (4) [15], as well as four previously reported helvolic acid derivatives, 16-O-propionyl-16-O-deacetylhelvolic acid (5), 6-O-propionyl-6-O-deacetylhelvolic acid (6) [16], helvolic acid (7), and 1,2-dihydrohelvolic acid (8) [17] (Figure 1). Herein, we report the isolation and structural determination of the new compounds 1 and 2. The antibacterial activities of the compounds were also investigated against a panel of both Gram-positive and Gram-negative bacteria, Mycobacterium bovis bacillus Calmette Guérin (BCG) and Candida albicans. Compounds 5–7 showed significant antibacterial activities against both Staphylococcus aureus and methicillin resistant S. aureus.
Figure 1.
Chemical structures of 1–8.
2. Results
2.1. Structure Elucidation
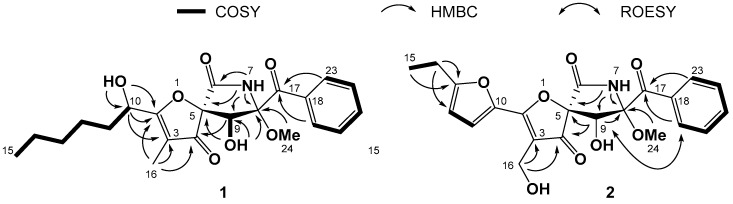
Compound 1 was obtained as pale yellow amorphous powder. Its molecular formula was determined as C22H27NO7 by HRESIMS m/z 440.1684 [M + Na]+ (calcd. for C22H27NO7Na 440.1680, Δmmu + 0.4) (Figure S1 in Supplementary Materials), which accounted for ten degrees of unsaturation. The 1H, 13C, and HSQC NMR spectra (Figures S2–S4 in Supplementary Materials) of compound 1 showed signals of two ketone carbonyls at δC 196.7 (C-4) and 196.4 (C-17), one amide carbonyl at δC 166.4 (C-6), a mono-substituted benzene ring (δC 133.4, δC 130.3/δH 8.25, δC 128.4/δH 7.53, δC 133.9/δH 7.64, δC 128.4/δH 7.53, 130.3/δH 8.25), two sp2 quaternary carbons at δC 188.5 (C-2) and 109.6 (C-3), four sp3 methylenes (δC 34.4/δH 1.64, δC 31.1/δH 1.24, δC 24.1/δH 1.37, and δC 22.0/δH 1.24), two sp3 oxygenated methines (δC 74.9/δH 4.38 and δC 67.0/δH 4.50), two sp3 oxygenated quaternary carbons at δC 91.2 (C-5) and δC 92.5 (C-8), and three methyls (δC 13.9/δH 0.84, δC 5.4/δH 1.64, and δC 51.7/δH 3.24) (Table 1). A comparison of 1H and 13C NMR data for compound 1 (Table 1) with those of previously reported pseurotin A (3) [14] revealed many similarities in their chemical structures, except for the oxygenated unsaturated side chain of 3 had been substituted with an oxygenated saturated fatty chain in 1. Compound 1 had the same skeleton as that of pseurotin A, while the side chain of 1 is an oxygenated saturated fatty chain. The COSY correlations (Figure 2, and Figure S5 in Supplementary Materials) from H-19 (δH 8.25) to H-23 (δH 8.25), through H-20 (δH 7.53), H-21 (δH 7.64) and H-22 (δH 7.53) identified the mono-substituted benzene ring. Likewise, the fatty acid side chain was confirmed by COSY correlations from H-10 (δH 4.50) to H3-15 (δH 0.84), through H-11 (δH 1.64), H-12 (δH 1.37), H-13 (δH 1.24), and H-14 (δH 1.24). The lactam ring was evidenced by the HMBC correlations (Figure 2, and Figure S6 in Supplementary Materials) from H-9-OH (δH 6.34) to C-5 (δC 91.2), C-8 (δC 92.5), and C-9 (δC 74.9), as well as from H-7-NH (δH 9.90) to C-5 (δC 91.2), C-6 (δC 166.4), C-8 (δC 92.5), and C-9 (δC 74.9). The phenylmethanone moiety was confirmed by the HMBC crossing peaks from H-19 (δH 8.25) and H-23 (δH 8.25) to C-17 (δC 196.4), and the connection from C-8 (δC 92.5) to C-17 (δC 196.4) was revealed by the HMBC correlation from H-9 (δH 4.38) to C-17 (δC 196.4). The HMBC correlation from H3-24 (δH 3.24) to C-8 (δC 92.5) confirmed the methoxy at C-8. The connection from C-10 to C-4 through C-2 and C-3 was confirmed by the HMBC correlations from H-10-OH (δH 5.62) to C-10 (δC 67.0) and C-2 (δC 188.5), from H-10 (δH 4.50) to C-2 (δC 188.5) and C-3 (δC 109.6), as well as from H3-16 (δH 1.64) to C-2 (δC 188.5), C-3 (δC 109.6), and C-4 (δC 196.7). In addition, the spirobicyclic moiety was suggested by the HMBC correlation from H-9 (δH 4.38) to C-4 (δC 196.7), the chemical shift of C-5 (δC 91.2) and the molecular formula. The cis configurations of 8-OCH3 and 9-OH were supported by the chemical shift of H-9 and the coupling constant (δH 4.38, J = 9.0 Hz) between H-9 and 9-OH [15,18]. The circular dichroism (CD) spectrum (Figure S7 in Supplementary Materials) of 1 showed negative Cotton effects at around 230, 280, and 345 nm, and positive Cotton effects at around 250 and 310 nm, which were consistent with the reported CD data for pseurotin A [18]. Thus, the structure of compound 1 was established, as shown in Figure 1, and was named cephalimysin M, its absolute configurations for C-5, C-8, and C-9 being assigned the same as those of pseurotin A. The absolute configuration of C-10 was not defined.
Table 1.
NMR data for 1 and 2 (DMSO-d6).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, mult (J in Hz) | δC, Type | δH, mult (J in Hz) | |
| 2 | 188.5, C | 172.2, C | ||
| 3 | 109.6, C | 111.6, C | ||
| 4 | 196.7, C | 193.4, C | ||
| 5 | 91.2, C | 92.1, C | ||
| 6 | 166.4, C | 166.4, C | ||
| 8 | 92.5, C | 92.6, C | ||
| 9 | 74.9, CH | 4.38, d (9.0) | 75.0, CH | 4.50, s |
| 10 | 67.0, CH | 4.50, td (7.2, 5.4) | 141.8, C | |
| 11 | 34.4, CH2 | 1.64, m (overlap) | 120.0, CH | 7.40, d (3.6) |
| 12 | 24.1, CH2 | 1.37, m | 108.6, CH | 6.52, d (3.6) |
| 13 | 31.1, CH2 | 1.24, m | 163.4, C | |
| 14 | 22.0, CH2 | 1.24, m | 21.0, CH2 | 2.76, q (7.8) |
| 15 | 13.9, CH3 | 0.84, t (7.2) | 11.8, CH3 | 1.22, t (7.8) |
| 16 | 5.4, CH3 | 1.64, s | 50.2, CH2 | 4.25, s |
| 17 | 196.4, C | 196.4, C | ||
| 18 | 133.4, C | 133.4, C | ||
| 19 | 130.3, CH | 8.25, dd (8.4, 1.2) | 130.4, CH | 8.27, d (7.2) |
| 20 | 128.4, CH | 7.53, ddd (8.4, 8.4, 1.2) | 128.4, CH | 7.53, dd (7.2, 7.2) |
| 21 | 133.9, CH | 7.64, dddd (8.4, 8.4, 1.2, 1.2) | 133.9, CH | 7.67, dd (7.2, 7.2) |
| 22 | 128.4, CH | 7.53, ddd (8.4, 8.4, 1.2) | 128.4, CH | 7.53, dd (7.2, 7.2) |
| 23 | 130.3, CH | 8.25, dd (8.4, 1.2) | 130.4, CH | 8.27, d (7.2) |
| 24 | 51.7, CH3 | 3.24, s | 51.7, CH3 | 3.26, s |
| 7-NH | 9.90, s | 9.98, s | ||
| 9-OH | 6.34, d (9.0) | |||
| 10-OH | 5.62, d (5.4) | |||
Figure 2.
Key two-dimensional (2D) NMR correlations for 1 and 2.
Compound 2 was obtained as pale yellow amorphous powder. Its molecular formula was determined as C22H21NO8 by HRESIMS m/z 450.1159 [M + Na]+ (calcd. for C22H21NO8Na 450.1159, Δmmu 0) (Figure S8 in Supplementary Materials), which accounted for thirteen degrees of unsaturation. The 1H, 13C, and HSQC NMR spectra (Figures S9–S11 in Supplementary Materials) of compound 2 showed signals of two ketone carbonyls at δC 193.4 (C-4) and 196.4 (C-17), one amide carbonyl at δC 166.4 (C-6), a mono-substituted benzene ring (δC 133.4, δC 130.4/δH 8.27, δC 128.4/δH 7.53, δC 133.9/δH 7.67, δC 128.4/δH 7.53, δC 130.4/δH 8.27), two sp2 methines (δC 120.1/δH 7.40, δC 108.6/δH 6.52), four sp2 quaternary carbons at δC 172.2 (C-2), 111.6 (C-3), 141.8 (C-10), and 163.4 (C-13), one sp3 methylene (δC 21.0/δH 2.76), one sp3 oxygenated methylene (δC 50.2/δH 4.25), one sp3 oxygenated methine (δC 75.0/δH 4.50), two sp3 oxygenated quaternary carbons (δC 92.1 and δC 92.6), and two methyls (δC 11.8/δH 1.22 and δC 51.7/δH 3.26) (Table 1). A comparison of 1H and 13C NMR data for compound 2 (Table 1) with that of the previously reported FD-838 (4) [15] revealed many similarities. Compound 2 had the same skeleton as that of FD-838, while methyl at C-3 of compound 4 was replaced by an oxygenated methylene in compound 2. The mono-substituted benzene ring was identified by the COSY correlations (Figure 2, and Figure S12 in Supplementary Materials) from H-19 (δH 8.27) to H-23 (δH 8.27), through H-20 (δH 7.53), H-21 (δH 7.67), and H-22 (δH 7.53), and the furan side chain was characterized by COSY correlations between H-11 (δH 7.40) and H-12 (δH 6.52), and between H2-14 (δH 2.76) and H3-15 (δH 1.22), along with the HMBC correlations (Figure 2, and Figure S13 in Supplementary Materials) from H3-15 to C-13, and from H2-14 to C-12 and C-13. The lactam ring was suggested by the HMBC correlations from H-9 (δH 4.50) to C-5 (δC 92.1) and C-8 (δC 92.6), as well as from H-7-NH (δH 9.98) to C-5 (δC 92.1), C-8 (δC 92.6) and C-9 (δC 75.0). HMBC crossing peaks from H-19 (δH 8.27) and H-23 (δH 8.27) to C-17 (δC 196.4) confirmed the phenylmethanone moiety, and the connection from C-8 (δC 92.6) to C-17 (δC 196.4) was revealed by the HMBC correlation from H-9 (δH 4.50) to C-17 (δC 196.4). The methoxy at C-8 was also confirmed by the HMBC correlation from H3-24 (δH 3.26) to C-8 (δC 92.6). The spirobicyclic moiety was indicated by the HMBC correlation from H-9 (δH 4.50) to C-4 (δC 193.4) and the chemical shift of C-5 (δC 92.1). The ROESY correlations (Figure 2, and Figure S14 in Supplementary Materials) from H-9 (δH 4.50) to H-19 (δH 8.27) and H-23 (δH 8.27) indicated the relative configurations of C-8 and C-9. The chemical shifts of C-5, C-8, and C-9 for 2 were much closer to those of 1 and FD-838 [15], which defined the relative configurations of C-5, C-8, and C-9. The circular dichroism (CD) spectrum (Figure S15 in Supplementary Materials) of 2 showed a negative Cotton effect at around 318 nm and a positive Cotton effect at around 355 nm, which were consistent with the reported CD data for FD-838 [15]. Therefore, the structure of compound 2 was established, as shown in Figure 1, where absolute configurations were assigned the same as those of FD-838 and named cephalimysin N.
In addition to compounds 1 and 2, known compounds were also identified in the fermentation products, such as pseurotin A (3) [14], FD-838 (4) [15], as well as four known helvolic acid derivatives, 16-O-propionyl-16-O-deacetylhelvolic acid (5), 6-O-propionyl-6-O-deacetylhelvolic acid (6) [16], helvolic acid (7), and 1,2-dihydrohelvolic acid (8) [17].
2.2. Biological Activity
Compounds 1–8 were tested against Gram positive bacteria S. aureus (ATCC 6538) and methicillin resistant S. aureus (MRSA) (ATCC 29213), Gram negative bacteria Escherichia coli (ATCC 11775) and Pseudomonas aeruginosa (ATCC 15692), BCG, and C. albicans. Compounds 5–7 showed antibacterial activities against both S. aureus and MRSA. Comparing the antibacterial activities of compounds 5–7 with the inactive analogue 8 indicated that the α,β-unsaturated ketone appears to be a key functional group for antibacterial activity (Table 2). None of the isolated compounds exhibited antimicrobial activities against E. coli, P. aeruginosa, C. albicans (MIC > 100 µg/mL), nor BCG (MIC > 10 µg/mL).
Table 2.
Antimicrobial activities of 1–8 (µg/mL).
| Compounds | S. aureus a | MRSA a |
|---|---|---|
| 1 | >100 | >100 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | >100 | >100 |
| 5 | 12.5 | 25 |
| 6 | 6.25 | 12.5 |
| 7 | 0.78 | 0.78 |
| 8 | >100 | >100 |
a Vancomycin was used as positive control with MIC value of 0.78 µg/mL.
3. Materials and Methods
3.1. General Experimental Procedures
The optical rotations ([α]D) were measured on Anton Paar MCP 200 Modular Circular Polarimeter (Austria) in a 100 × 2 mm cell at 22 °C. CD spectra were recorded on an Applied Photophysics Chirascan spectropolarimeter (UK). NMR spectra were obtained on a Bruker Avance DRX600 spectrometer with residual solvent peaks serving as references (DMSO-d6: δH 2.50, δC 39.52). High-resolution ESIMS measurements were obtained on a Bruker micrOTOF mass spectrometer by direct infusion in MeCN at 3 mL/min using sodium formate clusters as an internal calibrate. HPLC was performed using an Agilent 1200 Series separation module that was equipped with Agilent 1200 Series diode array and Agilent 1260 Series fraction collector, and Agilent SB-C18 column (250 × 9.4 mm, 5 µm).
3.2. Fungal Material
The Aspergillus fumigatus strain CUGBMF170049 was isolated from a sediment sample that was collected from the Bohai Sea, China and grown on a potato dextrose agar plate at 28 °C. This strain was identified as Aspergillus fumigatus based on DNA sequence analysis of its internal transcribed spacer (ITS) region (Figure S16) (GenBank accession number MK453215) using a conventional primer pair of ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′).
3.3. Fermenttion and Extraction
A small spoonful of Aspergillus fumigatus (CUGBMF170049) spores growing on a potato dextrose agar slant was inoculated into four 250 mL conical flasks, each containing 40 mL of liquid medium consisting of potato infusion (20%), glucose (2.0%), artificial sea salt (3.5%), and distilled water. The flasks were incubated at 28 °C for 3 d on a rotary shaker at 160 rpm. An aliquot (5 mL) of the resultant seed culture was inoculated into teen 1 L conical flasks, with each containing solid medium consisting of rice (120 g) and artificial seawater (3.5%; 80 mL), and the flasks were incubated stationary for 30 days at 28 °C. The cultures were extracted three times by EtOAc:MeOH (80:20), and the combined extracts were reduced to dryness in vacuo and the residue was partitioned between EtOAc and H2O. Subsequently, the EtOAc layer was dried in vacuo to yield a dark residue (11.3 g).
3.4. Isolation and Purification
The EtOAc fraction was fractionated by a reduced pressure silica gel chromatography (50 × 80 mm column, TLC H silica) using a stepwise gradient of 50–100% hexane/CH2Cl2 and then 0–100% MeOH/CH2Cl2 to afford 15 fractions. Fraction C was fractionated on a Sephadex LH-20 column (600 × 30 mm) while using an isocratic elution of hexane:CH2Cl2:MeOH (5:5:1) to give five subfractions (F1–F5). Subfraction F3 (102.3 mg after drying in vacuo) was further fractionated by HPLC (Agilent Zorbax SB-C18 250 × 9.4 mm, 5 μm column, 2.0 mL/min, isocratic 65% MeOH/H2O) to yield FD-838 (4; tR 10.4 min, 3.3 mg). Fraction J was fractionated on a Sephadex LH-20 column (600 × 30 mm) using an isocratic elution of CH2Cl2:MeOH (2:1), to give four subfractions (F1–F4). Subfraction F1 was further fractionated by HPLC (Agilent Zorbax SB-C18 250 × 9.4 mm, 5 μm column, 2.0 mL/min, isocratic 65% MeOH/H2O) to yield helvolic acid (7, tR 10.8 min, 1.3 mg), 16-O-propionyl-16-O-deacetylhelvolic acid (5, tR 11.9 min, 1.2 mg), and 6-O-propionyl-6-O-deacetylhelvolic acid (6, tR 12.4 min, 1.4 mg). Fraction K was fractionated on a Sephadex LH-20 column (600 × 30 mm) using an isocratic elution of CH2Cl2:MeOH (2:1) to give five subfractions (F1–F5). Subfraction F3 was further fractionated by HPLC (Agilent Zorbax SB-C18 250 × 9.4 mm, 5 μm column, 2.0 mL/min, isocratic 65% MeOH/H2O) to yield 1,2-dihydrohelvolic acid (8, tR 13.9 min, 1.6 mg). Fraction L was fractionated on a Sephadex LH-20 column (600 × 30 mm) using an isocratic elution of CH2Cl2:MeOH (2:1) to give five subfractions (F1–F5). Subfraction F4 was further fractionated by an ODS column, which was eluted by a stepwise gradient (0–100% MeOH/H2O) to afford five subfractions (F1–F5). Subfraction F4 was further fractionated by HPLC (Agilent Zorbax SB-C18 250 × 9.4 mm, 5 μm column, 2.0 mL/min, isocratic 65% MeOH/H2O) to yield pseurotin A (3, tR 7.1 min, 3.2 mg), cephalimysins M (1, tR 12.0 min, 1.5 mg), and N (2, tR 8.9 min, 3.6 mg).
3.4.1. Cephalimysin M (1)
Pale yellow amorphous powder; [α] –21.3 (MeOH, 0.1); UV (MeOH) λmax (logε) 196 (4.43), 254 (4.14), 277(3.96) nm; (+)-ESIMS m/z 418.1 [M + H]+; (+)-HRESIMS m/z 440.1684 [M + Na]+ (calcd. for C22H27NO7Na 440.1680); 1H and 13C NMR data: See Table 1.
3.4.2. Cephalimysin N (2)
Pale yellow amorphous powder; [α] –21.5 (MeOH, 0.1); UV (MeOH) 197 (4.43), 252 (4.12), 329(3.56) nm; (+)-ESIMS m/z 428.0 [M + H]+; (+)-HRESIMS m/z 450.1159 [M + Na]+ (calcd. for C22H21NO8Na 450.1159); 1H and 13C NMR data: See Table 1.
3.5. Antimicrobial Assays
The antimicrobial assays were performed according to the Antimicrobial Susceptibility Testing Standards that were outlined by the Clinical and Laboratory Standards Institute (CLSI) against S. aureus ATCC 6538, MRSA ATCC 29213, E. coli ATCC 11775, P. aeruginosa ATCC 15692, and C. albicans ATCC 10231 based on a 96 well microplate format in liquid growth. Briefly, the bacteria from glycerol stocks was inoculated on LB agar plate and cultured overnight at 37°C. The glycerol stock of C. albicans was prepared on Sabouraud dextrose agar at 28 °C for 24 h. Afterwards, single colonies were picked and adjusted to approximately 104 CFU/mL with Mueller–Hinton Broth as bacterial suspension and with RPMI 1640 media as fungal suspension. 2 μL of two-fold serial dilution of each compound (in DMSO) were added to each row on 96-well microplate, containing 78 μL of bacterial or fungal suspension in each well. (Vancomycin and Ciprofloxacin were used as positive controls; Amphotericin B was used as positive for fungi; DMSO as negative control). The 96-well plate was aerobically incubated at 37 °C for 16 h. The 96-well plate of antifungal was aerobically incubated at 35 °C for 24 h. Here, MIC is defined as the minimum concentration of compound at which no bacterial growth is observed.
3.6. Anti-Bacillus Calmette Guérin (BCG) Assay
The anti-BCG assay was carried out by using a constitutive GFP expression strain (pUV3583c-GFP), according to previous published procedure (isoniazid was used as positive control with MIC value of 0.05 µg/mL) [19]. The concentrations for the tested compounds were from 0.156 to 10 µg/mL by using two-fold diluted solutions.
4. Conclusions
As part of our ongoing research program to discover novel secondary metabolites from the marine environment, eight compounds were isolated from the rice solid medium culture of the marine derived fungus CUGBMF170049 isolated from a sediment sample that was collected from the Bohai Sea, China. Two novel compounds (1 and 2) were isolated and characterized along with the previously reported analogues pseurotin A (3) and FD-838 (4), as well as four known helvolic acid derivatives, namely 16-O-propionyl-16-O-deacetylhelvolic acid (5), 6-O-propionyl-6-O-deacetylhelvolic acid (6), helvolic acid (7), and 1,2-dihydrohelvolic acid (8). All of the structures were confirmed by detailed analysis of the spectroscopic data. Compounds 5–7 showed antibacterial activity against both S. aureus and MRSA. Aanalogue 8 did not exhibit antibacterial activities that indicated that the α,β-unsaturated ketone of 5–7 is the key functional group for antibacterial activity. Structurally, cephalimysins M (1) and N (2) belong to a family of rare natural products with diverse biological activities, which contain an unusual spiro-heterocyclic γ-lactam core. To the best of our knowledge, 28 natural products of this family have been reported, including pseurotin A [14], 8-O-demethylpseurotin A [20] pseurotins A1 and A2 [18,21], pseurotins B – E [22], pseurotins F1 and F2 [23], 14-norpseurotin A [24] synerazol [25], azaspirene [26], azaspirofurans A and B [27], and FD-838 and cephalimysins A–L [15,28,29]. 2 is the first cephalimysin analogue where the methyl of C-16 was oxidized to hydroxymethyl. The current research diversifies the structures of this class of natural products.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/5/289/s1, Figures S1–S15: The HRESIMS, UV, 1 D, 2D NMR, and CD spectra of compounds 1 and 2, Figure S16 the phylogenetic tree of strain CUGBMF170049.
Author Contributions
Data curation, X.X.; Investigation, J.H., Y.W., R.L. and H.Y.; Supervision, X.X. and F.S.; Writing—original draft, X.X. and F.S.; Writing—review & editing, X.X., R.L., J.L., S.W., S.W.P. and F.S.
Funding
This work was supported in part by the National Key Research and Development Program of China (2018YFC0311002, 2017YFD0201203, 2017YFC1601300), the National Natural Science Foundation of China (31600136). SWP was supported by the National Health and Medical Research Council of Australia (GN1147538).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liu S.Z., Yan X., Tang X.X., Lin J.G., Qiu Y.K. New bis-alkenoic acid derivatives from a marine-derived fungus Fusarium solani H915. Mar. Drugs. 2018;16:483. doi: 10.3390/md16120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N., Li C.W., Cui C.B., Cai B., Xu L.L., Zhu H.J. Four new antitumor metabolites isolated from a mutant 3-f-31 strain derived from Penicillium purpurogenum G59. Eur. J. Med. Chem. 2018:548–558. doi: 10.1016/j.ejmech.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Rai M., Gade A., Zimowska B., Ingle A.P., Ingle P. Marine-derived phoma-the gold mine of bioactive compounds. Appl. Microbiol. Biotechnol. 2018;102:9053–9066. doi: 10.1007/s00253-018-9329-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhu A., Zhang X.W., Zhang M., Li W., Ma Z.Y., Zhu H.J., Cao F. Aspergixanthones I-K, new anti-vibrio prenylxanthones from the marine-derived fungus Aspergillus sp. ZA-01. Mar. Drugs. 2018;16:312. doi: 10.3390/md16090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su D., Ding L., He S. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini. Rev. Med. Chem. 2018;18:1702–1713. doi: 10.2174/1389557518666180727130826. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P., Jia C., Lang J., Li J., Luo G., Chen S., Yan S., Liu L. Mono- and dimeric naphthalenones from the marine-derived fungus Leptosphaerulina chartarum 3608. Mar. Drugs. 2018;16:173. doi: 10.3390/md16050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Li D.H., Li Z.L., Sun Y.J., Hua H.M., Liu T., Bai J. Terpenoids from the marine-derived fungus Aspergillus fumigatus YK-7. Molecules. 2016;21:31. doi: 10.3390/molecules21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Li Z.L., Bai J., Zhang L.M., Wu X., Zhang L., Pei Y.H., Jing Y.K., Hua H.M. 2,5-diketopiperazines from the marine-derived fungus Aspergillus fumigatus YK-7. Chem. Biodivers. 2012;9:385–393. doi: 10.1002/cbdv.201100061. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W.Y., Zhu T.J., Fan G.T., Liu H.B., Fang Y.C., Gu Q.Q., Zhu W.M. Three new dioxopiperazine metabolites from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2010;24:953–957. doi: 10.1080/14786410902726134. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D., Satake M., Fukuzawa S., Sugahara K., Niitsu A., Shirai T., Tachibana K. Two new indole alkaloids, 2-(3,3-dimethylprop-1-ene)-costaclavine and 2-(3,3-dimethylprop-1-ene)-epicostaclavine, from the marine-derived fungus Aspergillus fumigatus. J. Nat. Med. 2012;66:222–226. doi: 10.1007/s11418-011-0565-3. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.X., Himaya S.W., Dewapriya P., Zhang C., Kim S.K. Fumigaclavine C from a marine-derived fungus Aspergillus fumigatus induces apoptosis in MCF-7 breast cancer cells. Mar. Drugs. 2013;11:5063–5086. doi: 10.3390/md11125063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afiyatullov S.S., Zhuravleva O.I., Antonov A.S., Kalinovsky A.I., Pivkin M.V., Menchinskaya E.S., Aminin D.L. New metabolites from the marine-derived fungus Aspergillus fumigatus. Nat. Prod. Commun. 2012;7:497–500. doi: 10.1177/1934578X1200700421. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W.Y., Zhu T.J., Han X.X., Fan G.T., Liu H.B., Zhu W.M., Gu Q.Q. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Nat. Prod. Res. 2009;23:203–207. doi: 10.1080/14786410600906970. [DOI] [PubMed] [Google Scholar]
- 14.Bloch P., Tamm C. Isolation and structure of Pseurotin A, a microbial metabolite of Pseudeurotium ovalis STOLK with an unusual heterospirocyclic system. Helv. Chim. Acta. 1981;64:304–315. doi: 10.1002/hlca.19810640131. [DOI] [Google Scholar]
- 15.Yamada T., Kitada H., Kajimoto T., Numata A., Tanaka R. The relationship between the CD Cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. J. Org. Chem. 2010;75:4146–4153. doi: 10.1021/jo100496f. [DOI] [PubMed] [Google Scholar]
- 16.Kong F.D., Huang X.L., Ma Q.Y., Xie Q.Y., Wang P., Chen P.W., Zhou L.M., Yuan J.Z., Dai H.F., Luo D.Q., Zhao Y.X. Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047. J. Nat. Prod. 2018;81:1869–1876. doi: 10.1021/acs.jnatprod.8b00382. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.Y., Kinoshita H., Ihara F., Igarashi Y., Nihira T. Identification of novel derivative of helvolic acid from Metarhizium anisopliae grown in medium with insect component. J. Biosci. Bioeng. 2008;105:476–480. doi: 10.1263/jbb.105.476. [DOI] [PubMed] [Google Scholar]
- 18.Yamada T., Ohshima M., Yuasa K., Kikuchi T., Tanaka R. Assignment of the CD cotton effect to the chiral center in pseurotins, and the stereochemical revision of Pseurotin A2. Mar. Drugs. 2016;14:74. doi: 10.3390/md14040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Song F., Xiao X., Huang P., Li L., Monte A., Abdel-Mageed W.M., Wang J., Guo H., He W., et al. Abyssomicins from the South China Sea deep-sea sediment Verrucosispora sp.: Natural thioether Michael addition adducts as antitubercular prodrugs. Angew. Chem. Int. Ed. Engl. 2013;52:1231–1234. doi: 10.1002/anie.201208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenke J., Anke H., Sterner O. Pseurotin A and 8-O-demethylpseurotin A from Aspergillus fumigatus and their inhibitory activities on chitin synthase. Biosci. Biotech. Bioch. 1993;57:961–964. doi: 10.1271/bbb.57.961. [DOI] [Google Scholar]
- 21.Wang F.Z., Li D.H., Zhu T.J., Zhang M., Gu Q.Q. Pseurotin A1 and A2, two new 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-diones from the holothurian-derived fungus Aspergillus fumigatus WFZ-25. Can. J. Chem. 2011;89:72–76. doi: 10.1139/V10-157. [DOI] [Google Scholar]
- 22.Breitenstein W., Chexal K.K., Mohr P., Tamm C. Pseurotin B, C, D, and E. further new metabolites of Pseudeurotium ovalis STOLK. Helv. Chim. Acta. 1981;64:379–388. doi: 10.1002/hlca.19810640203. [DOI] [Google Scholar]
- 23.Wink J., Grabley S., Gareis M., Thiericke R., Kirsch R. Pseurotin F1/F2, New Metabolites from Aspergillus fumigatus, Process for Their Preparation and Their Use as Apomorphine Antagonists. EP546474. European Patent Application. 1993
- 24.Zhang M., Wang W.L., Fang Y.C., Zhu T.J., Gu Q.Q., Zhu W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 25.Ando O., Satake H., Nakajima M., Sato A., Nakamura T., Kinoshita T., Furuya K., Haneishi T. Synerazol, a new antifungal antibiotic. J. Antibiot. (Tokyo) 1991;44:382–389. doi: 10.7164/antibiotics.44.382. [DOI] [PubMed] [Google Scholar]
- 26.Asami Y., Kakeya H., Onose R., Yoshida A., Matsuzaki H., Osada H. Azaspirene: A novel angiogenesis inhibitor containing a 1-oxa-7-azaspiro[4.4]non-2-ene-4,6-dione skeleton produced by the fungus Neosartorya sp. Org. Lett. 2002;4:2845–2848. doi: 10.1021/ol020104+. [DOI] [PubMed] [Google Scholar]
- 27.Ren H., Liu R., Chen L., Zhu T.J., Zhu W.M., Gu Q.Q. Two new hetero-spirocyclic gamma-Lactam derivatives from marine dediment-derived fungus Aspergillus sydowi D2-6. Arch. Pharm. Res. 2010;33:499–502. doi: 10.1007/s12272-010-0401-4. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T., Imai E., Nakatuji K., Numata A., Tanaka R. Cephalimysin A, a potent cytotoxic metabolite from an Aspergillus species separated from a marine fish. Tetrahedron Lett. 2007;48:6294–6296. doi: 10.1016/j.tetlet.2007.07.024. [DOI] [Google Scholar]
- 29.Yamada T., Kajimoto T., Kikuchi T., Tanaka R. Elucidation of the relationship between CD Cotton effects and the absolute configuration of sixteen stereoisomers of spiroheterocyclic-lactams. Mar. Drugs. 2018;16:223. doi: 10.3390/md16070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.