Abstract
Al resistance of signalgrass (Brachiaria decumbens Stapf cv Basilisk), a widely sown tropical forage grass, is outstanding compared with the closely related ruzigrass (Brachiaria ruziziensis Germain and Evrard cv Common) and Al-resistant genotypes of graminaceous crops such as wheat, triticale, and maize. Secretion of organic acids and phosphate by root apices and alkalinization of the apical rhizosphere are commonly believed to be important mechanisms of Al resistance. However, root apices of signalgrass secreted only moderately larger quantities of organic acids than did those of ruzigrass, and efflux from signalgrass apices was three to 30 times smaller than from apices of Al-resistant genotypes of buckwheat, maize, and wheat (all much more sensitive to Al than signalgrass). In the presence, but not absence, of Al, root apices of signalgrass alkalinized the rhizosphere more than did those of ruzigrass. The latter was associated with a shortening of the alkalinizing zone in Al-intoxicated apices of ruzigrass, indicating that differences in alkalinizing power were a consequence, not a cause of, differential Al resistance. These data indicate that the main mechanism of Al resistance in signalgrass does not involve external detoxification of Al. Therefore, highly effective resistance mechanisms based on different physiological strategies appear to operate in this species.
Al toxicity is one of the most important constraints to crop production on acid soils (Rao et al., 1993; De la Fuente-Martínez and Herrera-Estrella, 1999). Al3+ ions, the most toxic mononuclear Al species, inhibit root elongation by injuring the root apex, particularly the distal part of the transition zone (Ryan et al., 1993; Kinraide, 1997; Sivaguru and Horst, 1998). Despite considerable research, the mechanistic basis of Al toxicity is not well understood (Delhaize and Ryan, 1995; Horst, 1995; Kochian, 1995; Rengel, 1996; Kochian and Jones, 1997). It has been established that Al can cross the plasma membrane, although the rate of uptake into the symplasm remains a subject of controversy (Lazof et al., 1997; Rengel and Reid, 1997; Taylor et al., 2000). Al resistance, accordingly, can be achieved through “external” mechanisms, which minimize uptake of Al, or “internal” mechanisms, which detoxify Al within the symplasm.
Two external Al resistance mechanisms based on Al detoxification in the apoplast and rhizosphere have gained experimental support: (a) formation of non-toxic Al chelates with Al ligands secreted by root apices, and (b) alkalinization of the apical apoplast and rhizosphere, which shifts the concentrations of mononuclear Al species in favor of less toxic Al hydroxides.
Malate, citrate, and oxalate have been identified as important Al-chelating ligands secreted by roots (Miyasaka et al., 1991; Delhaize et al., 1993; Pellet et al., 1995; Ma et al., 1997, 2000; Ma and Miyasaka, 1998; Zheng et al., 1998a, 1998b; Li et al., 2000; Ma, 2000; Matsumoto, 2000). They are thought to be involved in Al resistance because their secretion tends to be localized to apices and associated with the level of Al resistance and the presence of genes conferring Al resistance (Delhaize et al., 1993; Pellet et al., 1995; Ryan et al., 1995b; Zheng et al., 1998a; Ma et al., 2000). Stimulation of organic-acid secretion by overexpression of citrate synthase, or as a result of mutagenesis, has confirmed this view (De la Fuente et al., 1997; Larsen et al., 1998; Koyama et al., 1999). Chelation of apoplastic and rhizospheric Al by phosphate appears to contribute to Al exclusion in certain cultivars or species (Lindberg, 1990; Millard et al., 1990; Pellet et al., 1996, 1997). The isolation of an Al-resistant Arabidopsis mutant that increases the pH of the apical rhizosphere under Al stress has confirmed the long-standing hypothesis that a stronger alkalinization of the rhizosphere can increase Al resistance (Taylor, 1991; Degenhardt et al., 1998).
To date, most studies of Al-resistance mechanisms have focused on Al-resistant genotypes of crops that have a long breeding history. Because past breeding programs have rarely selected for Al resistance, the single major resistance genes, frequently encountered in resistant cultivars, may have resulted from random fixation or be a by-product of selection for other agronomic features carried out on (moderately) acid soils (McNeilly, 1994). Although virtually nothing is known about its genetic basis in wild species (McNeilly, 1994), Al resistance is likely to be a genetically and physiologically complex trait (Taylor, 1991; Dvorák et al., 1992). Hence, physiological studies on bred crop varieties may have over-emphasized resistance mechanisms that confer a moderate level of Al resistance at best.
Signalgrass (Brachiaria decumbens Stapf cv Basilisk) is one of the most widely sown forage grasses in the tropics with 26.4 million ha in Brazil alone. Unlike most food crops, it is directly derived from a wild apomictic germplasm accession that is highly resistant to Al and well adapted to infertile acid soils (Sánchez and Salinas, 1981; Paulino et al., 1987; Keller-Grein et al., 1996; Rao et al., 1996). Ruzigrass (Brachiaria ruziziensis Germain and Evrard cv Common), a closely related species of the same agamic complex (Miles and do Valle, 1996), is less Al-resistant and can thus be used as a reference for comparative studies.
The aim of this study was to investigate whether Al resistance mechanisms found in bred crops are responsible for the high level of Al resistance in signalgrass. Our experimental set up was multi-tiered. First, we quantified Al resistance levels of the two Brachiaria species and compared them with Al-resistant genotypes of other plant species reported in the literature. We subsequently carried out a series of experiments to investigate whether Al resistance of signalgrass could be attributed to more effective external detoxification mechanisms.
RESULTS
Al Resistance of Brachiaria Spp.
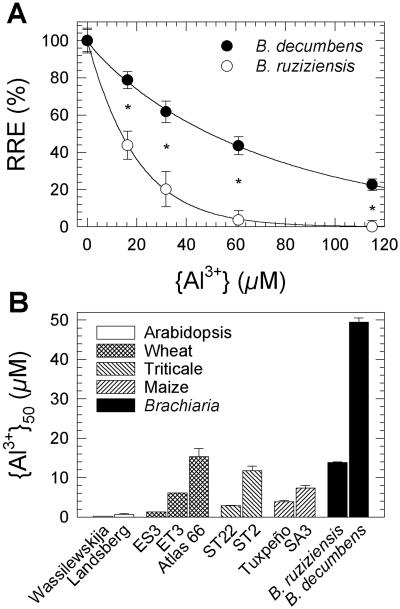
Signalgrass and ruzigrass showed a highly significant difference in Al resistance (P < 0.004 for species × Al level, and P < 10−11 for species; Fig. 1A). For quantitative comparison, the Al3+ activity inhibiting root elongation by 50% ({Al3+}50) was computed for each species and compared with {Al3+}50 of wheat, triticale, maize, and Arabidopsis genotypes, calculated from data in the literature. The {Al3+}50 value of signalgrass (49.5 ± 1.1 μm; mean ± se) was several times greater than that of Al-resistant genotypes of other species (Fig. 1B). Only limited root-elongation data for buckwheat (Fagopyrum esculentum), which has been described as “highly Al-resistant,” is available (see figs. 1 and 9 in Zheng et al., 1998a). However, growth of buckwheat was inhibited in an Al-containing nutrient solution that did not affect growth of ruzigrass, indicating that Al resistance of signalgrass would be significantly superior to that of buckwheat (Osaki et al., 1997).
Figure 1.
Al resistance of signalgrass (B. decumbens) and ruzigrass (B. ruziziensis), quantified with a root elongation assay in simple salt solutions. A, Relative root elongation (RRE; see Eq. 1 in “Materials and Methods”) of 4-d-old seedlings exposed to 200 μm CaCl2 (pH 4.2), containing 0 to 200 μm AlCl3, for 3 d (means ± se of 27–36 seedlings). RE0 was 40.8 mm (signalgrass) and 39.8 mm (ruzigrass). RE∞ was 9.8 mm for ruzigrass, but could not be measured for signalgrass because this would have required Al levels predicted to cause precipitation of Al(OH)3. RE∞ of signalgrass was thus estimated, assuming a constant RE0/RE∞ ratio for both species (10.0 mm). RRE values as a function of the Al3+ activity in the treatment solution were fitted to Equation 2 (solid lines). Asterisks denote statistically significant interspecific differences (P < 0.05). B, Comparison of Al resistance of Brachiaria spp. with contrasting genotypes of other species. RRE data were taken from the literature to determine {Al3+}50, the Al3+ activity causing a 50% inhibition in root elongation (Eqs. 3 and 4). Assay conditions for these genotypes were as follows: Arabidopsis, 0 to 2.5 μm AlCl3, 100 μm CaCl2 (pH 5.2) for 5 d (Toda et al., 1999); wheat, 0 to 10 μm AlCl3, 200 μm CaCl2 (pH 4.3) for 7 d (ES3 and ET3; Ryan et al., 1995b) or 0 to 150 μm AlCl3, 400 μm CaCl2 (pH 4.5) for 2 d (Atlas 66; Kinraide et al., 1992); triticale, 0 to 50 μm AlCl3, 500 μm CaCl2 (pH 4.5) for 1 d (Ma et al., 2000); and maize, 0 to 142 μm AlCl3, approximately 230 μm CaCl2 (pH 4.3) for 3 d (Pellet, 1993).
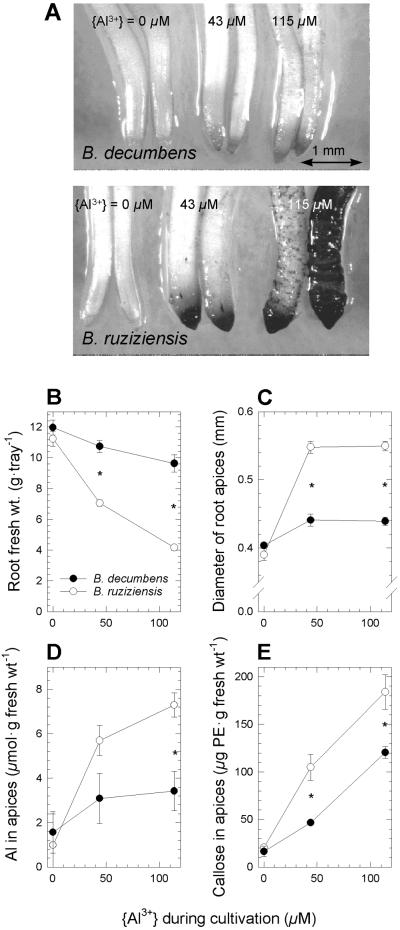
The effect of Al on root growth was also assessed in low-ionic-strength nutrient solutions that simulate soil solutions of acid soils in neotropical savannas. Growth in these solutions reflected the differential Al resistance detected in the short-term root-elongation assay. Fresh weight of roots of ruzigrass, classified as “Al-tolerant” by Osaki et al. (1997), was markedly more affected by Al than that of signalgrass (P < 10−5 for species × Al level; Fig. 2B). Roots of ruzigrass, but not of signalgrass, showed symptoms of Al injury at both Al levels, including stubby appearance, a large number of short laterals close to the apex of the main root axis, and deformed root apices that stained strongly with hematoxylin (Fig. 2A). The mean apical diameter of adventitious roots of ruzigrass increased from 0.39 to 0.55 mm, but the effect was significantly smaller in the case of signalgrass (from 0.40 to 0.44 mm; P < 10−5 for species × Al level, Fig. 2C). Furthermore, ruzigrass accumulated more Al (P < 0.03 for species × Al level and P < 10−4 for species) and callose (P < 10−19 for species) in apices of adventitious roots than did signalgrass (Fig. 2, D and E).
Figure 2.
Differential manifestation of Al stress symptoms in roots. Signalgrass (B. decumbens) and ruzigrass (B. ruziziensis) were cultivated at increasing levels of Al in low-ionic-strength nutrient solutions for 13 d. A, Al injury at apices of adventitious roots, visualized by hematoxylin staining. B, Root fresh weight per tray containing 200 seedlings (means ± se; n = 6–7). C, Apical diameter of adventitious roots (means ± se for three images containing 150–350 apices each). D, Al content of apices from adventitious roots (means ± se of 10–17 groups of five apices each). E, Callose concentration in apices of adventitious roots expressed as Pachymann equivalents (means ± se of 11 or 12 groups of five apices each). Asterisks denote statistically significant interspecific differences (P < 0.05).
Organic-Acid Exudation by Whole Roots
Whole-root exudates contained citrate, malate, and oxalate in addition to minor amounts of lactate, cis- and trans-aconitate, maleate, and fumarate. All acids, except cis-aconitate and fumarate, could also be detected in soil solutions extracted by centrifugation from a sandy-loam Oxisol in which ruzigrass had been cultivated (oxalate was not detectable because of baseline problems; data not shown). In addition to these acids, there were three unidentified peaks, for which pyruvate, tartrate, succinate, malonate, l-glycerate, glycolate, quinate, shikimate, phtalate, and ferulate were ruled out. Larger quantities of these unknown compounds were secreted by ruzigrass than by signalgrass, and Al stimulated their exudation in ruzigrass but not in signalgrass (data not shown).
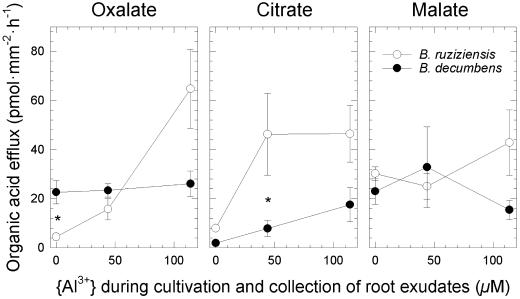
Although citrate exudation was stimulated in both species by approximately 5 times under Al stress (P < 0.001), ruzigrass secreted significantly larger amounts than signalgrass under all conditions (P < 0.002; Fig. 3). Al also triggered a 10-fold increase in oxalate exudation by roots of ruzigrass but had no effect on oxalate exudation from roots of signalgrass, which secreted oxalate at a higher basal level (P < 10−3 for species × Al level). Exudation of malate was not affected by Al in either species (P < 0.9; Fig. 3). Al stress stimulated the overall rate of organic-acid exudation of both species (P < 0.04), but the data suggest that on exposure to Al, ruzigrass secreted larger quantities than did signalgrass (P < 0.09).
Figure 3.
Efflux of oxalate, citrate, and malate from whole roots of signalgrass (B. decumbens) and ruzigrass (B. ruziziensis) (means ± se of five to six groups of five plants each). Plants were cultivated as in Figure 2, and exudates were collected during 1 h in the same nutrient solutions in which the plants had been cultivated. Asterisks denote statistically significant interspecific differences (P < 0.05).
Secretion of Al Ligands by Root Apices
Root apices released less citrate, oxalate, and malate (up to 17 pmol mm−2 h−1; Fig. 4) than whole roots (up to 65 pmol mm−2 h−1; Fig. 3). Levels of organic acids in apical exudates consequently were close to the limit of detection in some samples. Efflux of malate, only detectable in signalgrass, was greater than efflux of other acids. It was stimulated by Al present in the solution bathing root apices (P < 10−5). Apices from plants that had been cultivated in the presence of Al released greater amounts of oxalate (P < 10−3) and citrate (P < 10−9) but not malate (Fig. 4).
Figure 4.
Efflux of oxalate, citrate, and malate from root apices (means ± se of three to five groups of 30 apices each). Ruzigrass (upper row) and signalgrass (lower row) were cultivated as in Figure 2. Organic acids secreted by root apices were collected during a 2-h incubation of excised apices in 200 μm CaCl2 (pH 4.2), containing a varying level of Al. Dotted lines indicate approximate detection limits. Efflux of citrate from apices of plants cultivated in the absence of Al, and efflux of malate from apices of ruzigrass, were below the limit of detection.
Measurement of organically complexed monomeric Al (Alorg) confirmed that only a very small fraction of the monomeric Al in treatment solutions was complexed by chelating ligands secreted by root apices of either species (3 ± 2 μm; mean ± sd). The inferred efflux of Al-chelating equivalents (7 to 10 pmol mm−2 h−1) was somewhat smaller than the combined efflux of oxalate, citrate, and malate.
Phosphate efflux from root apices was smaller than organic-acid efflux (measured range in the presence of Al: 1.2–5.9 pmol mm−2 h−1). Apices of signalgrass tended to release less phosphate than those of ruzigrass. The presence of Al during cultivation of plants stimulated phosphate secretion from apices of ruzigrass up to 4-fold but had little effect in signalgrass (data not shown).
Alkalinization of the Apoplast and Rhizosphere of Root Apices
Apices of intact roots, as well as excised root apices, alkalinized the apoplast in a region up to approximately 1.2 mm from the tip, as indicated by the blue color of bromocresol green in the apoplast (data not shown). Alkalinization was strongest in the subapical region from approximately 0.4 to 1.2 mm, presumably the transition zone between the meristem and the elongation zone because cells started elongating just outside this zone. In contrast, acidification dominated in the elongation zone and more mature parts of root apices, as indicated by the yellow color of the dye (data not shown). The latter confirmed that the dye did not penetrate the symplasm because this would have caused a uniform blue color as a result of the high cytoplasmatic pH. Injured tissue at the site of excision accumulated more dye, but did not contribute to alkalinization, as indicated by the dye remaining yellowish green (data not shown).
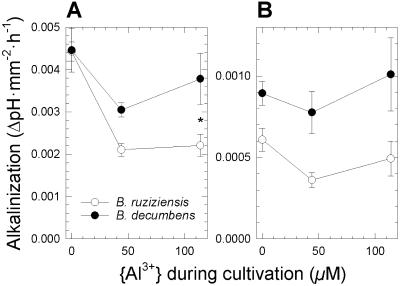
Excised root apices of both species, grown in the absence of Al, caused a pH increase in the Al-free treatment solution of 0.0045 per mm2 of apical surface area and hour (Fig. 5A, {Al3+} = 0 μm). This is equivalent to a net influx of H+ equivalents into root apices of 219 pmol mm−2 h−1 (see “Materials and Methods”). Exposure to Al during cultivation reduced the alkalinizing power of apices in ruzigrass more than in signalgrass (P < 10−5 for Al level and P < 0.002 for species; Fig. 5A). Furthermore, presence of Al during incubation of root apices appeared to inhibit alkalinization in ruzigrass, as indicated by the differential pH increase caused by apices of the two species grown in the absence of Al (Fig. 5B, {Al3+} = 0 μm). Under continuous exposure to Al, root apices of signalgrass therefore maintained a higher pH in the apoplast and rhizosphere than did the apices of ruzigrass (P < 10−3; Fig. 5B, {Al3+} > 0 μm).
Figure 5.
Surface area-based rates of alkalinization of solutions bathing excised root apices of signalgrass (B. decumbens) and ruzigrass (B. ruziziensis) (means ± se of four to six groups of 30 apices each). Plants were cultivated at increasing Al levels as in Figure 2. Excised root apices were incubated for 2 h in 200 μm CaCl2 (pH 4.2), containing either no Al or {Al3+} = 115 μm. A, Incubation of excised root apices without Al. B, Incubation of apices at {Al3+} = 115 μm. Asterisks denote statistically significant interspecific differences (P < 0.05).
DISCUSSION
Al Resistance of Signalgrass Is Outstandingly High Compared with Other Species
A widely used assay for quantifying Al resistance is based on the measurement of root elongation of seedlings in simple salt solutions in which the Al3+ activity {Al3+} can be predicted with precision (Kinraide et al., 1985). Using such an assay, we found Al resistance of signalgrass to be markedly superior than that of ruzigrass, previously classified as “Al-tolerant” (Osaki et al., 1997). Al resistance of signalgrass is clearly outstanding compared with Al-resistant genotypes of cereal crops (Fig. 1). The basal solution used for assaying Al resistance of the most resistant cereal genotypes, i.e. Atlas 66 (wheat) and ST2 (triticale), contained at least twice as much Ca2+ as the solution used for Brachiaria spp. (400–500 versus 200 μm; see legend to Fig. 1). It is well established that Ca2+ ions alleviate Al toxicity (Kinraide, 1998). Because of this and the significantly smaller size of Brachiaria seeds, which may therefore supply less nutrients to the elongating primary root, the level of Al resistance of signalgrass was almost certainly underestimated in the comparison.
The outstandingly high level of Al resistance of signalgrass was underscored by the finding that growth was only moderately inhibited in a low-ionic-strength nutrient solution at {Al3+} = 115 μm (Fig. 2B). This Al3+ activity, in terms of charge equivalents, is comparable with the sum of activities of all cationic macronutrients, plus Na+, in the nutrient solution (Table I). It should be noted that such high Al3+ activities are only rarely found in soil solutions of acid soils (Wright et al., 1989; Menzies et al., 1994). Interspecific differences in other well known Al-toxicity symptoms, such as hematoxylin staining, lateral swelling of root apices, and callose synthesis, also reflected the high resistance level of signalgrass compared with ruzigrass (Fig. 2, A, C, and E).
Table I.
Composition of the low-ionic-strength nutrient solutions used for plant cultivation and the simple salt solutions used for incubating excised root apices
| Ion | Nutrient Solutions
|
Solutions for Incubating Root Apices
|
||||
|---|---|---|---|---|---|---|
| No Al | Low Al | High Al | No Al | Low Al | High Al | |
| μm | ||||||
| {Al3+} | – | 43 | 115 | – | 43 | 115 |
| {AlOH2+} | – | 7 | 18 | – | 7 | 18 |
{Al(OH)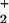 } } |
– | 0.9 | 2.3 | – | 0.9 | 2.3 |
{AlSO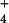 } } |
– | 10 | 22 | – | – | – |
{AlHPO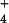 } } |
– | 0.5 | 0.7 | – | – | – |
{NH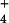 } } |
10 | 10 | 10 | – | – | – |
| {Ca2+} | 53 | 51 | 49 | 178 | 173 | 166 |
| {Mg2+} | 26 | 26 | 25 | – | – | – |
| {K+} | 58 | 58 | 57 | – | – | – |
| {Na+} | 155 | 154 | 153 | – | – | – |
{NO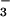 } } |
97 | 96 | 95 | – | – | – |
{H2PO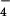 } } |
1.0 | 0.4 | 0.2 | – | – | – |
{SO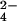 } } |
87 | 76 | 62 | – | – | – |
| {Cl−} | 169 | 389 | 784 | 389 | 583 | 954 |
Activities of major ions and ion complexes were predicted with GEOCHEM 2.0. The pH of all solutions was adjusted to 4.2 by adding calculated amounts of HCl.
A long evolutionary history of exposure to Al is likely to favor the development of highly adapted genotypes with polygenic control of resistance, because Al resistance can be modified by a considerable number of genes (Larsen et al., 1996; Schott and Gardner, 1997; Ezaki et al., 1999, 2000). Species of agricultural interest tend to be less Al-resistant than “wild species” (Wheeler et al., 1992; Crawford and Wilkens, 1998). Even an acid-soil adapted cultivar of rice, which is considered to be an Al-resistant species, was severely affected by Al toxicity when grown in the nutrient solution containing {Al3+} = 43 μm (data not shown). These findings suggest that agricultural species may not have been exposed to Al toxicity during their evolution. Resistance genes originally present in species derived from acid soils, alternatively, may have been lost in the course of genetic-improvement programs aiming at other breeding objectives. In contrast, the root elongation assay convincingly demonstrated that signalgrass has preserved the high level of Al resistance that characterized the original apomictic germplasm accession.
External Detoxification by Chelating Ligands Is Not Responsible for the High Level of Al Resistance in Signalgrass
Root apices of signalgrass accumulated less Al than those of ruzigrass (Fig. 2D). Whereas this might have been the result of a more pronounced growth-induced dilution of Al in apices of rapidly elongating signalgrass roots, it could also indicate that mechanisms excluding Al from root apices contribute to the greater Al resistance of signalgrass.
External Al detoxification by secreted organic acids is a well-documented mechanism excluding Al from root apices. Elevated rates of organic-acid secretion in response to Al stress can be detectable at the level of the whole root system (Miyasaka et al., 1991; Ma et al., 1997; Ma and Miyasaka, 1998; Zheng et al., 1998b; Li et al., 2000). This was also the case in the Brachiaria spp. (Fig. 3). Yet, the stronger stimulation of citrate exudation by Al in ruzigrass, the absence of a stimulatory effect of Al on oxalate exudation in signalgrass, and the larger overall amount of organic acids exuded by roots of ruzigrass at high Al levels (154 versus 59 pmol mm−2 h−1), indicate that a more intense exudation of organic acids by whole roots was not linked to a higher level of Al resistance in these species.
This conclusion is not invalidated by the theoretical possibility that organic-acid exudation may have been stimulated as a result of Al-induced P deficiency, rather than Al toxicity itself (Miyasaka et al., 1991), because control measurements showed that the P content of shoots of both species was above the critical internal level for Brachiaria (data not shown; Sánchez and Salinas, 1981). Furthermore, the phosphate concentration in nutrient solutions was probably too low (≤1 μm) to cause depletion of phosphate due to precipitation with Al. In addition, the more intense exudation of organic acids from roots of ruzigrass should have contributed to its overall Al resistance, irrespective of whether it was stimulated by Al itself or by Al-induced P deficiency.
Measurements of organic acids released by root apices ruled out the possibility that in signalgrass organic-acid efflux was more confined to root apices than in ruzigrass. Rates of organic-acid secretion, calculated on a surface-area basis, were only approximately one-fifth of those observed at the level of whole roots in either species, despite the possibility that organic acids may have been released from injured cells at the cut surface of excised apices (Figs. 3 and 4). This finding contrasts sharply with the Al-stimulated efflux of malate and citrate localized to root apices of wheat and maize, respectively (Delhaize et al., 1993; Pellet et al., 1995; Ryan et al., 1995a). Quantification of organically complexed Al (Alorg) in solutions bathing excised root apices demonstrated that they did not release appreciable amounts of unidentified Al ligands.
The absence of any apparent effect of excision on Al uptake by root apices (see “Materials and Methods”) suggests that the low rates of organic-acid secretion were not an artifact of the experimental protocol. This is because inhibition of an Al exclusion mechanism based on organic-acid secretion as a result of excision should have stimulated Al uptake. Low rates of organic-acid secretion from root apices coincided with high concentrations within apices, whereas the reverse was true at the whole-root level (P. Wenzl, A.L. Chaves, G.M. Patiño, J.E. Mayer, and I.M. Rao, unpublished data). This indicates that, in contrast to mature root sections, organic acids were selectively retained within root apices.
These data suggest that detoxification of extracellular Al by organic acids plays a secondary role in the outstanding resistance of signalgrass to Al. This conclusion is based on several lines of evidence. First, root apices of signalgrass secreted only moderately larger amounts of organic acids than did those of ruzigrass: The maximum efflux of Al-chelating equivalents was 26 pmol mm−2 h−1 in signalgrass and 10 pmol mm−2 h−1 in ruzigrass (0.5 × oxalate + 1 × citrate + 1 × malate; Fig. 4). Second, the moderately greater organic-acid efflux from apices of signalgrass was largely due to malate, which is a poorer Al chelator than citrate and oxalate. Third, maximum efflux of oxalate, citrate, and malate at root apices of signalgrass was 3.4, 12, and 30 times lower than in buckwheat, maize (cv South American 3), and wheat (near-isogenic line ET3), respectively, all considerably more sensitive to Al (Pellet et al., 1995; Ryan et al., 1995a; Zheng et al., 1998a). Finally, on exposure to Al, whole roots of ruzigrass exuded larger quantities of organic acids than did roots of signalgrass.
In certain Al-resistant plant varieties, phosphate released from root apices is thought to generate an apoplastic sink for Al3+ ions in a manner similar to organic acids (Lindberg, 1990; Millard et al., 1990; Pellet et al., 1996, 1997). The greatest efflux measured for apices of signalgrass in the presence of Al was 22 pmol apex−1 h−1. This is 30-fold lower than the value reported for the Al-resistant wheat cv Atlas 66 (680 pmol apex−1 h−1; Pellet et al., 1996). Root apices of less Al-resistant ruzigrass released similar or greater amounts of phosphate (data not shown). These findings rule out a significant contribution of phosphate efflux from root apices to Al resistance of signalgrass.
The Greater Alkalinizing Power of Root Apices of Signalgrass Is a Consequence, Not a Cause, of Its Greater Al Resistance
A long-standing hypothesis states that certain plants resist Al toxicity by increasing the pH around their roots (Taylor, 1991; Degenhardt et al., 1998). Using apices soaked in a pH sensitive dye, we found that the transition zone and, to a lesser extent, the meristematic zone alkalinize the apoplastic space (data not shown). This pH increase is likely to reflect a transcellular proton current, typically associated with polarized growth (Ryan et al., 1992). Although wounding can generate a positive inward current similar to that in the transition zone (Miller et al., 1988), the cut surface of excised Brachiaria root apices did not contribute to alkalinization (data not shown). Furthermore, the kinetics of alkalinization of the apoplast was identical for excised and nonexcised root apices (data not shown). We are therefore confident that alkalinization of solutions bathing excised root apices was principally caused by a proton influx in the transition zone, and was representative of intact root apices.
Given the close association of apical proton current with active growth of root apices, it has been argued that a greater H+ influx is a consequence, rather than a cause, of Al resistance (Miyasaka et al., 1989; Ryan et al., 1992; Kollmeier et al., 2000). Although this assumption is not generally valid, as exemplified by an Arabidopsis mutant (Degenhardt et al., 1998), it is consistent with the greater Al susceptibility of the influx of H+ equivalents into root apices of ruzigrass. The latter is evident from the weaker alkalinizing power of root apices from ruzigrass cultivated in the presence of Al (Fig. 5A, {Al3+} > 0 μm) and ruzigrass apices exposed to Al after excision (Fig. 5B, {Al3+} = 0 μm). A likely reason for the reduced alkalinizing power of Al-stressed root apices of ruzigrass was the shortening of the alkalinizing zone caused by Al stress, which was only observed in this species (data not shown; Kinraide, 1988). These data are therefore best explained by a greater Al susceptibility of the growth-associated influx of H+ equivalents into root apices of ruzigrass, a view that accords with its greater Al sensitivity.
External Al Detoxification Mechanisms May Not Be Efficient Enough for Highly Acidic Soils
The finding that Al resistance of signalgrass is independent of external detoxification of Al by organic acids or phosphate raises the question whether prolonged selective pressure exerted by Al-toxic soils has favored other resistance strategies, because external detoxification mechanisms are not sufficiently effective, or metabolically economical, for highly acidic soils. Stoichiometric considerations would indeed suggest that the amount of organic acids secreted by root apices of Al resistant wheat and maize genotypes might only be adequate to detoxify Al at concentrations well below those inhibiting root growth of signalgrass (Delhaize et al., 1993; Pellet et al., 1995, 1997; Ryan et al., 1995b; Parker and Pedler, 1998). The effectiveness of Al resistance mechanisms based on external chelation of Al3+ by chelating ligands may also be limited by other cell-surface ligands competing for Al ions (Parker and Pedler, 1998), the presence of other cations capable of forming complexes with organic acids (Jones and Darrah, 1994), adsorption of organic acids to the soil's exchange phase (Jones et al., 1996a), and rapid microbial degradation of organic acids in soils (Jones et al., 1996b). In addition, an Al exclusion mechanism based on the efflux of phosphate may be of questionable adaptive value in highly weathered acid soils where P is the principal limiting nutrient.
CONCLUSIONS
We have presented evidence that currently prevailing models of Al resistance mechanisms, based on external detoxification of Al by chelating ligands or alkalinization, do not adequately account for the outstanding resistance of signalgrass to Al. This suggests that mechanisms based on other physiological strategies, for example a low Al permeability of the plasma membrane, an active Al extrusion from the symplasm, or a greater tolerance to symplastic Al, could be responsible for the high resistance level of this species.
MATERIALS AND METHODS
Quantifying Al Resistance
Al resistance of signalgrass (Brachiaria decumbens Stapf cv Basilisk) and ruzigrass (Brachiaria ruziziensis Germain & Evrard cv Common) was quantified by measuring elongation of primary roots of seedlings cultivated in 200 μm CaCl2 (pH 4.2), containing 0, 25, 50, 100, or 200 μm AlCl3. Al3+ activities {Al3+} were calculated with GEOCHEM 2.0 (Parker et al., 1987), using the stability constants given by Nordstrom and May (1989) and the solubility constant for Al(OH)3 suggested by Kinraide and Parker (1989). The amount of HCl required for adjusting the pH to 4.2 (64.9, 61.5, 58.1, 51.6, and 39.2 μm for the control and the various Al treatments) was predicted with GEOCHEM 2.0 and confirmed empirically using high-quality de-ionized water (18 MΩ; measured range: 4.15–4.23).
Scarified seeds of signalgrass (tetraploid, apomictic; Agrosemillas, Medellín, Colombia) and ruzigrass (CIAT 654; diploid, sexual; CIAT, Cali, Colombia) were surface-sterilized in 70% (v/v) ethanol for 1 min and 2% (w/v) NaOCl, 0.1% (v/v) Triton X-100 for 15 min. After 4 d of germination in 200 μm CaCl2 (pH 4.2), uniform seedlings were transferred to constantly aerated control and Al solutions. The seedlings were left to grow in a growth chamber at 24°C and a 12-h diurnal cycle with a photon-flux density of approximately 110 μmol m−2 s−1. Root elongation was measured after 3 d, during which the pH of the control and Al treatments remained constant. Data from three independent experiments were pooled to compute relative root elongation (RRE) according to the following formula:
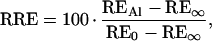 |
1 |
in which REAl is the root elongation in the presence of Al, RE∞ is the mean residual elongation at Al levels sufficient to saturate growth inhibition, and RE0 is the mean elongation in the Al-free control solution. To compare Al resistance of Brachiaria with that of other species, RRE values obtained in this and other studies conducted under comparable conditions (see legend to Fig. 1B) were fitted to a Weibull-type equation (Kinraide, 1997), using the Marquardt-Levenberg algorithm for non-linear regression (r2 > 0.99 in all cases):
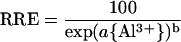 |
2 |
The activity of Al3+ required to decrease RRE by 50% ({Al3+}50) was calculated from fitted values of the empirical parameters a and b according to the formula:
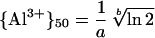 |
3 |
The se of {Al3+}50 was estimated by computing the propagation of the ses of a and b, estimated by the Marquardt-Levenberg algorithm, that is:
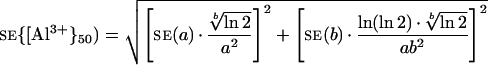 |
4 |
Al3+ activities in treatment solutions described in the literature were calculated, where necessary, with GEOCHEM 2.0 as outlined above, but disallowing precipitation of Al(OH)3, although some of the treatment solutions were over-saturated, according to the Kinraide and Parker constant (1989).
Obtaining Plant Materials
Cultivating Plants in Low-Ionic-Strength Nutrient Solutions
Plants were cultivated in large numbers in a glasshouse at CIAT (Cali, Colombia; 3° 30′ N, 76° 21′ W; altitude, 965 m; typical maximum/minimum temperature and relative humidity, 36/19°C and 96/48%; typical maximum photon-flux density during the day, 1,100 μmol m−2 s−1). For germination, sterilized seeds were distributed on mosquito nets glued to Styrofoam frames floating on 10 L of an Al-free low-ionic-strength nutrient solution (pH 4.2). The solution contained low nutrient concentrations, comparable with those found in soil solutions of the highly weathered Oxisols in the Colombian savannas (P. Wenzl, J.E. Mayer, R. Albert, and I.M. Rao, unpublished data). The composition was (in μm): 100 NO3−, 10 NH4+, 60 K+, 60 Ca2+, 30 Mg2+, 160 Na+, 1 H2PO4−, 100 SO42−, 5 Fe3+, 1 Mn2+, 1 Zn2+, 0.2 Cu2+, 6 H3BO3, 5 SiO32−, 0.001 MoO42−, 5 H2-EDTA2−, 108.4 Cl− (excluding HCl), and 65.4 HCl to adjust the pH to 4.2 (GEOCHEM 2.0).
After 5 d, groups of 200 seedlings were transferred to fresh floating devices and cultivated for another 12 d in continuously aerated nutrient solutions. These solutions contained identical nutrient concentrations as the solution used for germination and 0, 80, or 225 μm Al3+, 108.4, 348.4, or 783.4 μm Cl− and 65.4, 55.4, or 38.4 μm HCl, respectively. These Al concentrations resulted in {Al3+} = 0, 43, and 115 μm, respectively (GEOCHEM 2.0; Table I). Solutions were renewed 2, 4, 6, 8, 9, 10, and 11 d after transfer of seedlings. The pH of nutrient solutions showed a tendency to increase. The highest pH values were observed at harvest on d 13: 4.5 ± 0.1, 4.3 ± 0.1, and 4.4 ± 0.1 for signalgrass and 4.7 ± 0.4, 4.3 ± 0.1, and 4.3 ± 0.1 for ruzigrass grown at {Al3+} = 0, 43, or 115 μm, respectively (means ± sd).
For each combination of species × treatment, we used three independent cultivations with two to three containers, each holding 200 plants, resulting in 8,400 plants in all. The large number was needed to obtain adequate measurements, because primary roots were too thin to detect organic-acid efflux from apices, and only a part of the plants developed adventitious roots by harvest time.
Harvesting Roots and Root Apices
To collect exudates from whole roots, a group of five plants was selected at random from each container. Next, 3-mm apices were excised from adventitious roots of approximately 20 plants from each container and grouped for the following analyses: (a) to measure Al content, one to three groups of five apices each were dried at 70°C for 48 h, and (b) to quantify callose content, one to two groups of five apices each were collected in 500 μL 100% (v/v) ethanol and stored at 4°C. The remaining apices of adventitious roots (approximately 60 per container) were used to study both secretion of organic acids and phosphate and to investigate alkalinization of the apoplast and rhizosphere.
Physiological Studies
Collecting Root Exudates from Whole Roots
Roots were gently rinsed with sterile 200 μm CaCl2 (pH 4.2) and transferred to 40 mL of the same (sterile) nutrient solution in which plants had been cultivated. Root exudates, collected over 1 h under continuous aeration, were concentrated in two steps under reduced pressure at 40°C to 60°C and dissolved in 300 μL 0.3 n HCl to ensure that all Al and Ca salts present in the concentrated nutrient solution were soluble (GEOCHEM 2.0). Controls confirmed that this protocol did not degrade organic acids and that HPLC retention times of organic acids did not change in the presence of Al. Incubation tests similar to those of Zheng et al. (1998a) showed that organic-acid-degrading micro-organisms did not interfere with the collection of root exudates.
Collecting Organic Acids and Phosphate Released from Root Apices
To quantify organic acids secreted by root apices, apices of adventitious roots, previously washed in sterile nutrient solutions, were excised at 3 mm. They were collected in groups of 30 in 5-mL glass vials containing 1 mL of sterile 200 μm CaCl2 (pH 4.2) on a rotary shaker at 100 rpm to permit recovery from injury (Gronewald and Hanson, 1980). When excision of apices from a group of plants was complete (approximately 30 min), they were continued to incubate for another 30 min. They subsequently were rinsed once with 1 mL of sterile 200 μm CaCl2, containing 0, 68, or 200 μm AlCl3 at pH 4.2 (resulting in the same Al3+ activities as in nutrient solutions; Table I), and suspended in another 1 mL of either of these treatments. After a secretion period of 2 h, the treatment solutions were removed. Approximately two-thirds of them were dried in a SpeedVac centrifuge at 40°C and used to analyze secreted organic acids and phosphate. Some solutions were used in an incubation test (Zheng et al., 1998a) to ensure that microbial degradation of organic acids was not a problem.
Quantifying Secreted Organic Acids by HPLC
The residues containing root apical exudates were dissolved in 45 μL de-ionized water and centrifuged at 14,000g for 10 min. Aliquots (25 μL) were analyzed on an Aminex HPX 87H ion exclusion column (300 × 7.8 mm), using isocratic elution with 12 mm H2SO4 at 0.4 mL min−1 and 30°C. Organic acids were detected by monitoring absorption at 210 nm and identified and quantified by comparing retention times and peak areas with a mixture of standards composed of cis- and trans-aconitate, citrate, fumarate, lactate, maleate, malate, malonate, oxalate, succinate, and tartrate. Concentrated exudates collected from whole roots were analyzed under identical conditions after passing them through a 0.45-μm filter.
Quantifying Phosphate Secreted by Root Apices
Twelve microliters of the concentrated apical exudates was diluted with de-ionized water to 100 μL. Phosphate was quantified by measuring A622, after adding 10 μL of the Malachite green reagent described by Shimogawara and Usuda (1995). Control experiments demonstrated that Al, present in some solutions, did not interfere with this assay.
Investigating Alkalinization Caused by Root Apices
Intact adventitious roots, or excised root apices (3 mm) that had been washed in 200 μm CaCl2 (pH 4.2) for 1 h, were used to visualize apoplastic pH. After being soaked in 2 mm bromocresol green (pKa = 4.7), 200 μm CaCl2 (pH 4.2) for 15 min, the apices were transferred to the surface of slices of 0.7% (w/v) agarose, previously equilibrated with 200 μm CaCl2 (pH 4.2). They were photographed 20 min later when color development had reached its maximum. The pH of approximately one-third of the solutions containing apical exudates was determined using a pH electrode specifically designed for low-ionic-strength solutions (Markson LabSales, Hillsboro, OR; highest value measured in Al treatments: 4.54). In the case of the treatment solution lacking Al, the increase in pH was converted into a net influx of H+ equivalents by calculating the amount of protons that had to be removed from the solution to adjust the pH to the measured values (GEOCHEM 2.0).
Quantifying the Amount of Al Chelated by Ligands Released from Root Apices
The inorganic monomeric Al remaining in solutions after incubation of excised root apices was quantified with pyrocatechol violet (Kerven et al., 1989; maximum depletion: 44.0 μm, i.e. 22% of the initial 200 μm AlCl3). Total monomeric Al (Almono) was measured, using the pyrocatechol-violet assay described by Menzies et al. (1992). The concentration of monomeric Al complexed by Al ligands secreted from root apices (Alorg), was calculated from the difference between Almono and inorganic monomeric Al.
Measuring Al in Root Apices
Root apices were digested in a 2:1 mixture of 65% (w/w) HNO3 and 70% (w/w) HClO4 at 200°C for 2 h, and their Al content was analyzed by atomic absorption spectroscopy, using an UNICAM 969 spectrophotometer (UNICAM, Cambridge, UK) equipped with a graphite furnace. Adventitious roots, selected at random, were stained with hematoxylin according to Polle et al. (1978).
Testing the Effect of Excision of Root Apices on Al Uptake
Excised root apices have been widely used for physiological studies of Al uptake and organic-acid metabolism (Chang and Roberts, 1989; Ryan et al., 1995a; Pellet et al., 1996, 1997; Archambault et al., 1997; Pellet et al., 1996, 1997; Zheng et al., 1998a), but one report suggested that excision might artificially stimulate Al uptake by root apices (Samuels et al., 1997). Rates of Al uptake into intact root apices, estimated from the apical Al content and the rate of root elongation of adventitious roots measured during the last day of plant cultivation, were found to be statistically indistinguishable from rates of Almono depletion from solutions bathing excised root apices (data not shown). Excision therefore did not significantly stimulate Al uptake by root apices.
Determining Callose in Root Apices
Root apices, stored in ethanol at +4°C, were washed once with de-ionized water, and callose was determined according to Köhle et al. (1985), using Pachymann (Fluka, Milwaukee, WI) as a reference.
Root Image Analysis
We used root-image-analysis software (WinRHIZO; Régent Instruments, Québec, Canada) to measure the surface area of whole roots and root apices, as well as to determine the mean diameter and volume of root apices. The latter was converted into fresh weight, based on the density of water. To obtain images, excised root apices and whole roots used for collecting exudates were stained in 0.1% (w/v) methylene blue, 0.1% (w/v) neutral red for at least 24 h. They were then scanned with a flatbed scanner at optimized brightness and contrast settings, held constant throughout this study.
Statistical Analysis
Statistical analysis followed Sokal and Rohlf's (1995) recommendations. Standardized deviates of each set of data (e.g. RRE, root fresh weight, organic-acid efflux) were tested for normality, using the Kolmogorov-Smirnov test. Potential outliers within experimental groups (i.e. species × treatment) were winsorized if confirmed with Dixon's test (two individual values in total). Homogeneity of variance among experimental groups was tested, using Bartlett's test, and data were transformed, in some cases, to reduce heteroscedasticity (y = xa, with a = 0.3–0.5, for apical callose content, phosphate efflux, and alkalinization, and y = log(x) for organic-acid efflux from whole roots). Two-way (species × Al level during cultivation) or three-way (species × Al level during cultivation × Al level during incubation of apices) model I ANOVAs were computed, and differences among group means were tested for significance, using the T test of Spjotvoll/Stoline.
ACKNOWLEDGMENTS
We thank Dr. David R. Parker (University of California, Riverside) for providing us GEOCHEM 2.0. We are grateful to Dr. Emmanuel Delhaize (CSIRO, Canberra, ACT, Australia) for his critical review of the manuscript, and to Elizabeth de Páez for editorial improvements of the text.
Footnotes
This work was supported by the Kommission für Entwicklungsfragen of the Austrian Academy of Sciences, and by the Colombian Ministry of Agriculture and Rural Development.
LITERATURE CITED
- Archambault DJ, Zhang G, Taylor GJ. Spatial variation in the kinetics of aluminum (Al) uptake in roots of wheat (Triticum aestivum L.) exhibiting differential resistance to Al: evidence for metabolism-dependent exclusion of Al. J Plant Physiol. 1997;151:668–674. [Google Scholar]
- Chang K, Roberts JKM. Observation of cytoplasmic and vacuolar malate in maize root tips by 13C-NMR spectroscopy. Plant Physiol. 1989;89:197–203. doi: 10.1104/pp.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SA, Wilkens S. Effect of aluminum on root elongation in two Australian perennial grasses. Aust J Plant Physiol. 1998;25:165–171. [Google Scholar]
- De la Fuente JM, Ramírez-Rodríguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- De la Fuente-Martínez JM, Herrera-Estrella L. Advances in the understanding of aluminum toxicity and the development of aluminum-tolerant transgenic plants. Adv Agron. 1999;66:102–120. [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol. 1998;117:19–27. doi: 10.1104/pp.117.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorák J, Epstein E, Galvez A, Gulick P, Omielan JA. Genetic basis of plant tolerance of soil toxicity. In: Stalker HT, Murphy JP, editors. Plant Breeding in the 1990s. Wallingford: CAB International; 1992. pp. 201–217. [Google Scholar]
- Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 2000;122:657–665. doi: 10.1104/pp.122.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Sivaguru M, Ezaki Y, Matsumoto H, Gardner RC. Acquisition of aluminum tolerance in Saccharomyces cerevisiae by expression of the BCB or NtGDl1 gene derived from plants. FEMS Microbiol Lett. 1999;171:81–87. doi: 10.1111/j.1574-6968.1999.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Gronewald JW, Hanson JB. Sensitivity of the proton and ion transport mechanisms of corn roots to injury. Plant Sci Lett. 1980;18:143–150. [Google Scholar]
- Horst WJ. The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernaehr Bodenkd. 1995;158:419–428. [Google Scholar]
- Jones DL, Darrah PR. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil. 1994;166:247–257. [Google Scholar]
- Jones DL, Prabowo AM, Kochian LV. Aluminum-organic acid interactions in acid soils: II. Influence of solid phase sorption on organic acid-Al complexation and Al rhizotoxicity. Plant Soil. 1996a;182:229–237. [Google Scholar]
- Jones DL, Prabowo AM, Kochian LV. Kinetics of malate transport and decomposition in acid soils and isolated bacterial populations: the effect of microorganisms on root exudation of malate under Al stress. Plant Soil. 1996b;182:239–247. [Google Scholar]
- Keller-Grein G, Maass BL, Hanson J. Natural variation in Brachiaria and existing germplasm collections. In: Miles JW, Maass BL, do Valle CB, editors. Brachiaria: Biology, Agronomy, and Improvement. Centro Internacional de Agricultura Tropical, Cali, Colombia. 1996. pp. 16–35. [Google Scholar]
- Kerven GL, Edwards DG, Asher CJ, Hallman PS, Kokot S. Aluminum determination in soil solution: II. Short-term colorimetric procedures for the measurement of inorganic monomeric aluminum in the presence of organic acid ligands. Aust J Soil Res. 1989;27:91–102. [Google Scholar]
- Kinraide TB. Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiol. 1988;88:418–423. doi: 10.1104/pp.88.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Reconsidering the rhizotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminum. J Exp Bot. 1997;48:1115–1124. [Google Scholar]
- Kinraide TB. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998;118:513–520. doi: 10.1104/pp.118.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Arnold RC, Baligar VC. A rapid assay for aluminum phytotoxicity at submicromolar concentrations. Physiol Plant. 1985;65:245–250. [Google Scholar]
- Kinraide TB, Parker DR. Assessing the phytotoxicity of mononuclear hydroxy-aluminum. Plant Cell Environ. 1989;12:479–487. [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 1992;99:1461–1468. doi: 10.1104/pp.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Kochian LV, Jones DL. Aluminum toxicity and resistance in plants. In: Yokel RA, Golub MS, editors. Research Issues in Aluminum Toxicity. Washington, DC: Taylor and Francis; 1997. pp. 69–89. [Google Scholar]
- Kollmeier M, Felle H, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone: is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhle H, Jeblick W, Poten F, Blaschek W, Kauss H. Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol. 1985;77:544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Takita E, Kawamura A, Hara T, Shibata D. Over expression of mitochondrial citrate synthase gene improves the growth of carrot cells in Al-phosphate medium. Plant Cell Physiol. 1999;40:482–488. doi: 10.1093/oxfordjournals.pcp.a029568. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Degenhardt J, Tai CY, Stenzler LM, Howell SH, Kochian LV. Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol. 1998;117:9–18. doi: 10.1104/pp.117.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Tai CY, Kochian LV, Howell SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol. 1996;110:743–751. doi: 10.1104/pp.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazof DB, Goldsmith JG, Linton RW. The in situ analysis of intracellular aluminum in plants. Prog Bot. 1997;58:112–149. [Google Scholar]
- Li XF, Ma JF, Matsumoto H. Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol. 2000;123:1537–1543. doi: 10.1104/pp.123.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg S. Aluminum interactions with K+(86Rb+) and 45Ca2+ fluxes in three cultivars of sugar beet (Beta vulgaris) Physiol Plant. 1990;79:275–282. [Google Scholar]
- Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- Ma JF, Taketa S, Yang ZM. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in Triticale. Plant Physiol. 2000;122:687–694. doi: 10.1104/pp.122.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Specific secretion of citric acid induced by Al stress in Cassia tora L. Plant Cell Physiol. 1997;38:1019–1025. [Google Scholar]
- Ma Z, Miyasaka SC. Oxalate exudation by taro in response to Al. Plant Physiol. 1998;118:861–865. doi: 10.1104/pp.118.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- McNeilly T. Metal toxicity. In: Yeo AR, Flowers TJ, editors. Soil Mineral Stresses: Approaches to Crop Improvement: Monographs on Theoretical and Applied Genetics. Vol. 21. Berlin: Springer-Verlag; 1994. pp. 145–174. [Google Scholar]
- Menzies NW, Bell LC, Edwards DG. Exchange and solution phase chemistry of acid, highly weathered soils: II. Investigation of mechanisms controlling Al release into solution. Aust J Soil Res. 1994;32:269–283. [Google Scholar]
- Menzies NW, Kerven GL, Bell LC, Edwards DG. Determination of total soluble aluminum in soil solution using pyrocatechol violet, lanthanum, and iron to discriminate against micro-particulates and organic ligands. Commun Soil Sci Plant Anal. 1992;23:2525–2545. [Google Scholar]
- Miles JW, do Valle CB. Manipulation of apomixis in Brachiaria breeding. In: Miles JW, Maass BL, do Valle CB, editors. Brachiaria: Biology, Agronomy, and Improvement. Centro Internacional de Agricultura Tropical, Cali, Colombia. 1996. pp. 164–177. [Google Scholar]
- Millard MM, Foy CD, Coradetti CA, Reinsel MD. X-ray photoelectron spectroscopy surface analysis of aluminum ion stress in barley roots. Plant Physiol. 1990;93:578–583. doi: 10.1104/pp.93.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Shand E, Gow NAR. Ion currents associated with root tips, emerging laterals and induced wound sites in Nicotiana tabacum: spatial relationship proposed between resulting electrical fields and phytophtoran zoospore infection. Plant Cell Environ. 1988;11:21–25. [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka SC, Kochian LV, Shaff JE, Foy CD. Mechanisms of aluminum tolerance in wheat: an investigation of genotypic differences in rhizosphere pH, K+, and H+ transport, ad root-cell membrane potentials. Plant Physiol. 1989;91:1188–1196. doi: 10.1104/pp.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom DK, May HM. Aqueous equilibrium data for mononuclear aluminum species. In: Sposito G, editor. The Environmental Chemistry of Aluminum. Boca Raton, FL: CRC Press; 1989. pp. 29–53. [Google Scholar]
- Osaki M, Watanabe T, Tadano T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci Plant Nutr. 1997;43:551–563. [Google Scholar]
- Parker DR, Pedler JF. Probing the “malate hypothesis” of differential aluminum tolerance in wheat by using other rhizotoxic ions as proxies for Al. Planta. 1998;205:389–396. [Google Scholar]
- Parker DR, Zelazny LW, Kinraide TB. Improvements to the program GEOCHEM. Soil Sci Soc Am J. 1987;51:488–491. [Google Scholar]
- Paulino VT, Anton DP, Colozza MT. Problemas nutricionais do género Brachiaria e algumas relações com o comportamento animal. Zootecnia (São Paulo) 1987;25:215–263. [Google Scholar]
- Pellet DM. Mechanisms of aluminum tolerance in maize (Zea mays L.): root growth study and organic aid release. Progress Report to the Swiss National Science Foundation. Ithaca, NY: Cornell University; 1993. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Pellet DM, Papernik LA, Jones DL, Darrah PR, Grunes DL, Kochian LV. Involvement of multiple aluminum exclusion mechanisms in aluminum tolerance in wheat. Plant Soil. 1997;192:63–68. [Google Scholar]
- Pellet DM, Papernik LA, Kochian LV. Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol. 1996;112:591–597. doi: 10.1104/pp.112.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle E, Konzak CF, Kittrick JA. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci. 1978;18:823–827. [Google Scholar]
- Rao IM, Kerridge PC, Macedo MCM. Nutritional requirements of Brachiaria and adaptation to acid soils. In: Miles JW, Maass BL, do Valle CB, editors. Brachiaria: Biology, Agronomy, and Improvement. Centro Internacional de Agricultura Tropical , Cali, Colombia. 1996. pp. 53–71. [Google Scholar]
- Rao IM, Zeigler RS, Vera R, Sarkarung S. Selection and breeding for acid-soil tolerance in crops. BioScience. 1993;43:454–465. [Google Scholar]
- Rengel Z. Uptake of aluminum by plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Rengel Z, Reid RJ. Uptake of Al across the plasma membrane of plant cells. Plant Soil. 1997;192:31–35. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta. 1995a;196:103–110. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminum are highly correlated in wheat. Aust J Plant Physiol. 1995b;22:531–536. [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Ryan PR, Shaff JE, Kochian LV. Aluminum toxicity in roots: correlation among ionic currents, ion fluxes, and root elongation in aluminum-sensitive and aluminum-tolerant wheat cultivars. Plant Physiol. 1992;99:1193–1200. doi: 10.1104/pp.99.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels TD, Küçükakyüz K, Rincón-Zachary M. Al partitioning patterns and root growth as related to Al sensitivity and Al tolerance in wheat. Plant Physiol. 1997;113:527–534. doi: 10.1104/pp.113.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez PA, Salinas JG. Low-input technology for managing Oxisols and Ultisols in tropical America. Adv Agron. 1981;34:279–406. [Google Scholar]
- Schott EJ, Gardner RC. Aluminum-sensitive mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1997;254:63–72. doi: 10.1007/s004380050391. [DOI] [PubMed] [Google Scholar]
- Shimogawara K, Usuda H. Uptake of inorganic phosphate by suspension-cultured tobacco cells: kinetics and regulation by Pi starvation. Plant Cell Physiol. 1995;36:341–351. [Google Scholar]
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–163. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. Ed 3. New York: Freeman and Company; 1995. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response; the physiological basis of tolerance. Curr Top Plant Biochem Physiol. 1991;10:57–93. [Google Scholar]
- Taylor GJ, McDonald-Stephens, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Koyama H, Hori T, Hara T. Aluminum tolerance of Arabidopsis thaliana under hydroponic and soil culture conditions. Soil Sci Plant Nutr. 1999;45:419–425. [Google Scholar]
- Wheeler DM, Edmeades DC, Christie RA, Gardner R. Effect of aluminum on the growth of 34 plant species: a summary of results obtained in low ionic strength solution culture. Plant Soil. 1992;146:61–66. [Google Scholar]
- Wright RJ, Baligar VC, Ahlrichs JL. The influence of extractable and soil solution aluminum on root growth of wheat seedlings. Soil Sci. 1989;148:293–302. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. High aluminum resistance in buckwheat: I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998a;117:745–751. doi: 10.1104/pp.117.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. Continuous secretion of organic acids is related to aluminum resistance during relatively long-term exposure to aluminum stress. Physiol Plant. 1998b;103:209–214. [Google Scholar]