Abstract
Early detection is important for improving the survival rate of patients with gastric cancer (GC). Serum tumor markers have been widely used for detecting GC. However, their clinical values remain controversial. This study aims to investigate the role of serum cancer antigen 72-4 (CA72-4) in the diagnosis of GC in a healthy population. A total of 7757 adults who underwent upper gastrointestinal endoscopy and serum CA72-4 level measurement in multicenters in Taiwan from January 2006 to August 2016 were recruited in this retrospective study. Risk factors for GC, serum tumor markers, and esophagogastroduodenoscopy (EGD) findings were evaluated. High serum levels of CA72-4 were found in 7.2% of healthy adults. CA72-4 level showed lower sensitivity (33.3%) but higher specificity (92.8%); however, the positive predictive value was quite low (0.18%). After adjustment of clinical risk factors for GC using EGD findings, gastric ulcer (adjusted odds ratio (aOR) = 2.11), gastric polyps (aOR = 1.42), and atrophic gastritis (aOR = 1.27) were significantly associated with high serum CA72-4 levels. Furthermore, both age (OR = 1.01) and Helicobacter pylori infection (OR = 1.44) exhibited a significant association with high serum CA72-4 levels. These results indicate that routine screening of CA72-4 levels for diagnosing GC in asymptomatic patients may be ineffective due to low sensitivity and low positive predictive value. The clinical utility of EGD findings along with serum CA72-4 level for screening healthy individuals with GC is warranted.
Keywords: CA72-4, esophagogastroduodenoscopy, gastric cancer, Helicobacter pylori, tumor marker
1. Introduction
Gastric cancer (GC) is one of the most common malignancies and is a major cause of cancer-related deaths worldwide [1]. The incidence of GC has been declining in the past few decades due to improvements in hygiene practices and effective eradication of Helicobacter pylori [1,2]. However, the survival rate of GC patients remains low, mostly due to delayed diagnosis [2]. The 5-year survival rate in Japan is much better than that in the western world, which is attributed to annual endoscopic screening for persons aged over 40 years and consecutive early tumor resections [3].
Although endoscopic examination and biopsy are the primary tools for diagnosing GC, high cost and invasiveness prevents them from being used as routine screening methods for asymptomatic persons, especially in low-incidence regions [4]. Several tumor markers have been used to assess patients with GC, including carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), and CA72-4. Several studies indicated that a combination of these markers is useful in the diagnosis and prognosis of patients with GC [5,6].
CA72-4 was first described in the early 1980s as a novel antigen reactive to murine antibodies produced by mice that were immunized with membrane-enriched fractions of human metastatic mammary cancer cells [5,7,8]. It may be detected in patients with breast, colon, ovary, pancreatic, and gastric cancers [8]. Previous studies have reported that CA72-4 level was elevated in 29.9% (829/2774) of patients with GC and increased as the disease progressed [6]. However, its role as a screening marker for GS in healthy individuals is still under investigation. This study aims to assess serum CA72-4 as a screening tool for GC in asymptomatic populations.
2. Materials and Methods
2.1. Study Design and Population Collection
From January 2006 to August 2016, 7757 adults who had routine physical examinations, completed a self-administered questionnaire, and underwent esophagogastroduodenoscopy (EGD) [9] and serum CA72-4 measurement in multicenters, including Health Promotion Centers of Taipei Medical University Hospital, Wan Fang Hospital, Shuang Ho Hospital, and Everlife Health Services in Taiwan, were enrolled in this study. Demographic factors of the healthy individuals, including age, gender, weight and height (body mass index), smoking history, H. pylori infection, alpha-fetoprotein (AFP), CA72-4, CA19-9, and CEA, were recorded. The study participants were divided into abnormal group (CA72-4 > 6.9 ng/mL, n = 557) and normal group (CA72.4 ≤ 6.9 ng/mL, n = 7200) as suggested by Roche Diagnostics GmbH (Mannheim, Germany). The clinical risk factors for GC, other tumor markers, and EGD findings between the two groups were assessed. This study was approved by the Institutional Review Board of Taipei Medical University (no: N201702016). After providing a complete explanation of the study, a written informed consent was obtained from all participants. All clinical and biological samples were collected after obtaining patients’ consent. All the study methods were in accordance with the guidelines approved by the joint institutional review board and aforementioned governmental regulations.
2.2. Analysis of Serum Tumor Markers
Blood was obtained from all participants after fasting for 8 h. The CA72-4 level was assessed using a chemiluminescence kit (Roche Diagnostics GmbH). Serum CEA, CA19-9, and AFP levels were respectively measured using chemiluminescence assays (Beckman Coulter, Fullerton, CA, USA). The cutoff values for serum CEA, CA19-9, AFP, and CA72-4 were 5 ng/mL, 37 U/mL, 20 ng/mL, and 6.9 ng/mL, respectively, and were measured according to the manufacturer’s instructions.
2.3. Evaluation of EGD
The endoscopic examination was carried out using standard, forward-viewing video endoscopes by experienced gastroenterologists. The diagnosis and indications for biopsy were determined according to the American Society for Gastrointestinal Endoscopy guidelines [9]. The findings on EGD were classified as normal, benign (including reflux esophagitis, gastric ulcer, gastric polyp, gastric erosion, atrophic gastritis, and gastric submucosal tumor), or malignant (including esophageal cancer, gastric lymphoma, and GC) [9]. Each patient may have more than one EGD findings. All biopsy samples were evaluated by experienced pathologists, and the gastroenterologists applied the pathological report and made the definite EGD diagnosis.
2.4. Statistical Analysis
Categorical variables were compared with Pearson’s chi-square (χ2) test, and continuous variables were evaluated by Student’s t-test. Correlations between clinical risk factors for GC and CA72-4 level were further assessed by using multivariate logistic regression analyses. The logistic regression model was used to calculate odds ratios (OR) between both groups. Additional adjustments on clinical risk factors of GC, including age, gender, body mass index (BMI), smoking history, and H. pylori infection status were also carried out. All statistics analyses were performed by using SPSS statistical package (version 24.0, SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Demographic Characteristics of the Study Population
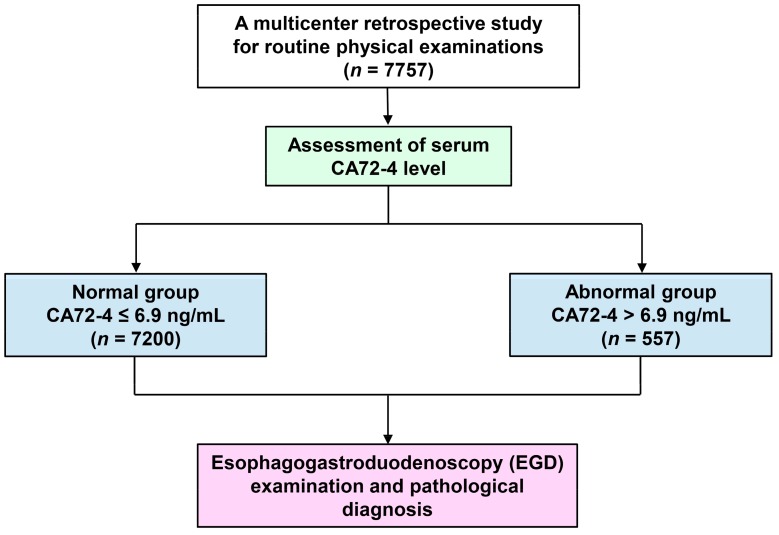
A total of 7757 adults who underwent health examinations from January 2006 to August 2016 in various health promotion centers in Taiwan were recruited in this study (Figure 1). The clinical profiles of the enrolled patients are shown in Table 1. The mean age was 45.6 ± 11.1 years (mean ± SD, range: 20–82 years); majority of the patients were men (n = 4704, 60.6%). A total of 1668 patients (21.9%) had a smoking history, and 1611 (21.1%) had H. pylori infection.
Figure 1.
Flowchart of the population selection, identification, and analysis in a multicenter retrospective study.
Table 1.
Demographic characteristics of patients who were recruited in this study.
| Parameter | Number (%) |
|---|---|
| Total population | 7757 |
| Age (mean ± SD) | 45.6 ± 11.1 |
| Gender | |
| Male, n (%) | 4704 (60.6%) |
| Female, n (%) | 3053 (39.4%) |
| Total number | 7757 |
| Smoking history | |
| Yes, n (%) | 1668 (21.9%) |
| No, n (%) | 5950 (78.1%) |
| Total number | 7618 |
| BMI † (mean ± SD), kg/m2 | 23.8 ± 3.6 |
| H. pylori infection | |
| Positive, n (%) | 1611 (21.1%) |
| Negative, n (%) | 6030 (78.9%) |
| Total number | 7641 |
| CA72-4 (mean ± SD), ng/mL | 2.8 ± 3.9 |
| Total number | 7757 |
| CA19-9 (mean ± SD), U/mL | 10.3 ± 8.3 |
| Total number CA19-9 positive, n (%) |
5989 52 (0.87%) |
| CEA (mean ± SD), ng/mL | 1.7 ± 1.2 |
| Total number CEA positive, n (%) |
7703 181 (2.35%) |
† BMI: Body mass index.
3.2. Measuring Serum CA72-4 in Healthy Population
About 557 patients had elevated CA72-4 levels (7.2%). As shown in Table 2, 181 patients (2.3%), had high serum CEA levels, while 52 (0.87%) had high serum CA19-9 levels. In the further analyses, three patients had GC based on the results of endoscopic examination and pathological findings. The CA72-4 levels of these three patients were 1.46, 1.69, and 7.47 ng/mL, respectively. In this study, only one patient had abnormal CEA, CA19-9, and CA72-4 levels and was found to have benign gastric disease.
Table 2.
Clinical risk factors of gastric cancer (GC) and tumor markers in high and normal CA72-4 level groups.
| Variable | High CA72-4 Level (n = 557) |
Normal CA72-4 Level (n = 7200) |
p-Value † |
|---|---|---|---|
| Age (years ± SD) | 46.8 ± 12.2 | 45.5 ± 11.0 | 0.007 |
| Male, n (%) | 346 (62.1%) | 4358 (60.5%) | 0.459 |
| Smoking history, % | 22.2% | 21.9% | 0.848 |
| BMI (mean ± SD), kg/m2 | 24.0 ± 3.6 | 23.8 ± 3.6 | 0.394 |
| H. pylori infection, % | 27.5% | 20.6% | <0.001 |
| CA72-4 (mean ± SD), ng/mL | 14.2 ± 7.1 | 2.0 ± 1.3 | <0.001 |
| CA19-9 (mean ± SD), U/mL | 10.1 ± 8.9 | 10.3 ± 8.3 | 0.744 |
| CEA (mean ± SD), ng/mL | 2.0 ± 1.2 | 1.7 ± 1.2 | <0.001 |
† Statistical significant difference is indicated by a bold number.
The mean value of CA72-4 values in high and normal CA72-4 groups were 14.2 ± 7.1 and 2.0 ± 1.3 ng/mL, respectively (p < 0.001). Our results showed that older age (p = 0.007) and presence of H. pylori infection (p < 0.001) presented in the high CA72-4 group. Age, male, smoking history, BMI, and H. pylori infection were further analyzed using a multivariate regression model. As shown in Table 3, both age (OR = 1.01; 95% confidence interval (CI) = 1.00–1.02) and H. pylori infection (OR = 1.44; 95% CI = 1.19–1.76) had significant association with high serum CA72-4 level.
Table 3.
Multivariate logistic regression analysis for independent predicators of high serum CA72-4 level.
| Variable ‡ | Multiple Analysis † | ||
|---|---|---|---|
| p-Value ¶ | OR | (95% CI) | |
| Age (Years) | 0.014 | 1.01 | 1.00–1.02 |
| Male | 0.476 | 1.07 | 0.89–1.29 |
| Smoking history | 0.911 | 0.99 | 0.80–1.22 |
| BMI (kg/m2) | 0.515 | 1.01 | 0.98–1.03 |
| H. pylori infection | <0.001 | 1.44 | 1.19–1.76 |
† OR: odds ratios; CI: confidence intervals; BMI: body mass index. ‡ Multivariate model included age, male, smoking history, BMI, and H. pylori infection. ¶ Statistical significant difference is indicated by a bold number.
3.3. Association between Serum CA72-4 and EGD Findings
We then correlated EGD findings with CA72-4 level. As shown in Table 4, subjects with gastric ulcer (p < 0.001), gastric polyps (p = 0.003), and atrophic gastritis (p = 0.047) tend to have high serum CA72-4 level. Univariate and multivariate binary logistic regression analyses were then performed to evaluate their correlations. Univariate analysis showed similar outcomes: gastric ulcer (OR = 2.20; CI = 1.63–3.99), gastric polyps (OR = 1.45; CI = 1.14–1.85), and atrophic gastritis (OR = 1.34; CI = 1.00–1.78). After adjusting by age, male, smoking history, BMI, and H. pylori infection, multivariate logistic regression revealed that gastric ulcer (adjusted OR (aOR) = 2.11; CI = 1.56–2.86) and gastric polyps (aOR = 1.42; CI = 1.11–1.81) possessed significant positive correlations with high CA72-4 level, while atrophic gastritis (aOR = 1.27; CI = 0.95–1.69) exhibited a negative correlation (Table 5).
Table 4.
Difference in esophagogastroduodenoscopy (EGD) findings between high and normal CA72-4 level groups.
| EGD Finding | High CA72-4 Level (n = 557) |
Normal CA72-4 Level (n = 7200) |
p-Value † |
|---|---|---|---|
| Normal | 214 (38.4%) | 3454 (48%) | |
| Reflux esophagitis | 143 (25.7%) | 1870 (26%) | 0.877 |
| Gastric ulcer | 53 (9.5%) | 328 (4.6%) | <0.001 |
| Gastric polyps | 83 (14.9%) | 776 (10.8%) | 0.003 |
| Gastric erosions | 74 (13.3%) | 792 (11%) | 0.099 |
| Atrophic gastritis | 57 (10.2%) | 566 (7.9%) | 0.047 |
| Gastric submucosal tumor | 23 (4.1%) | 291 (4.0%) | 0.920 |
| Esophageal cancer | 0 (0%) | 2 (0.03%) | 0.694 |
| Gastric cancer | 1 (0.18%) | 2 (0.03%) | 0.079 |
† Statistical significant difference is indicated by a bold number.
Table 5.
Univariate and multivariate logistic regression of high serum CA72-4 level for different EGD findings.
| Outcome of EGD Finding | Univariate † | Multivariate ‡ | ||
|---|---|---|---|---|
| OR | 95% CI | Adjusted OR | Adjusted 95% CI | |
| Reflux esophagitis | 0.99 | 0.81–1.20 | 0.98 | 0.80–1.20 |
| Gastric ulcer | 2.20 | 1.63–2.99 *** | 2.11 | 1.56–2.86 *** |
| Gastric polyps | 1.45 | 1.14–1.85 ** | 1.42 | 1.11–1.81 ** |
| Gastric erosions | 1.24 | 0.96–1.60 | 1.13 | 0.87–1.47 |
| Atrophic gastritis | 1.34 | 1.00–1.78* | 1.27 | 0.95–1.69 |
| Gastric submucosal tumor | 1.02 | 0.66–1.58 | 0.98 | 0.63–1.51 |
| Gastric cancer | 6.47 | 0.59–71.5 | 4.54 | 0.37–56.32 |
† OR: odds ratios; CI: confidence intervals; BMI: body mass index. ‡ Multivariate model included age, male, smoking history, BMI, and H. pylori infection, and serum CA72-4 level (high or normal). * p < 0.05; ** p < 0.01; *** p < 0.001.
3.4. Association between CA72-4 Serum Level and GC
Among 7757 enrolled objects, only 3 persons had GC as confirmed by EGD and pathology. One patient had a high CA72-4 level, with sensitivity of 33.3% and specificity of 92.8%. This finding indicated that CA72-4 had a very low positive predictive value (0.18%) of detecting GC in healthy population. In addition, the negative predictive value was 99.97%, and the total diagnostic accuracy rate was 92.8%.
4. Discussion
There are several important findings that may be helpful in clinical interpretation. Our results indicated that routine screening of CA72-4 for GC in asymptomatic population seems unnecessary due to extremely low positive predictive value (0.18%). High CA72-4 value is correlated with gastric ulcers, gastric polyps, and atrophic gastritis, but not with GC. In addition, old age, H. pylori infection, and high CEA value are positively associated with high CA72-4 level.
CA72-4 is commonly regarded as one of the most specific and sensitive markers for monitoring GC [6,10]. A systemic review performed by the Task Force of the Japanese Gastric Cancer Association in 2014 revealed that the overall positive rate of CA72-4 for GC was 16–70%, which makes it the most highly correlated serum tumor marker for GC, although the sensitivity remained low [6]. Liang reported that CA 72-4 was positive in 27.6% patients with GC, which was higher than that in the control (14.8%, p < 0.001) [11]. In the present study, we enrolled much more healthy subjects (n = 7757) than the previous studies and found a lower positive rate of CA72-4 (7.2%) in the general population.
The sensitivity and specificity of CA72-4 for GC were significantly higher in patients with advanced stage GC than in those with early stage GC [6,12]. In a retrospective study in Turkey, CA72-4 were detected in 82% of GC patients with liver metastasis [13]. Elevated CA72-4 level was associated with tumor stages, tumor depth, nodal involvement, and metastasis [14,15,16]. In a review study, Shimada found that CA72-4 positive rate increased as the disease progressed (stage I: 12%, stage II: 15.6%, stage III: 36.7%, and stage IV: 49.6%) [6]. This is because the CA72-4 antigen in cancer cells is not released into the circulation in the early stage and may enter the circulation as the lymph vessels or veins are invaded with cancer cells [17]. Together, CA 72-4 seems to be useful in assessing the extent of cancer invasion before operation; however, it is not qualified as a screening tool for GC in a healthy population.
Most of the study objects were limited to GC patients and indicated that CA72-4 may be helpful in disease monitoring and prognosis [6,8,12,18]. A meta-analysis conducted in Chinese population revealed that odds ratio of CA72-4 for GC was as high as 32.9 [18]. However, the value of CA72-4 in predicting GC in asymptomatic population remained unclear. Noticeably, the enrolled subjects were limited to patients with GC in the previous studies and the positive prediction rate could not be calculated accurately. Our study enrolled patients from multicenters in Taiwan. In this study, the total diagnostic accuracy rate was 92.8%, but it was not considered significant due to the low positive predictive value of CA72-4 for GC (0.18%). This was the first multicenter large-scale study to evaluate the clinical value of CA72-4 for screening of GC in the asymptomatic healthy population.
It has been known that a combination of multiple tumor markers is more effective in evaluating GC than a single tumor marker [11,19,20]. A retrospective study revealed that the combined detection of CEA, CA19-9, CA242, and CA72-4 possessed a sensitivity of 82.6% and a specificity of 83.3% [21]. Yu and Zeng found that the establishment of a GC screening system based on serum levels of CEA, CA19-9, and CA72-4 biomarkers seemed to be an effective approach [19]. However, in their study, the sample size was very limited and may not reach a solid conclusion. In this study, only one person was found with triple positive of CEA, CA19-9, and CA72-4, but was not diagnosed with GC.
Our study indicated that high CA72-4 value is correlated with H. pylori infection. H. pylori infection has been considered as a leading cause of gastric cancer [22]. Epigenetic reprogramming of host cell genome is initiated by H. pylori infection through a direct microbe–gastric epithelial cell interaction, and possibly plays a key role in gastric carcinogenesis [23]. However, whether epigenetic modification in gastric cancer correlated with the CA72-4 level, has not been well studied. We also investigated the association between serum CA72-4 level and benign gastric disease. Chronic gastric diseases are usually independent of gastric cancer occurrence; therefore, these populations are potentially GC patients in the long run. Comprehensive large-scale prospective studies are necessary to clarify this issue.
In Taiwan, GC is the seventh leading cause of cancer-related deaths [24]. The economic burden of advanced GC accounts for 0.08% of the Taiwanese medical expense [25]. Although endoscopic biopsy along with histopathological evaluation is the gold standard for diagnosing GC, its invasiveness and high expense precludes it as a routine screening method. Establishing a noninvasive and cost-effective comprehensive screening system is the major goal of future studies.
Although this study enrolled a larger proportion of healthy individuals who underwent routine physical examination from multicenters, some limitations might exist, including potential confounders, histopathological examination, and various cancer types. First, GC may be possibly missed by endoscopic examination, either by misinterpretation or inadequate sampling [26]. Second, in addition to GC, CA72-4 is also related to breast, colon, and pancreatic cancers [8]. In this study, we did not determine the presence of other cancers and focused only on GC. There may be some patients who had cancers other than GC.
5. Conclusions
In this study, we demonstrated that high serum CA72-4 level is positively associated with older age, H. pylori infection status, gastric ulcer, gastric polyps, and atrophic gastritis. The total diagnostic accuracy rate of CA72-4 for detecting GC was 92.8%; however, the positive predictive value was low (0.18%). Routine screening of CA72-4 for GC in asymptomatic patients may be ineffective due to the low positive predictive rate. A combination of EGD findings and serum CA72-4 for screening healthy individuals with GC is recommended.
Acknowledgments
The authors would like to thank the editor and reviewers for the editorial assistance and their valuable comments. The authors sincerely thank the following persons who help collecting data and editing this manuscript: Tsu-Chen Lin, Ming-Huei Yang, Yen-Kuang Lin, Sen-Te Wang, and Chi-Chih Wang.
Author Contributions
Conception or design of this work: H.-J.L.; conceived and designed the experiments: P.-J.H., C.-H.L., M.-Y.C., H.-J.L.; performed the experiments: M.-Y.C., M.-S.W., Y.-C.L., P.-H.S.; analyzed the data: P.-J.H.; wrote the manuscript: P.-J.H., C.-H.L., H.-J.L.; final approval: All authors.
Funding
This study is supported by Taipei Medical University (No: N201702016), Shung Ho Hospital, Wan Fang Hospital, Taipei Medical University hospital, Everlife Health Services, and Tomorrow Medical Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Laversanne M., Brewster D.H., Gombe Mbalawa C., Kohler B., Pineros M., Steliarova-Foucher E., Swaminathan R., Antoni S., et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer. 2015;137:2060–2071. doi: 10.1002/ijc.29670. [DOI] [PubMed] [Google Scholar]
- 2.Sitarz R., Skierucha M., Mielko J., Offerhaus G.J.A., Maciejewski R., Polkowski W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bickenbach K., Strong V.E. Comparisons of Gastric Cancer Treatments: East vs. West. J. Gastric Cancer. 2012;12:55–62. doi: 10.5230/jgc.2012.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dan Y.Y., So J.B., Yeoh K.G. Endoscopic screening for gastric cancer. Clin. Gastroenterol. Hepatol. 2006;4:709–716. doi: 10.1016/j.cgh.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.H., Oh S.J., Oh C.A., Choi M.G., Noh J.H., Sohn T.S., Bae J.M., Kim S. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J. Surg. Oncol. 2011;104:585–591. doi: 10.1002/jso.21919. [DOI] [PubMed] [Google Scholar]
- 6.Shimada H., Noie T., Ohashi M., Oba K., Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: A systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. 2014;17:26–33. doi: 10.1007/s10120-013-0259-5. [DOI] [PubMed] [Google Scholar]
- 7.Colcher D., Hand P.H., Nuti M., Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proc. Natl. Acad. Sci. USA. 1981;78:3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariampillai A.I., Cruz J.P.D., Suh J., Sivapiragasam A., Nevins K., Hindenburg A.A. Cancer Antigen 72-4 for the Monitoring of Advanced Tumors of the Gastrointestinal Tract, Lung, Breast and Ovaries. Anticancer Res. 2017;37:3649–3656. doi: 10.21873/anticanres.11735. [DOI] [PubMed] [Google Scholar]
- 9.Marshall B. Helicobacter pylori: 20 years on. Clin. Med. 2002;2:147–152. doi: 10.7861/clinmedicine.2-2-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehena Z., Ghosh C.K., Afroz F., Alam M.B., Ferdousi S., Mahmuduzzaman M., Sultana T., Ahmed A.N. Comparison of Serum CA72-4 and CEA Levels in Patient with Endoscopically Suspected Gastric Carcinoma. Mymensingh Med. J. 2015;24:542–549. [PubMed] [Google Scholar]
- 11.Liang Y., Wang W., Fang C., Raj S.S., Hu W.M., Li Q.W., Zhou Z.W. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7:49565–49573. doi: 10.18632/oncotarget.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goral V., Yesilbagdan H., Kaplan A., Sit D. Evaluation of CA 72-4 as a new tumor marker in patients with gastric cancer. Hepatogastroenterology. 2007;54:1272–1275. [PubMed] [Google Scholar]
- 13.Ucar E., Semerci E., Ustun H., Yetim T., Huzmeli C., Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv. Ther. 2008;25:1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar M.J., Arribas I., Coca M.C., Diez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour. Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 15.Aloe S., D’Alessandro R., Spila A., Ferroni P., Basili S., Palmirotta R., Carlini M., Graziano F., Mancini R., Mariotti S., et al. Prognostic value of serum and tumor tissue CA 72-4 content in gastric cancer. Int. J. Biol. Markers. 2003;18:21–27. doi: 10.1177/172460080301800104. [DOI] [PubMed] [Google Scholar]
- 16.Ikeguchi M., Katano K., Saitou H., Tsujitani S., Maeta M., Kaibara N. Pre-operative serum levels of CA72-4 in patients with gastric adenocarcinoma. Hepatogastroenterology. 1997;44:866–871. [PubMed] [Google Scholar]
- 17.Hamazoe R., Maeta M., Matsui T., Shibata S., Shiota S., Kaibara N. CA72-4 compared with carcinoembryonic antigen as a tumour marker for gastric cancer. Eur. J. Cancer. 1992;28A:1351–1354. doi: 10.1016/0959-8049(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen X.Z., Zhang W.K., Yang K., Wang L.L., Liu J., Wang L., Hu J.K., Zhang B., Chen Z.X., Chen J.P., et al. Correlation between serum CA72-4 and gastric cancer: Multiple analyses based on Chinese population. Mol. Biol. Rep. 2012;39:9031–9039. doi: 10.1007/s11033-012-1774-x. [DOI] [PubMed] [Google Scholar]
- 19.Yu J., Zheng W. An Alternative Method for Screening Gastric Cancer Based on Serum Levels of CEA, CA19-9, and CA72-4. J. Gastrointest. Cancer. 2018;49:57–62. doi: 10.1007/s12029-016-9912-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.C., Lee S.Y., Kim C.Y., Yang D.H. Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer. J. Korean Surg. Soc. 2013;85:283–289. doi: 10.4174/jkss.2013.85.6.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing J.X., Wang Y., Xu X.Q., Sun T., Tian B.G., Du L.L., Zhao X.W., Han C.Z. Tumor markers for diagnosis, monitoring of recurrence and prognosis in patients with upper gastrointestinal tract cancer. Asian Pac. J. Cancer Prev. 2014;15:10267–10272. doi: 10.7314/APJCP.2014.15.23.10267. [DOI] [PubMed] [Google Scholar]
- 22.Parkin D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 23.Chiariotti L., Angrisano T., Keller S., Florio E., Affinito O., Pallante P., Perrino C., Pero R., Lembo F. Epigenetic modifications induced by Helicobacter pylori infection through a direct microbe-gastric epithelial cells cross-talk. Med. Microbiol. Immunol. 2013;202:327–337. doi: 10.1007/s00430-013-0301-6. [DOI] [PubMed] [Google Scholar]
- 24.Taiwan’s Leading Causes of Death in 2016 (Last updated by 19 June, 2017) [(accessed on 26 April 2019)]; Available online: https://www.mohw.gov.tw/cp-3425-33347-2.html.
- 25.Hong J., Tsai Y., Novick D., Hsiao F.C., Cheng R., Chen J.S. The economic burden of advanced gastric cancer in Taiwan. BMC Health Serv. Res. 2017;17:663. doi: 10.1186/s12913-017-2609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon S., Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A meta-analysis. Endosc. Int. Open. 2014;2:E46–E50. doi: 10.1055/s-0034-1365524. [DOI] [PMC free article] [PubMed] [Google Scholar]