Abstract
The marine-sourced fungus Penicillium sp. ZZ380 was previously reported to have the ability to produce a series of new pyrrospirone alkaloids. Further investigation on this strain resulted in the isolation and identification of novel penicipyrroether A and pyrrospirone J. Each of them represents the first example of its structural type, with a unique 6/5/6/5 polycyclic fusion that is different from the 6/5/6/6 fused ring system for the reported pyrrospirones. Their structures were elucidated by extensive nuclear magnetic resonance (NMR) and high resolution electrospray ionization mass spectroscopy (HRESIMS) spectroscopic analyses, electronic circular dichroism (ECD) and 13C NMR calculations and X-ray single crystal diffraction. Penicipyrroether A showed potent antiproliferative activity against human glioma U87MG and U251 cells with half maximal inhibitory concentration (IC50) values of 1.64–5.50 μM and antibacterial inhibitory activity with minimum inhibitory concentration (MIC) values of 1.7 μg/mL against methicillin-resistant Staphylococcus aureus and 3.0 μg/mL against Escherichia coli.
Keywords: marine fungus, Penicillium sp. ZZ380, penicipyrroether A, pyrrospirone J, antiproliferative and antibacterial activities
1. Introduction
Hirsutellones and related compounds are a family of fungal secondary metabolites that bear a unique 13-membered ether ring, which is composed of the structural units of decahydrofluorene, para-cyclophane and pyrrolidinone [1,2]. This family of fungal metabolites is divided into four groups: GKK1032s, hirsutellones, pyrrocidines and pyrrospirones. To date, about 29 such metabolites have been isolated and identified from fungal sources of genera Cylindrocarpon, Embellisia, Hirsutella, Lewia, Neonectria, Penicillium and Trichoderma and some of these metabolites have proved to have cytotoxic, antifungal and antibacterial activities [1,2,3,4].
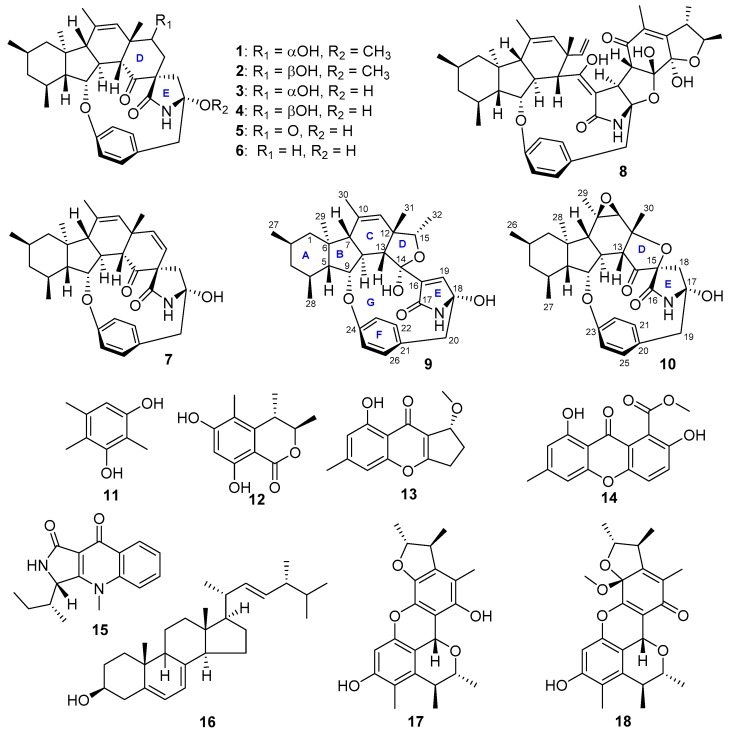
A marine fungus Penicillium sp. ZZ380 [2] was recently isolated from a wild sea crab Pachygrapsus crassipes during the course of our ongoing program to discover novel bioactive agents from marine microorganisms. This fungus in the BMPM liquid medium produced new pyrrospirones C–I (1–7) [2], penicipyrrodiether A (8) [3] and compound 10 (Figure 1), the structure of which was not possible to establish previously, due to its structural instability. Pyrrospirone G (5) was found to have potent activity against the proliferation of different glioma cells and pyrrospirones C (1), F (4), I (7) and penicipyrrodiether A (8) showed activities in inhibiting the growth of methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli.
Figure 1.
Structures of compounds 1–18 isolated from the cultures of Penicillium sp. ZZ380.
It is well known that OSMAC (One Strain, Many Compounds) has been used to activate the silent gene cluster of microbial secondary metabolic biosynthesis to express and then produce different novel bioactive natural products [5,6,7]. In order to obtain more bioactive compounds from this pyrrospirones-produced fungus ZZ380, a different liquid medium of PDB (potato dextrose broth) was used to culture strain ZZ380 in this study, resulting in the isolation of nine compounds, including novel penicipyrroether A (9) (Figure 1) but no pyrrospirones and penicipyrrodiether A. Herein, we report the culture of strain ZZ380 in PDB medium as well as the structural determination and bioactive evaluation of penicipyrroether A (9) and the previously unidentified compound 10.
2. Results and Discussion
An EtOAc extract prepared from a mass culture of Penicillium sp. ZZ380 in PDB medium was separated by ODS column chromatography, followed by HPLC purification, to give compounds 9 and 11–18. Compound 10 was isolated from a previous culture of ZZ380 in BMPM medium [2]. On the basis of the nuclear magnetic resonance (NMR) data and specific rotation, as well as the comparison with the reported data, eight known compounds were identified as 2,4,5-trimethylresorcinol (11) [8], stoloniferol B (12) [9], coniochaetone E (13) [10], pinselin (14) [11], quinolactacin A1 (15) [12], ergosterol (16) [13], penicitrinol A (17) [14] and B (18) [15]. Their 13C NMR data are presented in Tables S1 and S2 (Supplementary Materials).
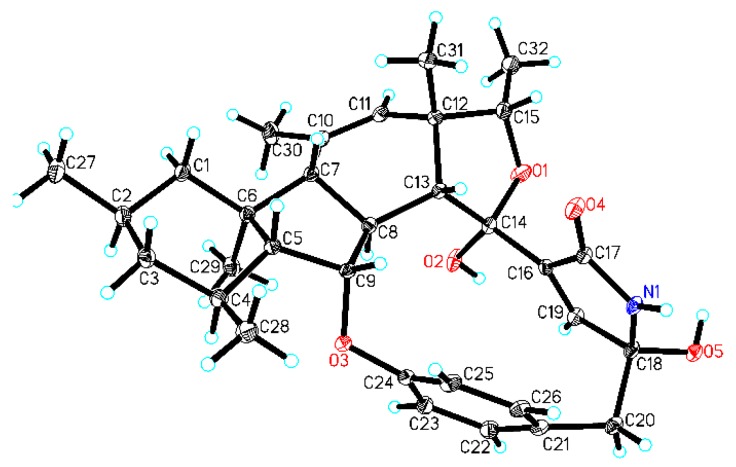
Compound 9 was obtained as colorless block crystals from MeOH and has a molecular formula C32H41NO5 deduced from its high resolution electrospray ionization mass spectroscopy (HRESIMS) ions at m/z [M + H]+ 520.3063 and [M + Na]+ 542.2877 as well as its 13C NMR data. Analyses of its 1H, 13C, 1H-1H COSY, HSQC and HMBC spectra (Figures S1–S17) indicated that the structural parts of rings A–C, F and G for 9 and pyrrospirones C–I (1–7) are the same. However, the structures of 9 and pyrrospirones have significant differences. The first difference is the ring D with a five-membered ether ring for 9 and a cyclohexanone for pyrrospirones. The second difference is that 9 has no spiro junction for rings D and E. The five-membered ether ring D and its connections with rings C and E were confirmed by 1H-1H COSY correlation (Table 1 and Figure 2) of H-8 (δH 3.12, m) with H-13 (δH 3.78, d, J = 5.7 Hz) and HMBC correlations (Table 1 and Figure 2) of H-13 with C-8 (δC 48.9), C-9 (δC 87.5), C-11 (δC 126.8), C-12 (δC 48.6), C-14 (δC 102.1), C-16 (δC 139.2) and C-31 (δC 22.0), H-15 (δH 4.35, q, J = 6.4 Hz) with C-11 and C-31 and H-19 (δH 6.95, d, J = 1.8 Hz) with C-14, C-17 (δC 172.7) and C-18 (δC 88.1). The locations of CH3-31, CH3-32 and OH-14 were also established by HMBC correlations of H3-31 (δH 1.35, s) with C-11, C-12, C-13 (δC 56.3) and C-15 (δC 79.4) and H3-32 (δH 1.26, d, J = 6.4 Hz) with C-12 and C-15, as well as OH-14 (δH 6.25, s) with C-14 and C-16. The planner structure of 9 was further confirmed by X-ray diffraction analysis (Cu Kα, CCDC deposition number 1868814, crystallized in MeOH, Figure 3).
Table 1.
13C and 1H NMR data of penicipyrroether A (9, in pyridine-d5).
| No. | δC, Type | δH (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 49.2, CH2 | βH: 0.76, t (12.0); αH: 1.88, dd (12.2, 3.3) |
H-2, αH-1; H-2, βH-1 |
C-27, C-29 |
| 2 | 28.5, CH | 1.76, m | H-1, H-3, H3-27 | |
| 3 | 46.1, CH2 | βH: 0.57, q (12.0); αH: 1.69, m |
H-2, αH-3, H-4; H-2, βH-3, H-4 |
C-27, C-28 |
| 4 | 28.0, CH | 1.97, m | H-3, H-5, H3-28 | |
| 5 | 62.0, CH | 1.22, dd (11.3, 7.6) | H-4, H-9 | C-4, C-6, C-29 |
| 6 | 41.3, C | – | ||
| 7 | 54.4, CH | 2.36, d (13.2) | H-8 | C-10 |
| 8 | 48.9, CH | 3.12, m | H-7, H-9, H-13 | C-7, C-9, C-13, C-14 |
| 9 | 87.5, CH | 5.01, dd (7.6, 4.8) | H-5, H-8 | C-6 |
| 10 | 139.9, C | – | ||
| 11 | 126.8, CH | 5.62, s | C-7, C-30 | |
| 12 | 48.6, C | – | ||
| 13 | 56.3, CH | 3.78, d (5.7) | H-8 | C-9, C-11, C-12, C-14, C-16, C-31 |
| 14 | 102.1, C | – | ||
| 15 | 79.4, CH | 4.35, q (6.4) | H3-32 | C-11, C-31 |
| 16 | 139.2, C | – | ||
| 17 | 172.7, C | – | ||
| 18 | 88.1, C | – | ||
| 19 | 147.3, CH | 6.95, d (1.8) | C-14, C-17, C-18 | |
| 20 | 45.7, CH2 | βH: 3.56, d (12.2); αH: 3.51, d (12.2) |
αH-20; βH-20 |
C-18, C-19, C-21, C-22, C-26 |
| 21 | 130.8, C | – | ||
| 22 | 132.7, CH | 7.31, dd (8.1, 1.9) | H-23 | C-20, C-24, C-26 |
| 23 | 122.4, CH | 7.13, dd (8.1, 2.4) | H-22 | C-21, C-25 |
| 24 | 159.6, C | – | ||
| 25 | 118.4, CH | 7.22 a | H-26 | C-21, C-23 |
| 26 | 130.2, CH | 7.38, dd (8.4, 1.9) | H-25 | C-22, C-24 |
| 27 | 23.4, CH3 | 0.91, d (6.4) | H-2 | C-1, C-2, C-3 |
| 28 | 20.2, CH3 | 1.18, d (6.3) | H-4 | C-3, C-4, C-5 |
| 29 | 16.8, CH3 | 1.31, s | C-1, C-5, C-6, C-7 | |
| 30 | 20.8, CH3 | 1.81, s | C-7, C-10, C-11 | |
| 31 | 22.0, CH3 | 1.35, s | C-11, C-12, C-13, C-15 | |
| 32 | 14.8, CH3 | 1.26, d (6.4) | H-15 | C-12, C-15 |
| OH-14 | – | 6.25, s | C-14, C-16 | |
| OH-18 | – | 8.35, s | ||
| NH-17 | – | 9.43, s | C-16, C-18, C-19 |
a The signal was overlapped with that of NMR solvent pyridine-d5.
Figure 2.
1H-1H COSY, key HMBC and NOE correlations of penicipyrroether A (9).
Figure 3.
X-ray crystal structure of penicipyrroether A (9, Cu Kα radiation).
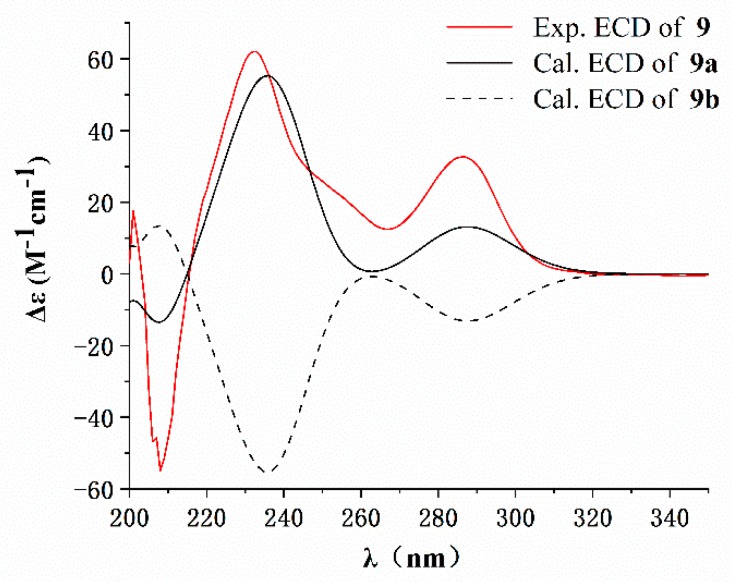
The relative stereochemistry of 9 was established based on the analyses of NOESY spectrum (Figures S18 and S19) and coupling constants. As depicted in Figure 2, NOE correlations of H-9 with H-5 and H-13, H3-31 with H-7, H-13 and H-15, βH-20 with NH-17 and H-26 and H-25 with H-9 and H-26 indicated a β-orientation for these protons. Similarly, NOE correlations of H3-29 with H-2, H-4 and H-8, H-8 with OH-14 and H-22 with αH-20 and H-23 were suggestive of an α-orientation for these protons. The large coupling constants of 3J4,5 (11.3 Hz) and 3J7,8 (13.2 Hz) also confirmed the trans-juncture for A/B and B/C rings [16]. NOE correlations of H-11 with H3-30 and H-19 with OH-14 indicated a Z-geometry for both of the double bonds at C10–11 and C16–19. The absolute configuration of 9 was determined as 2R,4S,5R,6S,7S,8S,9S,12S,13R,14R,18R based on the result of the X-ray diffraction analysis (Cu Kα, Flack parameter 0.02). A computational method was also applied to assign the absolute stereochemistry of 9 by comparing its experimental electronic circular dichroism (ECD) spectrum with the calculated ECD spectra. The result (Figure 4) showed that the calculated ECD spectrum of model molecule 9a was close to the experimental curve of 9. Based on the foregoing evidence, the structure of 9 was elucidated as a new alkaloid, named penicipyrroether A. Its 13C and 1H NMR data (Table 1) were assigned based on a combination of HSQC, 1H-1H COSY, HMBC and NOE spectroscopic analyses.
Figure 4.
Experimental electronic circular dichroism (ECD) spectrum of penicipyrroether A (9, 200–350 nm) in MeOH and the calculated ECD spectra of the model molecules 9a and 9b at the B3LYP/6-311+G(d,p) level (9a: 2R,4S,5R,6S,7S,8S,9S,12S,13R,14R,18R; 9b: 2S,4R,5S,6R,7R,8R,9R,12R,13S,14S,18S).
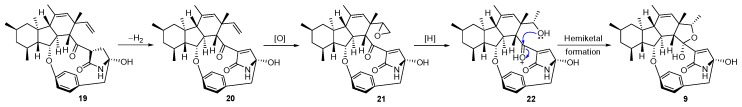
It has been reported that compound 20, a dehydro-derivative of GKK1032A2 (19) (Figure 5), might be a precursor of the fungal metabolites of GKK1032s, pyrrospirones and penicipyrrodiether A [1,2,3,17]. Similarly, penicipyrroether A (9) might be also derived from 20. A proposed biosynthetic pathway for penicipyrroether A (9) was depicted in Figure 5. After oxidation of the vinyl group of 20, the epoxide derivative (21) was transformed into 22 and then an intramolecular hemiketal formation [3,15] of 22 forms penicipyrroether A (9).
Figure 5.
Plausible biosynthetic pathway of penicipyrroether A (9).
Compound 10 was obtained as a colorless amorphous powder and has a molecular formula C30H37NO6 deduced from its HRESIMS ions at m/z [M + H]+ 508.2711 and [M + Na]+ 530.2531 as well as its 13C NMR data, which showed two fewer carbons, when compared to the structure of penicipyrroether A (9). Detailed interpretation of 1H, 13C, 1H-1H COSY, HSQC and HMBC spectra (Figures S23–S38) demonstrated that compound 10 is an analogue of penicipyrroether A (9) with the same structural part of rings A, B, F and G but possesses an epoxy moiety at C-10 and C-11 and a different five-membered ether ring D fused with ring E through a spiro carbon of C-15. In addition, the dehydro-pyrrolidinone moiety (ring E) in 9 was replaced by a pyrrolidinone moiety in 10. The presence of the epoxy moiety was confirmed by HMBC correlations (Table 2 and Figure 6) of H-7 (δH 1.55, d, J = 14.3 Hz) with C-10 (δC 58.8) and C-11 (δC 63.5), H-11 (δH 2.46, s) with C-10, C-12 (δC 81.3), C-13 (δC 44.5), C-29 (δC 21.0) and C-30 (δC 26.0) and H3-29 (δH 1.20, s) with C-7 (δC 49.9), C-10 and C-11. Similarly, the ether ring D and its connections with rings C and E was indicated by HMBC correlations of H-8 (δH 2.79, m) with C-13 and C-14 (δC 180.9), H-11 with C-12 and C-13, H-13 (δH 3.21, d, J = 8.0 Hz) with C-8 (δC 39.2), C-11, C-12, C-14 and C-30, H-18 (δH 1.99, 2.45, d, J = 12.1Hz, each) with C-14, C-15 (δC 79.7), C-16 (δC 172.1), C-17 (δC 86.2) and C-19 (δC 45.0), H3-30 (δH 1.59, s) with C-11, C-12 and C-13 and NH-16 (δH 8.76, s) with C-15, C-16, C-17 and C-18 (δC 40.9).
Table 2.
13C and 1H NMR data of pyrrospirone J (10, in DMSO-d6).
| No. | δC, Type | δH (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 47.3, CH2 | βH: 0.81, t (12.3); αH: 1.80, m |
H-2, αH-2; H-2, βH-2 |
C-26, C-28 |
| 2 | 27.4, CH | 1.82, m | H-1, H-3, H3-26 | C-6 |
| 3 | 45.5, CH2 | βH: 0.51, q (12.1); αH: 1.75, m |
H-2, αH, H-4 H-2, βH, H-4 |
C-2, C-27 |
| 4 | 26.8, CH | 1.78, m | H-3, H-5, H3-27 | C-2 |
| 5 | 59.5, CH | 1.22, dd (11.6, 8.8) | H-4, H-9 | C-4, C-6, C-28 |
| 6 | 42.5, C | – | ||
| 7 | 49.9, CH | 1.55, d (14.3) | H-8 | C-1, C-5, C-6, C-8, C-13, C-28 |
| 8 | 39.2, CH | 2.79, m | H-7, H-9, H-13 | C-7, C-9, C-10, C-13, C-14 |
| 9 | 85.0, CH | 4.82, dd (8.6, 7.0) | H-5, H-8 | C-5, C-6, C-13, C-23 |
| 10 | 58.8, C | – | ||
| 11 | 63.5, CH | 2.46, s | C-10, C-12, C-13, C-29, C-30 | |
| 12 | 81.3, C | – | ||
| 13 | 44.5, CH | 3.21, d (8.0) | C-8, C-9, C-11, C-12, C-14, C-30 | |
| 14 | 180.9, C | – | ||
| 15 | 79.7, C | – | ||
| 16 | 172.1, C | – | ||
| 17 | 86.2, C | – | ||
| 18 | 40.9, CH2 | βH: 1.99, d (12.1); αH: 2.45, d (12.1) |
αH-18; βH-18 |
C-14, C-15, C-16, C-17, C-19 |
| 19 | 45.0, CH2 | βH: 2.99, d (14.5); αH: 2.69, d (14.5) |
αH-19; βH-19 |
C-17, C-18, C-20, C-21, C-25 |
| 20 | 129.4, C | – | ||
| 21 | 133.3, CH | 6.92, dd (8.3, 2.1) | H-22 | C-19, C-23, C-25 |
| 22 | 124.4, CH | 6.82, dd (8.3, 2.7) | H-21 | C-20, C-23, C-24 |
| 23 | 158.7, C | – | ||
| 24 | 119.7, CH | 6.94, dd (8.8, 2.7) | H-25 | C-20, C-22, C-23 |
| 25 | 130.6, CH | 6.78, dd (8.8, 2.1) | H-24 | C-19, C-21, C-23 |
| 26 | 22.7, CH3 | 0.86, d (6.1) | H-2 | C-1, C-2, C-3 |
| 27 | 19.7, CH3 | 1.02, d (6.2) | H-4 | C-3, C-4, C-5 |
| 28 | 15.2, CH3 | 1.05, s | C-1, C-5, C-6, C-7 | |
| 29 | 21.0, CH3 | 1.20, s | C-7, C-10, C-11 | |
| 30 | 26.0, CH3 | 1.59, s | C-11, C-12, C-13 | |
| OH-17 | – | 6.24, s | C-17, C-18, C-19 | |
| NH-16 | – | 8.76, s | C-15, C-16, C-17, C-18 |
Figure 6.
1H-1H COSY, key HMBC and NOE correlations of pyrrospirone J (10).
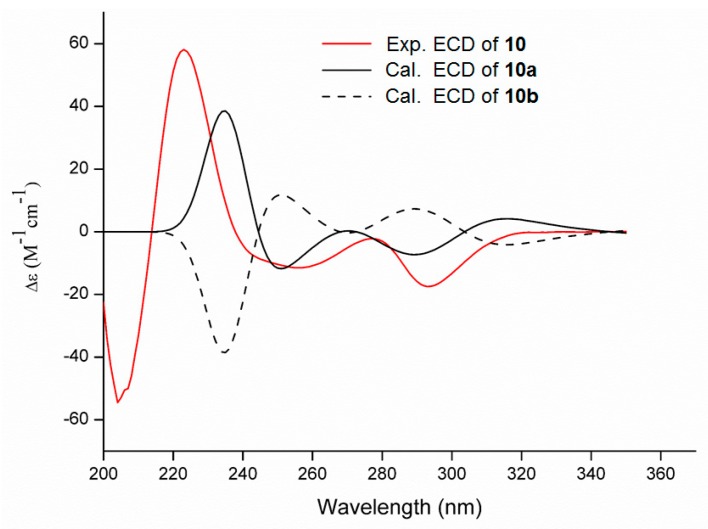
The relative stereochemistry of 10 was assigned by analyses of the NOESY spectrum (Figures S39 and S40) and coupling constants. NOE correlations (Figure 6) of H-5 (δH 1.22) with βH-3 (δH 0.51) and H-9 (δH 4.82), H-7 (δH 1.55) with βH-1 (δH 0.81) and H3-30 (δH 1.59), H-9 with H-13 (δH 3.21) and H-24 (δH 6.94), H-13 with H3-30, NH-16 (δH 8.76) with βH-19 (δH 2.99) and H-25 (δH 6.78) and H-25 with βH-19 and H-24 suggested a β-orientation for these protons; while NOE correlations of H-8 (δH 2.79) with H3-28 (δH 1.05), H3-28 with αH-1 (δH 1.80), H-2 (δH 1.82), H-4 (δH 1.78) and H3-29 (δH 1.20), H3-29 with H-11 (δH 2.46), H-21 (δH 6.92) with αH-19 (δH 2.69) and H-22 (δH 6.82) and H-18 (δH 1.99) with OH-17 (δH 6.24) proved an α-orientation for these protons. The trans-juncture for A/B and B/C rings was also confirmed by the large coupling constants of 3J4,5 (11.6 Hz) and 3J7,8 (14.3 Hz) [16]. The absolute configuration of 10 was determined as 2R,4S,5R,6R,7R,8S,9S,10R,11R,12R,13R,15R,17R based on the result from ECD calculation (Figure 7). Unfortunately, several efforts to obtain single crystal for X-ray diffraction were not successful because the structure of 10 was changed during the process of crystallization.
Figure 7.
Experimental ECD spectrum of pyrrospirone J (10, 200–400 nm) in MeOH and the calculated ECD spectra of the model molecules 10a and 10b at the B3LYP/6-311+G(d,p) level (10a: 2R,4S,5R,6R,7R,8S,9S,10R,11R,12R,13R,15R,17R; 10b: 2S,4R,5S,6S,7S,8R,9R,10S,11S,12S,13S,15S,17S).
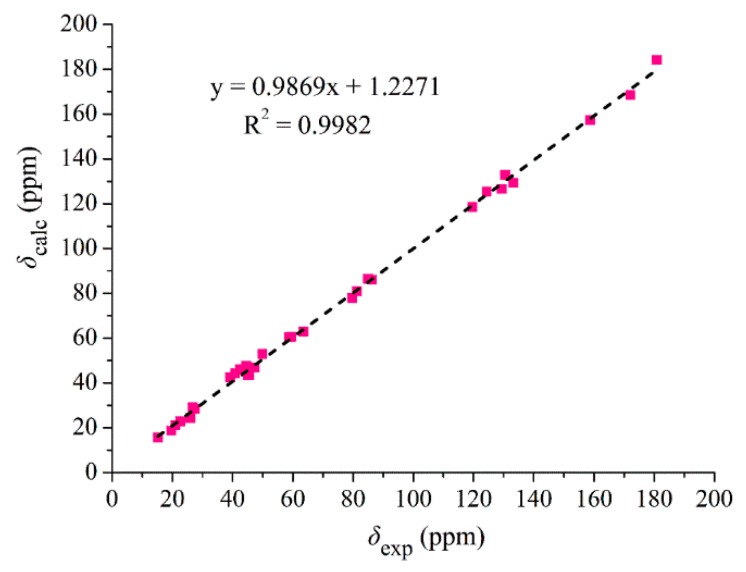
It was noted that the ketonic carbonyl at C-14 of 10 resonated at 180.9 ppm, which is unusual. In order to confirm this possibility, we conducted a computational calculation of its 13C chemical shifts [18,19,20]. The calculated 13C NMR data (Table S7) of 10 were in good agreement with its experimental values, with the corrected mean absolute error (CMAE) of 0.28 ppm and the correlation coefficient (R2) of 0.9982 (Figure 8) and the calculated 13C chemical shift for C-14 was 184.1 ppm, which was close to the experimental value of 180.9 ppm. Based on the foregoing evidence, the structure of 10 was determined as a new alkaloid, named pyrrospirone J. The full assignment of 13C and 1H NMR data (Table 2) was made based on HSQC, 1H-1H COSY, HMBC and NOE spectroscopic analyses.
Figure 8.
Regression analysis of experimental versus calculated 13C NMR chemical shifts of pyrrospirone J (10) at the mPW1PW91-SCRF (DMSO)/6-311+g(d,p) level and linear fitting is shown as a line.
New alkaloids—penicipyrroether A (9) and pyrrospirone J (10)—were evaluated for their activities in inhibiting the proliferation of human glioma U87MG and U251 cells through sulforhodamine B (SRB) assay [21]. Doxorubicin (DOX, a chemotherapeutic drug) was used as a positive control. It has been found that both compounds had activity against different glioma cells with IC50 values of 1.64–5.50 μM for 9 and 10.52–17.92 μM for 10 (Table 3). The cytotoxicities of penicipyrroether A (9) and DOX against normal human astrocytes were also tested and showed CC50 values of 23.28 ± 1.05 μM for 9 and 8.57 ± 0.16 μM for DOX. The data indicated that the antiglioma activity of 9 was equivalent to (or slightly stronger than) the activity of the positive control DOX and the selective index (CC50/IC50) of 4.2–14.2 for 9 was higher than that of 1.1–7.1 for DOX.
Table 3.
Antiglioma and antibacterial activities of penicipyrroether A (9) and pyrrospirone J (10).
| Compounds | Glioma Cells (μM) | Bacteria (μg/mL) | ||
|---|---|---|---|---|
| U87MG | U251 | MRSA | E. coli | |
| 9 | 1.64 ± 0.05 | 5.50 ± 0.12 | 1.7 | 3.0 |
| 10 | 10.52 ± 0.62 | 17.92 ± 0.93 | >50 | >50 |
| DOX | 1.20 ± 0.06 | 8.03 ± 1.20 | NT | NT |
| Gentamicin | NT | NT | 0.36 | 1.44 |
| Vancomycin | NT | NT | 0.20 | NT |
NT: No testing.
The antibacterial activities of penicipyrroether A (9) and pyrrospirone J (10) against MRSA and E. coli were also determined by the micro broth dilution method [22]. Gentamicin (an antibiotic against both Gram-positive and Gram-negative bacteria) and vancomycin (an antibiotic against MRSA) were used as positive controls. The results (Table 3) showed that penicipyrroether A (9) had good antibacterial activities with MIC values of 1.7 μg/mL against MRSA and 3.0 μg/mL against E. coli. However, pyrrospirone J (10) was inactive at a concentration of 50 μg/mL.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotation, ultraviolet-visible (UV) and electronic circular dichroism (ECD) were measured on an Autopol I polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA), a METASH UV-8000 spectrometer (Shanghai METASH Instruments Co. Ltd., Shanghai, China) and a JASCO J-815 spectropolarimeter (JASCO Co. Tokyo, Japan), respectively. Infrared radiation (IR) spectra were recorded on a Bruker TENSOR II high performance FT-IR spectrometer (Bruker, Karlsruhe, Germany). HRESIMS data was obtained from an Agilent 6230 time-of-flight liquid chromatography–mass spectrometry (TOF LC-MS, Agilent, CA, USA). NMR spectra were acquired on a Bruker 500 spectrometer and chemical shifts were expressed in δ (ppm). Octadecyl silane (ODS, Cosmosil 75C18-Prep, Nacalai Tesque Inc., Kyoto, Japan) was applied for column chromatography. HPLC separation was conducted on a SHIMADZU LC-20AP prepared HPLC system with column A (Welch-20, 250 × 21 mm, 5 μm, XB-C18) or column B (CT-30, 280 × 30 mm, 10 μm, Fuji-C18). All solvents were ordered from the Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Human glioma U87MG (JDS-2568) and U251 (XB-0439) cells were purchased from the Cell Bank of the Chinese Academy of Sciences and normal human astrocytes (HA, Cat. No. 1800) from the ScienCell. Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300 and Escherichia coli ATCC 25922 were gifts from Drs. Zhongjun Ma and Pinmei Wang, respectively. Gentamicin (99.6%) and vancomycin (>98.0%) were bought from Meilune Biotechnology Co. Ltd. (Dalian, China), doxorubicin (DOX, >98.0%) from Sigma-Aldrich, PDA (potato dextrose agar) from Baisi Biotechnology Co. Ltd. (Hangzhou, China) and MHB (Mueller-Hinton Broth) from Oxoid Ltd. PDB medium (potato dextrose broth, potato 100 g, glucose 10 g, sea salt 35 g, tap water 1000 mL) was made in the authors’ laboratory.
3.2. Marine Strain ZZ380
Strain ZZ380 was previously isolated from marine crab Pachygrapsus crassipes and assigned as Penicillium sp. ZZ380 by ITS DNA sequence analysis [2].
3.3. Mass Culture of Strain ZZ380
Colonies of the strain ZZ380 from the PDA slant were inoculated into 250 mL of PDB liquid medium in a 500 mL Erlenmeyer flask and then incubated at 28 °C for three days on a rotary shaker (180 rpm) to produce seed broth. The seed broth of 5 mL was inoculated into a 500 mL Erlenmeyer flask, containing 250 mL of PDB medium and then incubated at 28 °C for 30 days under stationary state. A total of 55 L of culture were prepared for this study.
3.4. Isolation of Compounds 9–18
The 55 L culture of ZZ380 in PDB medium was centrifuged to give mycelia and broth. The mycelia were extracted with MeOH three times to get a MeOH extract. The broth was partitioned with EtOAc three times to give an EtOAc extract. A mixture (23.0 g) of the MeOH and EtOAc extract was fractionated by an ODS column eluting successively with 40%, 60%, 80% and 100% MeOH to afford four fractions (Frs. 1–4). Fr. 1 was further separated by an ODS column eluting with 35%, 45% and 55% MeOH to give three sub fractions (SFrs. 1a–1c). Through purification using prepared HPLC with column A at a flow rate of 12 mL/min, compounds 11 (4.2 mg, tR: 22.0 min, mobile phase: MeOH/H2O, 38/62, detector: 254 nm) and 12 (6.7 mg, tR: 31.0 min, mobile phase: MeOH/H2O, 38/62, detector: 254 nm) were obtained from SFr. 1a; compounds 15 (4.0 mg, tR: 33.0 min, mobile phase: MeOH/H2O, 45/55, detector: 210 nm), 14 (20.5 mg, tR: 37.0 min, mobile phase: MeOH/H2O, 53/47, detector: 254 nm), 18 (7.0 mg, tR: 37.0 min, mobile phase: MeOH/H2O, 66/34, detector: 254 nm) were obtained from SFr. 1b, SFr. 1c and Fr. 2, respectively. Fr. 3 and Fr. 4 were also separated by ODS column eluting with 85% or 95% MeOH to furnish SFrs. 3a and 3b or SFrs. 4a and 4b. Through purification using prepared HPLC with column B at a flow rate of 15 mL/min, compounds 13 (43.0 mg, tR: 19.0 min, mobile phase: MeOH/H2O, 80/20, detector: 210 nm) and 17 (9.5 mg, tR: 45.0 min, mobile phase: MeOH/H2O, 83/17, detector: 254 nm) were obtained from SFrs. 3a and 3b, respectively; while compounds 9 (3.1 mg, tR: 41.0 min, mobile phase: MeOH/H2O, 91/9, detector: 210 nm) and 16 (50.0 mg, tR: 62.0 min, mobile phase: MeOH/H2O, 98/2; 280 nm; tR 62 min; 50.0 mg) were obtained from SFrs. 4a and 4b, respectively.
Compound 10 was isolated from a previous culture of strain ZZ380 in BMPM medium [2]. A crude extract was fractionated on an ODS column eluting successively with 80% MeOH, 90% MeOH and 100% MeOH to give six fractions (Frs. 1–6) based on the results of TLC analysis. Fr. 2 was re-separated by an ODS column eluting with 80% MeOH to give Frs. 2A and 2B. Fr. 2A was further separated by prepared HPLC with column B using mobile phase of acetonitrile/water (68:32) at a flow rate of 10 mL/min to give 10 (2.0 mg, tR: 60.0 min) and Frs. 2A1–2A3.
Penicipyrroether A (9): Colorless block crystals; molecular formula C32H41NO5; [α] + 71.4° (c 0.10, MeOH); ECD (10 mg/L, MeOH) λmax (Δε) 208 (−57.22), 233 (+64.65), 286 (+34.08) nm; UV (MeOH) λmax (log ε) 202 (4.20), 229 (3.66), 278 (2.67) nm; IR (MeOH) νmax 3375, 2924, 1672, 1437, 1408, 1239, 1098, 1013, 951 cm−1; 13C (125 MHz) and 1H (500 MHz) NMR data, see Table 1; HRESIMS m/z [M + H]+ 520.3063 (calcd. for C32H42NO5, 520.3063), [M + Na]+ 542.2877 (calcd. for C32H41NNaO5, 542.2882).
Pyrrospirone J (10): Colorless amorphous powder; molecular formula C30H37NO6; ECD (10 mg/L, MeOH) λmax (Δε) 204 (−55.93), 223 (+59.59), 256 (−11.80), 293 (−17.92) nm; UV (MeOH) λmax (log ε) 211 (4.40), 230 (4.21), 284 (3.36) nm; IR (MeOH) νmax 3347, 2923, 2851, 1748, 1686, 1505, 1460, 1371, 1239, 1169, 1074, 937 cm−1; 13C (125 MHz) and 1H (500 MHz) NMR data, see Table 2; HRESIMS m/z [M + H]+ 508.2711 (calcd. for C30H38NO6 508.2699) and [M + Na]+ 530.2531 (calcd. for C30H37NNaO6 530.2519).
Crystal data of penicipyrroether A (9): Colorless crystals of penicipyrroether A (9) was obtained from MeOH. X-ray diffraction analysis was performed on an Xcalibur Atlas Gemini Ultra diffractometer (Agilent Technologies, CA, USA) with Cu Kα radiation (λ = 1.54184 Å) at 100 K. Structure was solved by direct method (SHELXL-2018) and refined with full-matrix least-squares on F2 (ShelXL, Sheldrick, 2015). All non-hydrogen atoms were refined anisotropically and all hydrogen atoms were placed in idealized positions and refined as riding atoms with the relative isotropic parameters [2]. Crystal data of penicipyrroether A (9): C32H41NO5 (M = 519.68), orthorhombic crystal (0.13 × 0.12 × 0.09 mm), space group P212121 (no. 19), unit cell dimensions a = 6.32760(10) Å, b = 19.8687(2) Å, c = 25.0617(3) Å, V = 3150.79(7) Å3, α = 90°, β = 90°, γ = 90°; Z = 4; Dcalced = 1.231 g/cm3; μ = 0.684 mm−1; 18406 reflection measured (5.676° ≤ 2θ ≤ 147.088°); 6237 unique (Rint = 0.0244, Rsigma = 0.0209) which were used in all calculation; the final refinement ( I ≥ 2σ (I)) gave R1 = 0.0418 and wR2 = 0.1116 (all data); Flack parameter = 0.02 (5). Crystallographic data of penicipyrroether A (9) have been deposited at the Cambridge Crystallographic Data Centre (deposition number: CCDC 1868814). Copies of the crystallographic data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, U.K. [fax (+44)1223-336-033; or e-mail: data_request@ccdc.cam.ac.uk).
3.5. ECD Calculation
Monte Carlo conformational searches of compound 10 were conducted with the Spartan’ 10 software (v1.1.0, x 86, Wavefunction Inc., Irvine, CA, USA) using Merck Molecular Force Field (MMFF) and three conformers were obtained for ECD calculations. The X-ray CIF profile of 9 and the three conformer of 10 (Tables S3 and S4 and Figures S44 and S45) were initially optimized at B3LYP/6-31g (d,p) level in MeOH using the conductor-like polarizable continuum model (CPCM). The theoretical ECD calculation was carried out in MeOH using Time-dependent Density functional theory (TD-DFT) at the B3LYP/6-31+g (d,p) level for all conformers of 9 and 10. Rotatory strengths for a total of 60 excited states for 9 (or 30 for 10) were calculated. ECD spectra were generated using the program SpecDis 1.6 (University of Würzburg, Würzburg, Germany) and GraphPad Prism 5 (University of California San Diego, USA) from dipole-length rotational strengths by applying Gaussian band shapes with sigma = 0.2 eV for 9 (or 0.3 eV for 10).
3.6. 13C NMR Calculation
The previously described methods [19,20,21] were used for 13C NMR calculation. Briefly, Monte Carlo conformational searches were carried out by means of Spartan’s 10 software using MMFF. Four conformers (Table S8 and Figure S46) of compound 10 were obtained for NMR calculations. The conformers were initially optimized at B3LYP/6-31g (d,p) level in DMSO using the CPCM calculation model. Gauge-independent atomic orbital (GIAO) calculations of 13C NMR chemical shifts were performed by DFT at the mPW1PW91-SCRF (DMSO)/6-311+g(d,p) level with the CPCM calculation model in Gaussian 09 software (G09w, D01, Gaussian Inc., Wallingford, CT, USA). The calculated 13C NMR data of the lowest energy conformers for 10 were averaged based on the Boltzmann distribution theory and their relative Gibbs free energy.
3.7. Sulforhodamine B (SRB) Assay
The activity of the tested compounds in inhibiting the proliferation of human glioma U87-MG and U251 cells was determined by SRB assay [22]. Doxorubicin (DOX) was used as a positive control. U87MG cells were cultured in MEM (Minimum Essential Medium, Gibco, Grand Island, NY, USA) with 10% FBS (Fetal Bovine Serum, PAA Laboratories Inc., Toronto, ON, Canada), U251 in DMEM (Dulbecco’s Modified Eagle Medium, Gibco) and normal human astrocytes (HA) in AM (Astrocyte Medium, ScienCell, Cat. No. 1801). All cells were incubated at 37 °C in a 5% CO2 humidified incubator. The cultured cells after the third generation were used for experiment.
3.8. Antibacterial Activive Assay
The previously described micro broth dilution method [3,22] was used to evaluate the antibacterial activity of the tested compounds against the growth of MRSA and E. coli. Vancomycin (an antibiotic against MRSA) and gentamicin (an antibiotic against Gram-positive and Gram-negative bacteria) were used as positive controls. The microorganisms were cultured in MHB medium in 96-well plates at a concentration of 1 × 106 CFU/mL. The MIC was determined after 12 h incubation at 37 °C with tested compounds.
4. Conclusions
It was reported that the marine-derived Penicillium sp. ZZ380 produced a series of pyrrospirones C–I with a characteristic spiro conjunction for rings D and E in BMPM medium [2]. This study reported two new alkaloids of penicipyrroether A (9), produced by strain ZZ380 in PDB medium and pyrrospirone J (10), a previously unidentified compound isolated from a previous culture of ZZ380 in BMPM medium [2]. Both compounds 9 and 10 possess unique structures with a 6/5/6/5 fused ring system of rings A/B/C/D, rather than the 6/5/6/6 ring fusion for pyrrospirones C–I. Each of the two new alkaloids is the first compound of its structural type. Penicipyrroether A (9) is a cyclo-condensation product of GKK1032 analogue via the addition of a five-membered ether ring and exhibited potent inhibitory activity against the proliferation of glioma U87MG and U251 cells and the growth of MRSA and E. coli.
Supplementary Materials
The following is available online at https://www.mdpi.com/1660-3397/17/5/292/s1, Figures S1–S22: NMR, HRESIMS, UV and IR spectra of penicipyrroether A (9), Figures S23–S43: NMR, HRESIMS, UV and IR spectra of pyrrospirone J (10), Figure S44: The optimized geometry of conformer (9-1) of penicipyrroether A (9), Figure S45: The optimized geometry of conformers (10-1–10-3) of pyrrospirone J (10), Figure S46: Four conformations of the low-energy conformers of pyrrospirone J (10) calculated at B3LYP/6-31G(d) level, Table S1: 13C NMR data of known compounds 11–15, Table S2: 13C NMR data of known compounds 16–18, Table S3: Gibbs free energies and equilibrium populations of low-energy conformer of penicipyrroether A (9), Table S4: Cartesian coordinates for the low-energy reoptimized MMFF conformers of penicipyrroether A (9) at B3LYP/6-311+G(d,p) level of theory in CH3OH, Table S5: Gibbs free energies and equilibrium populations of low-energy conformers of pyrrospirone J (10), Table S6: Cartesian coordinates for the low-energy reoptimized MMFF conformers of pyrrospirone J (10) at B3LYP/6-311+G(d,p) level of theory in CH3OH, Table S7: Experimental and calculated 13C NMR data of pyrrospirone J (10), Table S8: Gibbs free energies and equilibrium populations of the low-energy conformers of pyrrospirone J (10) for 13C NMR calculation, Table S9: Cartesian coordinates for the low-energy reoptimized MMFF conformers of pyrrospirone J (10) at B3LYP/6-311+G(d,p) level of theory in DMSO for 13C NMR calculation.
Author Contributions
T.S. and M.C. conducted the isolation and culture of stain ZZ380 as well as the isolation and structural elucidation of compounds; M.T. and H.G. performed the bioactive assay; X.L. and Z.Z. designed and supervised the experiments and wrote the manuscript.
Funding
This research was supported by the National Key R&D Program of China (No. 2017YFE0102200) and the National Natural Science Foundation of China (Nos. 81773587 and 81773769).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Li X.W., Ear A., Nay B. Hirsutellones and beyond: Figuring out the biological and synthetic logics toward chemical complexity in fungal PKS-NRPS compounds. Nat. Prod. Rep. 2013;30:765–782. doi: 10.1039/c3np70016j. [DOI] [PubMed] [Google Scholar]
- 2.Song T.F., Chen M.X., Chai W.Y., Zhang Z.Z., Lian X.Y. New bioactive pyrrospirones C–I from a marine-derived fungus Penicillium sp. ZZ380. Tetrahedron. 2018;74:884–891. doi: 10.1016/j.tet.2018.01.015. [DOI] [Google Scholar]
- 3.Song T., Chen M.X., Ge Z.W., Chai W.Y., Li X.C., Zhang Z.Z., Lian X.Y. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018;83:13395–13401. doi: 10.1021/acs.joc.8b02172. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahim W., Aly A.H., Wray V., Mándi A., Teiten M.H., Gaascht F., Orlikova B., Kassack M.U., Lin W., Diederich M., et al. Embellicines A and B: Absolute configuration and NF-κB transcriptional inhibitory activity. J. Med. Chem. 2013;56:2991–2999. doi: 10.1021/jm400034b. [DOI] [PubMed] [Google Scholar]
- 5.Bode H.B., Bethe B., Höfs R., Zeeck A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem. 2002;3:619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 7.Yuan C., Guo Y.H., Wang H.Y., Ma X.J., Jiang T., Zhao J.L., Zou Z.M., Ding G. Allelopathic polyketides from an endolichenic fungus Myxotrichum sp. by using OSMAC strategy. Sci. Rep. 2016;6:19350. doi: 10.1038/srep19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Qin D., Zhang K., Huang Q., Liu S., Han M.J., Dong J.Y. Metabolites from the co-culture of nigranoic acid and Umbelopsis dimorpha SWUKD 3.1410, an endophytic fungus from Kadsura angustifolia. Nat. Prod. Res. 2017;31:1414–1421. doi: 10.1080/14786419.2016.1255891. [DOI] [PubMed] [Google Scholar]
- 9.Xin Z.H., Li T., Zhu T.J., Wang W.L., Du L., Fang Y.C., Gu Q.Q., Zhu W.M. Isocoumarin derivatives from the sea squirt-derived fungus Penicillium stoloniferum QY2-10 and the halotolerant fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007;30:816–819. doi: 10.1007/BF02978830. [DOI] [PubMed] [Google Scholar]
- 10.Trisuwan K., Rukachaisirikul V., Borwornwiriyapan K., Phongpaichit S., Sakayaroj J. Benzopyranone, benzophenone and xanthone derivatives from the soil fungus Penicillium citrinum PSU-RSPG95. Tetrahedron Lett. 2014;55:1336–1338. doi: 10.1016/j.tetlet.2014.01.017. [DOI] [Google Scholar]
- 11.Cui X.Q., Zhu G.L., Liu S.H., Jiang G.L., Wang Y., Zhu W.M. Diversity and function of the antarctic krill microorganisms from Euphausia superba. Sci. Rep. 2016;6:36496. doi: 10.1038/srep36496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim W.G., Song N.K., Yoo I.D. Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J. Antibiot. 2001;54:831–835. doi: 10.7164/antibiotics.54.831. [DOI] [PubMed] [Google Scholar]
- 13.Shirane N., Takenaka H., Ueda K., Hashimoto Y., Katoh K., Ishii H. Sterol analysis of DMI-resistant and sensitive strains of Venturia inaequalis. Phytochemistry. 1996;41:1301–1308. doi: 10.1016/0031-9422(95)00787-3. [DOI] [Google Scholar]
- 14.Wakana D., Hosoe T., Itabashi T., Okada K., de Campos Takaki G., Yaguchi T., Fukushima K., Kawai K. New citrinin derivatives isolated from Penicillium citrinum. J. Nat. Med. 2006;60:279–284. doi: 10.1007/s11418-006-0001-2. [DOI] [Google Scholar]
- 15.Lu Z.Y., Lin Z.J., Wang W.L., Du L., Zhu T.J., Fang Y.C., Gu Q.Q., Zhu W.M. Citrinin dimers from the halotolerant fungus Penicillium citrinum B-57. J. Nat. Prod. 2008;71:543–546. doi: 10.1021/np0704708. [DOI] [PubMed] [Google Scholar]
- 16.Shiono Y., Shimanuki K., Hiramatsu F., Koseki T., Tetsuya M., Fujisawa N., Kimura K. Pyrrospirones A and B, apoptosis inducers in HL-60 cells, from an endophytic fungus, Neonectria ramulariae Wollenw KS-246. Bioorg. Med. Chem. Lett. 2008;18:6050–6053. doi: 10.1016/j.bmcl.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Oikawa H. Biosynthesis of structurally unique fungal metabolite GKK1032A2: indication of novel carbocyclic formation mechanism in polyketide biosynthesis. J. Org. Chem. 2003;68:3552–3557. doi: 10.1021/jo0267596. [DOI] [PubMed] [Google Scholar]
- 18.Yang X.W., Yang J., Xu G. Skeleton reassignment of type C polycyclic polyprenylated acylphloroglucinols. J. Nat. Prod. 2017;80:108–113. doi: 10.1021/acs.jnatprod.6b00754. [DOI] [PubMed] [Google Scholar]
- 19.Gu B.B., Wu W., Jiao F.R., Jiao W.H., Li L., Sun F., Wang S.P., Yang F., Lin H.W. Asperflotone, an 8(14→15)-abeo-ergostane from the sponge-derived fungus Aspergillus flocculosus 16D-1. J. Org. Chem. 2019;84:300–306. doi: 10.1021/acs.joc.8b02679. [DOI] [PubMed] [Google Scholar]
- 20.Cui H., Liu Y., Li J., Huang X., Yan T., Cao W., Liu H., Long Y., She Z. Diaporindenes A-D: Four unusual 2,3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018;83:11804–11813. doi: 10.1021/acs.joc.8b01738. [DOI] [PubMed] [Google Scholar]
- 21.Xin W.X., Ye X.W., Yu S.R., Lian X.Y., Zhang Z.Z. New capoamycin-type antibiotics and polyene acids from marine Streptomyces fradiae PTZ0025. Mar. Drugs. 2012;10:2388–2402. doi: 10.3390/md10112388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye X.W., Anjum K., Song T.F., Wang W.L., Yu S.R., Huang H.C., Lian X.Y., Zhang Z.Z. A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat. Prod. Res. 2016;30:1156–1161. doi: 10.1080/14786419.2015.1047775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.