Abstract
An excessive requirement for methionine (MET), termed MET dependence, appears to be a general metabolic defect in cancer and has been shown to be a very effective therapeutic target. MET restriction (MR) has inhibited the growth of all major cancer types by selectively arresting cancer cells in the late-S/G2 phase, when they also become highly sensitive to cytotoxic agents. Recombinant methioninase (rMETase) has been developed to effect MR. The present review describes the efficacy of rMETase on patient-derived orthotopic xenograft (PDOX) models of recalcitrant cancer, including the surprising result that rMETase administrated orally can be highly effective.
Keywords: recombinant methioninase, methionine dependence, nude mice, orthotopic implantation, patient-derived tumor
1. Introduction
1.1. Methionine (MET)
Methionine (MET) is an essential amino acid, which is absorbed in the small intestine. The absorbed methionine is used for protein synthesis and converted to S-adenosylmethionine (SAM), which plays an important role in DNA methylation and metabolic reactions. SAM is converted to S-adenosylhomocysteine (SAH) during the methylation of DNA, various proteins and other molecules (Figure 1) [1].
Figure 1.
Schema of methionine (MET) metabolism.
1.2. MET Dependence in Cancer
In 1959, Sugimura et al. observed that rat tumor growth was slowed by a MET-restricted (MR) diet [2]. In 1973, it was observed that L5178Y mouse leukemia cells in culture required very high levels of MET to proliferate [3]. Subsequently, most cancer cell lines were found to be MET dependent [4,5]. These cell lines were derived from various cancer types including liver, pancreatic ovarian, submaxillary, brain, lung, bladder, prostate, breast, kidney, cervical, colon, fibrosarcoma, osteosarcoma, rhabdomyosarcoma, leiomyosarcoma, neuroblastoma, glioblastoma and melanoma. Normal unestablished cell strains, thus far characterized, grow well in MET-depleted medium. The very frequent occurrence of MET dependence among these diverse cancer types suggested that MET dependence may be a general phenomenon in cancer and thus an important target for cancer treatment [4].
MET dependence is due not to a deficit in MET synthesis in cancer cells [6] but to elevated MET utilization for aberrant methylation reactions [7,8]. The overuse of MET by cancer cells is termed the Hoffman effect, analogous to the Warburg effect of overutilization of glucose by cancer cells [9]. The Hoffman effect is observed in the clinic in [11C] MET-postiron emission tomography (PET) imaging, which gives a stronger signal than [18F] fluorodeoxyglucose (FDG) PET, thus indicating that the Hoffman effect is more pronounced than the Warburg effect [10].
1.3. Recombinant Methioninase (rMETase)
The enzyme L-methionine-α-amino-γ-mercaptoethane lyase, termed methioninase (METase), was developed to lower the MET level in vivo. METase, was initially purified from Clostridium sporogene and catabolized MET to α-ketobutyrate, methanethiol and ammonia [11]. METase suppressed the Walker-256 sarcoma tumor growing in rats more effectively than a MET-free diet [12]. Later, a more stable METase was cloned and purified from Pseudomonas putida [13]. METase purified from P. putida inhibited the growth of Yoshida sarcoma and lung cancer cells without any overt toxicity, such as body weight loss [14]. Our laboratory cloned and over-expressed the P. putida METase gene in Escherichia coli, producing high yields of recombinant methioninase (rMETase) [15]. rMETase was reported to have a broad selective efficacy for many cancer cell lines [5].
1.4. The Patient-Derived Orthotopic Xenograft (PDOX) Mouse Model
The transplantation of patient-derived tumors to mouse orthotopic sites can replicate the clinical pattern of metastasis [16]. Our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI). PDOX models were established from patients with colon [17,18,19], stomach [20], pancreas [21,22,23,24,25,26], breast [27], ovarian [28], lung [29], cervical [30], skin (melanoma) [31,32,33,34,35], bone and soft tissue sarcoma [36,37,38,39,40,41,42].
2. Materials and Methods
2.1. Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA, USA), 4–6 weeks old, were used. The mice were housed in a barrier facility on a high-efficacy particulate arrestance (HEPA)-filtered rack under standard conditions of 12 hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. All mouse surgical procedures and imaging were performed with the animals anesthetized by subcutaneous injection of a ketamine mixture (0.02 mL solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). The response of animals during surgery was monitored to ensure adequate depth of anesthesia. The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation if they met the following humane endpoint criteria: Severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion or body temperature drop.
2.2. Surgical Orthotopic Implantation (SOI)
For the establishment of PDOX model, patient-derived tumor fragments (5 mm3) were initially implanted subcutaneously in nude mice. After several weeks, the subcutaneously-implanted tumors grew to more than 10 mm in diameter. The subcutaneously-grown tumors were then harvested and cut into small fragments (3 mm3). After nude mice were anesthetized with the ketamine solution described above, single tumor fragments were implanted orthotopically into each original site or organ to establish the PDOX model.
2.3. Recombinant Methioninase (rMETase) Production
Recombinant L-methionine α-deamino-γ-mercaptomethane lyase (recombinant methioninase, referred to as rMETase), EC 4.4.1.11, from Pseudomonas putida has been previously cloned and was produced in Escherichia coli (AntiCancer, Inc., San Diego, CA, USA) and purified as previously described [15].
2.4. Preparation and Administration of Salmonella typhimurium A1-R
GFP-expressing S. typhimurium A1-R bacteria (AntiCancer, Inc., San Diego, CA, USA) were grown overnight on LB medium (Fisher Sci., Hanover Park, IL, USA) and then diluted 1:10 in LB medium. The bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS [43,44,45].
3. Results and Discussion
3.1. Intraperitoneal Injection of rMETase in PDOX Models of Cancer
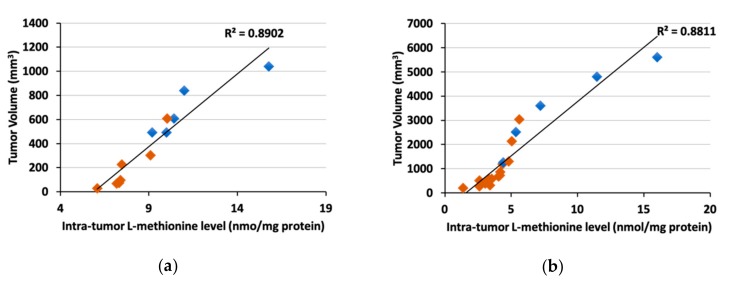
Initially, rMETase was administrated by intraperitoneal injection (i.p.-rMETase) in the PDOX model. i.p.-rMETase was absorbed into the blood circulation through the peritoneum and degraded MET in the blood directly. Kawaguchi et al. demonstrated that intra-tumoral MET levels highly correlated with tumor volume in both pancreatic cancer and melanoma PDOX models, indicating the high degree of MET dependence of the tumors [46]. Furthermore, tumors treated with i.p.-rMETase had a lower concentration of MET and were smaller in size than untreated controls (Figure 2). These results suggested that i.p.-rMETase decreases MET in the blood and suppresses the supply of MET to tumors, thereby inhibiting tumor growth.
Figure 2.
Correlation between tumor volume and methionine (MET) level in pancreatic cancer (a) and melanoma (b) patient-derived orthotopic xenograft (PDOX). Blue box: Untreated control, red box: Treated with recombinant methioninase (rMETase) [46].
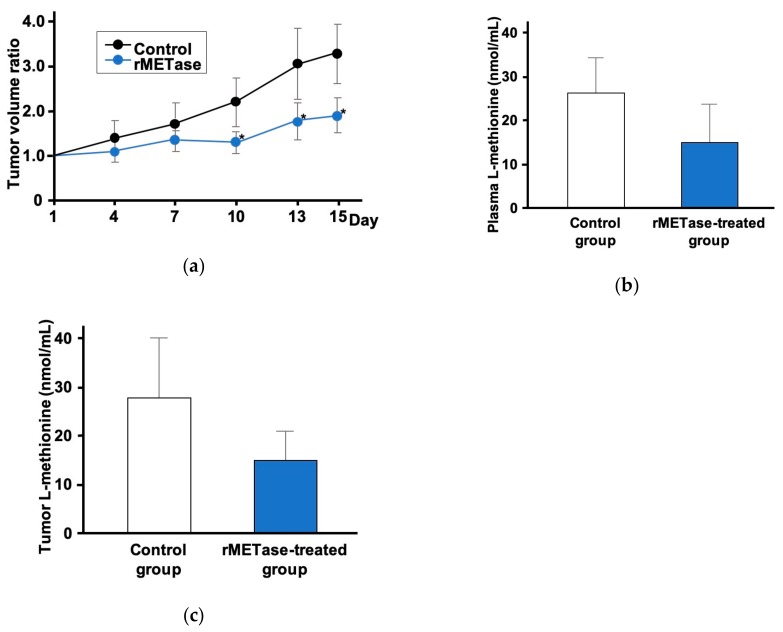
Our first experience with i.p.-rMETase on a PDOX model was conducted on Ewing’s sarcoma [41]. This study demonstrated that i.p.-rMETase could inhibit tumor growth (Figure 3). Based on this result, other PDOX tumor models were tested with rMETase and high efficacy was shown (Table 1).
Figure 3.
Intraperitoneal (i.p.) recombinant methioninase (i.p.-rMETase) for patient-derived orthotopic xenograft (PDOX). (a) Response of Ewing’s sarcoma patient-derived orthotopic xenograft (PDOX) to intraperitoneal injection (i.p.-rMETase). The plasma L-methionine level (b) and intra-tumoral L-methionine level (c) after i.p.-rMETase treatment. * p = <0.05, error bars show the standard deviation (SD) [41].
Table 1.
Recombinant methioninase (rMETase) for patient-derived orthotopic xenograft (PDOX).
| Cancer Type | Route | rMETase Combination | Reference | |
|---|---|---|---|---|
| Melanoma (BRAF mutant) |
i.p. | Alone | Arrest | [31] |
| + Temozolomide | Regress | |||
| Melanoma (BRAF mutant) |
Oral | Alone | Arrest | [47] |
| + i.p.-rMETase | Regress | |||
| Melanoma (BRAF wild) |
i.p. | Alone | Arrest | [48] |
| + Temozolomide | Arrest | |||
| Melanoma (BRAF wild) |
Oral | Alone | Arrest | [49] |
| + Temozolomide | Arrest | |||
| + S. typhimurium A1-R | Regress | |||
| Pancreatic cancer | i.p. | Alone | Arrest | [50] |
| + Gemcitabine | Regress | |||
| Pancreatic cancer | Oral | Alone | Arrest | [51] |
| + i.p.-rMETase | Regress | |||
| Osteosarcoma | i.p. | Alone | Arrest | [52] |
| + Cisplatinum | Arrest | |||
| + S. typhimurium A1-R | Arrest | |||
| + Cisplatinum+ S. typhimurium A1-R |
Arrest | |||
| Synovial sarcoma | i.p. | Alone | Arrest | [53] |
| + Doxorubicin | Arrest | |||
| Synovial sarcoma | Oral | Alone | Arrest | [54] |
| + Caffeine | Arrest | |||
| + Doxorubicin + Caffeine |
Regress | |||
| Liposarcoma | i.p. | Alone | Arrest | [55] |
| + Palbociclib | Regress | |||
| Spindle-cell sarcoma | i.p. | Alone | Arrest | [40] |
| Spindle-cell sarcoma | i.p. | Alone | Arrest | [56] |
| + Doxorubicin | Regress | |||
| Ewing’s sarcoma | i.p. | Alone | Arrest | [41] |
| Ewing’s sarcoma | Oral | Alone | Arrest | [42] |
| + S. typhimurium A1-R | Regress | |||
3.2. Oral administration of rMETase for PDOX
A very surprising result was recently observed that oral administration of rMETase (o-rMETase) was highly effective in a PDOX model. o-rMETase decreased plasma MET concentration and inhibited tumor growth to a greater extent than i.p.-rMETase in a melanoma PDOX (Figure 4) [47]. Subsequent studies showed that o-rMETase could significantly arrest tumor growth in pancreatic cancer PDOX [51]. These are the first reports on oral administration of rMETase. The studies showed that o-rMETase is effective on patient tumors in PDOX models. o-rMETase appears to restrict circulating and tumor MET by degrading MET in the gastrointestinal (GI) tract. Other PDOX studies also demonstrated the usefulness of o-rMETase (Table 1).
Figure 4.
The first report of oral administration of recombinant methioninase (o-rMETase) for melanoma patient-derived orthotopic xenograft (PDOX). (a) Comparison of treatment efficacy on oral administration of recombinant methioninase (o-rMETase) and intraperitoneal injection (i.p.-rMETase) for BRAF mutant melanoma PDOX. (b) Plasma methionine level treated after recombinant methioninase (rMETase). ** p < 0.01, * p < 0.05, error bars show the SD [47].
3.3. Combination of rMETase and Chemotherapy
In a very early study, we used MR in vitro to enhance the efficacy of doxorubicin (DOX) in a co-culture of cancer and normal cells. The MET-dependent cancer cells became blocked in the late S/G2 phase by MR. The addition of DOX during MR enhanced its activity as the cells were trapped in S/G2, where they are most sensitive to DOX [57]. In a subsequent in vivo study, using fluorescence ubiquitination-based cell cycle indicator (FUCCI)-expressing cancer cells—where color-coded genetic reporters indicate the phase of the cell cycle—rMETase was used to trap cells in S/G2 and enhanced the efficacy of the chemotherapy [58,59]. The growth arrest of MET-dependent cancer cells under MR resulted in a reduction in the percentage of mitotic cells in the cell population and the cancer cells were arrested in the S/G2 phases of the cell cycle under MR [58,59,60]. The S/G2 block by MR is responsible for the high efficacy of the combination of rMETase and chemotherapy.
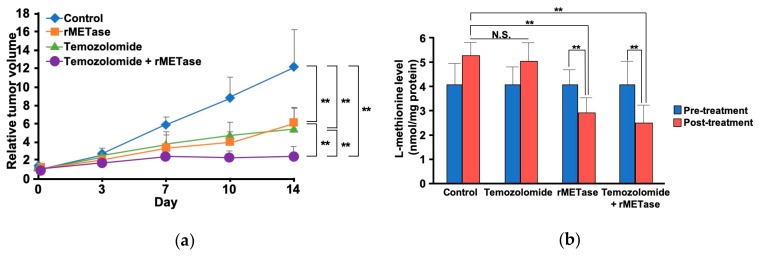
Kawaguchi et al. first reported the rMETase combination with chemotherapy on a melanoma PDOX [31]. Temozolomide (TEM), the first-line chemotherapy for advanced melanoma, and i.p.-rMETase had significantly better efficacy than either therapy alone on a BRAF-V600E mutant melanoma PDOX. The post-treatment L-MET levels in tumors treated with i.p.-rMETase alone, or along with TEM, were significantly decreased compared to untreated controls (Figure 5) [31].
Figure 5.
The first report of recombinant methioninase (rMETase) combined with chemotherapy on an orthotopic xenograft (PDOX) model. (a) Comparison of treatment. (b) Intra-tumoral methionine level after recombinant methioninase (rMETase). ** p < 0.01, error bars show the SD [31].
The effectiveness of the combination therapy of rMETase and chemotherapy was also shown in pancreatic cancer [50] and several types of sarcoma [52,53,54,55,56] in addition to BRAF-wild melanoma [48,49] (Table 1).
3.4. Combination Therapy of rMETase and Bacterial Therapy
Our laboratory developed Salmonella typhimurium A1-R (S. typhimurium A1-R) that is auxotrophic for Leu–Arg, which prevents it from mounting a continuous infection in normal tissues. S. typhimurium A1-R inhibited or eradicated primary and metastatic tumors as monotherapy in nude-mouse models of major cancers [61], including prostate [43,45], breast [62,63], lung [64,65], pancreatic [66,67,68,69,70], ovarian [71,72], stomach [73], cervical cancer [74], glioma [75,76], melanoma [77] as well as sarcoma [78,79,80,81,82,83], all of which are highly aggressive tumor models.
S. typhimurium A1-R decoyed cancer cells in tumors to cycle from the G0/G1 to S/G2/M phases. When the cancer cells were subsequently treated with rMETase, they were selectively trapped in S/G2. We showed using sequential treatment of tumors with S. typhimurium A1-R to decoy quiescent cancer cells to cycle and rMETase to selectively trap the decoyed cancer cells in the S/G2 phase, that subsequent chemotherapy could eradicate tumors in mouse models of human stomach cancer and a metastasis osteosarcoma PDOX model. These results demonstrated a new paradigm of “decoy, trap and shoot (kill)” chemotherapy [52].
Igarashi et al. first reported the i.p.-rMETase combination with S. typhimurium A1-R on an osteosarcoma cisplatinum-resistant lung metastasis PDOX model [52]. They showed that the combination of i.p.-rMETase and S. typhimurium A1-R could inhibit tumor growth significantly greater than either monotherapy on an osteosarcoma lung-metastasis PDOX. Another study reported that the combination of o-rMETase and S. typhimurium A1-R was also effective for a melanoma PDOX, as shown in Figure 6 [49]. These results showed that the decoy, trap and kill combination of S. typhimurium A1-R, rMETase and chemotherapy should be effective for chemo-resistant recalcitrant cancer.
Figure 6.
Combination therapy oral administration of recombinant methioninase (o-rMETase) and S. typhimurium A1-R. (a) Fluorescence imaging of S. typhimurium A1-R-GFP cultured from the melanoma patient-derived orthotopic xenograft (PDOX). (b) Comparison of treatment. ** p < 0.01, error bars show the SD. Obtained permission from [49].
4. Conclusions
Here we reviewed the usefulness of MET restriction (MR) therapy using rMETase on PDOX models. MET dependence may be the only known general metabolic defect in cancer. These results have important clinical implications.
Acknowledgments
This paper is dedicated to the memory of A.R. Moossa, Sun Lee and Li Jiaxi.
Author Contributions
Writing-Original Draft Preparation, K.K.; Major revisions, R.M.H.; Review, Q.H., S.L., Y.T., K.I., T.M., M.U.
Funding
This research received no external funding.
Conflicts of Interest
K.K., K.I., T.M. and R.M.H. are unsalaried associates of AntiCancer, Inc. Q.H., S.L. and Y.T. are or were employees of AntiCancer, Inc, who develop o-rMETase and use PDOX models for contract research. The authors declare no conflict of interest.
References
- 1.Zingg J.M., Jones P.A. Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997;18:869–882. doi: 10.1093/carcin/18.5.869. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura T., Birnbaum S.M., Winitz M., Greenstein J.P. Quantitative nutritional studies with water-soluble, chemically defined diets. VIII. The forced feeding of diets each lacking in one essential amino acid. Arch. Biochem. Biophys. 1959;81:448–455. doi: 10.1016/0003-9861(59)90225-5. [DOI] [PubMed] [Google Scholar]
- 3.Chello P.L., Bertino J.R. Dependence of 5-methyltetrahydrofolate utilization by L5178Y murine leukemia cells in vitro on the presence of hydroxycobalamin and transcobalamin II. Cancer Res. 1973;33:1898–1904. [PubMed] [Google Scholar]
- 4.Mecham J.O., Rowitch D., Wallace C.D., Stern P.H., Hoffman R.M. The metabolic defect of methionine dependence occurs frequently in human tumor cell lines. Biochem. Biophys. Res. Commun. 1983;117:429–434. doi: 10.1016/0006-291X(83)91218-4. [DOI] [PubMed] [Google Scholar]
- 5.Tan Y., Xu M., Hoffman R.M. Broad selective efficacy of recombinant methioninase and polyethylene glycol-modified recombinant methioninase on cancer cells In Vitro. Anticancer Res. 2010;30:1041–1046. [PubMed] [Google Scholar]
- 6.Hoffman R.M., Erbe R.W. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc. Natl. Acad. Sci. USA. 1976;73:1523–1527. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern P.H., Wallace C.D., Hoffman R.M. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J. Cell. Physiol. 1984;119:29–34. doi: 10.1002/jcp.1041190106. [DOI] [PubMed] [Google Scholar]
- 8.Judde J.G., Ellis M., Frost P. Biochemical analysis of the role of transmethylation in the methionine dependence of tumor cells. Cancer Res. 1989;49:4859–4865. [PubMed] [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman R.M. Is the Hoffman Effect for Methionine Overuse Analogous to the Warburg Effect for Glucose Overuse in Cancer? Methods Mol. Biol. 2019;1866:273–278. doi: 10.1007/978-1-4939-8796-2_21. [DOI] [PubMed] [Google Scholar]
- 11.Kreis W., Hession C. Isolation and purification of L-methionine-alpha-deamino-gamma-mercaptomethane-lyase (L-methioninase) from Clostridium sporogenes. Cancer Res. 1973;33:1862–1865. [PubMed] [Google Scholar]
- 12.Kreis W., Hession C. Biological effects of enzymatic deprivation of L-methionine in cell culture and an experimental tumor. Cancer Res. 1973;33:1866–1869. [PubMed] [Google Scholar]
- 13.Esaki N., Soda K. L-methionine gamma-lyase from Pseudomonas putida and Aeromonas. Methods Enzymol. 1987;143:459–465. doi: 10.1016/0076-6879(87)43081-4. [DOI] [PubMed] [Google Scholar]
- 14.Tan Y., Xu M., Guo H., Sun X., Kubota T., Hoffman R.M. Anticancer efficacy of methioninase in vivo. Anticancer Res. 1996;16:3931–3936. [PubMed] [Google Scholar]
- 15.Tan Y., Xu M., Tan X., Tan X., Wang X., Saikawa Y., Nagahama T., Sun X., Lenz M., Hoffman R.M. Overexpression and large-scale production of recombinant L-methionine-alpha-deamino-gamma-mercaptomethane-lyase for novel anticancer therapy. Protein Expr. Purif. 1997;9:233–245. doi: 10.1006/prep.1996.0700. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 17.Fu X.Y., Besterman J.M., Monosov A., Hoffman R.M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl. Acad. Sci. USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metildi C.A., Kaushal S., Luiken G.A., Talamini M.A., Hoffman R.M., Bouvet M. Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Surg. Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiroshima Y., Maawy A., Metildi C.A., Zhang Y., Uehara F., Miwa S., Yano S., Sato S., Murakami T., Momiyama M., et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J. Laparoendosc. Adv. Surg. Tech. 2014;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa T., Kubota T., Watanabe M., Kitajima M., Hoffman R.M. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int. J. Cancer. 1993;53:608–612. doi: 10.1002/ijc.2910530414. [DOI] [PubMed] [Google Scholar]
- 21.Hiroshima Y., Zhang Y., Murakami T., Maawy A., Miwa S., Yamamoto M., Yano S., Sato S., Momiyama M., Mori R., et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu X., Guadagni F., Hoffman R.M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl. Acad. Sci. USA. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiroshima Y., Maawy A., Zhang Y., Murakami T., Momiyama M., Mori R., Matsuyama R., Katz M.H., Fleming J.B., Chishima T., et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19-9-conjugated fluorophore. PLoS ONE. 2014;9:e114310. doi: 10.1371/journal.pone.0114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiroshima Y., Maawy A.A., Katz M.H., Fleming J.B., Bouvet M., Endo I., Hoffman R.M. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J. Surg. Oncol. 2015;111:311–315. doi: 10.1002/jso.23816. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi K., Igarashi K., Murakami T., Kiyuna T., Lwin T.M., Hwang H.K., Delong J.C., Clary B.M., Bouvet M., Unno M., et al. MEK inhibitors cobimetinib and trametinib, regressed a gemcitabine-resistant pancreatic-cancer patient-derived orthotopic xenograft (PDOX) Oncotarget. 2017;8:47490–47496. doi: 10.18632/oncotarget.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi K., Igarashi K., Miyake K., Lwin T.M., Miyake M., Kiyuna T., Hwang H.K., Murakami T., Delong J.C., Singh S.R., et al. MEK inhibitor trametinib in combination with gemcitabine regresses a patient-derived orthotopic xenograft (PDOX) pancreatic cancer nude mouse model. Tissue Cell. 2018;52:124–128. doi: 10.1016/j.tice.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Fu X., Le P., Hoffman R.M. A metastatic orthotopic-transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. [PubMed] [Google Scholar]
- 28.Fu X., Hoffman R.M. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. [PubMed] [Google Scholar]
- 29.Wang X., Fu X., Hoffman R.M. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int. J. Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 30.Hiroshima Y., Zhang Y., Zhang N., Maawy A., Mii S., Yamamoto M., Uehara F., Miwa S., Yano S., Murakami T., et al. Establishment of a patient-derived orthotopic Xenograft (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS ONE. 2015;10:e0117417. doi: 10.1371/journal.pone.0117417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi K., Igarashi K., Li S., Han Q., Tan Y., Kiyuna T., Miyake K., Murakami T., Chmielowski B., Nelson S.D., et al. Combination treatment with recombinant methioninase enables temozolomide to arrest a BRAF V600E melanoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2017;8:85516–85525. doi: 10.18632/oncotarget.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi K., Murakami T., Chmielowski B., Igarashi K., Kiyuna T., Unno M., Nelson S.D., Russell T.A., Dry S.M., Li Y., et al. Vemurafenib-resistant BRAF-V600E-mutated melanoma is regressed by MEK-targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. doi: 10.18632/oncotarget.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi K., Igarashi K., Murakami T., Chmielowski B., Kiyuna T., Zhao M., Zhang Y., Singh A., Unno M., Nelson S.D., et al. Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936. doi: 10.18632/oncotarget.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi K., Igarashi K., Murakami T., Zhao M., Zhang Y., Chmielowski B., Kiyuna T., Nelson S.D., Russell T.A., Dry S.M., et al. Tumor-targeting Salmonella typhimurium A1-R Sensitizes Melanoma with a BRAF-V600E Mutation to Vemurafenib in a Patient-derived Orthotopic Xenograft (PDOX) Nude Mouse Model. J. Cell. Biochem. 2017;118:2314–2319. doi: 10.1002/jcb.25886. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M., Zhao M., Hiroshima Y., Zhang Y., Shurell E., Eilber F.C., Bouvet M., Noda M., Hoffman R.M. Efficacy of Tumor-Targeting Salmonella A1-R on a Melanoma Patient-Derived Orthotopic Xenograft (PDOX) Nude-Mouse Model. PLoS ONE. 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igarashi K., Kawaguchi K., Kiyuna T., Murakami T., Miwa S., Nelson S.D., Dry S.M., Li Y., Singh A., Kimura H., et al. Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle. 2017;16:91–94. doi: 10.1080/15384101.2016.1252885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igarashi K., Kawaguchi K., Murakami T., Kiyuna T., Miyake K., Nelson S.D., Dry S.M., Li Y., Yanagawa J., Russell T.A., et al. Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle. 2017;16:1164–1170. doi: 10.1080/15384101.2017.1317417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi K., Igarashi K., Kiyuna T., Miyake K., Miyake M., Murakami T., Chmielowski B., Nelson S.D., Russell T.A., Dry S.M., et al. Individualized doxorubicin sensitivity testing of undifferentiated soft tissue sarcoma (USTS) in a patient-derived orthotopic xenograft (PDOX) model demonstrates large differences between patients. Cell Cycle. 2018;17:627–633. doi: 10.1080/15384101.2017.1421876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyake K., Murakami T., Kiyuna T., Igarashi K., Kawaguchi K., Li Y., Singh A.S., Dry S.M., Eckardt M.A., Hiroshima Y., et al. Eribulin regresses a doxorubicin-resistant Ewing’s sarcoma with a FUS-ERG fusion and CDKN2A-deletion in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Cell. Biochem. 2018;119:967–972. doi: 10.1002/jcb.26263. [DOI] [PubMed] [Google Scholar]
- 40.Igarashi K., Li S., Han Q., Tan Y., Kawaguchi K., Murakami T., Kiyuna T., Miyake K., Li Y., Nelson S.D., et al. Growth of doxorubicin-resistant undifferentiated spindle-cell sarcoma PDOX is arrested by metabolic targeting with recombinant methioninase. J. Cell. Biochem. 2018;119:3537–3544. doi: 10.1002/jcb.26527. [DOI] [PubMed] [Google Scholar]
- 41.Murakami T., Li S., Han Q., Tan Y., Kiyuna T., Igarashi K., Kawaguchi K., Hwang H.K., Miyake K., Singh A.S., et al. Recombinant methioninase effectively targets a Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2017;8:35630–35638. doi: 10.18632/oncotarget.15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake K., Kiyuna T., Li S., Han Q., Tan Y., Zhao M., Oshiro H., Kawaguchi K., Higuchi T., Zhang Z., et al. Combining Tumor-Selective Bacterial Therapy with Salmonella typhimurium A1-R and Cancer Metabolism Targeting with Oral Recombinant Methioninase Regressed an Ewing’s Sarcoma in a Patient-Derived Orthotopic Xenograft Model. Chemotherapy. 2019;63:278–283. doi: 10.1159/000495574. [DOI] [PubMed] [Google Scholar]
- 43.Zhao M., Geller J., Ma H., Yang M., Penman S., Hoffman R.M. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc. Natl. Acad. Sci. USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao M., Yang M., Ma H., Li X., Tan X., Li S., Yang Z., Hoffman R.M. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Tome Y., Suetsugu A., Zhang L., Zhang N., Hoffman R.M., Zhao M. Determination of the optimal route of administration of Salmonella typhimurium A1-R to target breast cancer in nude mice. Anticancer Res. 2012;32:2501–2508. [PubMed] [Google Scholar]
- 46.Kawaguchi K., Han Q., Li S., Tan Y., Igarashi K., Miyake K., Kiyuna T., Miyake M., Chemielwski B., Nelson S.D., et al. Intra-tumor L-methionine level highly correlates with tumor size in both pancreatic cancer and melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse models. Oncotarget. 2018;9:11119–11125. doi: 10.18632/oncotarget.24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igarashi K., Kawaguchi K., Kiyuna T., Miyake K., Miyaki M., Yamamoto N., Hayashi K., Kimura H., Miwa S., Higuchi T., et al. Metabolic targeting with recombinant methioninase combined with palbociclib regresses a doxorubicin-resistant dedifferentiated liposarcoma. Biochem. Biophys. Res. Commun. 2018;506:912–917. doi: 10.1016/j.bbrc.2018.10.119. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi K., Igarashi K., Li S., Han Q., Tan Y., Miyake K., Kiyuna T., Miyake M., Murakami T., Chmielowski B., et al. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2018;9:915–923. doi: 10.18632/oncotarget.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman R.M., Jacobsen S.J. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proc. Natl. Acad. Sci. USA. 1980;77:7306–7310. doi: 10.1073/pnas.77.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawaguchi K., Han Q., Li S., Tan Y., Igarashi K., Kiyuna T., Miyake K., Miyake M., Chmielowski B., Nelson S.D., et al. Targeting methionine with oral recombinant methioninase (o-rMETase) arrests a patient-derived orthotopic xenograft (PDOX) model of BRAF-V600E mutant melanoma: Implications for chronic clinical cancer therapy and prevention. Cell Cycle. 2018;17:356–361. doi: 10.1080/15384101.2017.1405195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Igarashi K., Kawaguchi K., Li S., Han Q., Tan Y., Murakami T., Kiyuna T., Miyake K., Miyake M., Singh A.S., et al. Recombinant methioninase in combination with doxorubicin (DOX) overcomes first-line DOX resistance in a patient-derived orthotopic xenograft nude-mouse model of undifferentiated spindle-cell sarcoma. Cancer Lett. 2018;417:168–173. doi: 10.1016/j.canlet.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 52.Igarashi K., Kawaguchi K., Kiyuna T., Miyake K., Miyake M., Li S., Han Q., Tan Y., Zhao M., Li Y., et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: Decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle. 2018;17:801–809. doi: 10.1080/15384101.2018.1431596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Igarashi K., Kawaguchi K., Li S., Han Q., Tan Y., Gainor E., Kiyuna T., Miyake K., Miyake M., Higuchi T., et al. Recombinant methioninase combined with doxorubicin (DOX) regresses a DOX-resistant synovial sarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2018;9:19263–19272. doi: 10.18632/oncotarget.24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higuchi T., Kawaguchi K., Miyake K., Han Q., Tan Y., Oshiro H., Sugisawa N., Zhang Z., Razmjooei S., Yamamoto N., et al. Oral Recombinant Methioninase Combined with Caffeine and Doxorubicin Induced Regression of a Doxorubicin-resistant Synovial Sarcoma in a PDOX Mouse Model. Anticancer Res. 2018;38:5639–5644. doi: 10.21873/anticanres.12899. [DOI] [PubMed] [Google Scholar]
- 55.Kawaguchi K., Miyake K., Han Q., Li S., Tan Y., Igarashi K., Lwin T.M., Higuchi T., Kiyuna T., Miyake M., et al. Targeting altered cancer methionine metabolism with recombinant methioninase (rMETase) overcomes partial gemcitabine-resistance and regresses a patient-derived orthotopic xenograft (PDOX) nude mouse model of pancreatic cancer. Cell Cycle. 2018;17:1–23. doi: 10.1080/15384101.2018.1445907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawaguchi K., Miyake K., Han Q., Li S., Tan Y., Igarashi K., Kiyuna T., Miyake M., Higuchi T., Oshiro H., et al. Oral recombinant methioninase (o-rMETase) is superior to injectable rMETase and overcomes acquired gemcitabine resistance in pancreatic cancer. Cancer Lett. 2018;432:251–259. doi: 10.1016/j.canlet.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Stern P.H., Hoffman R.M. Enhanced in vitro selective toxicity of chemotherapeutic agents for human cancer cells based on a metabolic defect. J. Natl. Cancer Inst. 1986;76:629–639. doi: 10.1093/jnci/76.4.629. [DOI] [PubMed] [Google Scholar]
- 58.Yano S., Li S., Han Q., Tan Y., Bouvet M., Fujiwara T., Hoffman R.M. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensitivity. Oncotarget. 2014;5:8729–8736. doi: 10.18632/oncotarget.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yano S., Takehara K., Zhao M., Tan Y., Han Q., Li S., Bouvet M., Fujiwara T., Hoffman R.M. Tumor-specific cell-cycle decoy by Salmonella typhimurium A1-R combined with tumor-selective cell-cycle trap by methioninase overcome tumor intrinsic chemoresistance as visualized by FUCCI imaging. Cell Cycle. 2016;15:1715–1723. doi: 10.1080/15384101.2016.1181240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawaguchi K., Higuchi T., Li S., Han Q., Tan Y., Igarashi K., Zhao M., Miyake K., Kiyuna T., Miyake M., et al. Combination therapy of tumor-targeting Salmonella typhimurium A1-R and oral recombinant methioninase regresses a BRAF-V600E-negative melanoma. Biochem Biophys. Res. Commun. 2018;503:3086–3092. doi: 10.1016/j.bbrc.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman R.M. Bacterial Therapy of Cancer. Methods in Molecular Biology. Volume 1409. Humana Press; New York, NY, USA: 2016. Future of Bacterial Therapy of Cancer; pp. 177–184. [DOI] [PubMed] [Google Scholar]
- 62.Zhao M., Yang M., Li X.M., Jiang P., Baranov E., Li S., Xu M., Penman S., Hoffman R.M. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc. Natl. Acad. Sci. USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y., Miwa S., Zhang N., Hoffman R.M., Zhao M. Tumor-targeting Salmonella typhimurium A1-R arrests growth of breast-cancer brain metastasis. Oncotarget. 2015;6:2615–2622. doi: 10.18632/oncotarget.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchugonova A., Zhao M., Zhang Y., Weinigel M., Konig K., Hoffman R.M. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res. 2012;32:4331–4337. [PubMed] [Google Scholar]
- 65.Liu F., Zhang L., Hoffman R.M., Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle. 2010;9:4518–4524. doi: 10.4161/cc.9.22.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiroshima Y., Zhao M., Zhang Y., Maawy A., Hassanein M.K., Uehara F., Miwa S., Yano S., Momiyama M., Suetsugu A., et al. Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle. 2013;12:2774–2780. doi: 10.4161/cc.25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiroshima Y., Zhao M., Maawy A., Zhang Y., Katz M.H., Fleming J.B., Uehara F., Miwa S., Yano S., Momiyama M., et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) J. Cell. Biochem. 2014;115:1254–1261. doi: 10.1002/jcb.24769. [DOI] [PubMed] [Google Scholar]
- 68.Nagakura C., Hayashi K., Zhao M., Yamauchi K., Yamamoto N., Tsuchiya H., Tomita K., Bouvet M., Hoffman R.M. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 69.Yam C., Zhao M., Hayashi K., Ma H., Kishimoto H., McElroy M., Bouvet M., Hoffman R.M. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J. Surg. Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawaguchi K., Miyake K., Zhao M., Kiyuna T., Igarashi K., Miyake M., Higuchi T., Oshiro H., Bouvet M., Unno M., et al. Tumor targeting Salmonella typhimurium A1-R in combination with gemcitabine (GEM) regresses partially GEM-resistant pancreatic cancer patient-derived orthotopic xenograft (PDOX) nude mouse models. Cell Cycle. 2018;17:2019–2026. doi: 10.1080/15384101.2018.1480223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumoto Y., Miwa S., Zhang Y., Hiroshima Y., Yano S., Uehara F., Yamamoto M., Toneri M., Bouvet M., Matsubara H., et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J. Cell. Biochem. 2014;115:1996–2003. doi: 10.1002/jcb.24871. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto Y., Miwa S., Zhang Y., Zhao M., Yano S., Uehara F., Yamamoto M., Hiroshima Y., Toneri M., Bouvet M., et al. Intraperitoneal administration of tumor-targeting Salmonella typhimurium A1-R inhibits disseminated human ovarian cancer and extends survival in nude mice. Oncotarget. 2015;6:11369–11377. doi: 10.18632/oncotarget.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yano S., Zhang Y., Zhao M., Hiroshima Y., Miwa S., Uehara F., Kishimoto H., Tazawa H., Bouvet M., Fujiwara T., et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle. 2014;13:3958–3963. doi: 10.4161/15384101.2014.964115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hiroshima Y., Zhang Y., Zhao M., Zhang N., Murakami T., Maawy A., Mii S., Uehara F., Yamamoto M., Miwa S., et al. Tumor-Targeting Salmonella typhimurium A1-R in Combination with Trastuzumab Eradicates HER-2-Positive Cervical Cancer Cells in Patient-Derived Mouse Models. PLoS ONE. 2015;10:e0120358. doi: 10.1371/journal.pone.0120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Momiyama M., Zhao M., Kimura H., Tran B., Chishima T., Bouvet M., Endo I., Hoffman R.M. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012;11:628–632. doi: 10.4161/cc.11.3.19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kimura H., Zhang L., Zhao M., Hayashi K., Tsuchiya H., Tomita K., Bouvet M., Wessels J., Hoffman R.M. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawaguchi K., Igarashi K., Murakami T., Kiyuna T., Zhao M., Zhang Y., Nelson S.D., Russell T.A., Dry S.M., Singh A.S., et al. Salmonella typhimurium A1-R targeting of a chemotherapy-resistant BRAF-V600E melanoma in a patient-derived orthotopic xenograft (PDOX) model is enhanced in combination with either vemurafenib or temozolomide. Cell Cycle. 2017;16:1288–1294. doi: 10.1080/15384101.2017.1314420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murakami T., DeLong J., Eilber F.C., Zhao M., Zhang Y., Zhang N., Singh A., Russell T., Deng S., Reynoso J., et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:12783–12790. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiroshima Y., Zhao M., Zhang Y., Zhang N., Maawy A., Murakami T., Mii S., Uehara F., Yamamoto M., Miwa S., et al. Tumor-Targeting Salmonella typhimurium A1-R Arrests a Chemo-Resistant Patient Soft-Tissue Sarcoma in Nude Mice. PLoS ONE. 2015;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Igarashi K., Kawaguchi K., Kiyuna T., Miyake K., Miyake M., Singh A.S., Eckardt M.A., Nelson S.D., Russell T.A., Dry S.M., et al. Tumor-targeting Salmonella typhimurium A1-R is a highly effective general therapeutic for undifferentiated soft tissue sarcoma patient-derived orthotopic xenograft nude-mouse models. Biochem. Biophys. Res. Commun. 2018;497:1055–1061. doi: 10.1016/j.bbrc.2018.02.174. [DOI] [PubMed] [Google Scholar]
- 81.Hayashi K., Zhao M., Yamauchi K., Yamamoto N., Tsuchiya H., Tomita K., Hoffman R.M. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J. Cell. Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayashi K., Zhao M., Yamauchi K., Yamamoto N., Tsuchiya H., Tomita K., Kishimoto H., Bouvet M., Hoffman R.M. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 83.Miwa S., Zhang Y., Baek K.E., Uehara F., Yano S., Yamamoto M., Hiroshima Y., Matsumoto Y., Kimura H., Hayashi K., et al. Inhibition of spontaneous and experimental lung metastasis of soft-tissue sarcoma by tumor-targeting Salmonella typhimurium A1-R. Oncotarget. 2014;5:12849–12861. doi: 10.18632/oncotarget.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]