Abstract
Rare actinomycetes are prolific in the marine environment; however, knowledge about their diversity, distribution and biochemistry is limited. Marine rare actinomycetes represent a rather untapped source of chemically diverse secondary metabolites and novel bioactive compounds. In this review, we aim to summarize the present knowledge on the isolation, diversity, distribution and natural product discovery of marine rare actinomycetes reported from mid-2013 to 2017. A total of 97 new species, representing 9 novel genera and belonging to 27 families of marine rare actinomycetes have been reported, with the highest numbers of novel isolates from the families Pseudonocardiaceae, Demequinaceae, Micromonosporaceae and Nocardioidaceae. Additionally, this study reviewed 167 new bioactive compounds produced by 58 different rare actinomycete species representing 24 genera. Most of the compounds produced by the marine rare actinomycetes present antibacterial, antifungal, antiparasitic, anticancer or antimalarial activities. The highest numbers of natural products were derived from the genera Nocardiopsis, Micromonospora, Salinispora and Pseudonocardia. Members of the genus Micromonospora were revealed to be the richest source of chemically diverse and unique bioactive natural products.
Keywords: rare actinomycetes, marine actinobacteria, cultivation, natural products, bioactive compounds
1. Introduction
Emerging infectious diseases and multidrug-resistant human pathogens are becoming a major threat to global health [1]. Therefore, there is an urgent need for new antibiotics to fight evolving bacterial infections. Despite the use of large synthetic combinatorial libraries of molecules to develop novel drugs, natural products and microbial metabolites, in particular, are a predominant source of bioactive scaffolds that represent the foundation for the development of life-saving antibiotics [2]. Nature encompasses millions of prokaryotes and eukaryotes with particularly high diversity in oceans and rainforests. However, so far only a small fraction (approximately 250,000–300,000 living species) of at least 1.5 million fungi, 0.5 million plant species and 1011–1012 microbial species on Earth have been documented [3,4]. Moreover, even the known species have only been explored for bioactivity or for natural product discovery up to a limited extent. Therefore, natural resources are virtually unlimited for natural product discovery. The phylum Actinobacteria represents one of the largest phyla among the 30 major phyla currently recognized within the domain Bacteria. There are 6 classes, 18 orders, 14 suborders, 63 families and 374 genera recorded in this phylum until October 2016 (http://www.bacterio.net/-classifphyla.html#actinobacteria). In this review paper the term “actinomycetes” (http://www.bacterio.net/-classifphyla.html#actinobacteria) will be used to refer the members of the order Actinomycetales of the phylum Actinobacteria. The members of actinomycetes have been characterized as the most important group of microorganisms in the field of biotechnology, as producers of bioactive secondary metabolites with medical, industrial and agricultural applications [5]. However, until now, only less than 1% of the actinomycetes have been identified, investigated and documented [3]. Out of 500,000 natural compounds reported worldwide from biological sources, approximately 70,000 are microbially-derived compounds (both from bacteria and fungi), of which 29% is derived from actinomycetes. Approximately 60% of antibiotics applied, were isolated from actinomycetes between 1950 and 1970, exclusively from the genus Streptomyces [3]. However, in more recent history, high replication of discovery of compounds has been reported from Streptomyces species, which diverted the attention to non-Streptomyces actinomycetes and a noteworthy renaissance in antibiotics development from microorganisms has come with the exploration of previously poorly assessed microorganisms from underexplored environments. The unexplored and underexplored environments including marine ecosystems are promising sources of rare actinomycetes that are believed to be rich sources of interestingly new compounds [6,7]. ‘Rare actinomycetes’ are defined as the actinomycete strains less frequently isolated than that of the ‘commonly’ isolated Streptomyces spp., even though they may not actually be rare in the environment.
Oceans occupy 71% of the Earth’s surface holding 97% of the planet’s water and nearly 87% of life with essentially untouched fauna and flora [3] and are a great source for undiscovered organisms including microorganisms and novel natural products. Marine-derived rare actinomycetes are reported to be a potentially rich source of diverse chemicals, structurally unique secondary metabolites and novel therapeutic compounds [2,6]. Only 11 rare actinomycetes genera had been reported by 1970, followed by 100 genera by 2005 and 220 genera by 2010 [7]. High-throughput metagenome sequencing methods have expanded our knowledge and revealed the presence of many novel actinomycetes that were not previously detected in cultivation studies [5,8,9]. The retrieval of rare actinomycetes in conventional cultivation experiments is generally lower than that of the streptomycete strains [5]. However, recent understanding of marine actinomycetes’ physiological, chemical, and structural features has enabled the design of selective isolation media [5]. A total of 13,700 bioactive metabolites were reported from actinomycetes up to 2010, of which 10,400 were derived from streptomycetes and 3300 from rare actinomycete strains [3]. In 1974, only 125 active metabolites had been isolated from rare actinomycetes, increasing to 361, 745, 1276, 2250, 2500 bioactive metabolites by 1980, 1984, 1988, 2005 and 2010, respectively [7]. In our previous review, we summarized the novel families, novel genera, and new species of rare actinomycetes from marine habitats including bioactive compounds reported from 2007 to mid-2013 [7]. The goal of this present review is to summarize new species of marine rare actinomycetes, and the bioactive compounds discovered between mid-2013 and 2017 and discuss their chemical diversity and biotechnological potential.
2. Isolation Methods for Marine Rare Actinomycetes
Members of the phylum Actinobacteria adapt well to and successfully colonize different extreme environments including the deep sea [10] and genera of this phylum exhibit huge diversity in terms of their morphology, physiology, and metabolic capabilities [5]. Marine rare actinomycetes generally require special growth conditions compared to terrestrial actinomycete species [11,12,13,14]. Notably, it has been observed that a large number of bacterial cells in under/unexplored environments are viable but not culturable (VBNC), as approximately 1% of bacterial cells can form colonies on isolation media by conventional methods [15]. Therefore, high throughput molecular techniques, including metagenomics, are increasingly favored to investigate microbial communities in the environment [16] for which culture-based approaches have been rather unsuccessful up to now. Concurrently, knowledge of functional characteristics of actinomycetes based on cultivation-independent studies, has led to improved strategies with respect to growth conditions and cultivation media to recover previously unculturable actinomycetes [5,17,18,19,20,21].
2.1. Basic Approaches for Isolation Media for Marine Rare Actinomycetes
Targeting unknown rare actinomycetes for isolation requires knowledge and experience of actinomycetes taxonomy, physiology and environmental factors, such as pH, cultivation temperature, oxygen, nutrient requirements etc. [22]. Sodium, is one of the most important medium components for growth of marine microorganisms including marine actinomycetes such as Salinispora spp. and therfore growth media should generally have osmotic values similar to seawater [13]. Besides, different carbon (soluble starch, glucose, dextrose, maltose, trehalose, mannitol, raffinose, fucose, chitin, glycerol and oatmeal) and combined carbon-nitrogen sources (peptone, yeast extract, casein, malt extract, meat extract, beef extract and tryptone) have been supplemented in isolation media for successful isolation of marine rare actinomycete taxa [23,24,25,26,27]. In addition, researchers have added sediment extracts, sponge extracts and natural seawater alone or as a supplement to mimic natural environmental conditions [12,28,29,30,31,32,33,34]. In general, low-nutrient media are more efficient than nutrient-rich media for isolation of marine rare actinomycetes [13,28,35]. Generally, some basic approaches may be followed for isolation of marine rare actinomycetes: 1, Three to five different isolation media with various components should be employed for any target genus of actinomycetes [9,11,28]; 2, The isolation media must meet the requirements of the target actinomycetes and at the same time should limit the growth of unwanted microbes [22,28]; 3, Growth inhibitors, in the form of antibiotics or chemicals, should be added into isolation media to inhibit or restrict the growth of Gram-negative bacteria and fungi [11,28]; 4, The medium should be so designed that it mimicks the microbe’s natural environmental conditions [12,28,29]; 5, The medium should also suppress the growth of fast-growing and common streptomycete strains [22].
2.2. Pretreatment of Marine Samples
Marine samples, particularly sediments used for the isolation of rare actinomycetes, may be treated prior to isolation to remove common terrestrial actinomycetes and unwanted microorganisms to reduce replication of isolation. Commonly used pre-treatment methods for the isolation of rare actinomycetes from marine samples generally include dilution and mixing with sterile natural seawater [25,36,37], artificial seawater [38,39,40], deionized/distilled water supplemented with NaCl [41,42], multi-salts [24,26,40], vitamin B mixtures [43], one-quarter Ringer’s solution [44] and saline solution [45] before transferring the inoculum to Petri dishes [44]. A variety of pre-treatment methods for selectively isolating actinomycetes has been applied (Table 1). However, the drying of the environmental sample using laminar air flow, dilution with seawater or saline prior to sample heating are most frequently employed pre-treatments (Table 1). Actinomycetes spores are generally resistant to desiccation and heating and can thus be used to select against other Gram positive bacteria [46]. Further, actinomycetes spores are resistant to a wide range of chemicals, such as benzethonium chloride, chlorhexidine gluconate, phenol, sodium dodecyl sulfate, and different antibiotics. These chemicals have been used to selectively isolate actinomycete taxa. Treatment with these chemicals for 30 min. can kill or inhibit aerobic Gram negative bacteria, endospore-forming bacilli and pseudomonads, thus increasing the chance of selectively isolating actinomycetes, and reduce other types of bacteria [22]. Additionally, ultrasonic waves can release actinomycetes propagules from sediment particles into suspension, thus also increasing the number of Actinobacterial strains and reducing unwanted bacteria [47].
Table 1.
Pre-treatment methods for the selective isolation of marine rare actinomycetes.
| Pre-treatment | Marine Source | Isolation Medium | Incubation Temperature/Time | Target Rare Genera | Ref. |
|---|---|---|---|---|---|
| Heat | |||||
| Incubation in water bath at 50 °C for 60 min | WS | Starch-casein agar + 10 µg nalidixic acid, 25 µg nystatin and 10 µg cycloheximide | 24 °C for 28 days | Micromonospora | [45] |
| WS | M1 agar + 75 µg cycloheximide | 20–24 °C for 14–28 days | Micromonospora | [49] | |
| 50 °C for 15 min | WS | Starch-casein agar + 10 µg nystatin and 25 µg cycloheximide | 28 °C for 7 days | Monashia, Microbacterium and Sinomonas | [23,50,51] |
| 55 °C for 20 min | WS | Glucose peptone tryptone agar + 50 mg nystatin, 50 mg cycloheximide, 25 mg novobiocin and 20 mg nalidixic acid | 28 °C for 21 days | Micromonospora | [52] |
| Incubation in water bath at 55 °C for 6 min | DS | M1–M5 agar + 100 µg cycloheximide and 5 µg rifampin | 25–28 °C for 2–6 weeks | Micromonospora and Salinispora | [11,53] |
| Incubation in water bath at 60 °C for 10 min | DS | M1–M12 agar + 100 µg cycloheximide and 50 µg nystatin | 28 °C for 3 months | Micromonospora and Salinispora | [28] |
| Speedvac 30 °C, 16 h; 120 °C, 60 min | DS | Different selective media + cycloheximide (50 μg/mL), nystatin (75 μg/mL) and nalidixic acid (30 μg/mL) | 20 °C for 2–6 weeks | Rare actinomycetes | [54] |
| 41 °C for 10, 30 and 60 days | DS | Different selective media | 28 °C for 2–3 weeks | Streptoverticillium, Catellatospora, Nocardia and Actinopolyspora | [55] |
| 70 °C for 15 min | WS | Different selective media | 25 °C for 4 weeks | Micromonospora, Microbispora, Actinoplanes and Actinomadura | [56] |
| 55 °C for 15 min | DS | Asparagine-glucose agar medium + nalidixic acid (25 μg/mL) and secnidazole (25 μg/mL) | 25 °C for 2 weeks | Pseudonocardia | [57] |
| 45, 55 or 65 °C for 30 min | WS | ISP-3 and ISP-4 + cycloheximide (50 μg/mL), nystatin (50 μg/mL), and nalidixic acid (20 μg/mL) | 27 °C for 3 weeks | Micromonospora | [58] |
| 60 °C for 6 min | WS | M1 medium and Glucose-yeast extract medium + nystatin (50 μg/mL), and nalidixic acid (10 μg/mL) | 25 °C for 6 weeks | Nocardia, Nonomuraea, Rhodococcus, Saccharopolyspora and Gordonia | [59] |
| Physical | |||||
| Dry in laminar air flow hood; Stamping | WS/DS | M1–M12 agar + 100 µg cycloheximide and 50 µg nystatin | 28 °C for 3 months | Micromonospora and Salinispora | [28,53] |
| Mechanic | |||||
| Shake with glass beads for 30 s and settled for 5 min | WS | Different selective media + cycloheximide (50 μg/mL), nystatin (75 μg/mL) and nalidixic acid (30 μg/mL) | 20 °C for 6 weeks | Rare actinomycetes | [54] |
| Chemical/+ Heat | |||||
| 1.5% phenol + 30 min at 30 °C | DS | Different selective media + cycloheximide (50 μg/mL), nystatin (75 μg/mL) and nalidixic acid (30 μg/mL) | 20 °C for 2–6 weeks | Micromonospora | [4] |
| 0.02% benzethonium chloride + 30 min at 30 °C | DS | Different selective media + cycloheximide (50 μg/mL), nystatin (75 μg/mL) and nalidixic acid (30 μg/mL) | 20 °C for 2–6 weeks | Rare actinomycetes | [54] |
| 0.05% SDS and 6% yeast extract (40 °C, 200 rpm, 30 min) | DS | Different selective media + cycloheximide (25–100 μg/mL) and nystatin (25–50 μg/mL) | 28 °C for 1–12 weeks | Actinomadura, Micromonospora, Nocardia, Nonomuraea, Rhodococcus and Verrucosispora | [60] |
| 1.5% phenol | DS | Different selective media + cycloheximide (50 μg/mL), nystatin (75 μg/mL) and nalidixic acid (30 μg/mL) | 20 °C for 2–6 weeks | Rare actinomycetes | [54] |
| Chloramine-T | DS | Different selective media + cycloheximide (25–100 μg/mL) and nystatin (25–50 μg/mL) | 28 °C for 1–12 weeks | Actinomadura, Micromonospora, Nocardia, Nonomuraea, Rhodococcus, Streptomyces and Verrucosispora | [60] |
| Centrifugation | |||||
| Differential centrifugation | WS | Selective media | 28 °C for 12 weeks | Micromonospora, Rhodococcus and Streptomyces | [30] |
| Freezing | |||||
| Freeze (−20 °C, 24 h), thawed, dilution | WS | M1-M12 agar + 100 µg cycloheximide and 50 µg nystatin | 28 °C for 3 months | Micromonospora and Salinispora | [28] |
| Freeze at −18 °C | WS | Different selective media + nystatin (50 μg/mL) and nalidixic acid (10 μg/mL) | 28 °C for 2–3 weeks | Nocardiopsis, Nocardia and Streptosporangium | [61] |
| Radiation | |||||
| UV irradiation for 30 s (distance 20 cm, 254 nm, 15 W) | WS | Different selective media + nystatin (50 μg/mL) and nalidixic acid (10 μg/mL) | 28 °C for 2–3 weeks | Nocardiopsis, Nocardia and Pseudonocardia | [61] |
| Superhigh frequency radiation inmicrowave oven for 45 s (2460 MHz, 80 W) | WS | Different selective media + nystatin (50 μg/mL) and nalidixic acid (10 μg/mL) | 28 °C for 2–3 weeks | Streptosporangium and Rhodococcus | [61] |
| Extremely high frequency radiation (1 kHz within wavelength band of 8–11.5 mm) | WS | Different selective media + nystatin (50 μg/mL) and nalidixic acid (10 μg/mL) | 28 °C for 2–3 weeks | Nocardiopsis, Nocardia and Streptosporangium | [61] |
WS: Wet Sediment; DS: Dried Sediment.
3. Marine Habitats: The Largest Reservoir for Rare Actinomycetes
The world’s oceans constitute more than 90% of the inhabitable space on the planet and it is the largest reservoir of life on Earth. Approximately 80% of all life on Earth lives in the ocean and the oceans harbour 32 out of 33 known animal phyla, of which 15 are exclusively marine [48].
Marine habitats are also a rich source of diverse and largely uncharacterized microbial communities including actinomycetes [62]. This habitat shows extreme variations in ecological pressure, including competition for space, predation, available nutrients, light, oxygen concentration and pressure. Marine organisms including actinomycetes have developed a diverse range of secondary metabolites with unique structural elements to ensure their survival in these habitats [63]. Diverse new rare species including novel genera and novel families of actinomycetes have been isolated from marine habitats, such as coastal, tidal and deep-sea sediments, marine organisms (sponges, corals and ascidians), seawater and also mangrove forests [7]. Approximately 220 genera of rare actinomycetes were reported from marine sources until 2010 [64] and in the following sections we summarize new rare actinomycete isolates from these habitats since then [7]. For this review we’ve applied a conservative threshold on labelling a species as “novel” when sharing less than 97% similarity of the 16S rRNA gene to known species [65,66,67,68,69,70]. For the labelling of genera and families as “novel” we followed Silva taxonomy [71].
3.1. Rare Actinomycetes from Marine Sediments, Seawater, Eukaryotic Hosts and Mangroves
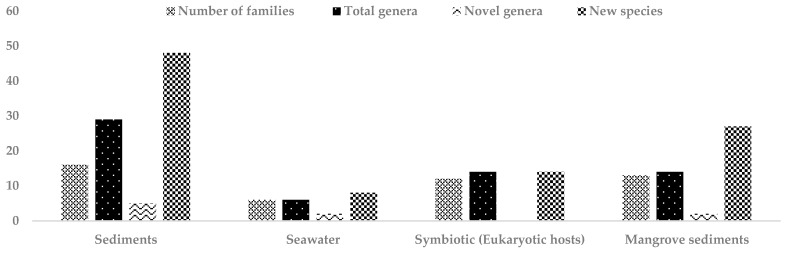
Approximately 83% of marine sediments are more than 1000 m below sea level, so most marine sediments are located in a cold, lightless, high pressure habitat where food is supplied from distant surface waters [72]. Deep-sea environments are divided into three zones: the bathyal (depth range between 200 and 2000 m), the abyssal (depth from 2000 to 6000 m) and the hadal (depth below 6000 m) [73]. Especially the abyssal and hadal zones are largely unexplored. Highest biodiversity has been recorded at a depth of 3000 m and the heterogeneity of biomass is expanding to 5000 m [74,75]. Cold deep-sea muds have an astounding species richness and diversity compared to tropical rain forests [76]. The majority of these species has not been isolated in the laboratory and an estimated 95% of these species are unidentified and mostly considered as new species [74]. Actinomycetes, including rare actinomycetes, are abundant in diverse marine sediments. A total of 48 new rare actinomycete species belonging to 16 different actinomycete families were isolated from marine sediments in the period from mid 2013 to 2017 (Table 2). Among them, 5 novel genera: Flaviflexus, Halopolyspora, Mariniluteicoccus, Sediminivirga and Haloactinomyces were described. The actinomycete families reported from marine sediments to which the novel species belong are Pseudonocardiaceae (8 new species), Nocardioidaceae (5 new species), Nocardiopsaceae (4 new species), Microbacteriaceae (4 new species), Micrococcaceae (4 new species), Propionibacteriaceae (4 new species), Micromonosporaceae (3 new species), Nocardiaceae (2 new species), Demequinaceae (2 new species), Intrasporangiaceae (2 new species), Bogoriellaceae (2 new species), Acidimicrobiaceae (2 new species), Brevibacteriaceae (2 new species), Actinopolysporaceae (2 new species), Actinomycetaceae (1 new species) and Cellulomonadaceae (1 new species).
Table 2.
New species of rare actinomycetes from marine sediments reported during the period of mid 2013–2017.
| Strain/Family | Nature of Sample | Isolation Medium | Ref. |
|---|---|---|---|
| Saccharomonospora amisosensis/Pseudonocardiaceae | Deep marine sediment at a depth of 60 m | SM3 medium (yeast nitrogen base 67.0 g, casamino acids 100 mg were added to a litre of distilled water and the solution sterilised using cellulose filters (0.20 mm) prior to the addition of sterilised di-potassium hydrogen phosphate (200 mL; 10%, w/v); 100 mL of this basal medium was added to 900 mL of sterilised molten agar (1.5%, w/v) followed by filter sterilised solutions of D(+) melezitose (1%, w/v), cycloheximide (50 µg mL−1), neomycin sulphate (4 µg mL−1) and nystatin (50 µg mL−1) | [82] |
| Saccharomonospora oceani/Pseudonocardiaceae | Marine sediment | Trypticase soy broth agar (DSMZ Medium 535) | [83] |
| Actinophytocola sediminis/Pseudonocardiaceae | Marine sediment at a depth of 2439 m | Starch casein nitrate agar medium (10.0 g soluble starch, 0.3 g casein, 2 g KNO3, 0.05 g MgSO4.7H2O, 35 g NaCl, 2 g K2HPO4, 0.02 g CaCO3, 10 mg FeSO4, 20 g agar, distilled water 1 L) | [84] |
| Pseudonocardia sediminis/Pseudonocardiaceae | Sea sediment at a depth of 652 m | DSMZ 621 medium (250 mg each of Bacto peptone (Difco), Bacto yeast extract and glucose, as well as 20 mL Hutner’s basal salts medium, 10 mL vitamin solution no. 6, 35 g NaCl and 1000 mL distilled water) | [85] |
| Amycolatopsis flava/Pseudonocardiaceae | Marine sediment | CMKA medium [(L−1) 0.5 g casein hydrolysate, 1.5 g mannitol, 1 g KNO3, 2 g (NH4)2SO4, 0.5 g K2HPO4, 0.5 g CaCO3, 20 g agar]. The multi-salts comprised of 49% (w/w) MgCl2.6H2O, 32% (w/w) NaCl, 14 % (w/w) CaCl2 and 5 % (w/w) KCl | [86] |
| Saccharopolyspora griseoalba/Pseudonocardiaceae | Marine sediment | CMKA medium [(L−1) 0.5 g casein hydrolysate, 1.5 g mannitol, 1 g KNO3, 2 g (NH4)2SO4, 0.5 g K2HPO4, 0.5 g CaCO3, 20 g agar]. The multi-salts comprised of 49% (w/w) MgCl2.6H2O, 32% (w/w) NaCl, 14 % (w/w) CaCl2 and 5 % (w/w) KCl | [87] |
| Amycolatopsis albispora/Pseudonocardiaceae | Deep-sea sediment at a depth of −2945 m | Modified Zobell 2216E agar (1.0 g yeast extract, 5.0 g tryptone, 34 g NaCl, 15 g agar and 1 L distilled water) | [88] |
| Pseudonocardia profundimaris/Pseudonocardiaceae | Marine sediment at a depth of −7118 m | Modified ZoBell 2216E agar plates (0.5% tryptone, 0.1% yeast extract, 3.4% sodium chloride and 1.8% agar) | [89] |
| Nocardioides pacificus/Nocardioidaceae | Deep sub-seafloor sediment at a depth of 107.3–107.4 m | Marine agar 2216 (Difco) | [90] |
| Nocardioides nanhaiensis/Nocardioidaceae | Sea sediment at a depth of 880 m | DSMZ 621 medium (250 mg each of Bacto peptone (Difco), Bacto yeast extract and glucose, as well as 20 mL Hutner’s basal salts medium, 10 mL vitamin solution no. 6, 35 g NaCl and 1000 mL distilled water) | [91] |
| Nocardioides antarcticus/Nocardioidaceae | Marine sediment | Marine agar 2216 (Becton Dickinson) | [92] |
| Nocardioides litoris/Nocardioidaceae | Marine beach sediment | Starch casein agar (1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar) | [93] |
| Nocardioides flavus/Nocardioidaceae | Marine sediment at a depth of −7068 m | Seawater agar (15.0 g agar and 1 L natural seawater) | [35] |
| Streptomonospora sediminis/Nocardiopsaceae | Marine sediment | Agar medium (glycerine 10.0 g, l-arginine 5.0 g, (NH4)2SO4 2.64 g; KH2PO4 2.38 g, K2HPO4 5.65 g, MgSO4.7H2O 1.0 g, CuSO4.5H2O 0.0064 g, FeSO4.7H2O 0.0011 g; MnCl2.4H2O 0.0079 g; ZnSO4.7H2O 0.0015 g, agar 15.0 g; distilled water 1.0 L) | [94] |
| Streptomonospora nanhaiensis/Nocardiopsaceae | Marine sediment at a depth of 2918 m | Agar medium (glycerine 10.0 g, l-arginine 5.0 g, (NH4)2SO4 2.64 g; KH2PO4 2.38 g, K2HPO4 5.65 g, MgSO4.7H2O 1.0 g, CuSO4.5H2O 0.0064 g, FeSO4.7H2O 0.0011 g; MnCl2.4H2O 0.0079 g; ZnSO4.7H2O 0.0015 g, agar 15.0 g; distilled water 1.0 L) | [94] |
| Nocardiopsis oceani/Nocardiopsaceae | Marine sediment at a depth of 2460 m | Gauze’s synthetic medium no. 1 (soluble starch 20.0 g, KNO3 1.0 g, NaCl 0.5 g, MgSO4.7H2O, 0.5 g, K2HPO4 0.5 g, FeSO4.7H2O 10.0 mg, agar 15.0 g and distilled water 1.0 L) | [95] |
| Nocardiopsis nanhaiensis/Nocardiopsaceae | |||
| Microbacterium hydrothermale/Microbacteriaceae | Hydrothermal sediment at a depth of 2943 m | Modified ZoBell 2216E agar plates (0.5% tryptone, 0.1% yeast extract, 3.4% sodium chloride and 1.8% agar) | [96] |
| Agromyces marinus/Microbacteriaceae | Sea sediment | NBRC medium 802 [Polypepton (Wako) 2 g, yeast extract 0.4 g, MgSO4.7H2O 0.2 g and agar 15 g in 1.0 L distilled water supplemented with NaCl (30 g−l), cycloheximide (50 mg−l) and nalidixic acid (20 mg−l)]. | [97] |
| Microbacterium enclense/Microbacteriaceae | Marine sediment | Marine agar (HiMedia) | [98] |
| Microbacterium nanhaiense/Microbacteriaceae | Sea sediment at a depth of 2093 m | Yeast extract/malt extract agar (1 L seawater, 0.5 g malt extract, 0.2 g yeast extract, 0.1 g glucose and 20 g agar) | [99] |
| Zhihengliuella flava/Micrococcaceae | Sea sediment | NBRC medium 802 (0.2% polypeptone, 0.04% yeast extract, 0.02% MgSO4.7H2O and 1.5% agar) | [100] |
| Kocuria indica/Micrococcaceae | Marine sediment | Marine agar 2216 (Difco) | [101] |
| Nesterenkonia alkaliphila/Micrococcaceae | Deep-sea sediment at a depth of 7118 m | Modified ISP 1 (1 L natural seawater, 10 g glucose, 5 g peptone, 5 g yeast extract, 0.2 g MgSO4.7H2O, 10 g NaHCO3, 27 g Na2CO3.10H2O and 15 g agar) | [102] |
| Kocuria subflava/Micrococcaceae | Marine sediment | No. 38 medium [(L−1) yeast extract 0.4 g; glucose 0.4 g; malt extract 0.4 g; B-vitamin trace 1 mL (0.5 mg each of thiamine-HCl (B1), riboflavin, niacin, pyridoxin, ca-pantothenate, inositol, p-aminobenzoic acid, and 0.25 mg of biotin, agar 15 g, distilled water 1000 mL] | [103] |
| Luteococcus sediminum/Propionibacteriaceae | Deep subseafloor sediment | Marine agar 2216 (Difco) | [104] |
| Mariniluteicoccus flavus (novel genus)/Propionibacteriaceae | Deep-sea sediment at a depth of 2439 m | HP agar medium (5 g fucose, 1 g proline, 1 g (NH4)2SO4, 2 g CaCl2, 1 g K2HPO4, B vitamin mixture (0.5 mg each thiamine hydrochloride, riboflavin, niacin, pyridoxine, calcium pantothenate, inositol and p-aminobenzoic acid and 0.25 mg biotin), 35 g NaCl, 12 g agar, 1000 mL distilled water) | [43] |
| Tessaracoccus lapidicaptus/Propionibacteriaceae | Deep subsurface sediment at a depth of 297 m | Anoxic F4 medium (0.4 g NaCl, 0.4 g NH4Cl, 0.3 g MgCl2.6H2O, 0.05 g CaCl2.2H2O, 1 g yeast extract, 2 g peptone, 1 g glucose, 1 g succinic anhydride, 7.5 g NaHCO3, 0.5 g KH2PO4, 0.5 g Na2S, 1 mg resazurin and 1 L distilled water) | [105] |
| Tessaracoccus arenae/Propionibacteriaceae | Sea sediment | Marine agar 2216 (Difco) | [106] |
| Rhodococcus enclensis/Nocardiaceae | Marine sediment | Marine agar 2216 (Difco) | [107] |
| Nocardia jiangsuensis/Nocardiaceae | Coastal sediment | Starch arginine agar (2.5 g soluble starch, 1.0 g arginine, 1.0 g (NH4)2SO4, 2.0 g CaCl2, 1.0 g K2HPO4, 0.2 g MgSO4.7H2O, 10 mg FeSO4.7H2O, 15.0 g agar supplemented with 3% (w/v) NaCl, nystatin and nalidixic acid) | [108] |
| Micromonospora fluostatini/Micromonosporaceae | Marine sediment | M1 medium (10 g soluble starch, 4 g yeast extract, 2 g peptone, 18 g agar, and 1 L of natural seawater) | [109] |
| Micromonospora yasonensis/Micromonosporaceae | Marine sediment at a depth of 45 m | SM3 medium (Gauze’s medium 2) [20 g casaminoacids, 20 g soluble starch, 4 g yeast extract, 15 g agar, 1 L distilled water] supplemented with filter sterilised cycloheximide (50 µg mL−1), nalidixic acid (10 µg mL−1), novobiocin (10 µg mL−1) and nystatin (50 µg mL−1) | [110] |
| Micromonospora profundi/Micromonosporaceae | Marine sediment at a depth of 45 m | ISP 2 medium (yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, distilled water 1 L and Bacto agar 20.0 g) | [111] |
| Demequina activiva/Demequinaceae | Tidal flat sediment | Marine agar 2216 (Becton Dickinson) | [112] |
| Demequina litorisediminis/Demequinaceae | Tidal flat sediment | Marine agar 2216 (Difco) | [113] |
| Janibacter cremeus/Intrasporangiaceae | Sea sediment | NBRC medium 802 (1.0% polypeptone, 0.2% yeast extract, 0.1% MgSO4.7H2O and 1.5% agar) | [114] |
| Janibacter indicus/Intrasporangiaceae | Hydrothermal sediment | ZoBell 2216E agar (0.5% tryptone, 0.1% yeast extract, 3.4%sodium chloride and 1.8% agar) | [115] |
| Georgenia sediminis/Bogoriellaceae | Marine Sediment at a depth of 141 m | Marine agar 2216 (Becton Dickinson) | [116] |
| Georgenia subflava/Bogoriellaceae | Deep sea sediment at a depth of 6310 m water depth | Modified ZoBell 2216E agar (1.0 g yeast extract, 5.0 g tryptone, 1 L of clarified seawater, 15.0 g agar) | [117] |
| Ilumatobacter nonamiense/Acidimicrobiaceae | Seashore sediment | Medium R (NaCl 25 g, MgSO4.7H2O 9 g, CaCl2.2H2O 0.14 g, KCl 0.7 g, Na2.HPO4.12H2O 0.25 g, Na2-EDTA 30 mg, H2BO3 34 mg, FeSO4.7H2O 10 mg, FeCl3.6H2O 1.452 mg, MnCl2.4H2O 4.32 mg, ZnCl2 0.312 mg, CoCl2.6H2O 0.12 mg, NaBr 6.4 mg, Na2MoO.2H2O 0.63 mg, SrCl2.6H2O 3.04 mg, RbCl 0.1415 mg, LiCl 0.61 mg, KI 0.00655 mg, V2O5 0.001785 mg, Cycloheximide 50 mg, Griseofulvin 25 mg, Nalidixic acid 20 mg, Aztreonam 40 mg, RPMI1640 500 mg, Eagle Medium 500 mg, l-Glutamine 15 mg, NaHCO3 100 mg, Agar 20 g and Distilled water 1 L) | [118] |
| Ilumatobacter coccineum/Acidimicrobiaceae | Seashore sand | ||
| Sediminivirga luteola (novel genus)/Brevibacteriaceae | Marine sediment at a depth of −5233 m | Isolation medium (10 g glucose, 5 g peptone, 5 g yeast extract, 0.2 g MgSO4.7H2O, 10 g NaHCO3, 27 g Na2CO3.10H2O, 20 g agar and 1 L natural seawater) | [25] |
| Brevibacterium sediminis/Brevibacteriaceae | Deep-sea sediment at a depth of −2461 m | ISP 2 medium (yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, distilled water 1 L and Bacto agar 20.0 g) | [119] |
| Halopolyspora alba (novel genus)/Actinopolysporaceae | Sea sediment | CMKA medium [(0.5 g casein acids hydrolysate, 1.5 g mannitol, 1 g KNO3, 2 g (NH4)2SO4, 0.5 g K2HPO4, 0.5 g CaCO3, 20 g agar and 20% (w/v) multi-salts]. The multi-salts comprised 49% (w/w) MgCl2, 32% (w/w) NaCl, 14% (w/w) CaCl2 and 5% (w/w) KCl | [24] |
| Haloactinomyces albus (novel genus)/Actinopolysporaceae | Marine sediment | CMKA medium [(0.5 g casein acids hydrolysate, 1.5 g mannitol, 1 g KNO3, 2 g (NH4)2SO4, 0.5 g K2HPO4, 0.5 g CaCO3, 20 g agar and 20% (w/v) multi-salts]. The multi-salts comprised 49% (w/w) MgCl2, 32% (w/w) NaCl, 14% (w/w) CaCl2 and 5% (w/w) KCl | [26] |
| Flaviflexus huanghaiensis (novel genus)/Actinomycetaceae | Coastal sediment | Marine agar 2216 (Difco) | [120] |
| Paraoerskovia sediminicola/Cellulomonadaceae | Sea sediment | NBRC medium 802 (1.0% polypeptone, 0.2% yeast extract, 0.1% MgSO4.7H2O and 1.5% agar) | [121] |
Although earlier culture-dependent studies have described microbial population size to be only a few hundred cells per mL of seawater, the staining of cells using fluorescence microscopy studies demonstrated nominal cell densities of >105 cells per mL of seawater [77,78], which anticipates that the ocean harbors 3.6 × 1029 microbial cells [79]. The microorganisms in the seawater play an important role in marine biogeochemical processes involved in cycling and decomposition of organic matter [80]. A total of 8 new rare actinomycete species were reported from seawater for the period mid-2013 to 2017 (Table 3), among which 2 novel genera, Pontimonas and Tamlicoccus. They belong to six actinomycete families: Nocardioidaceae (3 new species), Cellulomonadaceae (1 new species), Micrococcaceae (1 new species), Microbacteriaceae (1 new species), Dermacoccaceae (1 new species) and Dermabacteraceae (1 new species). From these studies, it is apparent that lower numbers of rare actinomycetes are isolated from seawater than from sediments. However, novel genera have been reported from seawater, which contribute to the extension of phylogenetic diversity of rare actinomycetes (Table 3) [7].
Table 3.
New species of rare actinomycetes from seawater reported during the period of mid 2013–2017.
| Strain/Family | Nature of Sample | Isolation Medium | Ref. |
|---|---|---|---|
| Nocardioides marinquilinus/Nocardioidaceae | Coastal seawater | Marine agar (Difco) | [122] |
| Nocardioides salsibiostraticola/Nocardioidaceae | Seawater at a depth of 1 m | R2A agar (Difco) | [123] |
| Nocardioides rotundus/Nocardioidaceae | Seawater at a depth of −7001 m | Modified ZoBell 2216E agar (1.0 g yeast extract, 5.0 g tryptone, 1 L clarificated seawater and 15.0 g agar) | [124] |
| Cellulomonas marina/Cellulomonadaceae | Deep-seawater at a depth of 2800 m | ISP 2 medium (yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, distilled water 1 L and Bacto agar 20.0 g) | [125] |
| Kocuria oceani/Micrococcaceae | Deep-sea hydrothermal plume water at a depth of 2800 m | ISP 2 medium (yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, distilled water 1 L and Bacto agar 20.0 g) and SMPS (0.1 g peptone, 0.5 g mannitol, 3 g sea salt, 1000 mL distilled water, pH 7.5) agar, supplemented with nalidixic acid, cycloheximide and nystatin (each at 25 μg mL−1). | [126] |
| Pontimonas salivibrio (novel genus)/Microbacteriaceae | Seawater | Marine agar (Difco) | [36] |
| Tamlicoccus marinus (novel genus)/Dermacoccaceae | Seawater | SC-SW agar (1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.2% KH2PO4, 0.002% CaCO3, 0.005% MgSO4.7H2O, 0.001% FeSO4.7H2O, 1.8% agar, 60% natural seawater and 40% distilled water) | [37] |
| Brachybacterium aquaticum/Dermabacteraceae | Seawater | Tryptic soy agar medium (HiMedia) | [127] |
A substantial number of rare actinomycetes were reported to be associated to various members of marine benthic communities, such as sponges, corals, ascidians, sea anemones, sea cucumbers, sea urchins and seaweeds [7,62,81]. Five novel genera, 17 new rare actinomycete species belonging to 11 different actinomycete families were reported from marine plants and animals between 2007 and mid-2013 [7]. A total of 14 new species of rare actinomycetes belonging to 12 different families have been reported from various sponges, corals, algae, sea urchin, jelly fish and fish between mid-2013 and 2017 (Table 4). The families of novel (potentially symbiotic) actinomycete species reported from mid-2013 to 2017 are Micromonosporaceae (2 new species), Pseudonocardiaceae (2 new species), Microbacteriaceae (1 new species), Mycobacteriaceae (1 new species), Nocardioidaceae (1 new species), Micrococcaeae (1 new species), Intrasporangiaceae (1 new species), Nocardiaceae (1 new species), Rubrobacteraceae (1 new species), Actinosynnemataceae (1 new species), Gordoniaceae (1 new species) and Promicromonosporaceae (1 new species). Thus, marine organisms remain a rich source of novel rare actinomycetes (Table 4) and a substantial number of host-associated rare actinomycete genera have not been reported from other marine habitats (Labedella, Phycicola, Iamia, Euzebya and Koreibacter) [7]. Interestingly, Microbacterium aureliae was reported for the first time from Aurelia aurita, the moon jellyfish.
Table 4.
New species of symbiotic rare actinomycetes from eukaryotic hosts reported during the period of mid 2013–2017.
| Strain/Family | Nature of Sample | Isolation Medium | Reference |
|---|---|---|---|
| Verrucosispora andamanensis/Micromonosporaceae | Marine sponge Xestospongia sp. | Starch-casein nitrate seawater agar (10 g soluble starch, 1 g sodium caseinate, 0.5 g KH2PO4, 0.5 g MgSO4 and 18 g agar in 1 L of seawater) | [130] |
| Micromonospora spongicola/Micromonosporaceae | Marine sponge at a depth of 5 m | Starch-casein nitrate agar (10 g soluble starch, 1 g sodium caseinate, 2 g KNO3, 0.5 g KH2PO4, 0.5 g MgSO4 and 18 g agar in 1 L seawater) | [131] |
| Prauserella coralliicola/Pseudonocardiaceae | Marine coral Galaxea fascicularis at a depth of 5 m | Isolation medium (yeast extract 0.25 g, K2HPO4 0.5 g, agar 12 g, 500 mL seawater and 500 mL distilled water) | [132] |
| Saccharopolyspora spongiae/Pseudonocardiaceae | Marine sponge Scopalina ruetzleri at depths between 20 and 30 m | M1 medium [1% starch, 0.4% yeast extract, 0.2% peptone, 2% agar containing artificial seawater (33 g red sea salt L−1) amended with cycloheximide and nystatin (each at 25 µg mL−1)] | [40] |
| Microbacterium aureliae/Microbacteriaceae | Moon jellyfish Aurelia aurita | Zobell marine agar (HiMedia) and Tryptic soy agar (HiMedia) | [133] |
| Mycobacterium stephanolepidis/Mycobacteriaceae | Marine teleost fish Stephanolepis cirrhifer | Middlebrook 7H11 agar with oleic albumin dextrose catalase (OADC) enrichment (Becton Dickinson) | [134] |
| Marmoricola aquaticus/Nocardioidaceae | marine sponge Glodia corticostylifera | M1 agar (soluble starch 10 g L−1, yeast extract 4 g L−1, peptone 2 g L−1, agar 15 g L−1, 80% artificial seawater) | [38] |
| Arthrobacter echini/Micrococcaceae | Purple sea urchin Heliocidaris crassispina | Marine agar 2216 (Difco) | [135] |
| Ornithinimicrobium algicola/Intrasporangiaceae | Marine green alga Ulva sp. | Modified R2A medium (yeast extract 0.5 g, peptone 0.5 g, casein enzyme hydrolysate 0.5 g, yeast extract 0.5 g, glucose 0.5 g, water soluble starch 0.5 g, dipotassium phosphate 0.3 g, magnesium sulphate 0.05 g, sodium pyruvate 0.3 g, sodium chloride 20.0 g and distilled water 1000 mL) | [41] |
| Nocardia xestospongiae/Nocardiaceae | Marine sponge Xestospongia sp. | Modified starch-casein nitrate seawater agar containing 10 g soluble starch, 1 g sodium caseinate, 0.5 g KH2PO4, 0.5 g MgSO4 and 18 g agar in 1 L seawater, pH 8.3, supplemented with 50 mg nalidixic acid L−1 and 200 mg nystatin L−1 | [136] |
| Rubrobacter aplysinae/Rubrobacteraceae | Marine sponge Aplysina aerophoba | Tryptone soy agar (Oxoid) | [137] |
| Actinokineospora spheciospongiae/Actinosynnemataceae | Marine sponge Spheciospongia vagabunda | ISP 2 medium (yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, distilled water 1 L and Bacto agar 20.0 g) | [138] |
| Williamsia spongiae/Gordoniaceae | Marine sponge Amphimedon viridis at depths of between 5 and 10 m | Tryptic Soy Agar [Oxoid; prepared with 80% (v/v) artificial seawater] | [39] |
| Myceligenerans cantabricum/Promicromonosporaceae | Marine coral at a depth of 1500 m | 1/3 Tryptic soy agar (Merck) and and 1/6 M-BLEB agar (9 g MOPS BLEB base (Oxoid) in 1 L Cantabrian seawater, containing the antifungal cycloheximide (80 µg mL−1) and anti-Gram-negative bacteria nalidixic acid (20 mg mL−1) | [139] |
Mangrove forests are highly dynamic ecosystems that cover and protect approximately 75% of the world’s tropical and subtropical coastal areas [128] and harbor a rich diversity of marine, freshwater and terrestrial flora and fauna. The diversity of the microbial community in mangrove environments is still rather unexplored [60]. The large fluctuation of salinity and tidal gradients make the mangrove forests unique environments that favors the production of unusual metabolites among the residing microorganisms [60]. Novel actinomycetes reported from different mangrove habitats including sediments, mangrove plant rhizosphere soil and mangrove endophytes are classified into 25 genera, 11 families and 8 suborders [7,129]. A total of 27 new species of rare actinomycetes belonging to 13 different families have been reported from mangrove habitats for the period mid-2013–2017 (Table 5). Among them, two novel genera, Mamia and Monashia, were reported to be isolated from the 20 cm top-layer of mangrove soil. The families reported in mangrove sediments between mid-2013 and 2017 are Demequinaceae (9 new species), Micromonosporaceae (5 new species), Nocardiopsaceae (2 new species), Micrococcaceae (2 new species), Nocardioidaceae (1 new species), Intrasporangiaceae (1 new species), Pseudonocardiaceae (1 new species), Microbacteriaceae (1 new species), Thermomonosporaceae (1 new species), Jiangellaceae (1 new species), Beutenbergiaceae (1 new species), Streptosporangiaceae (1 new species) and Kineosporiaceae (1 new species).
Table 5.
New species of rare actinomycetes from mangrove environment reported during the period of mid 2013–2017.
| Strain/Family | Nature of Sample | Isolation Medium | Ref. |
|---|---|---|---|
| Lysinimicrobiumaestuarii/Demequinaceae | Sediment of mangrove tidal flat | 1/5 NBRC medium 802 [0.2% (w/v) polypeptone, 0.04% (w/v) yeast extract, 0.02% (w/v) MgSO4.7H2O and 1.5% (w/v) agar; pH 7.0] supplemented with 5.0% (w/v) NaCl, 0.005% (w/v) cycloheximide and 0.002% (w/v) nalidixic acid | [140] |
| Lysinimicrobium flavum/Demequinaceae | Rhizosphere soil of mangrove | ||
| Lysinimicrobium gelatinilyticum/Demequinaceae | |||
| Lysinimicrobium iriomotense/Demequinaceae | |||
| Lysinimicrobium luteum/Demequinaceae | Soil of mangrove forest | ||
| Lysinimicrobium pelophilum/Demequinaceae | Mud of mangrove tidal flat | ||
| Lysinimicrobium rhizosphaerae/Demequinaceae | Rhizosphere soil of mangrove | ||
| Lysinimicrobium soli/Demequinaceae | Soil of mangrove forest | ||
| Lysinimicrobium subtropicum/Demequinaceae | Rhizosphere soil of mangrove | ||
| Micromonospora wenchangensis/Micromonosporaceae | Mangrove soil | Glucose-peptone-tryptone agar supplemented with 50 mg nystatin L−1, 50 mg cycloheximide L−1, 25 mg novobiocin L−1 and 20 mg nalidixic acid L−1 | [52] |
| Micromonospora zhanjiangensis/Micromonosporaceae | Mangrove soil | 1/10 ATCC 172 agar supplemented with nalidixic acid (10 µg mL−1), novobiocin (10 µg mL−1), nystatin (50 µg mL−1) and K2Cr2O7 (20 µg mL−1) | [141] |
| Micromonospora ovatispora/Micromonosporaceae | Mangrove soil | ATCC 172 agar | [142] |
| Micromonospora sediminis/Micromonosporaceae | Mangrove sediment | AV medium (1.0 g glucose, 1.0 g glycerol, 0.3 g L-arginine, 0.3 g K2HPO4, 0.2 g MgSO4.7H2O, 0.3 g NaCl, 18 g agar, artificial seawater added up to 1 L) | [42] |
| Micromonospora mangrovi/Micromonosporaceae | Mangrove soil | Glucose-peptone-tryptone agar (glucose 10 g, peptone 5 g, tryptone 3 g, NaCl 5 g, agar 15 g, ddH2O 1 L supplemented with 50 mg/L of nystatin, 50 mg/L of cycloheximide, 25 mg/L of novobiocin and 20 mg/L of nalidixic acid) | [143] |
| Nocardiopsis mangrovei/Nocardiopsaceae | Mangrove sediment | Humic acid vitamin agar (humic acid 1.0 g, KCl 1.7 g, Na2HPO4 0.5 g, MgSO4·7H2O 0.5 g, CaCO3 0.02 g, FeSO4·7H2O, 0.01 g, B vitamins (0.5 mg each of thiamin, riboflavin, niacin, pyridoxin, calcium d-pantothenate, inositol, p-aminobenzoic acid and 0.25 mg biotin), cycloheximide 25 mg; potassium dichromate 50 mg, nystatin 50 mg, agar 15.0 g per litre of distilled water) | [27] |
| Nocardiopsis sediminis/Nocardiopsaceae | Mangrove sediment | Starch casein agar (1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar) | [144] |
| Sinomonas humi/Micrococcaceae | Mangrove soil | Starch casein agar [1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar supplemented with cycloheximide (25 µg mL−1) and nystatin (10 µg mL−1)] | [51] |
| Kocuria pelophila/Micrococcaceae | Rhizosphere soil of mangrove | NBRC medium 802 [1.0% (w/v) polypeptone, 0.2% (w/v) yeast extract, 0.1% (w/v) MgSO4.7H2O and 1.5% (w/v) agar] | [145] |
| Mumia flava (novel genus)/Nocardioidaceae | Mangrove soil | ISP 2 medium [yeast extract 4.0 g, malt extract 10.0 g, dextrose 4.0 g, Distilled water 1 L and Bacto agar 20.0 g supplemented with cycloheximide (25 µg mL−1) and nystatin (10 µg mL−1)] | [146] |
| Monashia flava (novel genus)/Intrasporangiaceae | Mangrove soil | Starch casein agar [1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar supplemented with cycloheximide (25 µg mL−1) and nystatin (10 µg mL−1)] | [23] |
| Pseudonocardia nematodicida/Pseudonocardiaceae | Mangrove sediment | Modified gause inorganic agar (20 g soluble starch, 1 g KNO3, 0.5 g K2HPO4, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 15 g agar, 1 L aged seawater) | [147] |
| Microbacterium mangrovi/Microbacteriaceae | Mangrove soil | Starch casein agar [1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar supplemented with cycloheximide (25 µg mL−1) and nystatin (10 µg mL−1)] | [50] |
| Actinoallomurus acanthiterrae/Thermomonosporaceae | Rhizosphere soil of Acanthus ilicifolius | Oatmeal agar [Oatmeal 20.0 g, agar 18.0 g, supplemented with novobiocin (25 µg mL−1), nystatin (30 µg mL−1), nalidixic acid (10 µg mL−1) and K2Cr2O7 (20 mg mL−1] | [148] |
| Jiangella mangrovi/Jiangellaceae | Mangrove soil | Marine agar 2216 (Difco) | [149] |
| Serinibacter tropicus/Beutenbergiaceae | Rhizosphere soil of mangrove | NBRC medium 802 [0.2% (w/v) polypeptone, 0.04% (w/v) yeast extract, 0.02% (w/v) MgSO4.7H2O and 1.5% (w/v) agar] supplemented with 5.0% (w/v) NaCl, 0.005% (w/v) cycloheximide and 0.002% (w/v) nalidixic acid | [150] |
| Nonomuraea purpurea/Streptosporangiaceae | Mangrove sediment | Marine agar 2216 (Difco) | [151] |
| Kineococcus mangrovi/Kineosporiaceae | Mangrove sediment | Starch casein agar [1% soluble starch, 0.03% casein, 0.2% KNO3, 0.2% NaCl, 0.005% MgSO4.7H2O, 0.2% K2HPO4, 0.02% CaCO3, 0.001% FeSO4.7H2O, 1.8% agar supplemented with nalidixic acid (25 µg mL−1) and ketokonazole (100 µg mL−1)] | [152] |
3.2. Marine Rare Actinomycetes Diversity: A Decade of Experience (2007–2017)
In summary, a total of 97 new species belonging to 27 different rare actinomycete genera, of which 9 represent novel genera, were reported, from the marine environment between mid-2013 and 2017 (Table 2, Table 3, Table 4, Table 5 and Table 6; Figure 1). Furthermore, the families Pseudonocardiaceae, Demequinaceae, Micromonosporaceae and Nocardioidaceae were most frequently isolated from the marine environment. For the period 2007-mid 2013, 80 new species belonging to 23 families of marine rare actinomycetes were reported (Table 6). These data show that the discovery rate of new rare actinomycetes from marine habitats is steady. Interestingly, isolates from 10 actinomycete families, such as Actinomycetaceae, Actinopolysporaceae, Brevibacteriaceae, Rubrobacteraceae, Actinosynnemataceae, Gordoniaceae, Jiangellaceae, Kineosporiaceae, Dermacoccaceae and Dermabacteraceae were reported for the period between mid-2013 and 2017 that were not reported for the period 2007 to mid-2013. Cumulatively this means that a total of of 177 new species of rare actinomycetes representing 33 families including 3 novel families and 29 novel genera were reported from marine habitats in the last 10 years (Table 6). Actinomycete families such as Micromonosporaceae, Nocardioidaceae, Pseudonocardiaceae, Microbacteriaceae, Micrococcaceae, Demequinaceae, Nocardiopsaceae, Propionibacteriaceae and Intrasporangiaceae are the families most frequently reported from marine habitats during this period. However, no novel actinomycete family has been reported from marine habitats since mid-2013.
Table 6.
Number of new species of rare actinomycetes reported from marine environment between 2007 and 2017.
| Particular | 2007 to mid-2013 * | Mid-2013 to 2017 | Total (2007–2017) |
|---|---|---|---|
| New species reported | 80 | 97 | 177 |
| Novel families reported | 3 | - | 3 |
| Novel genera reported | 20 | 9 | 29 |
| Total families reported | 23 | 27 | 33 |
| No. of new species reported in each family | |||
| Micromonosporaceae | 13 | 10 | 23 |
| Nocardioidaceae | 10 | 9 | 19 |
| Pseudonocardiaceae | 6 | 11 | 17 |
| Microbacteriaceae | 5 | 8 | 13 |
| Micrococcaceae | 5 | 8 | 13 |
| Demequinaceae | 1 | 11 | 12 |
| Nocardiopsaceae | 4 | 6 | 10 |
| Micrococcineae | 10 | - | 10 |
| Propionibacteriaceae | 4 | 4 | 8 |
| Intrasporangiaceae | 4 | 4 | 8 |
| Nocardiaceae | 2 | 4 | 6 |
| Streptosporangiaceae | 3 | 1 | 4 |
| Promicromonosporaceae | 3 | 1 | 4 |
| Cellulomonadaceae | 1 | 2 | 3 |
| Acidimicrobiaceae | 1 | 2 | 3 |
| Bogoriellaceae | 1 | 2 | 3 |
| Beutenbergiaceae | 1 | 1 | 2 |
| Thermomonosporaceae | 1 | 1 | 2 |
| Actinopolysporaceae | - | 2 | 2 |
| Brevibacteriaceae | - | 2 | 2 |
| Alteromonadaceae | 1 | - | 1 |
| Tsukamurellaceae | 1 | - | 1 |
| Iamiaceae | 1 | - | 1 |
| Euzebyaceae | 1 | - | 1 |
| Geodermatophilaceae | 1 | - | 1 |
| Actinomycetaceae | - | 1 | 1 |
| Rubrobacteraceae | - | 1 | 1 |
| Actinosynnemataceae | - | 1 | 1 |
| Gordoniaceae | - | 1 | 1 |
| Jiangellaceae | - | 1 | 1 |
| Kineosporiaceae | - | 1 | 1 |
| Dermacoccaceae | - | 1 | 1 |
| Dermabacteraceae | - | 1 | 1 |
* Adapted from Subramani and Aalbersberg [7].
Figure 1.
Total number of families, novel genera and new species of rare actinomycetes reported from different marine habitats between mid-2013 and 2017.
4. Actinomycetes as Sources of Antibiotics
Actinomycetes has been one of the most fertile sources for the discovery of new antibiotics since they were first discovered and a number of the antibiotics currently in use are natural products or analogs of natural products from actinomycetes [153]. Actinomycin was the first antibiotic discovered from actinomycetes in 1940 from a culture of Streptomyces antibioticus [154], followed by streptothricin from Streptomyces lavendulae in 1942 [155], and streptomycin from Streptomyces griseus in 1944 [156]. Streptomyces species have been the key source of clinical antibiotics, and more than 80% of all antibiotics of actinomycetes origin have been derived from this single genus [3,157]. Out of all microbially-derived antibiotic classes, 10 classes are exclusively produced by actinomycetes. Those are polyene macrolides, oligomycin-type large-membered macrolides, daunomycin-type anthracyclines, nigericin-type polyether antibiotics, nonactin-type cyclopolylactones, aminoglycosides, anthracyclines, streptothricins, actinomycins and quinoxaline-peptides [3]. The antibiotics production of different actinomycete strains can vary enormously as some actinomycete species produce a single antibiotic, whereas some produce a wide-range of different compounds and compound classes [5]. A total of 30 new antibiotics have been launched worldwide since 2000. Of the 30 new antibiotics, 2 were natural products (NP), 12 were NP-derived and 16 were synthetic antibiotics [158]. Out of these 30 new antibiotics, 12 were reported from members of actinomycetes, either as natural product or natural product-derivatives representing 7 different antibiotic classes (Table 7). Due to the decline in the number of new chemical scaffolds and rediscovery of known molecules, the innovation in antibiotic development has slowed down. The exploration of alternative taxa, which have not been previously cultivated, could alleviate urgent needs related to resistance against currently used antibiotics.
Table 7.
Antibiotics of therapeutic natural products derived from actinomycetes.
| Drug Name/Compound | Natural Product (NP) or Derivative | Source Organism | Chemical Class | Therapeutic Activity | Clinical Status (Year) | Reference |
|---|---|---|---|---|---|---|
| Non-marine source | ||||||
| Telithromycin | Erythromycin (NP-derivative) | Saccharopolyspora erythraea | Macrolide | Antibacterial (G+ve/G−ve) | Approved (2001) | [158] |
| Biapenem | Thienamycin (NP-derivative) | Streptomyces cattleya | Carbapenem | Antibacterial (G+ve/G−ve) | Approved (2002) | [158] |
| Ertapenem | Thienamycin (NP-derivative) | Streptomyces cattleya | Carbapenem | Antibacterial (G+ve/G−ve) | Approved (2002) | [158] |
| Daptomycin | Natural product | Streptomyces roseosporus | Lipopeptide | Antibacterial (G+ve) | Approved (2003) | [158] |
| Doripenem | Thienamycin (NP-derivative) | Streptomyces sp. | Carbapenem | Antibacterial (G+ve/G−ve) | Approved (2005) | [158] |
| Tigecycline | Tetracycline (NP-derivative) | Streptomyces aureofaciens | Tetracycline | Antibacterial (G+ve/G−ve) | Approved (2005) | [158] |
| Tebipenem pivoxil | Thienamycin (NP-derivative) | Streptomyces sp. | Carbapenem | Antibacterial (G+ve/G−ve) | Approved (2009) | [158] |
| Telavancin | Vancomycin (NP-derivative) | Amycolatopsis orientalis | Glycopeptide | Antibacterial (G+ve) | Approved (2009) | [158] |
| Fidaxomicin | Natural product | Dactylosporangium aurantiacum | Tiacumicin | Antibacterial (G+ve) | Approved (2011) | [158] |
| Dalbavancin | Teicoplanin (NP-derivative) | Nonomuria sp. | Glycopeptide | Antibacterial (G+ve) | Approved (2014) | [158] |
| Oritavancin | Chloroeremomycin (NP-derivative) | Amycolatopsis orientalis | Glycopeptide | Antibacterial (G+ve) | Approved (2014) | [158] |
| Tazobactam | NP-derivative | Actinomycete strain | Penicillanic acid sulfone derivative and β-lactamase inhibitor | Antibacterial (G−ve) | Approved (2014) | [158] |
| Marine source | ||||||
| Salinosporamide A (Marizomib) | Natural product | Salinispora tropica | Beta-lactone-gamma lactam | Multiple cancer | Phase I | [159] |
| Arenamides A and B | Natural product | Salinispora sp. | Peptide | Inflammation | Pre-clinical | [160] |
| Anthracimycin | Natural product | Streptomyces sp. | Polyketide | Anthrax | Pre-clinical | [161] |
G+ve: Gram positive; G−ve: Gram negative.
4.1. Rare Actinomycetes: A Target for Future Drugs
As a result, rare actinomycetes are becoming an increasingly important focus of investigation in the search for novel natural products because (1) they occupy a poorly explored taxonomic and environmental space, which reduces the likelihood of replication of discovery, and (2) the phylum Actinobacteria is a rich source of bioactive secondary metabolites [46] that can be expected to yield novel chemical scaffolds for the development of new antibiotics.
4.2. Marine Rare Actinomycetes Is a Source of Antibiotics
Approximately 100 new bioactive compounds were reported from 38 rare actinomycete strains belonging to 15 genera described between 2007 and mid-2013. Out of these 15 different genera, Salinispora (20 new compounds), Verrucosispora (18 new compounds), Nocardiopsis (12 new compounds), Actinoalloteichus (11 new compounds), Marinispora (10 new compounds) and Micromonospora (9 new compounds) were predominant for discovery of novel secondary metabolites from 2007 to mid-2013 [7]. A total of 4 compounds derived from marine actinomycetes are currently in clinical trials (Table 7) of which 3 were obtained from marine Salinispora spp. indicating that Streptomyces spp. are no longer the most important biological resource for new antibiotics.
4.3. Novel/New Compounds from Marine Rare Actinomycetes between mid-2013 and 2017
A total of 167 different new bioactive compounds were reported from 58 rare actinomycete strains belonging to 24 genera from mid-2013 to 2017 (Table 8). Among them, genera such as Nocardiopsis (40 new compounds), Micromonospora (37 new compounds), Salinispora (21 new compounds) and Pseudonocardia (14 new compounds) are leading with respect to the number of novel secondary metabolites (Table 8). Among them, there are new/novel pyrones, structurally diverse natural products and unique chemical moieties (Figure 2, Figure 3, Figure 4 and Figure 5).
Table 8.
Numbers of new natural products/bioactive compounds produced by rare actinomycete genera from the marine environment between 2007 and 2017.
| Particular | 2007 to mid-2013 * | Mid-2013 to 2017 | Total (2007–2017) |
|---|---|---|---|
| Novel/new compounds reported | 100 | 167 | 267 |
| Total number of rare actinomycetes | 38 | 58 | 96 |
| Total genera reported | 15 | 24 | 28 |
| No. of new compounds reported in each genus | |||
| Nocardiopsis | 12 | 40 | 52 |
| Micromonospora | 9 | 37 | 46 |
| Salinispora | 20 | 21 | 41 |
| Pseudonocardia | 3 | 14 | 17 |
| Verrucosispora | 18 | 2 | 20 |
| Amycolatopsis | 1 | 3 | 4 |
| Serinicoccus | 1 | 1 | 2 |
| Kocuria | 1 | - | 1 |
| Actinoalloteichus | 11 | 5 | 16 |
| Actinomadura | 3 | 5 | 8 |
| Dermacoccus | 7 | 3 | 10 |
| Kitasatospora | 1 | - | 1 |
| Nocardia | 2 | - | 2 |
| Saccharomonospora | 1 | 5 | 6 |
| Marinispora | 10 | - | 10 |
| Actinokineospora | - | 2 | 2 |
| Solwaraspora | - | 2 | 2 |
| Micrococcus | - | 1 | 1 |
| Microbacterium | - | 3 | 3 |
| Rubrobacter | - | 2 | 2 |
| Saccharothrix | - | 4 | 4 |
| Actinomycetospora | - | 3 | 3 |
| Williamsia | - | 1 | 1 |
| Streptomonospora | - | 4 | 4 |
| Nesterenkonia | - | 1 | 1 |
| Kribbella | - | 4 | 4 |
| Streptosporangium | - | 3 | 3 |
| Saccharopolyspora | - | 1 | 1 |
* Adapted from Subramani and Aalbersberg [7].
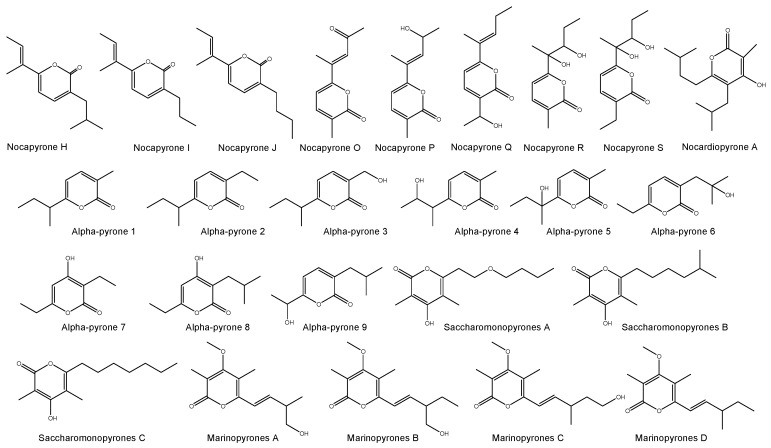
Figure 2.
New pyrones produced by marine rare Nocardiopsis spp., Streptomonospora sp. and Saccharomonospora sp. between mid-2013 and 2017.
Figure 3.
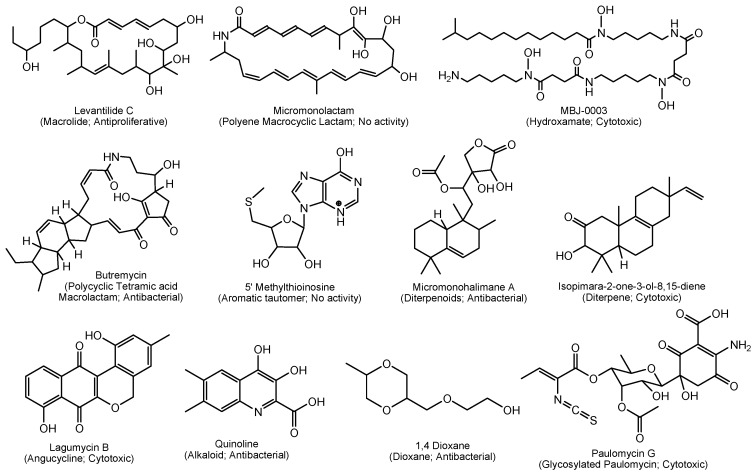
Some novel natural products from marine Micromonospora spp. reported from mid-2013 to 2017.
Figure 4.
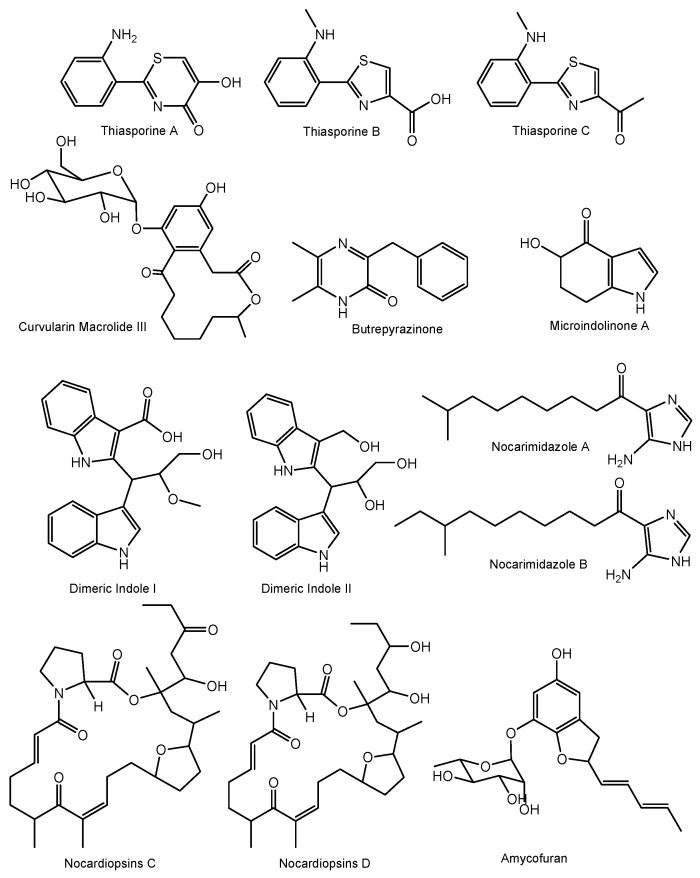
Some of the unique chemical moieties in natural products produced by marine rare actinomycetes between mid-2013 and 2017.
Figure 5.
Some unusual biologically active compounds produced by marine rare actinomycetes between mid-2013 and 2017.
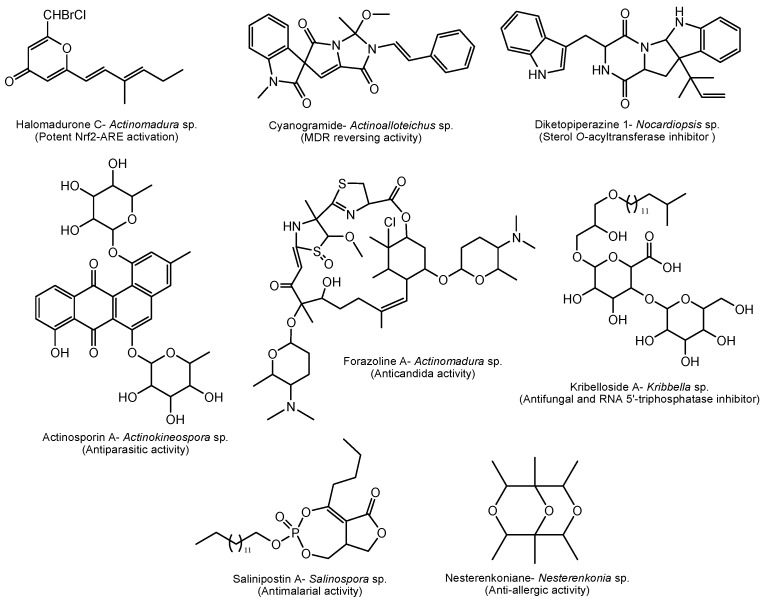
A total of 7 different chemical classes of natural products were reported from marine Nocardiopsis spp. between mid-2013 and 2017 of which, α-pyrones (18 out of 40 compounds) were predominant (Table 9; Figure 2). These molecules have a wide range of biological activities, such as pro-inflammatory activity (enhancing and stimulating the inflammatory response), anti-inflammatory activity, antibacterial and cytotoxic activities (Table 9). In addition, the genera Streptomonospora and Saccharomonospora also produce a substantial number of α-pyrones. Besides, nocarimidazoles from Nocardiopsis sp. possess a 4-aminoimidazole ring rarely found in microbial secondary metabolites [162] and rare prolinyl-macrolactam polyketides were isolated from Nocardiopsis sp. [163]. Sterol O-acyltransferase [SOAT, also known as acyl-CoA: cholesterol acyltransferase (ACAT)], an endoplasmic reticulum membrane protein, catalyzes the synthesis of cholesteryl ester from free cholesterol and long-chain fatty acyl-CoA. SOAT has been postulated as a target for modulation by a new type of antiatherosclerotic agent. Interestingly, a diketopiperazine derived from marine Nocardiopsis sp. was found to be an effective SOAT inhibitor [164].
Table 9.
Novel/new bioactive compounds produced by marine rare actinomycetes between mid 2013 and 2017.
| Compounds | Chemical Family/Class | Marine Source | Biological Activity | Reference |
|---|---|---|---|---|
| Halomadurones A–D | Halogenated electrophilic pyrones | Actinomadura sp. | Potent Nrf2-ARE activation | [176] |
| Levantilide C | 20-membered macrolide | Micromonospora sp. | Antiproliferative activity | [178] |
| Nocapyrones H–J | α-pyrones | Nocardiopsis sp. | Pro-inflammatory factor, stronger inhibitory effect on nitric oxide | [179] |
| Nocardiopsins C and D | Prolinyl-macrolactam polyketides | Nocardiopsis sp. | Not specified | [163] |
| Nocardiopyrone A | α-pyrone polyketide | Nocardiopsis sp. | Not specified | [163] |
| Nocardiamide A and B | Cyclic hexapeptides | Nocardiopsis sp. | Antimicrobial activity | [180] |
| Cyanosporasides C–F | Polyketides | Salinispora pacifica | Not specified | [181] |
| Micromonolactam | Polyene macrocyclic lactam | Micromonospora sp. | No antimicrobial activity | [165] |
| Cyanogramide | Spirocyclic alkaloid | Actinoalloteichus cyanogriseus | Multidrug-resistance (MDR) reversing activity | [177] |
| Actinosporins A and B | O-glycosylated angucyclines | Actinokineospora sp. | Moderate activity against Trypanosoma brucei | [182] |
| Solwaric acids A and B | Trialkyl-substituted aromatic acids | Solwaraspora sp. | Antibacterial activity against MDR pathogens | [183] |
| Seriniquinone | Quinones | Serinicoccus sp. | A selective anticancer agent | [184] |
| Cyanogrisides E–H | Acyclic bipyridine glycosides | Actinoalloteichus cyanogriseus | Cytotoxicity | [185] |
| Forazoline A | Polyketides | Actinomadura sp. | Anti-candida activity | [186] |
| Amycofuran | Benzofuran glycoside | Amycolatopsis sp. | Modest cytotoxicity | [175] |
| Amycolactam | Indole alkaloids | Amycolatopsis sp. | Cytotoxicity | [175] |
| Amycocyclopiazonic acid | Cyclopiazonic acid | Amycolatopsis sp. | Modest cytotoxicity | [175] |
| Dermacozines H–J | Heteroaromatic phenazines | Dermacoccus abyssi | Radical scavenging activity | [187] |
| Microluside A | O-glycosylated xanthone | Micrococcus sp. | Antibacterial activity | [188] |
| Nocapyrone R | α-pyrones | Nocardiopsis sp. | No cytotoxicity | [189] |
| Butremycin | Polycyclic tetramic acid macrolactams | Micromonospora sp. | Weak antibacterial activity | [167] |
| 5′-Methylthioinosine | Protonated aromatic tautomer | Micromonospora sp. | No antibacterial activity | [167] |
| Butrepyrazinone | Pyrazinone | Verrucosispora sp. | No antibacterial activity | [172] |
| MBJ-0003 | Hydroxamate | Micromonospora sp. | Moderate cytotoxicity | [190] |
| Microbacterins A and B | Peptaibols | Microbacterium sediminis | Potent cytotoxic activity | [191] |
| Salinipostins A–K | Bicyclic Phosphotriesters | Salinospora sp. | Antimalarial activity | [192] |
| Nocarimidazoles A and B | 4-aminoimidazole alkaloids | Nocardiopsis sp. | Weak antibacterial activity | [162] |
| Dimeric indole derivatives 1 and 2 | Dimeric indoles | Rubrobacter radiotolerans | Acetylcholinesterase (AchE) inhibitory activity | [174] |
| Saccharothrixones A–D | Aromatic polyketides | Saccharothrix sp. | Cytotoxic activity | [193] |
| Thiasporines A–C | Thiazine and Thiazole Derivatives | Actinomycetospora chlora | Cytotoxicity | [170] |
| Diketopiperazine 1 | Diketopiperazine | Nocardiopsis sp. | Sterol O-acyltransferase inhibitor | [164] |
| Isopimara-2-one-3-ol-8,15-diene | Pimarane Diterpene | Micromonospora sp. | Weak cytotoxicity | [169] |
| Lagumycin B, Dehydrorabelomycin, Phenanthroviridone, WS-5995 A | Angucyclines | Micromonospora sp. | Cytotoxicity | [169] |
| Micromonohalimane A and B | Halimane-type diterpenoids | Micromonospora sp. | Modest antibacterial activity against MRSA, bacteriostatic | [168] |
| Quinoline alkaloid | Alkaloid | Micromonospora sp. | Antibacterial activity | [194] |
| 1,4-dioxane derivative | Dioxane | Micromonospora sp. | Antibacterial activity | [194] |
| Pseudonocardides A–G | γ-butyrolactones | Pseudonocardia sp. | Antibacterial and cytotoxic activities | [195] |
| Curvularin macrolides 1–5 | Macrolides | Pseudonocardia sp. | Antibacterial and cytotoxic activities | [171] |
| α-pyrones 1–8 | α-pyrones | Nocardiopsis spp. | Moderate antibacterial activity | [196] |
| Compounds 1–12 | Benzamides, Indoles | Nocardiopsis sp. | Antibacterial, antifungal and cytotoxic activities | [197] |
| 3-benzyl-3α,4β-dihydroxypentan-2-one | Phenolics | Williamsia sp. | Not specified | [198] |
| Marinopyrones A–D | α-pyrones | Streptomonospora sp. | Inhibition of NO production | [199] |
| Glycerol 1-hydroxy-2,5-dimethyl benzoate | Salicylic derivative | Verrucosispora sp. | Anti-MRSA activity | [200] |
| Isomethoxyneihumicin | Lactam-lactim tautomers | Nocardiopsis alba | Strong cytotoxicity | [201] |
| Microindolinone A | Novel indole | Microbacterium sp. | No anti-allergic and anti-proliferative activities | [173] |
| Nesterenkoniane | Novel cyclic ether | Nesterenkonia flava | Moderate anti-allergic activity | [202] |
| Nocapyrones O–S | α-pyrones | Nocardiopsis sp. | Cytotoxicity | [203] |
| Paulomycin G | Glycosylated paulomycins | Micromonospora matsumotoense | Strong cytotoxic activity | [166] |
| Saccharomonopyrones A–C | α-pyrones | Saccharomonospora sp. | Weak antioxidant activity | [204] |
| Tetrocarcin Q | Spirotetronate glycoside | Micromonospora carbonacea | Moderate antibacterial activity | [205] |
| 22-dehydroxymethyl-kijanolide | Spirotetronate aglycone | Micromonospora harpali | No antibacterial activity | [206] |
| 8-hydroxy-22-dehydroxymethyl-kijanolide | Spirotetronate aglycone | Micromonospora harpali | No antibacterial activity | [206] |
| Microsporanates A–F | Spirotetronate glycosides | Micromonospora harpali | Antibacterial activity | [206] |
| Tetrocarcin P | Spirotetronate glycoside | Micromonospora harpali | Antibacterial activity | [206] |
| Nocazines F and G | Diketopiperazine | Nocardiopsis sp. | Excellent cytotoxicity | [207] |
| Kribellosides A-D | Alkyl glyceryl ethers | Kribbella sp. | Antifungal and RNA 5’-triphosphatase inhibitor | [208] |
| Branimycins B and C | Macrolide | Pseudonocardia carboxydivorans | Antibacterial activities | [209] |
| 1,2-naphthoquinone | Naphthalene derivative | Saccharopolyspora sp. | No cytotoxicity | [210] |
Marine Micromonospora spp. produced 37 out of the 167 compounds reported from mid-2013–2017 and were chemically diverse (Table 9; Figure 3). In total, 13 chemical classes, including macrolides, polyene macrocyclic lactams, polycyclic tetramic acid macrolactams, aromatic tautomers, hydroxamates, diterpenoids, diterpenes, angucyclines, quinolone alkaloids, dioxanes, glycosylated paulomycins, glycosides and aglycone spirotetrorates were identified in Micromonospora spp. during this period. Polyene macrolactams are an underexplored group of natural products that have only been found in actinomycetes. Micromonolactam is a new polyene macrocyclic lactam isolated from a marine Micromonospora sp. (Figure 3). However, micromonolactam did not show antibacterial activities against test pathogens [165]. Another interesting group of natural products, paulomycins, are glycosylated molecules containing a pauloate residue that are of pharmacological interest due to their strong antibiotic properties [166]. Paulomycin G is structurally unique because it is the smallest bioactive paulomycin in the paulomycin family of antibiotics, lacking the paulomycose moiety (Figure 3). Furthermore, a number of novel chemical skeletal structures are reported from marine Micromonospora spp. For example, polycyclic tetramic acid macrolactams of butremycin [167], halimane-type diterpenoids of micromonohalimanes [168] and a novel pimarane diterpene in isopimara-2-one-3-ol-8,15-diene [169] (Figure 3).
Additionally, other rare actinomycete genera have yielded a number of unique chemical moieties, which were not previously reported from microbially-derived natural products (Figure 4). For example, thiasporine A is the first natural product with a 5- hydroxy-4H-1,3-thiazin-4-one moiety, along with two new thiazole derivatives and were reported from Actinomycetospora chlora [170] (Figure 4). Other unusual structures include a curvularin macrolide with a rare α-D-glucopyranose substituent from Pseudonocardia sp. [171]; a butrepyrazinone, from Verrucosispora sp. that possesses an unusual methylation pattern on the pyrazinone ring [172], a novel indole microindolinone A from Microbacterium sp. [173], new dimeric indole derivatives with acetylcholinesterase (AchE) inhibitory activity from Rubrobacter radiotolerans [174] and a structurally new amycofuran bearing a rhamnose sugar from Amycolatopsis sp. [175].
Actinomadura sp. derived halomadurones (Figure 5) demonstrated potent nuclear factor E2-related factor antioxidant response element (Nrf2-ARE) activation, which is an important therapeutic approach for treatment of neurodegenerative diseases [176]. Cyanogramide obtained from Actinoalloteichus cyanogriseus showed efficient anticancer activity by reversing the adriamycin-induced resistance of K562/A02 and MCF-7/Adr cells, and the vincristine-induced resistance of KB/VCR cells [177].
A marine sponge-derived Actinokineospora sp. produces actinosporins with selective activity against the parasite Trypanosoma brucei brucei, the causative agent of sleeping sickness [182]. Fungal infections, particularly candidiasis, is one of the serious diseases worldwide. A novel antifungal polyketide, forazoline A isolated from Actinomadura sp. showing significant activity against Candida albicans works through a new mechanism of action by disrupting membrane integrity [186]. Another way of controlling candidiasis is by capping enzyme repressors. Inhibitors of the enzyme RNA 5’-triphosphatase in yeast may be used against pathogenic yeasts such as Candida. Interestingly, novel kribellosides from a marine Kribbella sp. inhibit activity of Cet1p (RNA 5’-triphosphatase) from Saccharomyces cerevisiae in vitro [208]. Another interesting biological activity is anti-allergic activity shown by nesterenkoniane, a novel cyclic ether isolated from the deep-sea-derived Nesterenkonia flava. Nesterenkoniane is the first report on secondary metabolites from the genus of Nesterenkonia [202]. Furthermore, discovery of anti-malarial drugs is one of the targets of research in pharma industries. Salinipostin A, isolated from the marine genus Salinispora shows potent antimalarial activity against Plasmodium falciparum growth (EC50 = 50 nM) and a high selectivity index (SI > 103) [192] (Figure 5).
4.4. Genome Mining of Marine Rare Actinomycetes
The rapid development of genome and metagenome sequencing methods including identification of secondary metabolite gene clusters has lead to the discovery of the genetic machinery encoding for novel natural products from microorganisms that have not yet been chemically identified [211]. The majority of these gene clusters encode for polyketides (PK), non-ribosomally synthesized peptides (NRP), ribosomally and post-translationally modified peptides (RiPPs) and aminoglycosides [211]. The bioinformatic analysis of genomes can also reveal silent secondary metabolite gene clusters, which are not expressed under standard laboratory conditions [212]. More than 23,000 PK and NRP have been reported so far, many of them found in actinomycetes, and they are being widely tested for pharmaceutical applications [213,214]. This approach has also been used for the identification of new antibiotic scaffolds from rare genera of actinomycetes from marine sediments [16]. Recently, Schorn and colleagues [215] have shown that rare marine actinomycetes-derived genomes demonstrated a high degree of novelty and diversity, with Corynebacterium, Gordonia, Nocardiopsis, Saccharomonospora and Pseudonocardia as genera representing the highest biosynthetic gene cluster diversity. A total of 13 new bioactive compounds have been derived from marine rare actinomycetes, such as Saccharomonospora sp., Salinispora spp., Micromonospora spp. and Streptosporangium sp. using genome-based approaches between mid 2013 and 2017 (Table 10).
Table 10.
New bioactive compounds discovered from marine rare actinomycetes using genome-based approaches between mid-2013 and 2017.
| Compounds | Chemical Class/Family | Marine Source | Biological Activity | Reference |
|---|---|---|---|---|
| Taromycin A | Dichlorinated lipopeptide | Saccharomonospora sp. | Moderate bioactivity against MDR pathogens | [217] |
| Retimycin A | Quinomycin-like depsipeptide | Salinispora sp. | Cytotoxicity against HCT-116 | [218] |
| Sioxanthin | Carotenoid | Salinispora sp. | Siderophore | [219] |
| Lobosamides A–C | Polyene macrolactams | Micromonospora sp. | Anti-protozoan parasite, Trypanosoma brucei | [220] |
| Hexaricins A–C | Pentangular polyphenols | Streptosporangium sp. | Not specified | [221] |
| Tetrocarcin N and O | Glycosidic spirotetronates | Micromonospora sp. | Modest antibacterial activity | [222] |
| Rifsaliniketal | Saliniketal | Salinispora sp. | Not specified | [223] |
| Nenestatin A | Benzofluorene | Micromonospora echinospora | Antibacterial activity | [224] |
These numbers of new biosynthetic gene clusters and corresponding compounds will undoubtly increase in the near future due to revolutionary developments in the genome- and metagenome-based approaches for drug discovery [215] and it likely that omics-based screening for novel bioactive compounds will prevail over microbial isolation as the most efficient method for first identification of bioactivity potential of strains and environmental samples [216].
5. Conclusions
In the last decade (2007–2017), a great range of diverse, new and rare actinomycetes have been isolated from the marine environment. Employment of heat-treatment of marine sediment samples, the use of low-nutrient agar medium (seawater agar) or a growth medium with natural seawater along with the use of antifungal agents, favor the isolation of marine rare actinomycetes. At least 177 new species, which represent 29 novel genera and 3 novel families, were obtained as pure cultures. Micromonosporaceae, Nocardioidaceae, Pseudonocardiaceae, Microbacteriaceae, Micrococcaceae, Demequinaceae, Nocardiopsaceae, Propionibacteriaceae and Intrasporangiaceae were the families most frequently isolated from the marine environment.
In total, 267 new natural products derived from 96 different marine rare actinomycete strains belonging to 28 genera have been reported from 2007 to 2017. Out of these 28 marine genera, Nocardiopsis, Micromonospora, Salinispora, Verrucosispora, Pseudonocardia and Actinoalloteichus are topmost producers of novel new secondary metabolites.
The rare actinomycetes isolated and biomolecules discovered represent most likely only the low-hanging fruits and the immense diversity of microorganisms in marine habitats as shown from large cultivation-independent studies [225,226] are the proof for the presence of an even larger diversity of currently uncultivable rare actinomycetes and putative secondary metabolites. This uncultured majority should be the target of future selective isolation strategies and procedures. In addition, genetic engineering of whole biosynthetic gene clusters is finally gaining ground [216] and may be the key to access hidden gene clusters from rare actinomycetes. A breakthrough in heterologous expression would herald ‘another golden age’ of novel bioactive natural product discovery, for which marine rare actinomycetes may be one of the important sources.
Acknowledgments
We are thankful to Steven Sutcliffe, the University of the South Pacific for English proofreading of the manuscript.
Author Contributions
R.S. conceived, designed the work and wrote the manuscript; D.S. conceived, critically analyzed the data, revised and corrected the manuscript.
Funding
D.S. acknowledges funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 679849 (SponGES).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Khan S.N., Khan A.U. Breaking the spell: Combating multidrug resistant ‘Superbugs’. Front. Microbiol. 2016;7:174. doi: 10.3389/fmicb.2016.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challinor V.L., Bode H.B. Bioactive natural products from novel microbial sources. Ann. N. Y. Acad. Sci. 2015;1354:82–97. doi: 10.1111/nyas.12954. [DOI] [PubMed] [Google Scholar]
- 3.Berdy J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012;65:385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- 4.Locey K.J., Lennon J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. USA. 2016;113:5970–5975. doi: 10.1073/pnas.1521291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barka E.A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Klenk H.-P., Clément C., Ouhdouch Y., van Wezel G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhakal D., Pokhrel A.R., Shrestha B., Sohng J.K. Marine rare actinobacteria: Isolation, characterization, and strategies for harnessing bioactive compounds. Front. Microbiol. 2017;8:1106. doi: 10.3389/fmicb.2017.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramani R., Aalbersberg W. Culturable rare actinomycetes: Diversity, isolation and marine natural product discovery. Appl. Microbiol. Biotechnol. 2013;97:9291–9321. doi: 10.1007/s00253-013-5229-7. [DOI] [PubMed] [Google Scholar]
- 8.Azman A.S., Othman I., Velu S.S., Chan K.G., Lee L.H. Mangrove rare actinobacteria: Taxonomy, natural compound and discovery of bioactivity. Front. Microbiol. 2015;6:856. doi: 10.3389/fmicb.2015.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arul Jose P., Jha B. Intertidal marine sediment harbours Actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 2017;7:10041. doi: 10.1038/s41598-017-09672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P., Zhang L., Guo X., Dai X., Liu L., Xi L., Wang J., Song L., Wang Y., Zhu Y., et al. Diversity, biogeography, and biodegradation potential of actinobacteria in the deep-sea sediments along the southwest indian ridge. Front Microbiol. 2016;7:1340. doi: 10.3389/fmicb.2016.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claverías F.P., Undabarrena A., González M., Seeger M., Cámara B. Culturable diversity and antimicrobial activity of Actinobacteria from marine sediments in Valparaíso bay, Chile. Front. Microbiol. 2015;6:737. doi: 10.3389/fmicb.2015.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hames-Kocabas E.E., Uzel A. Isolation strategies of marine-derived actinomycetes from sponge and sediment samples. J. Microbiol. Methods. 2012;88:342–347. doi: 10.1016/j.mimet.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado L.A., Fenical W., Jensen P.R., Kauffman C.A., Mincer T.J., Ward A.C., Bull A.T., Goodfellow M. Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int. J. Syst. Evol. Microbiol. 2005;55:1759–1766. doi: 10.1099/ijs.0.63625-0. [DOI] [PubMed] [Google Scholar]
- 14.Zotchev S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 2012;158:68–175. doi: 10.1016/j.jbiotec.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Overmann J., Lepleux C. Marine Bacteria and Archaea: Diversity, adaptations, and culturability. In: Stal L.J., Cretoiu M.S., editors. The Marine Microbiome: An Untapped Source of Biodiversity and Biotechnological Potential. Springer; Cham, Switzerland: 2016. pp. 21–55. [Google Scholar]
- 16.Schwager E., Luo C., Huttenhower C., Morgan X.C. Sequencing and other tools for studying microbial communities: Genomics and “meta’omic” tools are enabling us to explore the microbiome from three complementary perspectives—Taxonomic, functional and ecological. Microbe. 2015;10:419–425. doi: 10.1128/microbe.10.419.1. [DOI] [Google Scholar]
- 17.Kaeberlein T., Lewis K., Epstein S.S. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science. 2002;296:1127–1129. doi: 10.1126/science.1070633. [DOI] [PubMed] [Google Scholar]
- 18.Zengler K., Toledo G., Rappé M., Elkins J., Mathur E.J., Short J.M., Keller M. Cultivating the uncultured. Proc. Natl. Acad. Sci. USA. 2002;99:15681–15686. doi: 10.1073/pnas.252630999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vartoukian S.R., Palmer R.M., Wade W.G. Strategies for culture of ‘unculturable’ bacteria. FEMS Microbiol. Lett. 2010;309:1–7. doi: 10.1111/j.1574-6968.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart E.J. Growing unculturable bacteria. J. Bacteriol. 2012;194:4151–4160. doi: 10.1128/JB.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamagata Y. Keys to cultivating uncultured microbes: Elaborate enrichment strategies and resuscitation of dormant cells. Microbes Environ. 2015;30:289–290. doi: 10.1264/jsme2.ME3004rh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y., Li Q., Chen X., Jiang C. Isolation and cultivation methods of Actinobacteria. In: Dhanasekaran D., Jiang Y., editors. Actinobacteria-Basics and Biotechnological Applications. InTech; London, UK: 2016. pp. 39–57. Chapter 2. [Google Scholar]
- 23.Azman A.S., Zainal N., Mutalib N.A., Yin W.F., Chan K.G., Lee L.H. Monashia flava gen. nov., sp. nov., an actinobacterium of the family Intrasporangiaceae. Int. J. Syst. Evol. Microbiol. 2016;66:554–561. doi: 10.1099/ijsem.0.000753. [DOI] [PubMed] [Google Scholar]
- 24.Lai H., Wei X., Jiang Y., Chen X., Li Q., Jiang Y., Jiang C., Gillerman L. Halopolyspora alba gen. nov., sp. nov., isolated from sediment. Int. J. Syst. Evol. Microbiol. 2014;64:2775–2780. doi: 10.1099/ijs.0.057638-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G., Wang S., Wang L. Sediminivirga luteola gen. nov., sp. nov., a member of the family Brevibacteriaceae, isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2016;66:1494–1498. doi: 10.1099/ijsem.0.000909. [DOI] [PubMed] [Google Scholar]
- 26.Lai H., Jiang Y., Chen X., Li Q., Jiang C., Jiang Y., Wei X. Haloactinomyces albus gen. nov., sp. nov., isolated from the dead sea. Int. J. Syst. Evol. Microbiol. 2017;67:1163–1168. doi: 10.1099/ijsem.0.001783. [DOI] [PubMed] [Google Scholar]
- 27.Huang H.Q., Xing S.S., Yuan W.D., Wang Y., Liu M., Sun Q.G., Lin X.Z., Bao S.X. Nocardiopsis mangrovei sp. nov., isolated from mangrove sediment. Antonie Van Leeuwenhoek. 2015;107:1541–1556. doi: 10.1007/s10482-015-0447-x. [DOI] [PubMed] [Google Scholar]
- 28.Jensen P.R., Gontang E., Mafnas C., Mincer T.J., Fenical W. Culturable marine actinomycete diversity from tropical pacific ocean sediments. Environ. Microbiol. 2005;7:1039–1048. doi: 10.1111/j.1462-2920.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 29.Selvin J., Shanmughapriya S., Gandhimathi R., Kiran G.S., Ravji T.R., Natarajaseenivasan K., Hema T.A. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl. Microbiol. Biotechnol. 2009;83:435–445. doi: 10.1007/s00253-009-1878-y. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado L.A., Stach J.E.M., Pathom-aree W., Ward A.C., Bull A.T., Goodfellow M. Diversity of cultivable actinobacteria in geographically widespread marine Sediments. Antonie Van Leeuwenhoek. 2005;87:11–18. doi: 10.1007/s10482-004-6525-0. [DOI] [PubMed] [Google Scholar]
- 31.Mincer T.J., Fenical W., Jensen P.R. Cultured and culture-independent diversity within the obligate marine actinomycete genus Salinispora. Appl. Environ. Microbiol. 2005;71:7019–7028. doi: 10.1128/AEM.71.11.7019-7028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gontang E.A., Fenical W., Jensen P.R. Phylogenetic diversity of Gram-positive bacteria cultured from marine sediments. Appl. Environ. Microbiol. 2007;73:3272–3282. doi: 10.1128/AEM.02811-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy J., Baker P., Piper C., Cotter P.D., Walsh M., Mooij M.J., Bourke M.B., Rea M.C., O’Connor P.M., Ross R.P., et al. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar. Biotechnol. 2009;11:384–396. doi: 10.1007/s10126-008-9154-1. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado L.A., Frangoso-Yanez D., Perez-Garcia A., Rosellon-Druker J., Quintana E. Actinobacterial diversity from marine sediments collected in Mexico. Antonie Van Leeuwenhoek. 2009;95:111–120. doi: 10.1007/s10482-008-9294-3. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Zhou Y., Zhang G. Nocardioides flavus sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2016;66:5275–5280. doi: 10.1099/ijsem.0.001507. [DOI] [PubMed] [Google Scholar]
- 36.Jang G.I., Cho Y., Cho B.C. Pontimonas salivibrio gen. nov., sp. nov., a new member of the family Microbacteriaceae isolated from a seawater reservoir of a solar saltern. Int. J. Syst. Evol. Microbiol. 2013;63:2124–2131. doi: 10.1099/ijs.0.043661-0. [DOI] [PubMed] [Google Scholar]
- 37.Lee S.D. Tamlicoccus marinus gen. nov., sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 2013;63:1951–1954. doi: 10.1099/ijs.0.043117-0. [DOI] [PubMed] [Google Scholar]
- 38.De Menezes C.B., Tonin M.F., Silva L.J., de Souza W.R., Parma M., de Melo I.S., Zucchi T.D., Destéfano S.A., Fantinatti-Garboggini F. Marmoricola aquaticus sp. nov., an actinomycete isolated from a marine sponge. Int. J. Syst. Evol. Microbiol. 2015;65:2286–2291. doi: 10.1099/ijs.0.000254. [DOI] [PubMed] [Google Scholar]
- 39.Afonso de Menezes C.B., Afonso R.S., Souza W.R., Parma M., Melo I.S., Zucchi T.D., Fantinatti-Garboggini F. Williamsia spongiae sp. nov., an actinomycete isolated from the marine sponge Amphimedon viridis. Int. J. Syst. Evol. Microbiol. 2017;67:1260–1265. doi: 10.1099/ijsem.0.001796. [DOI] [PubMed] [Google Scholar]
- 40.Souza D.T., Silva F.S.P.D., Silva L.J.D., Crevelin E.J., Moraes L.A.B., Zucchi T.D., Melo I.S. Saccharopolyspora spongiae sp. nov., a novel actinomycete isolated from the marine sponge Scopalina ruetzleri (Wiedenmayer, 1977) Int. J. Syst. Evol. Microbiol. 2017;67:2019–2025. doi: 10.1099/ijsem.0.001912. [DOI] [PubMed] [Google Scholar]
- 41.Ramaprasad E.V., Sasikala C., Ramana C.V. Ornithinimicrobium algicola sp. nov., a marine actinobacterium isolated from the green alga of the genus Ulva. Int. J. Syst. Evol. Microbiol. 2015;65:4627–4631. doi: 10.1099/ijsem.0.000624. [DOI] [PubMed] [Google Scholar]
- 42.Phongsopitanun W., Kudo T., Ohkuma M., Pittayakhajonwut P., Suwanborirux K., Tanasupawat S. Micromonospora sediminis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2016;66:3235–3240. doi: 10.1099/ijsem.0.001175. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D.F., Wang H.F., Xiong Z.J., Tian X.P., Liu L., Zhang X.M., Jiang Z., Zhang S., Li W.J. Mariniluteicoccus flavus gen. nov., sp. nov., a new member of the family Propionibacteriaceae, isolated from a deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2014;64:1051–1056. doi: 10.1099/ijs.0.058404-0. [DOI] [PubMed] [Google Scholar]
- 44.Pathom-aree W., Stach J.E.M., Ward A.C., Horikoshi K., Bull A.T., Goodfellow M. Diversity of actinomycetes isolated from Challenger deep sediment (10,898 m) from the Mariana Trench. Extremophiles. 2006;10:181–189. doi: 10.1007/s00792-005-0482-z. [DOI] [PubMed] [Google Scholar]
- 45.Takizawa M., Colwell R.R., Hill R.T. Isolation and diversity of actinomycetes in the Chesapeake Bay. Appl. Environ. Microbiol. 1993;59:997–1002. doi: 10.1128/aem.59.4.997-1002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenical W., Jensen P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y., Cao Y.R., Zhao L.X., Wang Q., Jin R.X., He W.X., Xue Q.H. Treatment of ultrasonic to soil sample for increase of the kind of rare actinomycetes. Acta Microbiol. Sin. 2010;50:1094–1097. [PubMed] [Google Scholar]
- 48.Margulis L., Chapman M.J. An Illustrated Guide to the Phyla of Life on Earth. 1st ed. Elsevier Science, Marine Biological Laboratory; Woods Hole, MA, USA: 2009. Kingdoms and domains; p. 732. [Google Scholar]
- 49.Jensen P.R., Dwight R., Fenical W. Distribution of actinomycetes in near-shore tropical marine sediments. Appl. Environ. Microbiol. 1991;57:1102–1108. doi: 10.1128/aem.57.4.1102-1108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee L.H., Azman A.S., Zainal N., Eng S.K., Ab Mutalib N.S., Yin W.F., Chan K.G. Microbacterium mangrovi sp. nov., an amylolytic actinobacterium isolated from mangrove forest soil. Int. J. Syst. Evol. Microbiol. 2014;64:3513–3519. doi: 10.1099/ijs.0.062414-0. [DOI] [PubMed] [Google Scholar]
- 51.Lee L.H., Azman A.S., Zainal N., Yin W.F., Mutalib N.S., Chan K.G. Sinomonas humi sp. nov., an amylolytic actinobacterium isolated from mangrove forest soil. Int. J. Syst. Evol. Microbiol. 2015;65:996–1002. doi: 10.1099/ijs.0.000053. [DOI] [PubMed] [Google Scholar]
- 52.Ren J., Li L., Wei B., Tang Y.L., Deng Z.X., Sun M., Hong K. Micromonospora wenchangensis sp. nov., isolated from mangrove soil. Int. J. Syst. Evol. Microbiol. 2013;63:2389–2395. doi: 10.1099/ijs.0.045476-0. [DOI] [PubMed] [Google Scholar]
- 53.Mincer T.J., Jensen P.R., Kauffman C.A., Fenical W. Widespread and persistent populations of a major new marine actinomycete taxon in ocean sediments. Appl. Environ. Microbiol. 2002;68:5005–5011. doi: 10.1128/AEM.68.10.5005-5011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bredholdt H., Tjaervik E., Johnsen G., Zotchev S.B. Actinomycetes from sediments in the Trondheim fjord, Norway: Diversity and biological activity. Mar. Drugs. 2008;6:12–24. doi: 10.3390/md6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokare C.R., Mahadik K.R., Kadam S.S. Isolation of bioactive marine Actinomycetes from sediments isolated from Goa and Maharashtra coastlines (west coast of India) Indian J. Mar. Sci. 2004;33:248–256. [Google Scholar]
- 56.Naikpatil S.V., Rathod J.L. Selective isolation and antimicrobial activity of rare Actinomycetes from mangrove sediment of Karwar. J. Ecobiotechnol. 2011;3:48–53. [Google Scholar]
- 57.Mangamuri U.K., Muvva V., Poda S., Kamma S. Isolation, identification and molecular characterization of rare Actinomycetes from mangrove ecosystem of Nizampatnam. Malays. J Microbiol. 2012;8:83–91. doi: 10.21161/mjm.03212. [DOI] [Google Scholar]
- 58.Terahara T., Kobayashi T., Imada C. An effective method based on wet-heat treatment for the selective isolation of Micromonospora from estuarine sediments. World J. Microbiol. Biotechnol. 2013;29:1677–1684. doi: 10.1007/s11274-013-1330-4. [DOI] [PubMed] [Google Scholar]
- 59.Solano G., Rojas-Jiménez K., Jaspars M., Tamayo-Castillo G. Study of the diversity of culturable Actinomycetes in the North Pacific and Caribbean coasts of Costa Rica. Antonie Van Leeuwenhoek. 2009;96:71–78. doi: 10.1007/s10482-009-9337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong K., Gao A.H., Xie Q.Y., Gao H., Zhuang L., Lin H.P., Yu H.P., Li J., Yao X.S., Goodfellow M., et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs. 2009;7:24–44. doi: 10.3390/md7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bredholdt H., Galatenko O.A., Engelhardt K., Tjaervik E., Terekhova L.P., Zotchev S.B. Rare actinomycete bacteria from the shallow water sediments of the Trondheim fjord, Norway: Isolation, diversity and biological activity. Environ. Microbiol. 2007;9:2756–2764. doi: 10.1111/j.1462-2920.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- 62.Cumsille A., Undabarrena A., González V., Claverías F., Rojas C., Cámara B. Biodiversity of actinobacteria from the South Pacific and the assessment of Streptomyces chemical diversity with metabolic profiling. Mar. Drugs. 2017;15:286. doi: 10.3390/md15090286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skropeta D., Wei L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014;31:999–1025. doi: 10.1039/C3NP70118B. [DOI] [PubMed] [Google Scholar]
- 64.Tiwari K., Gupta R.K. Diversity and isolation of rare actinomycetes: An overview. Crit. Rev. Microbiol. 2012;39:256–294. doi: 10.3109/1040841X.2012.709819. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Murcia A.J., Collins M.D. A phylogenetic analysis of the genus Leuconostoc based on reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol. Lett. 1990;70:73–83. doi: 10.1111/j.1574-6968.1990.tb03780.x. [DOI] [PubMed] [Google Scholar]
- 66.Collins M.D., Rodrigues U., Ash C., Aguirre M., Farrow J.A.E., Martinez-Murcia A., Phillips B.A., Williams A.M., Wallbanks S. Phylogenetic analysis of the genus Lactobacillus and related lactic acid bacteria as determined by reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol. Lett. 1991;77:5–12. doi: 10.1111/j.1574-6968.1991.tb04313.x. [DOI] [Google Scholar]
- 67.Amann R.I., Lin C., Key R., Montgomery L., Stahl D.A. Diversity among Fibrobacter isolates: Towards a phylogenetic classification. Syst. Appl. Microbiol. 1992;15:23–31. doi: 10.1016/S0723-2020(11)80133-5. [DOI] [Google Scholar]
- 68.Fox G.E., Wisotzkey J.D., Jurtshuk P., Jr. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Murcia A.J., Benlloch S., Collins M.D. Phylogenetic interrelationships of members of the genera Aeromonas and Pleisiomonas as determined by 16S ribosomal DNA sequencing: Lack of congruence with results of DNA-DNA hybridizations. Int. J. Syst. Bacteriol. 1992;42:412–421. doi: 10.1099/00207713-42-3-412. [DOI] [PubMed] [Google Scholar]
- 70.Schlaberg R., Simmon K.E., Fisher M.A. A systematic approach for discovering novel, clinically relevant bacteria. Emerg. Infect. Dis. 2012;18:422–430. doi: 10.3201/eid1803.111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yilmaz P., Parfrey L.W., Yarza P., Gerken J., Pruesse E., Quast C., Schweer T., Peplies J., Ludwig W., Glöckner F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42:643–648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snelgrove P., Blackburn T., Hutchings P.A., Alongi D.M., Grassle J.F., Hummel H., King G., Koike I., Lambshead P.J.D., Ramsing N.B., et al. The importance of marine sediment biodiversity in ecosystem processes. Ambio. 1997;26:578–583. [Google Scholar]
- 73.Harino H., Arai T., Ohji M., Miyazaki N. Organotin contamination in deep sea environment. In: Arai T., Harino H., Ohji M., Langston W.J., editors. Ecotoxicology of Antifouling Biocides. Springer; New York, NY, USA: 2009. pp. 95–97. [Google Scholar]
- 74.Skropeta D. Deep-sea natural products. Nat. Prod. Rep. 2008;25:1131–1166. doi: 10.1039/b808743a. [DOI] [PubMed] [Google Scholar]
- 75.Durden J.M., Bett B.J., Jones D.O.B., Huvenne V.A.I., Ruhl H.A. Abyssal hills hidden source of increased habitat heterogeneity, benthic megafaunal biomass and diversity in the deep sea. Prog. Oceanogr. 2015;137:209–218. doi: 10.1016/j.pocean.2015.06.006. [DOI] [Google Scholar]
- 76.Haefner B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today. 2003;8:536–544. doi: 10.1016/S1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 77.Hobbie J.E., Daley R.J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porter K.G., Feig Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–948. doi: 10.4319/lo.1980.25.5.0943. [DOI] [Google Scholar]
- 79.Whitman W.B., Coleman D.C., Wiebe W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das S., Mangwani N. Ocean acidification and marine microorganisms: Responses and consequences. Oceanologia. 2015;57:349–361. doi: 10.1016/j.oceano.2015.07.003. [DOI] [Google Scholar]
- 81.Vicente J., Stewart A., Song B., Hill R.T., Wright J.L. Biodiversity of actinomycetes associated with caribbean sponges and their potential for natural product discovery. Mar. Biotechnol. 2013;15:413–424. doi: 10.1007/s10126-013-9493-4. [DOI] [PubMed] [Google Scholar]
- 82.Veyisoglu A., Sazak A., Cetin D., Guven K., Sahin N. Saccharomonospora amisosensis sp. nov., isolated from deep marine sediment. Int. J. Syst. Evol. Microbiol. 2013;63:3782–3786. doi: 10.1099/ijs.0.051516-0. [DOI] [PubMed] [Google Scholar]
- 83.Zhang D.F., Chen W., He J., Zhang X.M., Xiong Z.J., Sahu M.K., Sivakumar K., Li W.J. Saccharomonospora oceani sp. nov. isolated from marine sediments in Little Andaman, India. Antonie Van Leeuwenhoek. 2013;103:1377–1384. doi: 10.1007/s10482-013-9918-0. [DOI] [PubMed] [Google Scholar]
- 84.Zhang D.F., Jiang Z., Zhang X.M., Yang L.L., Tian X.P., Long L.J., Zhang S., Li W.J. Actinophytocola sediminis sp. nov., an actinomycete isolated from a marine sediment. Int. J. Syst. Evol. Microbiol. 2014;64:2834–2840. doi: 10.1099/ijs.0.062638-0. [DOI] [PubMed] [Google Scholar]
- 85.Zhang D.F., Jiang Z., Li L., Liu B.B., Zhang X.M., Tian X.P., Zhang S., Li W.J. Pseudonocardia sediminis sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2014;64:745–750. doi: 10.1099/ijs.0.057844-0. [DOI] [PubMed] [Google Scholar]
- 86.Wei X., Jiang Y., Chen X., Jiang Y., Lai H. Amycolatopsis flava sp. nov., a halophilic actinomycete isolated from dead sea. Antonie Van Leeuwenhoek. 2015;108:879–885. doi: 10.1007/s10482-015-0542-z. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Y., Wei X., Chen X., Jiang Y., Xue Q., Lai H., Jiang C. Saccharopolyspora griseoalba sp. nov., a novel actinomycete isolated from the dead sea. Antonie Van Leeuwenhoek. 2016;109:1635–1641. doi: 10.1007/s10482-016-0763-9. [DOI] [PubMed] [Google Scholar]
- 88.Zhang G., Wang L., Li J., Zhou Y. Amycolatopsis albispora sp. nov., isolated from deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2016;66:3860–3864. doi: 10.1099/ijsem.0.000846. [DOI] [PubMed] [Google Scholar]
- 89.Zhang G., Wang L., Li J., Zhou Y. Pseudonocardia profundimaris sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2017;67:1693–1697. doi: 10.1099/ijsem.0.001849. [DOI] [PubMed] [Google Scholar]
- 90.Fan X., Qiao Y., Gao X., Zhang X.H. Nocardioides pacificus sp. nov., isolated from deep sub-seafloor sediment. Int. J. Syst. Evol. Microbiol. 2014;64:2217–2222. doi: 10.1099/ijs.0.059873-0. [DOI] [PubMed] [Google Scholar]
- 91.Zhang D.F., Zhong J.M., Zhang X.M., Jiang Z., Zhou E.M., Tian X.P., Zhang S., Li W.J. Nocardioides nanhaiensis sp. nov., an actinobacterium isolated from a marine sediment sample. Int. J. Syst. Evol. Microbiol. 2014;64:2718–2722. doi: 10.1099/ijs.0.062851-0. [DOI] [PubMed] [Google Scholar]
- 92.Deng S., Chang X., Zhang Y., Ren L., Jiang F., Qu Z., Peng F. Nocardioides antarcticus sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2015;65:2615–2621. doi: 10.1099/ijs.0.000309. [DOI] [PubMed] [Google Scholar]
- 93.Lee D.W., Lee A.H., Lee H., Kim J.J., Khim J.S., Yim U.H., Kim B.S. Nocardioides litoris sp. nov., isolated from the Taean seashore. Int. J. Syst. Evol. Microbiol. 2017;67:2332–2336. doi: 10.1099/ijsem.0.002188. [DOI] [PubMed] [Google Scholar]
- 94.Zhang D.F., Pan H.Q., He J., Zhang X.M., Zhang Y.G., Klenk H.P., Hu J.C., Li W.J. Description of Streptomonospora sediminis sp. nov. and Streptomonospora nanhaiensis sp. nov., and reclassification of Nocardiopsis arabia Hozzein & Goodfellow 2008 as Streptomonospora arabica comb. nov. and emended description of the genus Streptomonospora. Int. J. Syst. Evol. Microbiol. 2013;63:4447–4455. doi: 10.1099/ijs.0.052704-0. [DOI] [PubMed] [Google Scholar]
- 95.Pan H.Q., Zhang D.F., Li L., Jiang Z., Cheng J., Zhang Y.G., Wang H.F., Hu J.C., Li W.J. Nocardiopsis oceani sp. nov. and Nocardiopsis nanhaiensis sp. nov., actinomycetes isolated from marine sediment of the South China Sea. Int. J. Syst. Evol. Microbiol. 2015;65:3384–3391. doi: 10.1099/ijsem.0.000425. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y., Ren H., Zhang G. Microbacterium hydrothermale sp. nov., an actinobacterium isolated from hydrothermal sediment. Int. J. Syst. Evol. Microbiol. 2014;64:3508–3512. doi: 10.1099/ijs.0.061697-0. [DOI] [PubMed] [Google Scholar]
- 97.Hamada M., Shibata C., Tamura T., Suzuki K. Agromyces marinus sp. nov., a novel actinobacterium isolated from sea sediment. J. Antibiot. 2014;67:703–706. doi: 10.1038/ja.2014.60. [DOI] [PubMed] [Google Scholar]
- 98.Mawlankar R.R., Mual P., Sonalkar V.V., Thorat M.N., Verma A., Srinivasan K., Dastager S.G. Microbacterium enclense sp. nov., isolated from sediment sample. Int. J. Syst. Evol. Microbiol. 2015;65:2064–2070. doi: 10.1099/ijs.0.000221. [DOI] [PubMed] [Google Scholar]
- 99.Yan L., Wang J., Chen Z., Guan Y., Li J. Microbacterium nanhaiense sp. nov., an actinobacterium isolated from sea sediment. Int. J. Syst. Evol. Microbiol. 2015;65:3697–3702. doi: 10.1099/ijsem.0.000480. [DOI] [PubMed] [Google Scholar]
- 100.Hamada M., Shibata C., Tamura T., Suzuki K. Zhihengliuella flava sp. nov., an actinobacterium isolated from sea sediment, and emended description of the genus Zhihengliuella. Int. J. Syst. Evol. Microbiol. 2013;63:4760–4764. doi: 10.1099/ijs.0.053272-0. [DOI] [PubMed] [Google Scholar]
- 101.Dastager S.G., Tang S.K., Srinivasan K., Lee J.C., Li W.J. Kocuria indica sp. nov., isolated from a sediment sample. Int. J. Syst. Evol. Microbiol. 2014;64:869–874. doi: 10.1099/ijs.0.052548-0. [DOI] [PubMed] [Google Scholar]
- 102.Zhang G., Zhang Y., Yin X., Wang S. Nesterenkonia alkaliphila sp. nov., an alkaliphilic, halotolerant actinobacteria isolated from the western Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2015;65:516–521. doi: 10.1099/ijs.0.065623-0. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Z., Zhang W.H., Yuan C.G., Chen J.Y., Cao L.X., Park D.J., Xiao M., Kim C.J., Li W.J. Kocuria subflava sp. nov., isolated from marine sediment from the Indian Ocean. Antonie Van Leeuwenhoek. 2015;108:1349–1355. doi: 10.1007/s10482-015-0587-z. [DOI] [PubMed] [Google Scholar]
- 104.Fan X., Zhang Z., Li Z., Zhang X.H. Luteococcus sediminum sp. nov., isolated from deep subseafloor sediment of the South Pacific Gyre. Int. J. Syst. Evol. Microbiol. 2014;64:2522–2527. doi: 10.1099/ijs.0.058529-0. [DOI] [PubMed] [Google Scholar]
- 105.Puente-Sánchez F., Sánchez-Román M., Amils R., Parro V. Tessaracoccus lapidicaptus sp. nov., an actinobacterium isolated from the deep subsurface of the Iberian pyrite belt. Int. J. Syst. Evol. Microbiol. 2014;64:3546–3552. doi: 10.1099/ijs.0.060038-0. [DOI] [PubMed] [Google Scholar]
- 106.Thongphrom C., Kim J.H., Bora N., Kim W. Tessaracoccus arenae sp. nov., isolated from sea sand. Int. J. Syst. Evol. Microbiol. 2017;67:2008–2013. doi: 10.1099/ijsem.0.001907. [DOI] [PubMed] [Google Scholar]
- 107.Dastager S.G., Mawlankar R., Tang S.K., Krishnamurthi S., Ramana V.V., Joseph N., Shouche Y.S. Rhodococcus enclensis sp. nov., a novel member of the genus Rhodococcus. Int. J. Syst. Evol. Microbiol. 2014;64:2693–2699. doi: 10.1099/ijs.0.061390-0. [DOI] [PubMed] [Google Scholar]
- 108.Bai J.L., Wang Y., Qin S., Ding P., Xing K., Yuan B., Cao C.L., Huang Y., Zhang Y.Q., Jiang J.H. Nocardia jiangsuensis sp. nov., an actinomycete isolated from coastal soil. Int. J. Syst. Evol. Microbiol. 2016;66:4633–4638. doi: 10.1099/ijsem.0.001402. [DOI] [PubMed] [Google Scholar]
- 109.Phongsopitanun W., Kudo T., Mori M., Shiomi K., Pittayakhajonwut P., Suwanborirux K., Tanasupawat S. Micromonospora fluostatini sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2015;65:4417–4423. doi: 10.1099/ijsem.0.000589. [DOI] [PubMed] [Google Scholar]
- 110.Veyisoglu A., Carro L., Guven K., Cetin D., Spröer C., Schumann P., Klenk H.P., Goodfellow M., Sahin N. Micromonospora yasonensis sp. nov., isolated from a black sea sediment. Antonie Van Leeuwenhoek. 2016;109:1019–1028. doi: 10.1007/s10482-016-0701-x. [DOI] [PubMed] [Google Scholar]
- 111.Veyisoglu A., Carro L., Cetin D., Guven K., Spröer C., Pötter G., Klenk H.P., Sahin N., Goodfellow M. Micromonospora profundi sp. nov., isolated from deep marine sediment. Int. J. Syst. Evol. Microbiol. 2016;66:4735–4743. doi: 10.1099/ijsem.0.001419. [DOI] [PubMed] [Google Scholar]
- 112.Park S., Jung Y.T., Won S.M., Lee J.S., Yoon J.H. Demequina activiva sp. nov., isolated from a tidal flat. Int. J. Syst. Evol. Microbiol. 2015;65:2042–2047. doi: 10.1099/ijs.0.000217. [DOI] [PubMed] [Google Scholar]
- 113.Park S., Jung Y.T., Won S.M., Yoon J.H. Demequina litorisediminis sp. nov., isolated from a tidal flat, and emended description of the genus Demequina. Int. J. Syst. Evol. Microbiol. 2016;66:4197–4203. doi: 10.1099/ijsem.0.001335. [DOI] [PubMed] [Google Scholar]
- 114.Hamada M., Shibata C., Tamura T., Yamamura H., Hayakawa M., Suzuki K. Janibacter cremeus sp. nov., an actinobacterium isolated from sea sediment. Int. J. Syst. Evol. Microbiol. 2013;63:3687–3690. doi: 10.1099/ijs.0.051532-0. [DOI] [PubMed] [Google Scholar]
- 115.Zhang G., Ren H., Wang S., Chen X., Yang Y., Zhang Y., Jiang Y. Janibacter indicus sp. nov., isolated from hydrothermal sediment of the Indian Ocean. Int. J. Syst. Evol. Microbiol. 2014;64:2353–2357. doi: 10.1099/ijs.0.059527-0. [DOI] [PubMed] [Google Scholar]
- 116.You Z.Q., Li J., Qin S., Tian X.P., Wang F.Z., Zhang S. Georgenia sediminis sp. nov., a moderately thermophilic actinobacterium isolated from sediment. Int. J. Syst. Evol. Microbiol. 2013;63:4243–4247. doi: 10.1099/ijs.0.051714-0. [DOI] [PubMed] [Google Scholar]
- 117.Wang S., Xu X., Wang L., Jiao K., Zhang G. Georgenia subflava sp. nov., isolated from a deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2015;65:4146–4150. doi: 10.1099/ijsem.0.000553. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto A., Kasai H., Matsuo Y., Shizuri Y., Ichikawa N., Fujita N., Omura S., Takahashi Y. Ilumatobacter nonamiense sp. nov. and Ilumatobacter coccineum sp. nov., isolated from seashore sand. Int. J. Syst. Evol. Microbiol. 2013;63:3404–3408. doi: 10.1099/ijs.0.047316-0. [DOI] [PubMed] [Google Scholar]
- 119.Chen P., Zhang L., Wang J., Ruan J., Han X., Huang Y. Brevibacterium sediminis sp. nov., isolated from deep-sea sediments from the Carlsberg and southwest Indian ridges. Int. J. Syst. Evol. Microbiol. 2016;66:5268–5274. doi: 10.1099/ijsem.0.001506. [DOI] [PubMed] [Google Scholar]
- 120.Du Z.J., Miao T.T., Lin X.Z., Liu Q.Q., Chen G.J. Flaviflexus huanghaiensis gen. nov., sp. nov., an actinobacterium of the family Actinomycetaceae. Int. J. Syst. Evol. Microbiol. 2013;63:1863–1867. doi: 10.1099/ijs.0.042044-0. [DOI] [PubMed] [Google Scholar]
- 121.Hamada M., Tamura T., Shibata C., Yamamura H., Hayakawa M., Schumann P., Suzuki K. Paraoerskovia sediminicola sp. nov., an actinobacterium isolated from sea sediment, and emended description of the genus Paraoerskovia. Int. J. Syst. Evol. Microbiol. 2013;63:2637–2641. doi: 10.1099/ijs.0.043745-0. [DOI] [PubMed] [Google Scholar]
- 122.Cho Y., Jang G.I., Cho B.C. Nocardioides marinquilinus sp. nov., isolated from coastal seawater. Int. J. Syst. Evol. Microbiol. 2013;63:2594–2599. doi: 10.1099/ijs.0.047902-0. [DOI] [PubMed] [Google Scholar]
- 123.Cho Y., Jang G.I., Hwang C.Y., Kim E.H., Cho B.C. Nocardioides salsibiostraticola sp. nov., isolated from biofilm formed in coastal seawater. Int. J. Syst. Evol. Microbiol. 2013;63:3800–3806. doi: 10.1099/ijs.0.051037-0. [DOI] [PubMed] [Google Scholar]
- 124.Wang L., Li J., Zhang G. Nocardioides rotundus sp. nov., isolated from deep seawater. Int. J. Syst. Evol. Microbiol. 2016;66:1932–1936. doi: 10.1099/ijsem.0.000966. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L., Xi L., Qiu D., Song L., Dai X., Ruan J., Huang Y. Cellulomonas marina sp. nov., isolated from deep-sea water. Int. J. Syst. Evol. Microbiol. 2013;63:3014–3018. doi: 10.1099/ijs.0.048876-0. [DOI] [PubMed] [Google Scholar]
- 126.Zhang L., Xi L., Ruan J., Huang Y. Kocuria oceani sp. nov., isolated from a deep-sea hydrothermal plume. Int. J. Syst. Evol. Microbiol. 2017;67:164–169. doi: 10.1099/ijsem.0.001713. [DOI] [PubMed] [Google Scholar]
- 127.Kaur G., Kumar N., Mual P., Kumar A., Kumar R.M., Mayilraj S. Brachybacterium aquaticum sp. nov., a novel actinobacterium isolated from seawater. Int. J. Syst. Evol. Microbiol. 2016;66:4705–4710. doi: 10.1099/ijsem.0.001414. [DOI] [PubMed] [Google Scholar]
- 128.Yang J., Gao J., Cheung A., Liu B., Schwendenmann L. Vegetation and sediment characteristics in expanding mangrove forest in New Zealand. Eastuar. Coast. Shelf. Sci. 2013;134:11–18. doi: 10.1016/j.ecss.2013.09.017. [DOI] [Google Scholar]
- 129.Hong K. Actinomycetes from mangrove and their secondary metabolites. Wei Sheng Wu Xue Bao. 2013;53:1131–1141. [PubMed] [Google Scholar]
- 130.Supong K., Suriyachadkun C., Suwanborirux K., Pittayakhajonwut P., Thawai C. Verrucosispora andamanensis sp. nov., isolated from a marine sponge. Int. J. Syst. Evol. Microbiol. 2013;63:3970–3974. doi: 10.1099/ijs.0.050906-0. [DOI] [PubMed] [Google Scholar]
- 131.Supong K., Suriyachadkun C., Pittayakhajonwut P., Suwanborirux K., Thawai C. Micromonospora spongicola sp. nov., an actinomycete isolated from a marine sponge in the Gulf of Thailand. J. Antibiot. 2013;66:505–509. doi: 10.1038/ja.2013.35. [DOI] [PubMed] [Google Scholar]
- 132.Wu J.F., Li J., You Z.Q., Zhang S. Prauserella coralliicola sp. nov., isolated from the coral Galaxea fascicularis. Int. J. Syst. Evol. Microbiol. 2014;64:3341–3345. doi: 10.1099/ijs.0.062679-0. [DOI] [PubMed] [Google Scholar]
- 133.Kaur G., Mual P., Kumar N., Verma A., Kumar A., Krishnamurthi S., Mayilraj S. Microbacterium aureliae sp. nov., a novel actinobacterium isolated from Aurelia aurita, the moon jellyfish. Int. J. Syst. Evol. Microbiol. 2016;66:4665–4670. doi: 10.1099/ijsem.0.001407. [DOI] [PubMed] [Google Scholar]
- 134.Fukano H., Wada S., Kurata O., Katayama K., Fujiwara N., Hoshino Y. Mycobacterium stephanolepidis sp. nov., a rapidly growing species related to Mycobacterium chelonae, isolated from marine teleost fish, Stephanolepis cirrhifer. Int. J. Syst. Evol. Microbiol. 2017;67:2811–2817. doi: 10.1099/ijsem.0.002028. [DOI] [PubMed] [Google Scholar]
- 135.Lee J.Y., Hyun D.W., Soo Kim P., Sik Kim H., Shin N.R., Yun J.H., Jung M.J., Kim M.S., Woong Whon T., Bae J.W. Arthrobacter echini sp. nov., isolated from the gut of a purple sea urchin, Heliocidaris crassispina. Int. J. Syst. Evol. Microbiol. 2016;66:1887–1893. doi: 10.1099/ijsem.0.000965. [DOI] [PubMed] [Google Scholar]
- 136.Thawai C., Rungjindamai N., Klanbut K., Tanasupawat S. Nocardia xestospongiae sp. nov., isolated from a marine sponge in the Andaman sea. Int. J. Syst. Evol. Microbiol. 2017;67:1451–1456. doi: 10.1099/ijsem.0.001736. [DOI] [PubMed] [Google Scholar]
- 137.Kämpfer P., Glaeser S.P., Busse H.J., Abdelmohsen U.R., Hentschel U. Rubrobacter aplysinae sp. nov., isolated from the marine sponge Aplysina aerophoba. Int. J. Syst. Evol. Microbiol. 2014;64:705–709. doi: 10.1099/ijs.0.055152-0. [DOI] [PubMed] [Google Scholar]
- 138.Kämpfer P., Glaeser S.P., Busse H.J., Abdelmohsen U.R., Ahmed S., Hentschel U. Actinokineospora spheciospongiae sp. nov., isolated from the marine sponge Spheciospongia vagabunda. Int. J. Syst. Evol. Microbiol. 2015;65:879–884. doi: 10.1099/ijs.0.000031. [DOI] [PubMed] [Google Scholar]
- 139.Sarmiento-Vizcaíno A., González V., Braña A.F., Molina A., Acuña J.L., García L.A., Blanco G. Myceligenerans cantabricum sp. nov., a barotolerant actinobacterium isolated from a deep cold-water coral. Int. J. Syst. Evol. Microbiol. 2015;65:1328–1334. doi: 10.1099/ijs.0.000107. [DOI] [PubMed] [Google Scholar]
- 140.Hamada M., Shibata C., Saitou S., Tamura T., Komaki H., Ichikawa N., Oguchi A., Hosoyama A., Fujita N., Yamamura H., et al. Proposal of nine novel species of the genus Lysinimicrobium and emended description of the genus Lysinimicrobium. Int. J. Syst. Evol. Microbiol. 2015;65:4394–4402. doi: 10.1099/ijsem.0.000587. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L., Li L., Deng Z., Hong K. Micromonospora zhanjiangensis sp. nov., isolated from mangrove forest soil. Int. J. Syst. Evol. Microbiol. 2015;65:4880–4885. doi: 10.1099/ijsem.0.000667. [DOI] [PubMed] [Google Scholar]
- 142.Li L., Hong K. Micromonospora ovatispora sp. nov. isolated from mangrove soil. Int. J. Syst. Evol. Microbiol. 2016;66:889–893. doi: 10.1099/ijsem.0.000808. [DOI] [PubMed] [Google Scholar]
- 143.Xie Q.Y., Ren J., Li L., Li Y., Deng Z.X., Hong K. Micromonospora mangrovi sp. nov., isolated from mangrove soil. Antonie Van Leeuwenhoek. 2016;109:483–491. doi: 10.1007/s10482-015-0641-x. [DOI] [PubMed] [Google Scholar]
- 144.Muangham S., Suksaard P., Mingma R., Matsumoto A., Takahashi Y., Duangmal K. Nocardiopsis sediminis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2016;66:3835–3840. doi: 10.1099/ijsem.0.001273. [DOI] [PubMed] [Google Scholar]
- 145.Hamada M., Shibata C., Tamura T., Nurkanto A., Ratnakomala S., Lisdiyanti P., Suzuki K.I. Kocuria pelophila sp. nov., an actinobacterium isolated from the rhizosphere of a mangrove. Int. J. Syst. Evol. Microbiol. 2016;66:3276–3280. doi: 10.1099/ijsem.0.001186. [DOI] [PubMed] [Google Scholar]
- 146.Lee L.H., Zainal N., Azman A.S., Mutalib N.S., Hong K., Chan K.G. Mumia flava gen. nov., sp. nov., an actinobacterium of the family Nocardioidaceae. Int. J. Syst. Evol. Microbiol. 2014;64:1461–1467. doi: 10.1099/ijs.0.058701-0. [DOI] [PubMed] [Google Scholar]
- 147.Liu M., Xing S.S., Yuan W.D., Wei H., Sun Q.G., Lin X.Z., Huang H.Q., Bao S.X. Pseudonocardia nematodicida sp. nov., isolated from mangrove sediment in Hainan, China. Antonie Van Leeuwenhoek. 2015;108:571–577. doi: 10.1007/s10482-015-0512-5. [DOI] [PubMed] [Google Scholar]
- 148.Tang Y.L., Lin H.P., Xie Q.Y., Li L., Peng F., Deng Z., Hong K. Actinoallomurus acanthiterrae sp. nov., an actinomycete isolated from rhizosphere soil of the mangrove plant Acanthus ilicifolius. Int. J. Syst. Evol. Microbiol. 2013;63:1874–1879. doi: 10.1099/ijs.0.043380-0. [DOI] [PubMed] [Google Scholar]
- 149.Suksaard P., Duangmal K., Srivibool R., Xie Q., Hong K., Pathom-aree W. Jiangella mangrovi sp. nov., isolated from mangrove soil. Int. J. Syst. Evol. Microbiol. 2015;65:2569–2573. doi: 10.1099/ijs.0.000303. [DOI] [PubMed] [Google Scholar]
- 150.Hamada M., Shibata C., Nurkanto A., Ratnakomala S., Lisdiyanti P., Tamura T., Suzuki K. Serinibacter tropicus sp. nov., an actinobacterium isolated from the rhizosphere of a mangrove, and emended description of the genus Serinibacter. Int. J. Syst. Evol. Microbiol. 2015;65:1151–1154. doi: 10.1099/ijs.0.000068. [DOI] [PubMed] [Google Scholar]
- 151.Suksaard P., Mingma R., Srisuk N., Matsumoto A., Takahashi Y., Duangmal K. Nonomuraea purpurea sp. nov., an actinomycete isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2016;66:4987–4992. doi: 10.1099/ijsem.0.001457. [DOI] [PubMed] [Google Scholar]
- 152.Duangmal K., Muangham S., Mingma R., Yimyai T., Srisuk N., Kitpreechavanich V., Matsumoto A., Takahashi Y. Kineococcus mangrovi sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2016;66:1230–1235. doi: 10.1099/ijsem.0.000860. [DOI] [PubMed] [Google Scholar]
- 153.Genilloud O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017;34:1203–1232. doi: 10.1039/C7NP00026J. [DOI] [PubMed] [Google Scholar]
- 154.Waksman S.A., Woodruff H.B. Bacteriostatic and bactericidal substances produced by a soil actinomyces. Proc. Soc. Exp. Biol. Med. 1940;45:609. doi: 10.3181/00379727-45-11768. [DOI] [Google Scholar]
- 155.Waksman S.A., Woodruff H.B. Selective antibiotic action of various substances of microbial origin. J. Bacteriol. 1942;44:373–384. doi: 10.1128/jb.44.3.373-384.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schatz A., Waksman S.A. Effect of streptomycin and other antibiotic substances upon Mycobacterium tuberculosis and related organisms. Proc. Soc. Exp. Biol. Med. 1944;57:244–248. doi: 10.3181/00379727-57-14769. [DOI] [Google Scholar]
- 157.Subramani R., Aalbersberg W. Marine actinomycetes: An ongoing source of novel bioactive metabolites. Microbiol. Res. 2012;167:571–580. doi: 10.1016/j.micres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 158.Butler M.S., Blaskovich M.A., Cooper M.A. Antibiotics in the clinical pipeline at the end of 2015. J. Antibiot. 2017;70:3–24. doi: 10.1038/ja.2016.72. [DOI] [PubMed] [Google Scholar]
- 159.Feling R.H., Buchanan G.O., Mincer T.J., Kauffman C.A., Jensen P.R., Fenical W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. Engl. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 160.Asolkar R.N., Freel K.C., Jensen P.R., Fenical W., Kondratyuk T.P., Park E.J., Pezzuto J.M. Arenamides A-C, cytotoxic NFκB Inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2009;72:396–402. doi: 10.1021/np800617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jang K.H., Nam S.J., Locke J.B., Kauffman C.A., Beatty D.S., Paul L.A., Fenical W. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew. Chem. Int. Ed. Engl. 2013;52:7822–7824. doi: 10.1002/anie.201302749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leutou A.S., Yang I., Kang H., Seo E.K., Nam S.J., Fenical W. Nocarimidazoles A and B from a marine-derived actinomycete of the genus Nocardiopsis. J. Nat. Prod. 2015;78:2846–2849. doi: 10.1021/acs.jnatprod.5b00746. [DOI] [PubMed] [Google Scholar]
- 163.Raju R., Piggott A.M., Quezada M., Capon R.J. Nocardiopsins C and D and nocardiopyrone A: New polyketides from an Australian marine-derived Nocardiopsis sp. Tetrahedron. 2013;69:692–698. doi: 10.1016/j.tet.2012.10.104. [DOI] [Google Scholar]
- 164.Kobayashi K., Fukuda T., Terahara T., Harunari E., Imada C., Tomoda H. Diketopiperazines, inhibitors of sterol O-acyltransferase, produced by a marine-derived Nocardiopsis sp. KM2-16. J. Antibiot. 2015;68:638–641. doi: 10.1038/ja.2015.38. [DOI] [PubMed] [Google Scholar]
- 165.Skellam E.J., Stewart A.K., Strangman W.K., Wright J.L. Identification of micromonolactam, a new polyene macrocyclic lactam from two marine Micromonospora strains using chemical and molecular methods: clarification of the biosynthetic pathway from a glutamate starter unit. J. Antibiot. 2013;66:431–441. doi: 10.1038/ja.2013.34. [DOI] [PubMed] [Google Scholar]
- 166.Sarmiento-Vizcaíno A., Braña A.F., Pérez-Victoria I., Martín J., de Pedro N., Cruz M., Díaz C., Vicente F., Acuña J.L., Reyes F., et al. Paulomycin G, a new natural product with cytotoxic activity against tumor cell lines produced by deep-sea sediment derived Micromonospora matsumotoense M-412 from the Avilés Canyon in the Cantabrian sea. Mar. Drugs. 2017;15:271. doi: 10.3390/md15090271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kyeremeh K., Acquah K.S., Sazak A., Houssen W., Tabudravu J., Deng H., Jaspars M. Butremycin, the 3-hydroxyl derivative of ikarugamycin and a protonated aromatic tautomer of 5′-methylthioinosine from a Ghanaian Micromonospora sp. K310. Mar. Drugs. 2014;12:999–1012. doi: 10.3390/md12020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y., Adnani N., Braun D.R., Ellis G.A., Barns K.J., Parker-Nance S., Guzei I.A., Bugni T.S. Micromonohalimanes A and B: Antibacterial halimane-type diterpenoids from a marine Micromonospora species. J. Nat. Prod. 2016;79:2968–2972. doi: 10.1021/acs.jnatprod.6b00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mullowney M.W., ÓhAinmhire E., Tanouye U., Burdette J.E., Pham V.C., Murphy B.T. A pimarane diterpene and cytotoxic angucyclines from a marine-derived Micromonospora sp. in Vietnam’s east sea. Mar. Drugs. 2015;13:5815–5827. doi: 10.3390/md13095815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fu P., MacMillan J.B. Thiasporines A-C, thiazine and thiazole derivatives from a marine-derived Actinomycetospora chlora. J. Nat. Prod. 2015;78:548–551. doi: 10.1021/np500929z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ye X., Anjum K., Song T., Wang W., Yu S., Huang H., Lian X.Y., Zhang Z. A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat. Prod. Res. 2016;30:1156–1161. doi: 10.1080/14786419.2015.1047775. [DOI] [PubMed] [Google Scholar]
- 172.Kyeremeh K., Acquah K.S., Camas M., Tabudravu J., Houssen W., Deng H., Jaspars M. Butrepyrazinone, a new pyrazinone with an unusual methylation pattern from a Ghanaian Verrucosispora sp. K51G. Mar. Drugs. 2014;12:5197–5208. doi: 10.3390/md12105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Niu S., Zhou T.T., Xie C.L., Zhang G.Y., Yang X.W. Microindolinone A, a novel 4,5,6,7-tetrahydroindole, from the deep-sea-derived actinomycete Microbacterium sp. MCCC 1A11207. Mar. Drugs. 2017;15:230. doi: 10.3390/md15070230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li J.L., Huang L., Liu J., Song Y., Gao J., Jung J.H., Liu Y., Chen G. Acetylcholinesterase inhibitory dimeric indole derivatives from the marine actinomycetes Rubrobacter radiotolerans. Fitoterapia. 2015;102:203–207. doi: 10.1016/j.fitote.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 175.Kwon Y., Kim S.H., Shin Y., Bae M., Kim B.Y., Lee S.K., Oh K.B., Shin J., Oh D.C. A new benzofuran glycoside and indole alkaloids from a sponge-associated rare actinomycete, Amycolatopsis sp. Mar. Drugs. 2014;12:2326–2340. doi: 10.3390/md12042326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wyche T.P., Standiford M., Hou Y., Braun D., Johnson D.A., Johnson J.A., Bugni T.S. Activation of the nuclear factor E2-related factor 2 pathway by novel natural products halomadurones A-D and a synthetic analogue. Mar. Drugs. 2013;11:5089–5099. doi: 10.3390/md11125089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fu P., Kong F., Li X., Wang Y., Zhu W. Cyanogramide with a new spiro[indolinone-pyrroloimidazole] skeleton from Actinoalloteichus cyanogriseus. Org. Lett. 2014;16:3708–3711. doi: 10.1021/ol501523d. [DOI] [PubMed] [Google Scholar]
- 178.Peng F., Wang C., Xie Y., Jiang H., Chen L., Uribe P., Bull A.T., Goodfellow M., Jiang H., Lian Y. A new 20-membered macrolide produced by a marine-derived Micromonospora strain. Nat. Prod. Res. 2013;27:1366–1371. doi: 10.1080/14786419.2012.740038. [DOI] [PubMed] [Google Scholar]
- 179.Kim M.C., Kwon O.W., Park J.S., Kim S.Y., Kwon H.C. Nocapyrones H-J, 3,6-disubstituted α-pyrones from the marine actinomycete Nocardiopsis sp. KMF-001. Chem. Pharm. Bull. 2013;61:511–515. doi: 10.1248/cpb.c12-00956. [DOI] [PubMed] [Google Scholar]
- 180.Wu Z.C., Li S., Nam S.J., Liu Z., Zhang C. Nocardiamides A and B, two cyclohexapeptides from the marine-derived actinomycete Nocardiopsis sp. CNX037. J. Nat. Prod. 2013;76:694–701. doi: 10.1021/np400009a. [DOI] [PubMed] [Google Scholar]
- 181.Lane L., Nam S.-J., Fukuda T., Yamanaka K., Kauffman C.A., Jensen P.R., Fenical W., Moore B.S. Structures and comparative characterization of biosynthetic gene clusters for cyanosporasides, enediyne-derived natural products from marine actinomycetes. J. Am. Chem. Soc. 2013;135:4171–4174. doi: 10.1021/ja311065v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abdelmohsen U.R., Cheng C., Viegelmann C., Zhang T., Grkovic T., Ahmed S., Quinn R.J., Hentschel U., Edrada-Ebel R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs. 2014;12:1220–1244. doi: 10.3390/md12031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ellis G.A., Wyche T.P., Fry C.G., Braun D.R., Bugni T.S. Solwaric acids A and B, antibacterial aromatic acids from a marine Solwaraspora sp. Mar. Drugs. 2014;12:1013–1022. doi: 10.3390/md12021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Trzoss L., Fukuda T., Costa-Lotufo L.V., Jimenez P., La Clair J.J., Fenical W. Seriniquinone, a selective anticancer agent, induces cell death by autophagocytosis, targeting the cancer-protective protein dermcidin. Proc. Natl. Acad. Sci. USA. 2014;111:14687–14692. doi: 10.1073/pnas.1410932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fu P., Zhu Y., Mei X., Wang Y., Jia H., Zhang C., Zhu W. Acyclic congeners from Actinoalloteichus cyanogriseus provide insights into cyclic bipyridine glycoside formation. Org. Lett. 2014;16:4264–4267. doi: 10.1021/ol5019757. [DOI] [PubMed] [Google Scholar]
- 186.Wyche T.P., Piotrowski J.S., Hu Y., Braun D., Deshpande R., Mcllwain S., Ong I.M., Myers C.L., Guzei I.A., Westler W.M., et al. Forazoline A: Marine-derived polyketide with antifungal in vivo efficacy. Angew. Chem. Int. Ed. Engl. 2014;53:11583–11586. doi: 10.1002/anie.201405990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wagner M., Abdel-Mageed W.M., Ebel R., Bull A.T., Goodfellow M., Fiedler H.P., Jaspars M. Dermacozines H-J isolated from a deep-sea strain of Dermacoccus abyssi from Mariana Trench sediments. J. Nat. Prod. 2014;77:416–420. doi: 10.1021/np400952d. [DOI] [PubMed] [Google Scholar]
- 188.Eltamany E.E., Abdelmohsen U.R., Ibrahim A.K., Hassanean H.A., Hentschel U., Ahmed S.A. New antibacterial xanthone from the marine sponge-derived Micrococcus sp. EG45. Bioorg. Med. Chem. Lett. 2014;24:4939–4942. doi: 10.1016/j.bmcl.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 189.Kim Y., Ogura H., Akasaka K., Oikawa T., Matsuura N., Imada C., Yasuda H., Igarashi Y. Nocapyrones: α- and γ-pyrones from a marine-derived Nocardiopsis sp. Mar. Drugs. 2014;12:4110–4125. doi: 10.3390/md12074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kawahara T., Itoh M., Izumikawa M., Kozone I., Sakata N., Tsuchida T., Shin-ya K. New hydroxamate metabolite, MBJ-0003, from Micromonospora sp. 29867. J. Antibiot. 2014;67:261–263. doi: 10.1038/ja.2013.124. [DOI] [PubMed] [Google Scholar]
- 191.Liu D., Lin H., Proksch P., Tang X., Shao Z., Lin W. Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T) Org. Lett. 2015;17:1220–1223. doi: 10.1021/acs.orglett.5b00172. [DOI] [PubMed] [Google Scholar]
- 192.Schulze C.J., Navarro G., Ebert D., DeRisi J., Linington R.G. Salinipostins A-K, long-chain bicyclic phosphotriesters as a potent and selective antimalarial chemotype. J. Org. Chem. 2015;80:1312–1320. doi: 10.1021/jo5024409. [DOI] [PubMed] [Google Scholar]
- 193.Gan M., Liu B., Tan Y., Wang Q., Zhou H., He H., Ping Y., Yang Z., Wang Y., Xiao C. Saccharothrixones A-D, tetracenomycin-type polyketides from the marine-derived actinomycete Saccharothrix sp. 10-10. J. Nat. Prod. 2015;78:2260–2265. doi: 10.1021/acs.jnatprod.5b00577. [DOI] [PubMed] [Google Scholar]
- 194.Thi Q.V., Tran V.H., Maia H.D., Le C.V., Hong MLe T., Murphy B.T., Chau V.M., Pham V.C. Antimicrobial metabolites from a marine-derived actinomycete in Vietnam’s east sea. Nat. Prod. Commun. 2016;11:49–51. doi: 10.1177/1934578X1601100116. [DOI] [PubMed] [Google Scholar]
- 195.Zhang X.M., Zhang D.F., Li W.J., Lu C.H. Pseudonocardides A-G, new γ-butyrolactones from marine-derived Pseudonocardia sp. YIM M13669. Helv. Chim. Acta. 2016;99:191–196. doi: 10.1002/hlca.201500109. [DOI] [Google Scholar]
- 196.Zhang H., Saurav K., Yu Z., Mándi A., Kurtán T., Li J., Tian X., Zhang Q., Zhang W., Zhang C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016;79:1610–1618. doi: 10.1021/acs.jnatprod.6b00175. [DOI] [PubMed] [Google Scholar]
- 197.Thi Q.V., Tran V.H., Mai H.D., Le C.V., Hong M.L.T., Murphy B.T., Chau V.M., Pham V.C. Secondary metabolites from an actinomycete from Vietnam’s east sea. Nat. Prod. Commun. 2016;11:401–404. doi: 10.1177/1934578X1601100320. [DOI] [PubMed] [Google Scholar]
- 198.Xie C.L., Niu S.W., Zhou T.T., Zhang G.Y., Yang Q., Yang X.W. Chemical constituents and chemotaxonomic study on the marine actinomycete Williamsia sp. MCCC 1A11233. Biochem. Syst. Ecol. 2016;67:129–133. doi: 10.1016/j.bse.2016.06.004. [DOI] [Google Scholar]
- 199.Lee J., Han C., Gu Lee T., Chin J., Choi H., Lee W., Jeong Paik M., Hwan Won D., Jeong G., Ko J., et al. Marinopyrones A-D, α-pyrones from marine-derived actinomycetes of the family Nocardiopsaceae. Tetrahedron Lett. 2016;57:1997–2000. doi: 10.1016/j.tetlet.2016.03.084. [DOI] [Google Scholar]
- 200.Huang P., Xie F., Ren B., Wang Q., Wang J., Wang Q., Abdel-Mageed W.M., Liu M., Han J., Oyeleye A., et al. Anti-MRSA and anti-TB metabolites from marine-derived Verrucosispora sp. MS100047. Appl. Microbiol. Biotechnol. 2016;100:7437–7447. doi: 10.1007/s00253-016-7406-y. [DOI] [PubMed] [Google Scholar]
- 201.Fukuda T., Takahashi M., Nagai K., Harunari E., Imada C., Tomoda H. Isomethoxyneihumicin, a new cytotoxic agent produced by marine Nocardiopsis alba KM6-1. J. Antibiot. 2017;70:590–594. doi: 10.1038/ja.2016.152. [DOI] [PubMed] [Google Scholar]
- 202.Xie C.L., Liu Q., Xia J.M., Gao Y., Yang Q., Shao Z.Z., Liu G., Yang X.W. Anti-allergic compounds from the deep-sea-derived actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs. 2017;15:71. doi: 10.3390/md15030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang X.M., Sun M.W., Shi H., Lu C.H. α-pyrone derivatives from a marine actinomycete Nocardiopsis sp. YIM M13066. Nat. Prod. Res. 2017;31:2245–2249. doi: 10.1080/14786419.2017.1299730. [DOI] [PubMed] [Google Scholar]
- 204.Yim C.Y., Le T.C., Lee T.G., Yang I., Choi H., Lee J., Kang K.Y., Lee J.S., Lim K.M., Yee S.T., et al. Saccharomonopyrones A-C, New α-pyrones from a marine sediment-derived bacterium Saccharomonospora sp. CNQ-490. Mar. Drugs. 2017;15:239. doi: 10.3390/md15080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gong T., Zhen X., Li X.L., Chen J.J., Chen T.J., Yang J.L., Zhu P. Tetrocarcin Q, a new spirotetronate with a unique glycosyl group from a marine-derived actinomycete Micromonospora carbonacea LS276. Mar. Drugs. 2017;16:74. doi: 10.3390/md16020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gui C., Zhang S., Zhu X., Ding W., Huang H., Gu Y.C., Duan Y., Ju J. Antimicrobial spirotetronate metabolites from marine-derived Micromonospora harpali SCSIO GJ089. J. Nat. Prod. 2017;80:1594–1603. doi: 10.1021/acs.jnatprod.7b00176. [DOI] [PubMed] [Google Scholar]
- 207.Sun M., Chen X., Li W., Lu C., Shen Y. New diketopiperazine derivatives with cytotoxicity from Nocardiopsis sp. YIM M13066. J. Antibiot. 2017;70:795–797. doi: 10.1038/ja.2017.46. [DOI] [PubMed] [Google Scholar]
- 208.Igarashi M., Sawa R., Yamasaki M., Hayashi C., Umekita M., Hatano M., Fujiwara T., Mizumoto K., Nomoto A. Kribellosides, novel RNA 5′-triphosphatase inhibitors from the rare actinomycete Kribbella sp. MI481-42F6. J. Antibiot. 2017;70:582–589. doi: 10.1038/ja.2016.161. [DOI] [PubMed] [Google Scholar]
- 209.Braña A.F., Sarmiento-Vizcaíno A., Pérez-Victoria I., Otero L., Fernández J., Palacios J.J., Martín J., de la Cruz M., Díaz C., Vicente F., et al. Branimycins B and C, antibiotics produced by the abyssal actinobacterium Pseudonocardia carboxydivorans M-227. J. Nat. Prod. 2017;80:569–573. doi: 10.1021/acs.jnatprod.6b01107. [DOI] [PubMed] [Google Scholar]
- 210.Sun M., Ou J., Li W., Lu C. Quinoline and naphthalene derivatives from Saccharopolyspora sp. YIM M13568. J. Antibiot. 2017;70:320–322. doi: 10.1038/ja.2016.142. [DOI] [PubMed] [Google Scholar]
- 211.Ziemert N., Alanjaryab M., Weber T. The evolution of genome mining in microbes-a review. Nat. Prod. Rep. 2016;33:988–1005. doi: 10.1039/c6np00025h. [DOI] [PubMed] [Google Scholar]
- 212.Hug J.J., Bader C.D., Remškar M., Cirnski K., Müller R. Concepts and methods to aaccess novel antibiotics from actinomycetes. Antibiotics. 2018;7:44. doi: 10.3390/antibiotics7020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wei Y., Zhang L., Zhou Z., Yan X. Diversity of gene clusters for polyketide and nonribosomal peptide biosynthesis revealed by metagenomic analysis of the yellow sea sediment. Front. Microbiol. 2018;9:295. doi: 10.3389/fmicb.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Katz L., Baltz R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016;43:155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 215.Schorn M.A., Alanjary M.M., Aguinaldo K., Korobeynikov A., Podell S., Patin N., Lincecum T., Jensen P.R., Ziemert N., Moore B.S. Sequencing rare marine actinomycete genomes reveals high density of unique natural product biosynthetic gene clusters. Microbiology. 2016;162:2075–2086. doi: 10.1099/mic.0.000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Loureiro C., Medema M.H., van der Oost J., Sipkema D. Exploration and exploitation of the environment for novel specialized metabolites. Curr. Opin. Biotechnol. 2018;50:206–213. doi: 10.1016/j.copbio.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 217.Yamanaka K., Reynolds K.A., Kersten R.D., Ryan K.S., Gonzalez D.J., Nizet V., Dorrestein P.C., Moore B.S. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA. 2014;111:1957–1962. doi: 10.1073/pnas.1319584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Duncan K.R., Crüsemann M., Lechner A., Sarkar A., Li J., Ziemert N., Wang M., Bandeira N., Moore B.S., Dorrestein P.C., et al. Molecular networking and pattern-based genome mining improves discovery of biosynthetic gene clusters and their products from Salinispora species. Chem. Biol. 2015;22:460–471. doi: 10.1016/j.chembiol.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Richter T.K., Hughes C.C., Moore B.S. Sioxanthin, a novel glycosylated carotenoid, reveals an unusual subclustered biosynthetic pathway. Environ. Microbiol. 2015;17:2158–2171. doi: 10.1111/1462-2920.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schulze C.J., Donia M.S., Siqueira-Neto J.L., Ray D., Raskatov J.A., Green R.E., McKerrow J.H., Fischbach M.A., Linington R.G. Genome-directed lead discovery: Biosynthesis, structure elucidation, and biological evaluation of two families of polyene macrolactams against Trypanosoma brucei. ACS Chem. Biol. 2015;10:2373–2381. doi: 10.1021/acschembio.5b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tian J., Chen H., Guo Z., Liu N., Li J., Huang Y., Xiang W., Chen Y. Discovery of pentangular polyphenols hexaricins A-C from marine Streptosporangium sp. CGMCC 4.7309 by genome mining. Appl. Microbiol. Biotechnol. 2016;100:4189–4199. doi: 10.1007/s00253-015-7248-z. [DOI] [PubMed] [Google Scholar]
- 222.Tan Y., Hu Y., Wang Q., Zhou H., Wang Y., Gan M. Tetrocarcins N and O, glycosidic spirotetronates from a marine-derived Micromonospora sp. identified by PCR-based screening. RSC Adv. 2016;6:91773–91778. doi: 10.1039/C6RA17026A. [DOI] [Google Scholar]
- 223.Feng Y., Liu J., Carrasco Y.P., MacMillan J.B., De Brabander J.K. Rifamycin biosynthetic congeners: Isolation and total synthesis of rifsaliniketal and total synthesis of salinisporamycin and saliniketals A and B. J. Am. Chem. Soc. 2016;138:7130–7142. doi: 10.1021/jacs.6b03248. [DOI] [PubMed] [Google Scholar]
- 224.Jiang X., Zhang Q., Zhu Y., Nie F., Wu Z., Yang C., Zhang L., Tian X., Zhang C. Isolation, structure elucidation and biosynthesis of benzo[b]fluorine nenestatin A from deep-sea derived Micromonospora echinospora SCSIO 04089. Tetrahedron. 2017;73:3585–3590. doi: 10.1016/j.tet.2017.03.054. [DOI] [Google Scholar]
- 225.Thomas T., Moitinho-Silva L., Lurgi M., Björk J.R., Easson C., Astudillo-García C., Olson J.B., Erwin P.M., López-Legentil S., Luter H., et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016;7:11870. doi: 10.1038/ncomms11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sunagawa S., Coelho L.P., Chaffron S., Kultima J.R., Labadie K., Salazar G., Djahanschiri B., Zeller G., Mende D.R., Alberti A., et al. Ocean plankton. Structure and function of the global ocean microbiome. Science. 2015;348:1261359. doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]