Abstract
Three GTPases, RAC, RHO, and Cdc42, play essential roles in coordinating many cellular functions during embryonic development, both in healthy cells and in disease conditions like cancers. We have presented patterns of distribution of the frequency of RAC1-alteration(s) in cancers as obtained from cBioPortal. With this background data, we have interrogated the various functions of RAC1 in tumors, including proliferation, metastasis-associated phenotypes, and drug-resistance with a special emphasis on solid tumors in adults. We have reviewed the activation and regulation of RAC1 functions on the basis of its sub-cellular localization in tumor cells. Our review focuses on the role of RAC1 in cancers and summarizes the regulatory mechanisms, inhibitory efficacy, and the anticancer potential of RAC1-PAK targeting agents.
Keywords: RAC1, sub-cellular signaling, solid tumors, resistance
1. Prologue
GTPases act as molecular switches. In the GTP (Guanosine triphosphate)-bound active form, RAC1 interacts with effector molecules to initiate signaling, while in the GDP (Guanosine diphosphate)-bound inactive form the signal is terminated. In the unstimulated condition, RAC GTPase exists as a complex with GDP dissociation inhibitor in the cytosol. When activated through the action of soluble chemoattractant, chemokines, phagocytic particles, or any upstream regulator, RAC dissociates from GDI (guanine nucleotide dissociation inhibitors), GDP is exchanged for GTP through the action of membrane-localized guanine nucleotide exchange factors, and RAC, now in its GTP-bound active form, becomes membrane-associated [1,2]. Over 80 GEFs (guanine nucleotide exchange factors) and more than 70 GAPs (GTPase-activating proteins) have been reported, suggesting that Rho-family GTPase regulation is complex and that activity and localization can be modulated by a multitude of signaling pathways depending on the spatiotemporal context [3]. All three GTPases (RHO, RAC, and Cdc42) have been shown to be activated at the front of migrating cells, but the spatiotemporal coordination between them is unknown [4]. RAC1 (Ras-related C3 botulinum toxin substrate1), one of the best characterized RHO GTPases, is an established effector of receptors and an important hub in signaling networks critical for tumorigenesis and metastasis. The RAC1 gene is present on chromosome 7 (7p22) and comprises 7 exons over a length of 29KB [5]. RAC1, but not RAC2 or RAC3 genes, contains an additional exon 3b that is included by alternative splicing in the variant of RAC1b, a constitutively active mutant that is expressed primarily in colon and breast cancer [6]. RAC1 is ubiquitously expressed, and its inactivation by gene targeting in mice leads to embryonic lethality [7].
RAC1 hyper activation is common in human cancers and could be the consequence of overexpression, abnormal upstream inputs, deregulated degradation, and/or anomalous intracellular localization. More recently, cancer-associated gain-of-function mutations in RAC1 have been identified which contribute to tumor phenotypes and confer resistance to targeted therapies [8]. Deregulated expression/activity of RAC guanine nucleotide exchange factors responsible for RAC activation has been largely associated with a metastatic phenotype and drug resistance.
2. RAC1 and Solid Tumors
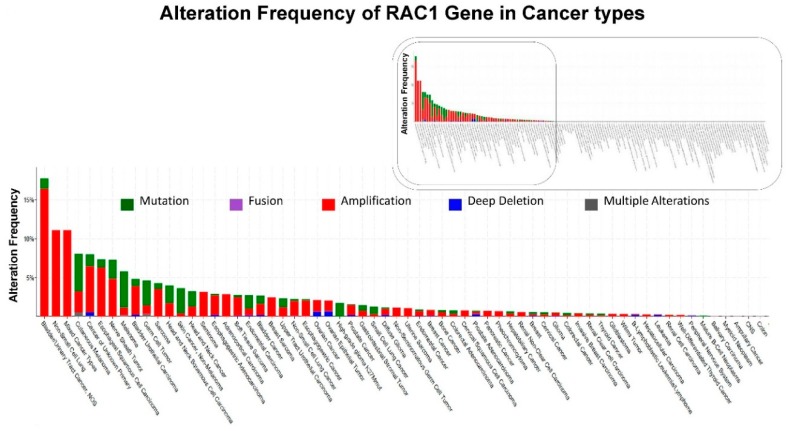
Unlike RAS proteins, which are frequently mutated in cancer (around 30%), RAC proteins (RAC1, RAC2 or RAC3) themselves are generally not found to be mutated in cancers at such a high frequency. Both typical Rho GTPases (RhoA, RAC1 and Cdc42 which cycle between an active GTP-bound and inactive GDP-bound conformation and are regulated by GEFs (guanine nucleotide exchange factors), GAPs (GTPase-activating proteins), and GDIs (guanine nucleotide dissociation inhibitors)) and atypical Rho GTPases (GTPase-deficient mutations and those that generally do not hydrolyze GTP) contribute to cancer progression. The “gain-of-function” substitution mutation of RAC1 (e.g., P29S) maintains its intrinsic GTP-hydrolysis property and acquires an inherent GDP-GTP nucleotide exchange capability leading to its “spontaneously activated” state. RhoA or RAC1 are mutated in a few cancers, but in most cancers, expression levels and/or activity of Rho GTPases is altered via a number of regulatory mechanisms [9]. Figure 1 shows the alteration frequency of RAC1 gene in different cancer types. The data was obtained from cBioPortal (January 2019) representing a combined study of 74247 samples. The presence of a long-tail and absence of alteration of RAC1 in more than half of the cancer types is the characteristic feature of alterations of the RAC1 gene in cancers. Bladder/urinary tract cancers, lung cancers, and melanoma are the cancers among which a higher frequency of alterations of the RAC1 gene has been identified, in contrast to other solid tumors like breast, colorectal, and endometrial cancers.
Figure 1.
Alterations of RAC1 Gene: Data has been queried from cBioPortal. Figure 1 shows the alteration frequency of RAC1 gene in different cancer types: Data obtained from c-BioPortal (January 2019) representing a combined study of 74247 samples, querying 71857 patients/74247 samples in 240 studies (http://www.cbioportal.org). Y-axis represents alteration frequency of RAC1 gene (colors represent the type of alterations as shown in the figure). The frequency of alterations is sorted on X-axis according to cancer types. Inset shows the entire distribution of the frequency of alterations of the RAC1 gene while the boxed portion of the inset represents only the cancers where the frequency of alterations occurred (boxed in the inset).
Recent studies, enabled by next-generation exome sequencing, report activating point mutations in RAC1 GTPases as driver mutations in melanoma, as well as breast, head, and neck cancers. Aberrant activation of RAC1 is implicated in numerous aspects of tumor development and progression and is the subject of several recent peer-reviewed articles [10,11,12,13]. Recently, a recurrent somatic missense mutation at codon 29 of RAC1 in the highly conserved switch I domain that results in the substitution of proline to a serine residue (RAC1 P29S) was discovered in up to 9% of sun-exposed melanomas. This discovery makes RAC1 the third most commonly mutated (somatic) proto-oncogene in melanoma after BRAF and NRAS [14,15,16]. Importantly, the RAC1 P29S mutation was more frequent in melanomas that were wild type for both NRAS and BRAF. There was a similar frequency of the RAC1 P29S mutation in primary (9.2%) and metastatic tumors (8.6%), which is consistent with this mutation occurring early in tumorigenesis. RAC1 P29S was significantly more prevalent in male patients (present in 12.8% of males, compared to 2.4% of females, P = 0.01) [14], which is consistent with these mutations being induced by UV exposure. Biochemical and cell-based assays demonstrated that RAC1 P29S is activated and has increased binding activity toward RAC1 effectors, and expression of mutant protein confers increased cell proliferation, altered cell migration, and stimulated membrane ruffling and MAPK signaling [17]. Activating mutations have also been identified in other RAC family members, such as RAC2-P29L and RAC2-P29Q [18]. The COSMIC database show that more than one RAC1 mutation can occur in different cancers types, which includes the large intestine, cervix, liver, endometrium, stomach, esophagus, lung, upper aero-digestive tract, hematopoietic/lymphoid, and breast. The MSK-IMPACT Clinical Sequencing Cohort, which is the most recent large-scale genomic study by the Memorial Sloan-Kettering Cancer Centre that sequenced tumors from more than 10,000 patients, identified many hotspot mutations involving the P29 residue (e.g., P29S, P29F, P29L, and P29T) in melanoma, Merkel cell carcinoma, squamous cell carcinoma, anaplastic thyroid cancer, and breast invasive ductal carcinoma using the cBioPortal [19,20,21]. Although the RAC1 P29S mutation is oncogenic and biochemically active, its clinical relevance in melanoma remains unclear. It has been recently demonstrated that shortening of the 3′ untranslated regions (3′UTR) of mRNA is an important mechanism for oncogene activation including RAC1. Chen et al. recently demonstrated that short 3’UTR isoform of RAC1 substantially upregulated RAC1 expression by escaping from miRNA-targeted repression and played an essential oncogenic role in urothelial carcinoma of the bladder pathogenesis [22].
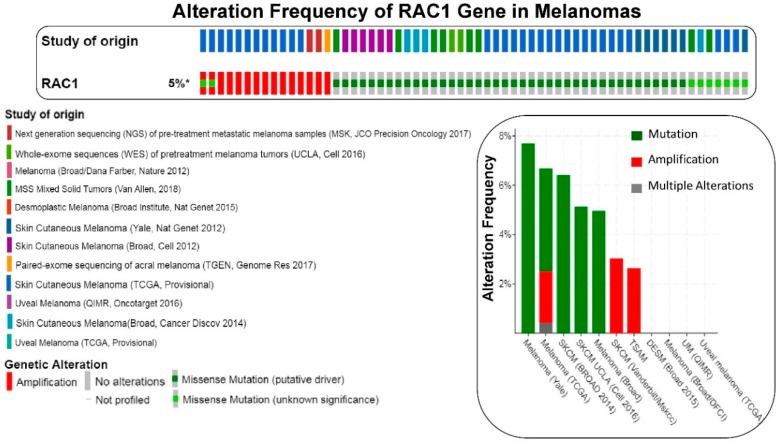
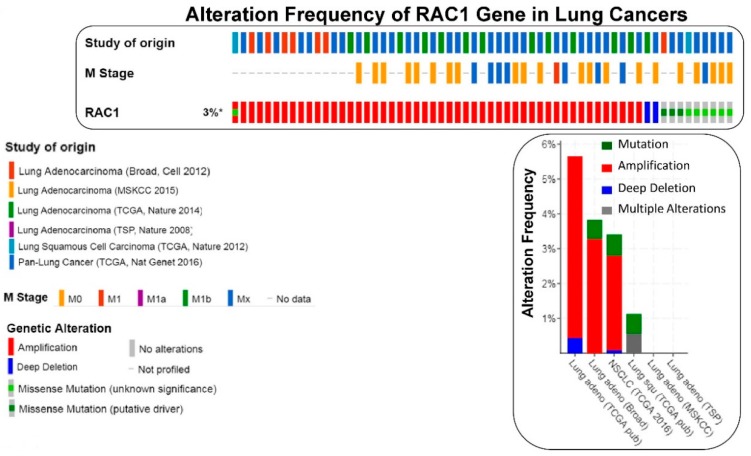
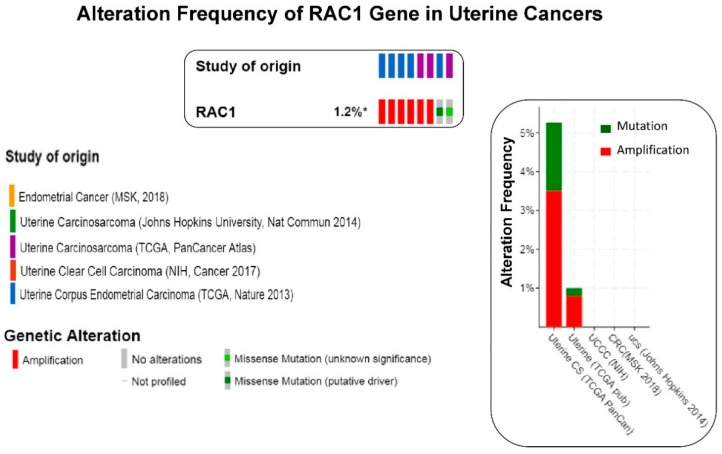
We have presented alteration frequencies of RAC1 gene in melanomas, lung cancers, and uterine cancers as queried from the cBioPortal (http://www.cbioportal.org). Figure 2 shows the frequency of alteration of the RAC1 gene in melanomas. The oncoprint presents data obtained from cBioPortal (February 2019) representing a combined study of 1315 samples (http://www.cbioportal.org; querying 1273 patients/1315 samples in 12 studies). The bar diagram represents the frequency of alterations in the RAC1 gene in a few individual melanoma studies where alterations was identified. Figure 3 shows the frequency of alteration of the RAC1 gene in lung cancers. The oncoprint presents data obtained from cBioPortal (February 2019) representing a combined study of 1933 samples (http://www.cbioportal.org). The oncoprint represents the types of alterations of the RAC1 gene in samples as shown under “Genetic Alteration” in the figure and the distribution of metastatic stages of the patients where alterations of the RAC1 gene was identified. The bar-diagram represents the frequency of alterations in the RAC1 gene in a few individual lung cancer studies where alterations was identified. Figure 4 shows the frequency of alteration of the RAC1 gene in uterine cancers. The oncoprint presents data obtained from cBioPortal (February 2019) representing a combined study of 792 samples (http://www.cbioportal.org). The oncoprint represents the types of alterations of the RAC1 gene in samples as shown under “Genetic Alteration” in the figure. The bar diagram represents the frequency of alterations in the RAC1 gene in a few individual uterine cancer studies where alteration was identified. It is evident from the data that although the predominant alteration in RAC1 gene is amplification (Figure 1, Figure 3, and Figure 4), melanoma represents cancer wherein most of the alterations observed are mutations of the RAC1 gene (Figure 2). In summary, Figure 1 demonstrates that alteration in the RAC1 gene occurs in only a few of the organ-type cancers, and the frequency never reaches more than 15%. Furthermore, the predominant form of alteration is the amplification (as in bladder and urinary tract cancer) of the gene, followed by mutation (as in melanoma and germ cell tumor). Figure 2 shows the predominant form of alteration occurring in melanoma is mutation (maximum 7.5%). It also shows that the predominant form of the alteration is center-dependent or the study of origin. In contrast to melanoma, amplification of the RAC1 is predominant in lung adenocarcinoma (Figure 3). Interestingly, both amplification and mutation of the RAC1 gene occur in uterine cancers (Figure 4). In melanoma, lung, and uterine cancers, although the percentage of total alteration of the RAC1 gene is around 5–7%, the type of alteration varied depending on the organ-type.
Figure 2.
The frequency of alteration of RAC1 gene in melanomas: The oncoprint presents data obtained from c-BioPortal (February 2019) representing a combined study of 1315 samples (http://www.cbioportal.org), querying 1273 patients/1315 samples in 12 studies, as shown in the different color codes of the oncoprint (upper panel) under “Study of origin” in the figure. The percentage (5%*) represents the alteration of the RAC1 gene in patients with melanomas. The lower panel of the oncoprint represents the types of alterations of RAC1 gene in samples as shown under “Genetic Alteration” in the figure. Unaltered cases (RAC1 gene) were not included in the oncoprint. The bar diagram (figure on the right panel) represents the frequency of alterations in RAC1 gene in a few individual melanoma studies where alterations were identified. The Y-axis of the bar diagram represents the alteration frequency of the RAC1 gene (colors represent the types of alterations as shown in the figure). The frequencies of the alterations are sorted on the X-axis according to “Cancer Study”. MSS Mixed Solid Tumors (Van Allen, 2018) is not represented in the bar-diagram.
Figure 3.
The frequency of alteration of the RAC1 gene in lung cancers: The oncoprint presents data obtained from c-BioPortal (February 2019) representing a combined study of 1933 samples (http://www.cbioportal.org), querying 1933 patients/samples in six studies as shown in the different color codes of the oncoprint (upper panel) under “Study of origin” in the figure. The percentage (3%*) represents the alteration of the RAC1 gene in patients with lung cancers. The lower panel of the oncoprint represents the types of alterations of RAC1 gene in samples as shown under “Genetic Alteration” in the figure. The middle panel of the oncoprint presents distribution of metastatic stage (M Stage as shown in the figure) of the patients where alteration of the RAC1 gene was identified. Unaltered cases (RAC1 gene) were not included in the oncoprint. The bar diagram (figure on the right panel) represents the frequency of alterations in the RAC1 gene in a few individual lung cancer studies where alteration was identified. The Y-axis of the bar diagram represents alteration frequency of the RAC1 gene (colors represent the type of alteration as shown in the figure). The frequencies of alterations are sorted on the X-axis according to “Cancer Study”.
Figure 4.
The frequency of alteration of RAC1 gene in uterine cancers: The oncoprint presents data obtained from c-BioPortal (February 2019) representing a combined study of 792 samples (http://www.cbioportal.org), querying 784 patients/792 samples in five studies as shown in different color codes of the oncoprint (upper panel) under “Study of origin” in the figure. The percentage (1.2%*) represents the alteration of the RAC1 gene in patients with uterine cancers. The lower panel of the oncoprint represents the types of alterations of RAC1 gene in samples as shown under “Genetic Alteration” in the figure. Unaltered (RAC1 gene) cases were not included in the oncoprint. The bar diagram (figure on the right panel) represents the frequency of alterations in the RAC1 gene in a few individual uterine cancer studies where alteration was identified. The Y-axis of the bar diagram represents alteration frequency of the RAC1 gene (colors represent the type of alteration as shown in the figure). The frequencies of alterations are sorted on X-axis according to “Cancer Study”.
3. Sub-Cellular Location-Based Activation and Regulation of RAC1 Functions in Tumor Cells
Cellular processes orchestrated by RAC1 in tumor cells are achieved via the spatiotemporal activation of RAC1 and the regulation of RAC1 activity, switching between active and inactive states at various subcellular locations, including the plasma membrane, nucleus, and mitochondria [23,24]. This compartmentalization of RAC1 signaling is regulated by a large number of proteins that fine-tune RAC1 functions by promoting/reducing its recruitment, activation, and stability, as well as its deactivation by its regulators via various posttranslational modifications. For example, localization of GEFs and GAPs has been identified as one of the modes of regulation of RAC1 activity. Regulation of plasma membrane-associated processes such as lamellipodia formation and membrane ruffling [25,26], focal contact formation [26], and E-cadherin-mediated cell-to-cell contacts [27] by RAC1 in tumor cells is one of the earliest known functions of RAC1 which occurs through its recruitment and subsequent regulation at the plasma membrane.
RAC1 function is initiate following its localization to the plasma membrane. Interaction of negatively charged PIP2/PIP3 with the positively charged polybasic sequence of small GTPases targets small GTPases to the plasma membrane [28]. In migrating cells, RAC1 localizes at the leading edge following its interaction with PIP3 [29]. Furthermore, phosphatidylserine is a bona fide binding partner of the polybasic region of RAC1 [30]. RAC1 can be brought to its site of activation, the plasma membrane, upon canonical activation of integrin(s), receptor tyrosine kinases (RTKs), and scaffold proteins [31]. In colon cancer cells, the transmembrane protein LGR5 recruits IQGAP together with RAC1 to the plasma membrane to enhance cell-to-cell adhesion [32]. LGR5 is a target gene of the Wnt-beta-catenin pathway. One of the most frequently mutated oncogenes found in hepatocellular carcinoma and colorectal cancers is beta-catenin. In addition to geranylgeranyl type of prenylation, RAC1 also undergoes reversible palmitoylation at cysteine-178, which eventually stabilizes RAC1 at actin cytoskeleton-linked membrane regions during spreading and migration [33]. Gradients of RAC1 nanoclusters support spatial patterns of RAC1 signaling [34]. Recently, Remorino et al. reported that RAC1 forms nanoclusters of 50–100 molecules which are increased upon RAC1 activation and through interaction with GEFs, GAPs, and effectors via the polybasic region at the plasma membrane of migrating fibroblasts [34]. This selective lipid sorting of small GTPases to form nanometer-scale lipid domains on the plasma membrane has been shown to drive RAC1 signaling [35].
RAC1 localization in the nucleus can be RTK mediated. Activated ERK following EGFR stimulation phosphorylates RAC1 at threonine108 leading to increased nuclear RAC1 [36]. Nuclear localization (nuclear localization signals identified in the C-terminal polybasic region of RAC1) of RAC1 is cell cycle dependent. High RAC1 is observed in the nucleus in late G2 phase, and nuclear exclusion of RAC1 occurs during early G1 phase [37]. RAC1 is also found to localize to centrosomes at G2 phase, prophase, and early prometaphase regulating centrosome separation and mitotic entry [38,39]. Nuclear RAC1 directly interacts with nucleophosmin1 (NPM1), which in addition to acting as a RAC1 chaperone also forms a nuclear protein complex with RAC1, its GEF, ECT2, and the nucleolar transcription factor to promote ribosomal DNA transcription during KRAS-TP53-induced lung tumorigenesis [40]. In the nucleus of colorectal cancer cells, TIAM1, a RAC1-GEF, negatively regulates YAP/TAZ transcriptional activity, hence suppressing their invasiveness [41]. Nuclear accumulation of RAC1-beta-catenin complex following Wnt or RAC1 activation helps in the formation of nuclear beta-catenin-lymphoid enhancer factor (LEF-1) complexes, necessary for transactivation of Wnt-dependent genes [42]. Nucleocytoplasmic shuttling of RAC1 has been reported to drive nuclear shape changes and tumor invasion [43]. Nuclear accumulation of RAC1 increases nuclear plasticity leading to a depletion of cytoplasmic, active RAC1 with a concomitant increase in RhoA signaling driving actomyosin-mediated cell shape changes; the two properties combine to enhance the tumor cell’s invasiveness [44].
RAC1 interacts with BCL2 (a co-localization and physical interaction between the two proteins) at the mitochondrial membrane of BCL2-overexpressing B-cell lymphoma cells [45], which stabilizes the anti-apoptotic, BCL2-mediated superoxide production. Inhibition of the BCL2-RAC1 interaction or blocking superoxide production, sensitizes lymphoma cells to apoptosis [45]. Interestingly, such interaction was only reported in primary cells derived from patients with B-cell lymphoma overexpressing BCL2 but not in noncancerous tissue.
4. Cellular Signaling of RAC1 in Solid Tumors
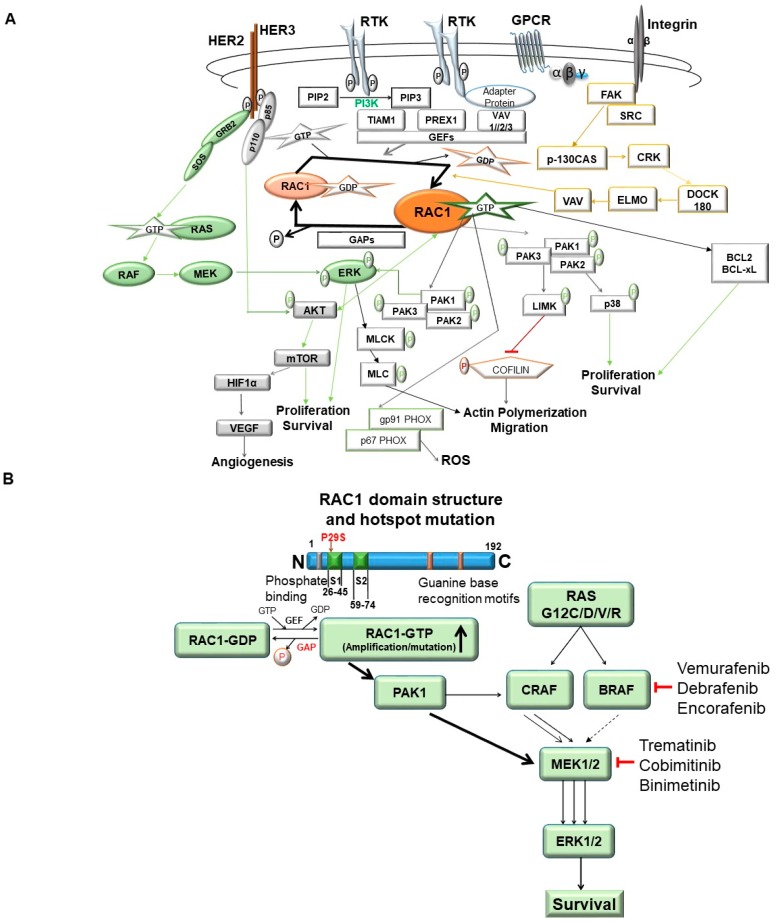
RAC1 GTPases, small G-proteins widely implicated in tumorigenesis and metastasis, transduce signals from receptor tyrosine-kinases (e.g., EGFR/ HER2) or non-receptor tyrosine kinases (e.g., SRC, FAK), G-protein-coupled receptors (GPCRs), and integrins (e.g., alpha/beta) and control a number of essential cellular functions, including motility, adhesion, and proliferation. Figure 5A illustrates RAC1 signaling pathways and functions of its effectors. Deregulation of RAC1 signaling in cancer is generally a consequence of enhanced upstream inputs from tyrosine-kinase receptors, PI3K, or GEFs (e.g., VAV, PREX, TIAM) or reduced RAC1 inactivation by GAPs (e.g., RhoGAP). In breast cancer cells RAC1 is a downstream effector of ERBB receptors via GRB7-VAV2 and mediates migratory responses by ERBB1/EGFR ligands, such as EGF or TGFα, and ERBB3 ligands, such as heregulins [46]. Recent developments in the field led to the identification of the RAC1-GEF PREX1 as an essential mediator of RAC1 responses in breast cancer cells. PREX1 is activated by the PI3K product PIP3 and Gβγ subunits and integrates signals from ERBB2 receptors and GPCRs. Most notably, PREX1 is highly overexpressed and amplified in human luminal breast tumors, particularly those expressing ERBB2 and estrogen receptor (ER). The HER2-PREX1-RAC1 signaling pathway may represent an attractive target for breast cancer therapy [47]. Similarly, RAC1 GTPase was found to signal the Wnt-beta-catenin pathway-mediated tumor cell phenotypes in triple negative breast cancers [48].
Figure 5.
RAC1 signaling in tumor cells: RAC1 signaling in tumors cells and the involvement of the RAC1 protein in tumorigenesis and drug-resistance as presented in the review. (A) represents the RAC1 signaling pathway. RAC1 cycles between an inactive-GDP-bound form, and an active-GTP-bound form. Signals from cell surface receptors, for example, G-protein coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), and integrins (alpha, beta) converge on the guanine nucleotide exchange factors (GEFS: VAV, TIAM, PREX), which converts RAC1 into its active state. Once activated, RAC1 can bind to a wide range of effectors, which in turn influence both a variety of oncogenic phenotypes like proliferation/survival, actin remodeling/migration, metastasis, and angiogenesis in tumor cells. In the process of functions mentioned above within tumor cells, RAC1 signals are closely knit to both the PI3K-AKT and the RAS-MAPK pathways integrating extracellular stimulations with oncogenic alterations. Other mechanisms, such as the stabilization of the active form of RAC1 by sumoylation or sequestration of the inactive form by guanine nucleotide dissociation factors (GDIs), can also influence the level of RAC1 signaling. Schematic illustration of RAC1 signaling pathways and functions of its effectors are presented. Green font represents signaling events following phosphorylation-mediated activation of the protein. The red font represents signaling events following phosphorylation-mediated inactivation of the protein. The blue border represents adaptor protein, the orange border represents GDP state/inactivating phosphorylation, the green border represents activated phosphorylation, the yellow border represents metastatic pathway, the yellow arrows represent metastatic pathway network, the green arrows represent proliferation/survival network, and the red line represents inhibition of signals. (B) illustrates the involvement of RAC1 in tumorigenesis and drug-resistance: RAS signaling-mediated tumorigenesis and the RAC1-PAK1 pathway-mediated resistance against RAS–RAF-MEK pathway-targeted drugs in cancers has been illustrated. RAC1 plays a cardinal role in the development of drug-resistance, especially in BRAF-mediated cancers like melanoma in which the development of resistance to BRAF inhibitors like vemurafenib, debrafenib, and encorafenib for a certain time leads to the hyper-activation of RAC1. The hyper-activation of RAC1 may either occur in the presence of upstream RTK-activation or due to the accompanying amplification or hot-stop mutation of RAC1. Under hyper-activation of RAC1, the RAC1-GTP activates PAK1, leading to the downstream activation of MEK and bypassing the upstream BRAF inhibition.
The most established mechanism for RAC1-mediated cytoskeleton reorganization is through PAK (p21-activated kinase), a family that comprises six isoforms (PAK1–6). PAKs have an N-terminal GTPase binding domain (GBD) where RAC1-GTP or Cdc42-GTP binds and a C-terminal serine/threonine kinase domain. Binding of active Cdc42 or RAC1 to the GBD domain of group I PAKs (PAK 1, 2, and 3) releases auto-inhibition and enhances kinase activity. Overexpression and/or hyperactivation of PAK isoforms have been detected in several cancer types, including breast cancer [49]. PAK1 is implicated in the regulation of cytoskeleton dynamics and cell motility, transcription, survival, and cell-cycle progression and also activates RAF-MEK signaling [50]. Upregulation of RAC1-PAK1 signaling exerts its effects on cytoskeleton organization, ROS production, and proliferation/survival through phosphorylation of various proteins, including LIMK (LIM kinase)/co-filin, gp91PHOX/p67 PHOX and AKT/BCL2/BCL-xL respectively [51,52,53] (see Figure 5A).
5. RAC1 Signaling in Tumor Cell Proliferation
Several studies, including ours [48], demonstrated that RAC1 GTPase is upregulated/hyperactivated in breast cancer cells (in all three subsets like luminal, HER2-enriched, and TNBC) and is associated with poor prognosis. Studies by Hein et al. revealed that RAC1-GTP activity is necessary for G2/M cell cycle activation and cell survival in response to ionizing radiation of breast cancer cells. Using a RAC1-specific inhibitor (NSC23766, albeit in high concentration, 100 µM) and the dominant negative mutant N17RAC1, they demonstrated that RAC1 inhibition decreased the phosphorylation of ERK1/2 and IκBα, as well as the protein expression levels of BCL-xL and MCL-1 protein in the HER-selected breast cancer cells along with clonogenic growth survival [54]. Active RAC1 was also required for RICTOR-mTORC2-dependent invasion and motility [55], essential for metastasis. Phosphatidylinositol 3,4,5-triphosphate (PIP3) also plays an important role in the activation of guanine nucleotide exchange factors for the activation of small GTPases, including RAC1. GEFs (e.g., VAV1/2/3, PREX1, TIAM) contain a PH domain and once they are bound to PIP3 (in the membrane) enhance small GTPase activity [56]. Although VAV1 alterations have been historically regarded as predominantly associated with hematopoietic malignancies, certain oncogenic mutation(s) of VAV1 in solid tumors have also been reported [57,58]. VAV1 mutations have been identified in human cancers of various origins in the context of different oncogenic phenotypes. Recently, Shalom et al. tested the transforming potential of three mutations (E59K, D517E, and L801P) reported in human lung adenocarcinoma. Studies demonstrated that mutations like E59K and D517E are highly transforming indicating that specific mutations of VAV1 can be recognized as a bona fide oncogene in human cancers [59]. GAPs are multi-domain proteins characterized by the presence of various functional domains including the conserved GAP domain which is triggering the intrinsic GTP hydrolysis. Increased GDP→GTP nucleotide exchange by RAC1-GEF in the context of the event of the activation of RAC1 mediates oncogenic effects, while alterations in RAC1-GAPs are not generally cancer associated [47,60]. Overexpression and/or hyperactivation of RAC1’s immediate effector molecule, PAK, has been detected in several cancer types, including in breast cancer [49]. PAK1 is implicated in the regulation of cytoskeleton dynamics and cell motility, transcription, survival, cell-cycle progression, and resistance to hormonal therapy (e.g., tamoxifen) in ER+ breast cancer [48,61]. NF2-knockout mouse embryonic fibroblasts (MEF) exhibit increased RAC1 activity (RAC1 activity is inversely regulated by NF2), loss of contact inhibition, and significantly increased canonical Wnt signaling [62].
RAC1 controls cancer cell invasion by regulating the production of matrix metalloproteinases, MMPs (RAC1/PAK1/p38/MMP-2 axis regulated angiogenesis), and their natural inhibitors, the tissue-specific inhibitors of MMP (TIMPs) [63,64]. Degradation of the extracellular matrix (ECM) by MMPs is essential for this kind of collective cell migration [65,66]. It is also known that colorectal cancer cells express an alternatively spliced RAC1b and depend on RAC1b signaling for survival via NF-kB activation [67]. Other studies found that RAC1b increased cell-cycle progression of G1-S phase and survival and transformation of NIH3T3 fibroblasts [68,69], promoted epithelial-mesenchymal transition of mouse mammary epithelial cells [70], and supported the further stimulation of Wnt signaling in HCT116 colorectal cells [71]. In a conditional lung cancer mouse model, RAC1 function was needed for KRAS-mediated cell proliferation and tumorigenicity [72]. Similarly, mice lacking the RAC-specific GEF Tiam1 are protected from RAS-driven skin cancer, developing fewer tumors [73]. These results strongly indicate that RAC1-GTP has the capacity to regulate tumorigenicity and increase tumor cell proliferation even in RAS-driven cancers.
6. RAC1 Signaling in Tumor Cells Migration
The small GTPase RAC1 has been implicated in the rearrangement of the actin cytoskeleton and remodeling of the plasma membrane in response to extracellular stimuli, a process known as “membrane ruffling” [25]. The RAC/RHO GTPases have been found to play a role in signaling that activates a variety of transcription factors, cell cycle progression, and integrin-mediated cell migration [74,75,76]. Because aberrant RAC1 GTPase signaling activities are widely associated with human cancer, key components of RAC1 GTPase signaling pathways have attracted increasing interest as potential therapeutic targets.
Hall, Ridley, and Nobes demonstrated that the RAC1 initiates lamellipodia formation in downstream of PDGF stimulation in contrast to the fact that RhoA stimulates the formation of contractile actomyosin fibers (i.e., stress fibers) following LPA signaling [25], and Cdc42 promotes filopodia [26]. RAC1-mediated lamellopodia formation involves binding to the SCAR/WAVE regulatory complex (WRC) components of Sra1 and WAVE1. An effector molecule ELMO, a downstream of RhoG, controls RAC-driven actin remodeling and migration [77,78,79]. Epithelial–mesenchymal transition (EMT) is one of the cardinal features associated with the metastatic process in tumor cells wherein these cells lose their polarity, shape (especially in the case of epithelial tumors; from epithelioid to mesenchymal), and cell-to-cell adhesion in order to acquire migratory and invasive potential as well as stem-like features [80]. Understandably, RAC1 plays an important role in EMT by virtue of its property to regulate cell polarity, migration, invasion, and stemness [81] in different cancers including gastric adenocarcinoma [82], squamous lung cancer [83,84], and colorectal cancers [85]. A mechanism involving RAC1 has been reported to involve the PI3K/AKT-RAC1-JNK axis in gastric adenocarcinoma [82], PI3K in squamous lung cancer [83,84], and the activation of STAT3 in colorectal cancer [85], highlighting the direct association of RAC1 activation and tumor aggressiveness.
7. RAC1 Signaling in Tumor Angiogenesis and Resistance
Tumor-induced angiogenesis supplies necessary nutrients and fosters tumor growth [86]. RAC1 is involved in angiogenesis and required for vascular integrity and the sprouting blood vessels (associated with tumor-induced angiogenesis), as demonstrated in a conditional RAC1 knockout mouse model [87]. RAC1 is activated by various angiogenic factors, e.g., vascular endothelial growth factor (VEGF)-A, angiopoietin 1, basic fibroblast growth factor (FGF), and others [88]. Activation of RAC1 and also RAC2 in endothelial cells regulates adhesion, filopodia formations, morphogenesis, cell proliferation, and integrin-directed endothelial migration [89,90,91,92]. Van Allen et al., in their clinical study on patients with BRAF V600-mutant metastatic melanoma who received vemurafenib or dabrafenib monotherapy, found early disease progression in 14 patients (out of 45 patients). No patients who demonstrated a sustained response to therapy (≥12 weeks of therapy) exhibited RAC1 P29S mutation [93]. Following these clinical studies, several other investigators have confirmed that the RAC1 P29S hotspot mutation in melanoma may be an important predictor for vemurafenib and dabrafenib resistance in patients [8]. Genetic deletion or pharmacological inhibition of RAC1 in NRAS Q61K-induced melanoma has been shown to suppresses tumor growth, lymph node spread, and tumor cell invasiveness, suggesting a potential value for RAC1 inhibition in this type of tumors [94].
RAC1 has been identified as a major mediator of chemo-resistance [95,96,97]. This role of RAC1 has been suggested in both treatment resistance and compensatory mechanisms following conventional chemotherapy (in diseases including chronic lymphocytic leukemia and squamous cell carcinoma) as well as targeted therapy (anti-EGFR for lung, anti-HER2/estrogen targeted therapies in breast cancers, BRAF protein inhibitors in melanoma, and anti-angiogenic therapies in prostate cancers). Translational laboratory-based data showed that in many cases sensitivity to the targeted therapeutic is restored upon RAC1 inhibition. Figure 5B summarizes the involvement of RAC1 in tumorigenesis and drug-resistance. RAS signaling-mediated tumorigenesis and RAC1-PAK1 pathway-mediated resistance against RAS–RAF pathway-targeted drugs in cancers have been of particular interest. Mechanistic involvement of RAC1 in the development of chemo-resistance, radio-resistance, resistance to targeted therapies and immune evasion opened the opportunities for interfering RAC1 signaling pathway in cancer therapeutics [95].
Furthermore, RAC1 signaling provides a novel insight into the mechanisms of cancer cell survival under ER stress (endoplasmic reticulum stress). N92I mutation of RAC1 is oncogenic in the sarcoma model [18]. RAC1 promotes cell survival under ER stress in cells with an oncogenic N92I RAC1 mutation. Bright et al. have identified a novel connection between the UPR (unfolded protein response) and N92I RAC1, whereby RAC1 attenuates phosphorylation of EIF2S1 under ER stress and drives overexpression of ATF4 in basal conditions. The above-mentioned finding is important since oncogenesis and uncontrolled cancer cell division often induce ER stress, and cell fate under ER stress is controlled by UPR. Their study demonstrated that oncogenic mutant RAC1 and NRAS drive resistance to ER stress by activating MEK/ERK signaling [98].
Interestingly, a study from Prof. Lewis Cantley’s laboratory reported that RAC1 drives macropinocytosis of an extracellular protein and regulates the adaptive metabolic pathway. In a subset of non-small cell lung cancer (NSCLC) cell lines (including H1299, H441, H1975, H1781, and HCC4006), it was demonstrated that cells survived in the absence of glucose by internalizing and metabolizing extracellular protein via macropinocytosis. There are glucose-independent cells (glucose-independent NSCLC cells require extracellular protein for growth during glucose withdrawal) wherein macropinocytosis is increased and is regulated by phosphoinositide 3-kinase (PI3K) activation of RAC-PAK signaling [99]. The exact role of RAC1-mediated micropinocytosis in tumorigenesis and drug resistance has not been established.
8. Epilogue
One can expect a merge between the mechanistic evidence for the involvement of RAC1 in the regulation of different tumorigenic phenotypes and the utilization of the evidence-based knowledge in clinics. Studies have provided evidence that RAC1 is involved in tumorigenesis, proliferation, metastatic events, and the development of resistance. RAC1 has been associated with the expression of PD-L1 in melanomas carrying RAC1 P29 mutations. Through reverse phase protein array (RPPA) analysis, Aplin et al. found that PD-L1 was significantly upregulated along with other cell cycle proteins like CYCLIN B1 following RAC1 P29S expression. Western blot and flow cytometry analyses revealed a robust increase in PD-L1 expression, specifically with RAC1 P29S expression. Using the Skin Cutaneous Melanoma (SKCM) database in the TCGA, they also demonstrated that PD-L1 expression was significantly increased in conditions of RAC1 P29S compared to RAC1 WT in melanoma patients [100,101]. Considering the relationship between the functional PD1-PD-L1 axis and the efficacy of immunotherapy drugs, PD-L1 positive tumors (melanomas) expressing oncogenic RAC1 P29S hotspot mutation may be a suitable situation to test immunotherapy drugs. A number of inhibitors of RAC1 are being tested in several laboratories. PAK1 is identified as an interactor of the Rho GTPases RAC1 and Cdc42. PAK1 was later shown to play a diverse role in cell signaling using its catalytic and scaffolding activities [102]. The consensus from published studies is that targeting PAK proteins provides the best potential treatment for RAC1 mutant melanomas. The first structure-based virtual screening led to the discovery of NSC23766, the first selective RAC1 inhibitor with high micromolar IC50 [103]. Araiza-Oliviera et al. showed that use of PAK inhibitors (e.g., Frax-1036 and PF3758309) or the MEK inhibitor PD325901 almost completely prevented aberrant embryonic abnormalities in RAC1P29S-injected zebrafish embryos; in contrast, it was only partly prevented with the RAC inhibitor NSC23766 [101]. To date, several PAK1-targeting compounds have been developed as preclinical agents, including one (PF-03758309) that has been evaluated in a clinical trial in a variety of solid tumors (NCT00932126). In the future, it is expected that the scientific knowledge will be put into action in clinics towards designing personalized clinical trials for the therapeutic management of cancers in which a specific involvement of RAC1 has been identified. As GTPase, RAC1 is not an easy target for treatment; studies have been conducted to inhibit its downstream PAK1. Since mutant allele-specific covalent inhibitor of oncogenic variants of KRAS-G12C (not KRAS G12D or KRAS G12V), ARS1620, and MRTX849 are in the clinical development (recently presented at the NCI/NIH-sponsored session titled New NCI Collaborative Initiatives on Rare Tumors and RASopathies, presentation by Prof. McCormick, AACR Annual Meeting, Atlanta 2019), a similar allele-specific covalent inhibitor(s) of oncogenic variants of RAC1-P29S may have a significant clinical application. The chemistry and technical hurdles of the problem are beyond the scope of the review.
Acknowledgments
The authors acknowledge Avera Cancer Institute, Sioux Falls, SD for their support.
Funding
This research received no external funding and was carried out by the internal funding from Avera Cancer Institute, SD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Yamauchi A., Marchal C.C., Molitoris J., Pech N., Knaus U., Towe J., Aktinson S.J., Dinauer M.C. Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo. Role of the C-terminal polybasic domain. J. Biol. Chem. 2005;280:953–964. doi: 10.1074/jbc.M408820200. [DOI] [PubMed] [Google Scholar]
- 2.Roberts A.W., Kim C., Zhen L., Lowe J.B., Kapur R., Petryniak B., Spaetti A., Pollock J.D., Borneo J.B., Bradford G.B., et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/S1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez D.E., Agaisse H. A role for the small GTPase Rac1 in vaccinia actin-based motility. Small GTPases. 2014;5:e29038. doi: 10.4161/sgtp.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X., Herbst-Robinson K.J., Zhang J. Visualizing dynamic activities of signaling enzymes using genetically encodable FRET-based biosensors from designs to applications. Methods Enzymol. 2012;504:317–340. doi: 10.1016/B978-0-12-391857-4.00016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matos P., Skaug J., Marques B., Beck S., Verissimo F., Gespach C., Boavida M.G., Scherer S.W., Jordan P. Small GTPase Rac1: Structure, localization, and expression of the human gene. Biochem. Biophys. Res. Commun. 2000;277:741–751. doi: 10.1006/bbrc.2000.3743. [DOI] [PubMed] [Google Scholar]
- 6.Jordan P., Brazao R., Boavida M.G., Gespach C., Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–6839. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara K., Nakatsuji N., Nakamura K., Nakao K., Hashimoto R., Otani H., Sakagami H., Kondo H., Nozama S., Aiba A., et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 8.Watson I.R., Li L., Cabeceiras P.K., Mahdavi M., Gutschner T., Genovese G., Wang G., Fang Z., Tepper J.M., Stemke-Hale K.Y., et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Res. 2014;74:4845–4852. doi: 10.1158/0008-5472.CAN-14-1232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haga R.B., Ridley A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansen S., Gosens R., Wieland T., Schmidt M. Paving the Rho in cancer metastasis: Rho GTPases and beyond. Pharmacol. Ther. 2018;183:1–21. doi: 10.1016/j.pharmthera.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Lawson C.D., Ridley A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018;217:447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson L.G., Gillette J.M., Kang H., Rivera M.R., Wandinger-Ness A. Ovarian Tumor Microenvironment Signaling: Convergence on the Rac1 GTPase. Cancers. 2018;10:358. doi: 10.3390/cancers10100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado M.D.M., Dharmawardhane S. Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018;78:3101–3111. doi: 10.1158/0008-5472.CAN-18-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauthammer M., Kong Y., Ha B.H., Evans P., Bacchiocchi A., McCusker J.P., Cheng E., Damis M.J., Goh G., Choi M., et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.P., Nickerson E., Auclair D., Li L., Place C., et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A., Machesky L.M. Rac1 cycling fast in melanoma with P29S. Pigment Cell Melanoma Res. 2013;26:289–290. doi: 10.1111/pcmr.12074. [DOI] [PubMed] [Google Scholar]
- 17.Davis M.J., Ha B.H., Holman E.C., Halaban R., Schlessinger J., Boggon T.J. RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc. Natl. Acad. Sci. USA. 2013;110:912–917. doi: 10.1073/pnas.1220895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawazu M., Ueno T., Kontani K., Ogita Y., Ando M., Fukumura K., Yamato A., Soda M., Takeuchi K., Miki Y., et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc. Natl. Acad. Sci. USA. 2013;110:3029–3034. doi: 10.1073/pnas.1216141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Zhang J.X., Luo J.H., Wu S., Yuan G.J., Ma N.F., Feng Y., Cai M.Y., Chen R.X., Lu J., et al. CSTF2-Induced Shortening of the RAC1 3’UTR Promotes the Pathogenesis of Urothelial Carcinoma of the Bladder. Cancer Res. 2018;78:5848–5862. doi: 10.1158/0008-5472.CAN-18-0822. [DOI] [PubMed] [Google Scholar]
- 23.Payapilly A., Malliri A. Compartmentalisation of RAC1 signalling. Curr. Opin. Cell Biol. 2018;54:50–56. doi: 10.1016/j.ceb.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Bosco E.E., Mulloy J.C., Zheng Y. Rac1 GTPase: A “Rac” of all trades. Cell. Mol. Life Sci. CMLS. 2009;66:370–374. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 26.Nobes C.D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 27.Braga V.M., Machesky L.M., Hall A., Hotchin N.A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heo W.D., Inoue T., Park W.S., Kim M.L., Park B.O., Wandless T.J., Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S., Yin T., Yang Q., Zhang J., Wu Y.I., Yu J. Single-molecule tracking of small GTPase Rac1 uncovers spatial regulation of membrane translocation and mechanism for polarized signaling. Proc. Natl. Acad. Sci. USA. 2015;112:E267–E276. doi: 10.1073/pnas.1409667112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkielstein C.V., Overduin M., Capelluto D.G. Cell migration and signaling specificity is determined by the phosphatidylserine recognition motif of Rac1. J. Biol. Chem. 2006;281:27317–27326. doi: 10.1074/jbc.M605560200. [DOI] [PubMed] [Google Scholar]
- 31.Fritz R.D., Pertz O. The dynamics of spatio-temporal Rho GTPase signaling: Formation of signaling patterns. F1000Research. 2016:5. doi: 10.12688/f1000research.7370.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmon K.S., Gong X., Yi J., Wu L., Thomas A., Moore C.M., Masuho I., Timson D.J., Martemyanov K.A., Liu Q.J. LGR5 receptor promotes cell-cell adhesion in stem cells and colon cancer cells via the IQGAP1-Rac1 pathway. J. Biol. Chem. 2017;292:14989–15001. doi: 10.1074/jbc.M117.786798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro-Lerida I., Sanchez-Perales S., Calvo M., Rentero C., Zheng Y., Enrich C., Del Pozo M.A. A palmitoylation switch mechanism regulates Rac1 function and membrane organization. Embo J. 2012;31:534–551. doi: 10.1038/emboj.2011.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remorino A., De Beco S., Cayrac F., Di Federico F., Cornilleau G., Gautreau A., Parrini M.C., Masson J.B., Dahan M., Coppey M. Gradients of Rac1 Nanoclusters Support Spatial Patterns of Rac1 Signaling. Cell Rep. 2017;21:1922–1935. doi: 10.1016/j.celrep.2017.10.069. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell K.N., Zhou Y., Hancock J.F. Clustering of Rac1: Selective Lipid Sorting Drives Signaling. Trends Biochem. Sci. 2018;43:75–77. doi: 10.1016/j.tibs.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Tong J., Li L., Ballermann B., Wang Z. Phosphorylation of Rac1 T108 by extracellular signal-regulated kinase in response to epidermal growth factor: A novel mechanism to regulate Rac1 function. Mol. Cell. Biol. 2013;33:4538–4551. doi: 10.1128/MCB.00822-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelson D., Abidi W., Guardavaccaro D., Zhou M., Ahearn I., Pagano M., Phillips M.R. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J. Cell Biol. 2008;181:485–496. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May M., Schelle I., Brakebusch C., Rottner K., Genth H. Rac1-dependent recruitment of PAK2 to G2 phase centrosomes and their roles in the regulation of mitotic entry. Cell Cycle. 2014;13:2211–2221. doi: 10.4161/cc.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodcock S.A., Rushton H.J., Castaneda-Saucedo E., Myant K., White G.R., Blyth K., Sansom O.J., Malliri A. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle assembly to facilitate chromosome congression. Curr. Biol. 2010;20:669–675. doi: 10.1016/j.cub.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justilien V., Ali S.A., Jamieson L., Yin N., Cox A.D., Der C.J., Murray N.R., Fields A.P. Ect2-Dependent rRNA Synthesis Is Required for KRAS-TRP53-Driven Lung Adenocarcinoma. Cancer Cell. 2017;31:256–269. doi: 10.1016/j.ccell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diamantopoulou Z., White G., Fadlullah M.Z.H., Dreger M., Pickering K., Maltas J., Ashton G., MacLeod R., Baillie G.S., Kouskoff V., et al. TIAM1 Antagonizes TAZ/YAP Both in the Destruction Complex in the Cytoplasm and in the Nucleus to Inhibit Invasion of Intestinal Epithelial Cells. Cancer Cell. 2017;31:621–634. doi: 10.1016/j.ccell.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamieson C., Lui C., Brocardo M.G., Martino-Echarri E., Henderson B.R. Rac1 augments Wnt signaling by stimulating beta-catenin-lymphoid enhancer factor-1 complex assembly independent of beta-catenin nuclear import. J. Cell Sci. 2015;128:3933–3946. doi: 10.1242/jcs.167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro-Lerida I., Pellinen T., Sanchez S.A., Guadamillas M.C., Wang Y., Mirtti T., Calvo E., DelPozo M.A. Rac1 nucleocytoplasmic shuttling drives nuclear shape changes and tumor invasion. Dev. Cell. 2015;32:318–334. doi: 10.1016/j.devcel.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Disanza A., Scita G. Nuclear and cellular plasticity: Nuclear RAC1 takes center stage. Dev. Cell. 2015;32:261–263. doi: 10.1016/j.devcel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Velaithan R., Kang J., Hirpara J.L., Loh T., Goh B.C., Le Bras M., Brenner C., Clement M.V., Pervaiz S. The small GTPase Rac1 is a novel binding partner of Bcl-2 and stabilizes its antiapoptotic activity. Blood. 2011;117:6214–6226. doi: 10.1182/blood-2010-08-301283. [DOI] [PubMed] [Google Scholar]
- 46.Pradip D., Bouzyk M., Dey N., Leyland-Jones B. Dissecting GRB7-mediated signals for proliferation and migration in HER2 overexpressing breast tumor cells: GTP-ase rules. Am. J. Cancer Res. 2013;3:173–195. [PMC free article] [PubMed] [Google Scholar]
- 47.Wertheimer E., Gutierrez-Uzquiza A., Rosemblit C., Lopez-Haber C., Sosa M.S., Kazanietz M.G. Rac signaling in breast cancer: A tale of GEFs and GAPs. Cell. Signal. 2012;24:353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De P., Carlson J.H., Jepperson T., Willis S., Leyland-Jones B., Dey N. RAC1 GTP-ase signals Wnt-beta-catenin pathway mediated integrin-directed metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget. 2017;8:3072–3103. doi: 10.18632/oncotarget.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dummler B., Ohshiro K., Kumar R., Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shrestha Y., Schafer E.J., Boehm J.S., Thomas S.R., He F., Du J., Wang S., Barretina J., Weir B.A., Zhao J.J., et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 2012;31:3397–3408. doi: 10.1038/onc.2011.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bokoch G.M. Regulation of cell function by Rho family GTPases. Immunol. Res. 2000;21:139–148. doi: 10.1385/IR:21:2-3:139. [DOI] [PubMed] [Google Scholar]
- 52.Edwards D.C., Sanders L.C., Bokoch G.M., Gill G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 53.Vadlamudi R.K., Li F., Barnes C.J., Bagheri-Yarmand R., Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. Embo Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hein A.L., Post C.M., Sheinin Y.M., Lakshmanan I., Natarajan A., Enke C.A., Bartra S.K., Ouellete M.M., Yan Y. RAC1 GTPase promotes the survival of breast cancer cells in response to hyper-fractionated radiation treatment. Oncogene. 2016;35:6319–6329. doi: 10.1038/onc.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison Joly M., Williams M.M., Hicks D.J., Jones B., Sanchez V., Young C.D., Sarbassov D.D., Muller W.J., Brantely-Sieders D., Cook R.S. Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis. Breast Cancer Res. BCR. 2017;19:74. doi: 10.1186/s13058-017-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goncalves M.D., Hopkins B.D., Cantley L.C. Phosphatidylinositol 3-Kinase, Growth Disorders, and Cancer. New Engl. J. Med. 2018;379:2052–2062. doi: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 57.Katzav S. Vav1: A Dr. Jekyll and Mr. Hyde protein--good for the hematopoietic system, bad for cancer. Oncotarget. 2015;6:28731–28742. doi: 10.18632/oncotarget.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han J., Luby-Phelps K., Das B., Shu X., Xia Y., Mosteller R.D., Krisna U.M., Falck J.R., White M.A., Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 59.Shalom B., Farago M., Pikarsky E., Katzav S. Vav1 mutations identified in human cancers give rise to different oncogenic phenotypes. Oncogenesis. 2018;7:80. doi: 10.1038/s41389-018-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vega F.M., Ridley A.J. Rho GTPases in cancer cell biology. Febs Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 61.Rayala S.K., Molli P.R., Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006;66:5985–5988. doi: 10.1158/0008-5472.CAN-06-0978. [DOI] [PubMed] [Google Scholar]
- 62.Bosco E.E., Nakai Y., Hennigan R.F., Ratner N., Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29:2540–2549. doi: 10.1038/onc.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lozano E., Betson M., Braga V.M. Tumor progression: Small GTPases and loss of cell-cell adhesion. Bioessays. 2003;25:452–463. doi: 10.1002/bies.10262. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Villasana V., Fuentes-Mattei E., Ivan C., Dalton H.J., Rodriguez-Aguayo C., Fernandez-de Thomas R.J., Aslan B., Cel C., Monroig P., Velazquez-Torres G., et al. Rac1/Pak1/p38/MMP-2 Axis Regulates Angiogenesis in Ovarian Cancer. Clin. Cancer Res. 2015;21:2127–2137. doi: 10.1158/1078-0432.CCR-14-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf K., Wu Y.I., Liu Y., Geiger J., Tam E., Overall C., Stack M.S., Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 66.Dey N., Young B., Abramovitz M., Bouzyk M., Barwick B., De P., Leyland-Jones B. Differential activation of Wnt-beta-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS ONE. 2013;8:e77425. doi: 10.1371/journal.pone.0077425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matos P., Jordan P. Increased Rac1b expression sustains colorectal tumor cell survival. Mol. Cancer Res. 2008;6:1178–1184. doi: 10.1158/1541-7786.MCR-08-0008. [DOI] [PubMed] [Google Scholar]
- 68.Singh A., Karnoub A.E., Palmby T.R., Lengyel E., Sondek J., Der C.J. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene. 2004;23:9369–9380. doi: 10.1038/sj.onc.1208182. [DOI] [PubMed] [Google Scholar]
- 69.Matos P., Jordan P. Rac1, but not Rac1B, stimulates RelB-mediated gene transcription in colorectal cancer cells. J. Biol. Chem. 2006;281:13724–13732. doi: 10.1074/jbc.M513243200. [DOI] [PubMed] [Google Scholar]
- 70.Radisky D.C., Levy D.D., Littlepage L.E., Liu H., Nelson C.M., Fata J.E., Leake D., Godden E.L., Albertson D.G., Nieto M.A., et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esufali S., Charames G.S., Pethe V.V., Buongiorno P., Bapat B. Activation of tumor-specific splice variant Rac1b by dishevelled promotes canonical Wnt signaling and decreased adhesion of colorectal cancer cells. Cancer Res. 2007;67:2469–2479. doi: 10.1158/0008-5472.CAN-06-2843. [DOI] [PubMed] [Google Scholar]
- 72.Kissil J.L., Walmsley M.J., Hanlon L., Haigis K.M., Bender Kim C.F., Sweet-Cordero A., Eckman M.S., Tuveson D.A., Capobianco A.J., Tybulewicz V., et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–8094. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 73.Malliri A., van der Kammen R.A., Clark K., van der Valk M., Michiels F., Collard J.G. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 74.Pradip D., Peng X., Durden D.L. Rac2 specificity in macrophage integrin signaling: Potential role for Syk kinase. J. Biol. Chem. 2003;278:41661–41669. doi: 10.1074/jbc.M306491200. [DOI] [PubMed] [Google Scholar]
- 75.Dey N., Howell B.W., De P.K., Durden D.L. CSK negatively regulates nerve growth factor induced neural differentiation and augments AKT kinase activity. Exp. Cell Res. 2005;307:1–14. doi: 10.1016/j.yexcr.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 76.Dey N., De P.K., Wang M., Zhang H., Dobrota E.A., Robertson K.A., Drudern D.L. CSK controls retinoic acid receptor (RAR) signaling: A RAR-c-SRC signaling axis is required for neuritogenic differentiation. Mol. Cell. Biol. 2007;27:4179–4197. doi: 10.1128/MCB.01352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eden S., Rohatgi R., Podtelejnikov A.V., Mann M., Kirschner M.W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z., Borek D., Padrick S.B., Gomez T.S., Metlagel Z., Ismail A.M., Umetani J., Billadeau D.D., Otwinowski Z., Rosen M.K. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katoh H., Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 80.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Orgaz J.L., Herraiz C., Sanz-Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. doi: 10.4161/sgtp.29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon C., Cho S.J., Chang K.K., Park D.J., Ryeom S.W., Yoon S.S. Role of Rac1 Pathway in Epithelial-to-Mesenchymal Transition and Cancer Stem-like Cell Phenotypes in Gastric Adenocarcinoma. Mol. Cancer Res. 2017;15:1106–1116. doi: 10.1158/1541-7786.MCR-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Bonelli M.A., Cavazzoni A., Saccani F., Alfieri R.R., Quaini F., La Monica S., Galetti M., Cretella D., Caffarra C., Madeddu D., et al. Inhibition of PI3K Pathway Reduces Invasiveness and Epithelial-to-Mesenchymal Transition in Squamous Lung Cancer Cell Lines Harboring PIK3CA Gene Alterations. Mol. Cancer Ther. 2015;14:1916–1927. doi: 10.1158/1535-7163.MCT-14-0892. [DOI] [PubMed] [Google Scholar]
- 84.Cavazzoni A., La Monica S., Alfieri R., Ravelli A., Van Der Steen N., Sciarrillo R., Madeddu D., Lagrasta C.A.M., Quaini F., Bonelli M., et al. Enhanced efficacy of AKT and FAK kinase combined inhibition in squamous cell lung carcinomas with stable reduction in PTEN. Oncotarget. 2017;8:53068–53083. doi: 10.18632/oncotarget.18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou K., Rao J., Zhou Z.H., Yao X.H., Wu F., Yang J., Yang L., Zhang X., Cui Y.H., Bian X.W., et al. RAC1-GTP promotes epithelial-mesenchymal transition and invasion of colorectal cancer by activation of STAT3. Lab Invest. 2018;98:989–998. doi: 10.1038/s41374-018-0071-2. [DOI] [PubMed] [Google Scholar]
- 86.Bid H.K., Roberts R.D., Manchanda P.K., Houghton P.J. RAC1: An emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol. Cancer Ther. 2013;12:1925–1934. doi: 10.1158/1535-7163.MCT-13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nohata N., Uchida Y., Stratman A.N., Adams R.H., Zheng Y., Weinstein B.M., Mukouyama Y.S., Gutkind J.S. Temporal-specific roles of Rac1 during vascular development and retinal angiogenesis. Dev. Biol. 2016;411:183–194. doi: 10.1016/j.ydbio.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Galan Moya E.M., Le Guelte A., Gavard J. PAKing up to the endothelium. Cell. Signal. 2009;21:1727–1737. doi: 10.1016/j.cellsig.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Abraham S., Scarcia M., Bagshaw R.D., McMahon K., Grant G., Harvey T., Yeo M., Estevens F.O., Thygesen H.H., Jones P.F., et al. A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat. Commun. 2015;6:7286. doi: 10.1038/ncomms8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fryer B.H., Field J. Rho, Rac, Pak and angiogenesis: Old roles and newly identified responsibilities in endothelial cells. Cancer Lett. 2005;229:13–23. doi: 10.1016/j.canlet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Xue Y., Bi F., Zhang X., Pan Y., Liu N., Zheng Y., Fan D. Inhibition of endothelial cell proliferation by targeting Rac1 GTPase with small interference RNA in tumor cells. Biochem. Biophys. Res. Commun. 2004;320:1309–1315. doi: 10.1016/j.bbrc.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 92.De P., Peng Q., Traktuev D.O., Li W., Yoder M.C., March K.L., Durden D.L. Expression of RAC2 in endothelial cells is required for the postnatal neovascular response. Exp. Cell Res. 2009;315:248–263. doi: 10.1016/j.yexcr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Allen E.M., Wagle N., Sucker A., Treacy D.J., Johannessen C.M., Goetz E.M., Place C.S., Taylor-Weiner A., Whittaker S., Kryukov G.V., et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li A., Ma Y., Jin M., Mason S., Mort R.L., Blyth K., Larue L., Sansom O.J., Machesky L.M. Activated mutant NRas(Q61K) drives aberrant melanocyte signaling, survival, and invasiveness via a Rac1-dependent mechanism. J. Investig. Dermatol. 2012;132:2610–2621. doi: 10.1038/jid.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cardama G.A., Alonso D.F., Gonzalez N., Maggio J., Gomez D.E., Rolfo C., Menna P.L. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: Opportunities in cancer therapeutics. Crit. Rev. Oncol./Hematol. 2018;124:29–36. doi: 10.1016/j.critrevonc.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 96.Hofbauer S.W., Krenn P.W., Ganghammer S., Asslaber D., Pichler U., Oberascher K., Henschler R., Wallner M., Kerschbaum H., Greil R., et al. Tiam1/Rac1 signals contribute to the proliferation and chemoresistance, but not motility, of chronic lymphocytic leukemia cells. Blood. 2014;123:2181–2188. doi: 10.1182/blood-2013-08-523563. [DOI] [PubMed] [Google Scholar]
- 97.Ikram M., Lim Y., Baek S.Y., Jin S., Jeong Y.H., Kwak J.Y., Yoon S. Co-targeting of Tiam1/Rac1 and Notch ameliorates chemoresistance against doxorubicin in a biomimetic 3D lymphoma model. Oncotarget. 2018;9:2058–2075. doi: 10.18632/oncotarget.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bright M.D., Clarke P.A., Workman P., Davies F.E. Oncogenic RAC1 and NRAS drive resistance to endoplasmic reticulum stress through MEK/ERK signalling. Cell. Signal. 2018;44:127–137. doi: 10.1016/j.cellsig.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hodakoski C., Hopkins B.D., Zhang G., Su T., Cheng Z., Morris R., Rhee K.Y., Gonclaves M.D., Cantley L.C. Rac-Mediated Macropinocytosis of Extracellular Protein Promotes Glucose Independence in Non-Small Cell Lung Cancer. Cancers. 2019;11:37. doi: 10.3390/cancers11010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vu H.L., Rosenbaum S., Purwin T.J., Davies M.A., Aplin A.E. RAC1 P29S regulates PD-L1 expression in melanoma. Pigment Cell Melanoma Res. 2015;28:590–598. doi: 10.1111/pcmr.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Araiza-Olivera D., Feng Y., Semenova G., Prudnikova T.Y., Rhodes J., Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene. 2018;37:944–952. doi: 10.1038/onc.2017.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manser E., Leung T., Salihuddin H., Zhao Z.S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 103.Marei H., Malliri A. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases. 2017;8:139–163. doi: 10.1080/21541248.2016.1211398. [DOI] [PMC free article] [PubMed] [Google Scholar]