Abstract
Sexual reproduction in filamentous ascomycete fungi results in the production of highly specialized sexual tissues, which arise from relatively simple, vegetative mycelia. This conversion takes place after the recognition of and response to a variety of exogenous and endogenous cues, and relies on very strictly regulated gene, protein, and metabolite pathways. This makes studying sexual development in fungi an interesting tool in which to study gene–gene, gene–protein, and protein–metabolite interactions. This review provides an overview of some of the most important genes involved in this process; from those involved in the conversion of mycelia into sexually-competent tissue, to those involved in the development of the ascomata, the asci, and ultimately, the ascospores.
Keywords: sexual reproduction, fungi, filamentous ascomycetes, gene expression, regulatory networks, functional characterisation
1. Introduction
Fungi represent the most diverse of the eukaryotic kingdoms, with more than 100,000 species described to date [1]. While there is some disagreement regarding the actual number of fungal species present globally, this value is generally accepted to be in the millions [2,3]. Such a rich diversity has led to an incredible variety of life cycles as well as a high level of reproductive plasticity. As such, fungal species exhibit some of the most interesting mechanisms of propagation, both sexual and asexual [4].
Sexual reproduction in filamentous ascomycetes (Figure 1) is initiated upon the recognition of a variety of factors that cause the conversion of vegetative mycelia into sexually-competent tissue (reviewed in [5]). This is followed by gamete fertilization, and the production of a dikaryotic cell and the protoascomata, which mature into fully developed fruiting structures, known as ascomata. These structures harbour the sac-shaped asci that produce and protect the eight internal ascospores [6,7]. The production of these highly specialized sexual tissue types is a morphological outcome that relies upon the initiation and control of gene, protein, and secondary metabolite networks [8]. These networks interact with one another to ensure the correct spatiotemporal expression of gene products, which enables the process of sexual reproduction to take place under the most suitable environmental conditions.
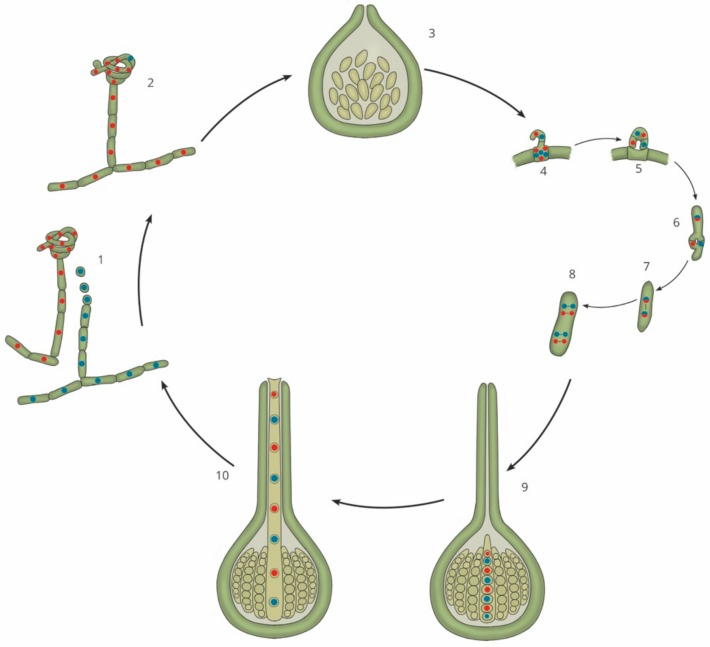
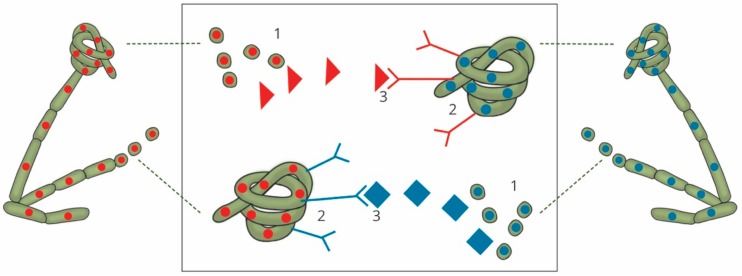
Figure 1.
Generalized sexual cycle of filamentous ascomycetes. Mycelial strands (1) recognize a variety of signals before being converted into sexually competent tissue. One such signal is the recognition of a suitable mating partner. One partner produces the female structure, an ascogonium (indicated by the structure with red nuclei), while the second partner produces fertilizing spermatia (indicated by the single cells with blue nuclei). Gamete fertilization (2) occurs when the spermatia physically interact with the ascogonium. This is followed by the production of the protoascomata (3). Stages (4) to (8) occur within the immature ascomata and the developing asci. This includes the development of a crozier, nuclear migration, karyogamy, meiosis, and mitosis. This entire process culminates in a fully mature ascoma (9), which can release ascospores (10). These spores will then germinate and begin the cycle again.
In this review, we consider the many genes that play pivotal roles during the process of sexual reproduction in filamentous ascomycete fungi. To this end, three broadly divided sections are presented. These cover the major morphological changes that occur during protoascomatal development, ascus formation, and ascospore production.
2. Protoascomatal Development
The conversion of vegetative mycelia into sexually-competent, ascomatal-forming tissue is the first step in sexual reproduction. This relies on the recognition of various endogenous and exogenous cues, each of which uniquely contributes to whether sexual structures are produced.
2.1. The Mating Type Genes
The master regulators of sex in most fungi are the mating type, or MAT, genes. These genes, harboured at the MAT locus, encode proteins that typically possess DNA binding domains [9]. The MAT proteins act as transcription factors that regulate the expression of genes related to sex, such as those involved in mate recognition, cellular differentiation, and meiosis [10,11]. It is the MAT genes that determine the mating strategies employed by a fungal species, as sexual reproduction typically requires the expression of MAT1-1 and MAT1-2 genes (Figure 2). In heterothallic fungi, individuals possess either the MAT1-1 or MAT1-2 genes, which confer the MAT1-1 and MAT1-2 mating types, respectively. This system requires opposite partners to physically interact in order to produce sexual offspring. In contrast, homothallic species typically possess both types of genes [12] and are thus able to sexually reproduce in complete isolate or with any other individual of the same species [4,13,14]. Until Turgeon and Yoder [15] proposed a revised naming system in 2000, MAT gene nomenclature was species-specific, making comparisons of gene content and function difficult. Thus, for the sake of this review, genes are referred to by the names that fulfil the requirements of the universal nomenclature system (Table 1), as revised and updated in 2017 by Wilken et al. [16].
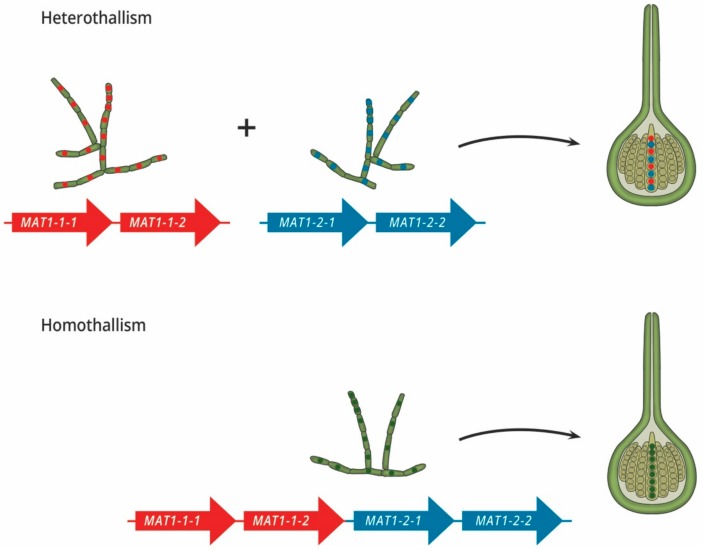
Figure 2.
The sexual strategies of filamentous ascomycetes. Heterothallism: Two isolates of an opposite mating type need to physically interact in order to produce sexual structures. These mating types are genetically determined by genes at the MAT locus, either encoding the MAT1-1 genes (red) or the MAT1-2 genes (blue). These genes confer the MAT1-1 and MAT1-2 mating identities, respectively. Homothallism: Sexual reproduction can either occur within a single isolate that expresses both the MAT1-1 and MAT1-2 genes (as illustrated) or between any two individuals of the same species.
Table 1.
The gene name equivalents of the MAT, pheromone, and pheromone receptor genes.
| Species | Sexual Strategy | MAT1-1 Idiomorph | Universal Name | MAT1-1 Genes | α-Factor Pheromone | α-Factor Receptor | MAT1-2 Idiomorph | Universal Name | MAT1-2 Genes | a-Factor Pheromone | a-Factor Receptor |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryphonectria parasitica | Heterothallic | MAT1-1 | MAT1-1-1 | MAT1-1-1 | Mf1/1 | - | MAT1-2 | MAT1-2-1 | MAT1-2-1 |
Mf2/1
Mf2/2 |
- |
| MAT1-1-2 | MAT1-1-2 | ||||||||||
| MAT1-1-3 | MAT1-1-3 | ||||||||||
| Magnaporthe grisea | Heterothallic | MAT1-1 a | MAT1-1-1 | MAT1-1 | MF2-1 | ste3-like | MAT1-2 a | MAT1-2-1 | MAT1-2 | MF1-1 | ste2-like |
| Neurospora crassa | Heterothallic | mat A | MAT1-1-1 | matA-1 | ccg4 | pre2 | mat a |
MAT1-2-1
MAT1-2-2 |
mata-1
mata-2 |
mfa-1 | pre1 |
| MAT1-1-2 | matA-2 | ||||||||||
| MAT1-1-3 | matA-3 | ||||||||||
| Trichoderma reesei | Heterothallic | MAT1-1 | MAT1-1-1 | mat1-1-1 | ppg1 c | pre2 | MAT1-2 | MAT1-2-1 | mat1-2-1 | hpp1 c | pre1 |
| MAT1-1-2 | mat1-1-2 | ||||||||||
| MAT1-1-3 | mat1-1-3 | ||||||||||
| Podospora anserina | Pseudo-homothallic | mat- | MAT1-1-1 | FMR1 | mfm | pre2 | mat+ | MAT1-2-1 | FPR1 | mfp | pre1 |
| MAT1-1-2 | SMR1 | ||||||||||
| MAT1-1-3 | SMR2 | ||||||||||
| Sordaria macrospora | Homothallic b | mat A | MAT1-1-1 | SmtA-1 | ppg1 | pre2 | mat a | MAT1-2-1 | Smta-1 | ppg2 | pre1 |
| MAT1-1-2 | SmtA-2 | ||||||||||
| MAT1-1-3 | SmtA-3 | ||||||||||
| Aspergillus nidulans | Homothallic b | MAT-1 | MAT1-1-1 | MAT1-1 | ppgA | preB | MAT-2 | MAT1-2-1 | MAT2-1 | - | preA |
| Fusarium graminearum | Homothallic b | MAT1-1 | MAT1-1-1 | MAT1-1-1 | ppg1 | pre2 | MAT1-2 | MAT1-2-1 | MAT1-2-1 | ppg2 | pre1 |
| MAT1-1-2 | MAT1-1-2 |
a Prior to the revised nomenclature of mating type genes, the MAT loci of Magnaporthe spp. were arbitrarily named Mat1-2 and Mat1-1. While these names have since been changed to comply with the naming system, the pheromone gene names still reflect the original MAT gene names. b In homothallic fungi, the mating type genes are all found within a single genome. Thus, it is not necessary to classify the locus as MAT1-1 or MAT1-2. However, these species do still harbour MAT genes with homology to those found in heterothallic species. c T. reesei harbours the h-type pheromone in place of the a-factor pheromone found in many other species. Furthermore, the two pheromones of T. reesei are not expressed in a mating-type dependant manner. This is covered in the section regarding pheromones.
In Podospora anserina, the sexual cycle is controlled by a variety of proteins that regulate the MAT genes. One of the most prominent MAT-regulating proteins is encoded by pro1, a gene that is very well characterized in a number of model fungi [17,18,19,20]. In P. anserina, this protein regulates similar pathways to those of other fungal species, but also plays an integral role in sexual reproduction [21]. Pro1 acts as a positive regulator upstream of a high mobility group (HMG) box protein named PaHMG8, which itself positively regulates the expression of both MAT1-1-1 and MAT1-2-1 [21,22]. In addition to PaHMG8, two other HMG box proteins, PaHMG5 and PaHMG6, are also responsible for the tight regulation of the two MAT genes and their transcription networks [22]. Transcription factors that possess the HMG box domain are involved in a multitude of biological processes, with a particular importance in pathways associated with sexual reproduction in fungi [23].
Transcriptional control of the mating genes in Trichoderma reesei is achieved by a complex of proteins that respond to the availability of light [24,25]. In this species, two photoreceptors are encoded by the blr1 and blr2 genes, while a third regulatory protein is encoded by the env1 gene [26,27,28]. Together, these three proteins regulate sexual reproduction in a mating-type dependent manner, by controlling the expression of MAT1-2-1 [25]. The deletion of env1 has a more pronounced effect on sexual reproduction than that of either of the photoreceptors, suggesting that BLR1 and BLR2 exert their effect via ENV1. Expression of MAT1-2-1 is also controlled by VEL1, yet another protein involved in light-dependent sexual development in T. reesei [24].
The two primary MAT genes, MAT1-1-1 and MAT1-2-1, have been functionally characterized in a variety of heterothallic and homothallic species. Unsurprisingly, both genes are typically essential for successful sexual reproduction, though their exact functions may differ from species to species. In P. anserina, Aspergillus nidulans, and Neurospora crassa, both genes are essential for fertilization and thus, while both female and male structures are often still produced in strains where either MAT gene has been deleted, further ascomatal development never takes place [11,29,30,31]. In addition to its role in fertilization, the P. anserina MAT1-1-1 gene is also essential for ascospore production [29]. In Fusarium graminearum, both genes are essential and the deletion of either results in mycelia that is unable to form even immature sexual structures [32]. In Penicillium chrysogenum, the MAT1-1-1 gene is not involved in the development of the ascoma, however, ΔMAT1-1-1 strains do not produce ascospores and this gene is consequently essential for sexual reproduction [33]. The P. chrysogenums MAT1-2-1, however, has a much earlier role in sexual development as ΔMAT1-2-1 strains do not form sexual structures, even in the presence of a suitable mating partner [34]. In contrast, MAT1-1-1 is dispensable for sexual reproduction in Sordaria macrospora, while MAT1-2-1 remains essential [35].
In addition to the primary MAT genes, other secondary genes have also been described from the MAT loci. Currently, six other MAT1-1 genes (MAT1-1-2 to MAT1-1-7) and 10 other MAT1-2 genes (MAT1-2-2 to MAT1-2-11) are known from various fungi (as reviewed in [16]). Although these genes often have no known functional domains and are not typically conserved beyond genus or family boundaries [12], a number of them have been at least partially characterized.
The secondary MAT1-1 genes that have been shown to be essential to sexual reproduction include the MAT1-1-2 genes of S. macrospora [10], F. graminearum [36], and P. anserina [37], as well as the MAT1-1-5 genes of Sclerotinia sclerotiorum [38] and Botrytis cinerea [39]. Deletion of any of these genes results in the complete inability to produce sexual ascospores. While the exact function of the S. macrospora and S. sclerotiorum genes remain unknown, the P. anserina MAT1-1-2 gene is directly responsible for ascus production, nuclear recognition, and cellular division [37,40]. The F. graminearum MAT1-1-2 and the B. cinerea MAT1-1-5 are both specifically involved in the development of the maturing protoascomata. Consequently, while mutant strains of both species produce immature sexual structures, asci-bearing ascomata never develop [36,39]. The P. anserina MAT1-1-3 gene, although not essential for sexual reproduction, helps to determine the nuclear identity in sexually reproducing cultures [37]. Thus, this gene, in conjunction with MAT1-1-1, is responsible for nuclear recognition early in the sexual process, when the protoascoma is developing [37,41].
In addition to these MAT1-1 genes, a variety of secondary MAT1-2 gene are also essential for sexual reproduction. These include the MAT1-2-4 genes of S. sclerotiorum [38] and A. fumigatus [42] as well as the MAT1-2-10 gene of B. cinerea [39]. Interestingly, despite being homologs, the MAT1-2-4 of S. sclerotiorum is important for ascomatal development [38], while the A. fumigatus MAT1-2-4 gene appears to play a more global role in sexual reproduction by directly affecting the expression of a variety of different sex-related genes [42]. Lastly, the B. cinerea MAT1-2-10 plays an important role in ascomatal development, with deletion strains unable to produce asci-harbouring fruiting bodies [39].
2.2. Important Signalling Pathways
One of the major protein complexes that control sexual development in fungi is the COP9 signalosome (CSN). This complex of proteins is involved in a huge diversity of processes because it plays an integral role in post-translational processes, including protein ubiquitination and phosphorylation [43]. In the most typical cases, the complex harbours up to eight individual subunits. One such example is encountered in A. nidulans, where genes CsnA through to CsnH are encoded by the genome [44]. The importance of this signalling pathway in sexual development is supported by the fact that mutant strains lacking the CsnA, CsnB, CsnD, and CsnE genes are unable to produce protoascomatal structures, despite initiating sexual development [44,45]. The deletion of a single Csn gene can affect the assembly of the entire signalosome, explaining the disrupted sexual development seen even in single gene knockouts [45].
The second important protein complex that is involved in sexual reproduction is known as STRIPAK, the striatin-interacting phosphatase and kinase complex (reviewed in [46]). This complex was first discovered in humans [47] and homologous complexes have since been identified in a huge variety of eukaryotic organisms, including Drosophila melanogaster [48]. The STRIPAK complex has been fairly well characterized in S. macrospora, where it is known to influence the production of the ascomata [49,50,51]. Three of the core proteins of this complex, PRO11, MOB3, and PRO22, have been studied in S. macrospora and are all essential for sexual reproduction.
The first of the STRIPAK genes to be characterized was pro11, which encodes a protein homologous to the mammalian striatin [51]. This gene is up-regulated during sexual reproduction [49], but is only involved in this process once the protoascomata have formed. Thus, while Δpro11 mutants are sterile, ascogonia and immature ascomata are produced [51]. PRO11 interacts with a second protein, encoded by the mob3 gene [49]. MOB3 is a phocein protein and like PRO11, is also up-regulated during sexual reproduction in S. macrospora. It is thus not surprising that Δmob3 mutants are also sterile. However, the phenotypic disruption seen in mob3 deletion mutants is more severe than that of Δpro11 mutants, as they are unable to produce any sexual tissues [49]. The third protein, PRO22, interacts directly with PRO11 in the STRIPAK complex, and is involved in sexual development [50]. In Δpro22 mutants, immature sexual tissue is formed, but does not develop further than the protoascomatal stage, which is similar to the Δpro11 mutants [50,51].
2.3. Nutrient Requirements for the Induction of Sexual Reproduction
One of the most important sex-regulating factors that fungi are able to recognize and respond to is nutrient availability. Before sexual reproduction can occur, vegetative mycelia need to acquire nutrients that will support the energetically-intense process of sexual reproduction [52]. Nutrient availability is one of the environmental factors that influences the expression of the MAT genes [46]. In P. anserina, it is hypothesized that nutrient starvation activates pro1, which in turn regulates PaHMG8. As previously discussed, PaHMG8 positively influences the expression of both MAT1-1-1 and MAT1-2-1, thereby acting as an intermediate between nutrient availability signalling and the onset of sexual reproduction [21,53]. While the nutrient requirements for sexual reproduction differ amongst species, a few nutrients are essential to most. These are treated individually in the sections that follow.
2.3.1. Sugars
Glucose and other sugars are the primary energy source for most living cells, including those of microorganisms [54]. Given that sexual reproduction is an energy-intensive process, carbon availability plays a significant role in its initiation. To this end, genes putatively encoding sugar-sensing proteins have been discovered in the genomes of many different fungi.
In A. nidulans, two of these proteins, gprD and gprH, have been partially characterized. The former shows significant similarity to the Saccharomyces cerevisiae gpr1 [55] and the Schizosaccharomyces pombe git3 [56] genes, both of which encode sugar sensing proteins. The latter, gprH, has been shown to recognize and respond to exogenous glucose levels [57]. Additionally, ΔgprH strains of A. nidulans undergo sexual reproduction without proper induction, while ΔgprD mutants display sexual reproduction under prohibitive conditions [57,58]. Furthermore, both mutants also show an increase in the expression of the mating pheromone receptors [57]. Taken collectively, these results indicate that the regulation of sex due to carbon availability is the result of a combined response by gprD and gprH [57].
The A. nidulans gprH in particular responds to periods of low carbon availability, in turn activating the cAMP-PKA (cyclic AMP- protein kinase A) pathway, an intracellular signalling pathway that ultimately initiates the transcriptional changes associated with the inhibition of sex [57]. During short-term starvation, nosA, a positive regulator of sex, is repressed [18,57]. This protein’s repression in turn activates a related protein, rosA, a negative regulator of sex [19,57]. Sexual reproduction is thus precluded until the stress associated with carbon starvation is alleviated.
2.3.2. Amino Acids
Amino acids are crucial nutrients that determine whether sexual reproduction will take place (Figure 3). In A. nidulans, sensing and responding to exogenously available amino acids is achieved via the cross-pathway regulatory network (CPRN). This pathway integrates the amino acid biosynthesis pathways with other important cellular processes, such as sexual reproduction and virulence. The two major genes involved in this pathway are cpcA and cpcB, the cross-pathway control genes. While cpcA responds to amino acid deficiencies, cpcB recognizes exogenous amino acid availability [59].
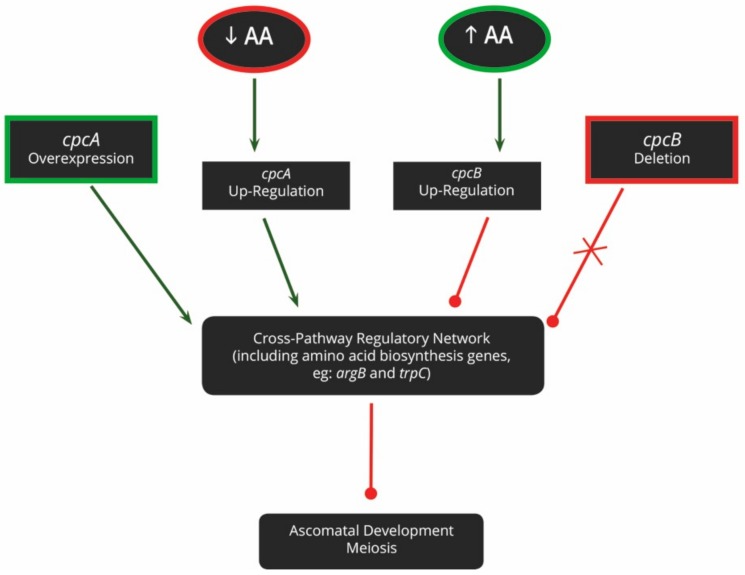
Figure 3.
The involvement of the cross-pathway regulatory network (CPRN) during sexual reproduction in A. nidulans. In the absence of amino acids, cpcA is up-regulated, thereby activating the CPRN. This pathway subsequently inhibits the onset of sexual reproduction. In contrast, when amino acids are available, cpcB is up-regulated and the CPRN is inhibited. This results in sexual development. Either the overexpression of cpcA or the deletion of cpcB can activate the CPRN, by simulating amino acid deficiency, and thus also inhibit sexual reproduction. Shapes with green outlines indicate conditions under which sexual reproduction is directly or indirectly activated. Those with red outlines indicate conditions which directly or indirectly repress sexual reproduction. Green arrows indicate activation of a particular pathway, while red lines terminating in circles indicate repression.
The CPRN has been experimentally elucidated by generating a variety of mutants, including cpcA over-expression, cpcB deletion, and amino acid auxotrophic mutants. Both the cpcA and cpcB mutants experience amino acid starvation, regardless of the actual amino acid availability and consequently, sexual reproduction cannot take place [59,60]. These phenotypes can also not be rescued by the exogenous provision of amino acids, given their inability to recognize that amino acids are indeed available. In contrast, the phenotype of ΔtrpA-D auxotrophic mutants, which are unable to produce their own tryptophan and are thus sterile, can be rescued in the presence of an exogenous amino acid source [61,62]. Furthermore, ΔargB auxotrophic mutants are also sterile and produce only immature ascomata [63]. This phenotype can, however, not be rescued by an exogenous arginine supply, indicating that argB may play a greater role in sexual reproduction than simply aiding in the production of arginine.
2.3.3. Calcium
As an essential element for most living organisms, calcium plays a variety of biological roles in the lives of many eukaryotes, especially during signal transduction [64,65]. To ensure consistent calcium availability, fungi utilize two different calcium uptake systems. The high-affinity calcium uptake system (HACS) functions when there are low levels of exogenous calcium and active uptake is required [66,67]. This system is discussed in more detail in the section dealing with ascospore discharge (see below). In contrast, the low-affinity calcium uptake system (LACS) is utilized when exogenous levels of calcium are high and active uptake is not necessary. This system is comprised of at least FIG1, a transmembrane calcium channel [68].
The LACS gene, fig1, has been extensively studied with regards to its role during sexual reproduction in F. graminearum [69,70], A. nidulans [71], and N. crassa [69]. Interestingly, while the importance of the gene is obvious in all three species, its actual function depends on the sexual strategy being employed by the species. For example, in both F. graminearum and A. nidulans, fig1 is essential for the production of ascomata during homothallic mating [69,70,71]. However, fig1 is completely dispensable in A. nidulans when an isolate undergoes outcrossing [71]. In contrast, the gene is important for heterothallic mating in N. crassa, particularly in MAT1-2 isolates, where Δfig1 mutants are female sterile. Interestingly, this mutant phenotype is not seen in Δfig1 MAT1-1 isolates, indicating a mating-type specific interaction [69].
2.4. Other Environmental Triggers
Once the critical nutrient and energy requirements have been met in the vegetative mycelium, a variety of other signals are needed to successfully initiate sexual reproduction.
2.4.1. Light
Light can act as either a positive or negative regulator of sexual reproduction, depending on the species and the wavelength [72]. In order to respond to light-based signals, fungi express a variety of light-sensing molecules, which range from small peptides to giant protein complexes. This allows for the recognition of wavelengths across the visible light spectrum, from near-ultraviolet to red [72]. Two of the primary photo-recognition systems in fungi, the white collar system and the velvet complex system, have been intensively studied in N. crassa and A. nidulans, respectively.
In N. crassa, the blue light response relies on the recognition of blue light by WC-1, a white collar protein, which harbours the light-recognizing LOV (light oxygen voltage sensing) domain [73]. The WC-1 protein also harbours PAS (Per-Arnt-Sim) domains, which allows dimerization with both itself and a second white collar protein, WC-2 [74,75]. Additionally, both proteins possess zinc finger DNA binding motifs, which bind to the promoters of various light-inducible genes [75,76]. Thus, these domains allow the two proteins to form the entire signal transduction pathway associated with blue light illumination in N. crassa, from light recognition to transcriptional regulation.
Blue light enhances the production and development of ascomata in N. crassa. Thus, although not essential for protoascomatal production, illumination significantly increases sexual competency and the number of protoascomata produced [77]. Furthermore, upon fertilization and subsequent development, the ascomatal necks orient themselves towards the source of the blue light illumination [78], a form of phototropism that may act to enhance spore dispersal. Thus, while Δwc1/2 mutants are not sexually defective, they produce far fewer protoascomata, most of which are unable to orient their necks correctly [77,78].
Light has a more direct effect on the sexual cycle of A. nidulans than that of N. crassa (Figure 4), with sexual reproduction being entirely precluded in the presence of light [79]. Furthermore, light sensing and its signal transduction pathway is more complex in A. nidulans, involving many proteins that form a variety of multi-peptide complexes [80,81]. The majority of these complexes are made up of the velvet proteins, which form different dimers and trimers during the A. nidulans life cycle, each of which elicits a unique response [81,82,83,84].
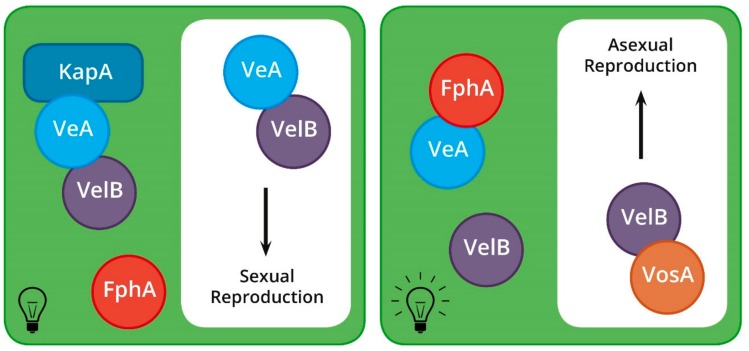
Figure 4.
Sexual reproduction and light sensing in A. nidulans. Darkness: VeA and VelB interact with the importin protein, KapA. This allows their transport into the nucleus where they regulate gene expression and initiate sexual development. Light: FphA, the red light sensor, interacts with VeA, putatively inhibiting its interaction with KapA and VelB. This prevents the transport of VeA into the nucleus. Instead, VelB interacts with other proteins, activating asexual reproduction.
The first velvet gene, veA, is constitutively expressed in A. nidulans and displays a major up-regulation during sexual reproduction [82]. The gene is essential for mating, and, if overexpressed, leads to the production of ascomata under normally restrictive conditions [82]. A second velvet protein, VelB, which interacts with VeA in the cytoplasm, is also essential for sexual reproduction [81]. The VeA–VelB dimer interacts with a KapA importin protein during periods of darkness to bring about sexual reproduction [83]. The COP9 signalosome discussed above has also been shown to play an important role in regulating the recognition of light in A. nidulans. It is thought that this protein complex may be involved in the same pathway as veA, given that ΔcsnD mutants are unable to respond to light [45].
During periods of red light illumination, the light sensing phytochrome, FphA, is activated and binds to VeA, thereby preventing its transport into the nucleus [85,86]. Thus, ΔfphA strains are sexually competent even in the presence of light. However, ΔfphA mutants also produce significantly fewer ascomata than wild type isolates when grown in the dark [85]. This, in addition to the fact that FphA possesses kinase activity, indicates that the protein is most likely involved in additional functions not linked to light-sensing during sexual reproduction.
2.4.2. Reactive Oxygen Species
Reactive oxygen species (ROS) are produced as by-products of aerobic respiration [87]. These radicals are known to cause damage to various cellular components, and thus most organisms express antioxidant systems for protection. The production of ROS is, however, increasingly being recognized as an important regulator of cellular processes, especially in cell differentiation and development [87,88].
In A. nidulans, genes involved in both ROS neutralization and production play integral roles in sexual development [89,90]. One such gene, cpeA, encodes a catalase-peroxidase that converts hydrogen peroxide into water. While cpeA is not expressed in vegetative mycelia, its expression dramatically increases during the onset of sexual reproduction [89]. It is hypothesized that cpeA is expressed as a mechanism to prevent ROS-induced damage to the developing fruiting structures. A. nidulans also expresses the ROS-producing enzyme NADPH oxidase [90]. This protein, encoded by noxA, produces superoxide molecules and is up-regulated in cultures induced to undergo sexual reproduction. The importance of ROS production in sexual reproduction has been illustrated by ΔnoxA mutants, which are unable to undergo homothallic mating, with sexual reproduction being blocked before protoascomata formation [90]. Interestingly, this knockout does not affect outcrossing, provided that the wild type partner can produce its own noxA and thus superoxide. It is thought that the ROS is responsible for signalling during cell differentiation prior to sexual reproduction and can be utilized by both interacting partners, regardless of which isolate produces it [90].
N. crassa expresses two NADPH oxidases that are essential for sexual reproduction. The first, nox-1, is a homolog of the A. nidulans noxA and is also significantly up-regulated during sexual development [91]. NOX-1 is specifically required for female fertility, and while Δnox-1 strains are sexually incompetent if used as the female partner, these strains are able to fertilize wild type female structures [91]. N. crassa also expresses the nox-2 gene, which, in contrast to nox-1, is highly expressed in asexual cells and prior to ascomatal development. This gene plays an important role in ascospore germination, despite the fact that Δnox-2 strains produce normal sexual spores [91]. Although its exact role in germination is not understood, it is reasonable to speculate that the ROS produced by NOX-2 may be involved in a signalling pathway that initiates ascospore germination.
NADPH oxidases are also important regulators of sexual reproduction in P. anserina, both in early ascomatal production and later during ascus development and ascospore germination [53]. There are two NADPH oxidase encoding genes in the P. anserina genome, nox1 and nox2. The two proteins are responsible for superoxide and peroxide secretion, a process which co-localizes with the developing protoascomata of sexually-competent isolates [53]. It is thought that nox1 is responsible for a type of ROS-mediated signalling, which induces cell wall degradation of the surrounding cells and provides nutrients to the developing ascomata [53]. It is consequently not surprising that in Δnox1 mutants, the number and size of protoascomata is greatly reduced. Furthermore, those that are fertilized take much longer to reach maturity than wild type protoascomata [53]. Given that a constant supply of fresh nutrients can rescue the mutant phenotype, the sexual defect is likely due to nutrient starvation.
2.4.3. Pheromones
Pheromones are a broad class of chemical signals that are involved in the mating of species as diverse as mammals [92], insects [93], and reptiles [94]. These biologically active compounds are secreted into the environment and are recognized by an individual of the same species, but different gender, sex, or mating type [95]. Fungi utilize sex pheromones in the form of diffusible peptides that allow for mate recognition and attraction (extensively reviewed by [96,97]).
Genes encoding mating pheromones have been found in the genomes of many ascomycete fungi, including Cryphonectria parasitica [98], Magnaporthe grisea [99], N. crassa [100], P. anserina [101], and various Fusarium species [102]. As with the MAT genes, however, these genes have been given species-specific names. These genes mostly fall into one of two categories; those with similarity to the pheromone expressed by S. cerevisiae a-cells and those to the pheromone expressed by S. cerevisiae α-cells. For the purpose of this review, these pheromones will thus be termed the a- and α-factor pheromones, respectively (Table 1).
In many filamentous fungi, the a-factor and the α-factor pheromone genes are transcriptionally controlled by the MAT genes. Consequently, in heterothallic species, they are expressed in a mating-type dependent manner (Figure 5). In such species, the MAT1-1-1 gene controls the expression of the α-factor, while the MAT1-2-1 gene is responsible for a-factor expression. As a direct result, MAT1-1 individuals express only the α-factor and MAT1-2 individuals express only the a-factor [98,99,100,103]. Homothallic species, which typically possess both the MAT1-1-1 and MAT1-2-1 genes, are often able to express both pheromones [104,105].
Figure 5.
Pheromone signalling in heterothallic filamentous ascomycetes. (1) Pheromones are expressed, with spermatia of MAT1-1 isolates expressing the α-factor and spermatia of MAT1-2 isolates expressing the a-factor pheromone. (2) The ascogonia of these isolates also express the pheromone receptors, which recognize the pheromones. (3) Recognition of the pheromones by their receptors results in a variety of physiological changes, including growth towards the suitable partner as well as the transcriptional regulation of sex-related genes.
Given that the pheromones are typically expressed by the male cells, either spermatia or conidia, it is not surprising that both pheromones play an essential role in the male fertility of P. anserina and N. crassa [101,106]. Deleting either of the factors results in isolates that cannot fertilize female isolates and are thus male sterile. Interestingly, while a complete knockout of the a-factor does not affect female fertility in N. crassa, disruption of the 3′ non-coding region greatly reduces protoascomatal production [107]. Additionally, the α-factor is also essential for male fertility in C. parasitica [108]. In contrast, the C. parasitica a-factor has been implicated in female fertility, where a-factor knockout strains produce only empty ascomata, but fully functional male structures [108,109]. Thus, while clearly important for the sexual process, the actual function of the pheromones differs slightly from species to species.
A third type of pheromone has been identified in certain heterothallic Fusarium species, as well as in the heterothallic T. reesei [102,110]. This pheromone harbours a number of repeating units as well as the terminal CaaX domain, and thus has characteristics of both the α- and a-factors, respectively. This hybrid pheromone has thus been termed the h-type factor [110]. Given its genomic location, it is thought that the gene encoding this factor assumed the function of the a-factor pheromone after the loss of the original a-factor-encoding gene. Furthermore, a typical α-factor pheromone gene has been found in the genomes of the species harbouring this h-type factor [102,110]. This illustrates the presence of a pheromone response pathway that, at least partially, resembles the typical α/a system. It is surprising, however, that the α-factor and h-type pheromones are not expressed in a mating-type dependant manner as seen in typically heterothallic species [110].
In order to recognise these pheromones, the female structures of these fungi express receptors that are able to specifically recognize each pheromone (Figure 5) [95]. These receptors belong to the class of seven transmembrane G-protein coupled receptors, which, when activated, initiate an MAP kinase signal transduction pathway (reviewed by [111]). Recognition of the pheromone by the receptor therefore initiates a transduction pathway, which in turn activates a variety of networks that lead to sexual reproduction. The pheromone receptors are also important regulators of pheromone expression and female fertility in N. crassa. Thus, the deletion of the a-factor receptor results in a decrease in α-factor expression in a MAT1-1 background [112] and deletion of the α-factor receptor results in the down-regulation of the a-factor in a MAT1-2 background [113]. Protoascomata are still produced by these mutants, indicating at least a partial activation of the female fertility pathway. However, these mutants are not able to recognize, grow towards, or fuse with fertilizing spores from a male isolate, thereby precluding successful fertilization and thus sexual reproduction [112,113].
The F. graminearum α-factor receptor also plays a role in female fertility [104]. Although it is not essential for sexual reproduction, female fertility is significantly reduced in gene deletion strains. Contrastingly, deletion of the a-factor receptor results in no observable sexual defects [104]. Given that F. graminearum is homothallic, it is perhaps not entirely surprising that a system with the primary function of mate seeking is no longer essential for sexual reproduction. This, however, is not true for all homothallic species, where expression of the pheromones and their receptors is indeed essential for the production of sexual spores [114,115,116].
Despite their apparent primary role in mate recognition, pheromones are hypothesized to play a role in some of the downstream processes associated with sexual reproduction [97,113]. This is substantiated by the fact that pheromone expression is not limited to heterothallic species, but is an important sex-promoting pathway in some homothallic species. Furthermore, the expression of pheromone pathway genes is not limited to the initiation of sexual reproduction and instead often continues throughout the entire process. Genes encoding both the pheromones and their receptors have been identified in the homothallic species, S. macrospora [105,115] and F. graminearum [104,117]. The genes encoding the α-factor pheromone as well as both the pheromone receptors have also been found in A. nidulans [118]. These genes are expressed and functional, despite the absence of mate seeking behaviour and are thus likely to have been co-opted into other functions.
In F. graminearum, the α-factor pheromone and its receptor are not essential for self-fertility, but single deletion mutants produce far fewer mature ascomata than wildtype isolates [104]. This indicates that this pheromone/receptor pair plays a role in ascomatal production or development. Unexpectedly, deletion of the α-factor gene also enhanced outcrossing events, indicating that α-factor expression may promote selfing, rather than outcrossing as in other species. This effect, however, is abolished if its receptor is also deleted [104].
Both pheromone receptor genes play an important role in homothallic mating in A. nidulans [116]. Deletion of either receptor results in a significant decrease in fertility, with mutants producing small ascomata that house a limited number of ascospores. Deletion of both the receptors results in an even more severe phenotype, where mutants are unable to produce ascomata at all [116]. Similar to F. graminearum, however, the disruption of either or both receptors does not affect outcrossing [116]. These genes are thus only important for selfing and play no role in outcrossing in this species.
In some species, pheromone expression has been proposed to facilitate post-fertilization events, particularly before karyogamy and meiosis in both homothallic and heterothallic species [119]. In N. crassa, the pheromones as well as their receptors are required for the production of the ascospores [107,113]. For example, forced mating interactions between a wild type isolate and an a-factor mutant result in the production of ascomata harbouring very few ascospores, while forced matings between mutants results in completely barren ascomata. Additionally, deletion of both a pheromone and its receptor in N. crassa results in protoascomata that never mature. Similarly, the deletion of the α-factor pheromone in C. parasitica results in the production of mature, but barren, ascomata [108,109]. Taken together, these results illustrate a role for both the pheromones and their response pathway in post-fertilization events, such as ascomatal, ascal, and ascospore development.
A combination of the above mentioned environmental and physiological factors ultimately lead to asexual reproduction, sexual development, or simply the continuation of vegetative growth. If all the requirements are met and sexual reproduction can take place, an immature fruiting body will form and develop into an ascoma. Subsequently, the asci can begin to form within the mature sexual structure.
3. Ascus Production
One of the defining characteristics of ascomycete fungi is the ascus, a sac-like structure in which the ascospores are produced and housed [6]. The wall of the ascus is unique compared to all other tissues formed by fungi, different even from others formed during sexual reproduction [120]. As such, there are a number of genes that are expressed almost exclusively by the ascus [121], ensuring that the most appropriate environment is created for the developing ascospores.
In F. graminearum, amd1 is one such gene and is essential for the correct development of the ascus [120,122]. The gene encodes a transmembrane protein, which localizes to the ascus membrane and possesses a domain associated with transmembrane transport [122]. While the AMD1 protein has no apparent function in the production of normal ascomata, Δamd1 strains are unable to produce stable ascus walls. Consequently, the wall degrades before the ascospores are ready for discharge, prompting their germination within the ascomata, and precluding effective dispersal [120,121,122]. Interestingly, the deletion of amd1 results in the differential expression of many genes, including the up-regulation in membrane transport and the down-regulation of genes involved in cell-wall synthesis and cell-wall integrity [122]. This suggests that amd1 is a master regulator of ascus wall synthesis and ensures cell wall integrity. This may be achieved by minimising cross-membrane transport and allowing the generation of turgor pressure in the ascus.
Ascus development genes have also been described from N. crassa, with functions mostly linked to gene regulation [123,124]. The ASD4 GATA DNA binding protein [123], the SMS-2 meiotic silencing Argonaute protein [125], and the STC1-like RNAi and chromatin remodelling protein [126] are all involved in regulating the production of the ascus. While ASD4 likely acts as a transcription factor, the other two proteins are intimately involved in the RNA silencing pathway [125,126]. The genes encoding these proteins are essential for this process, which is evident from the fact that mutant strains of asd4, sms-2, or stc1-like produce only empty ascomata that are incapable of forming asci. This extreme phenotype is thought to be due to the deregulation of the entire ascus development pathway, given the important role each protein plays in regulation [123,125,126].
In addition to its role in ascomatal development, ROS metabolism is also essential for ascus production in P. anserina [127]. The car1 gene, now known as pex2, which encodes a peroxisomal assembly factor, ensures that peroxisomes are formed in abundance during asci maturation as well as ascospore delineation [127,128]. These peroxisomes play an important role in ensuring the sexual tissue is supplied with sufficient nutrients during sexual development [129]. In pex2 mutants, these peroxisomes are not produced and the isolate is unable to undergo nuclear fusion prior to meiosis [127], precluding the development of the ascus. It is currently unclear whether this gene is essential for karyogamy itself, or whether it is involved in a process just upstream of nuclear fusion [129].
4. Ascospore Production
The production of ascospores represents the final step in sexual reproduction. If this process has been successful, these newly-produced spores will be discharged into the environment, where they germinate and initiate a new life cycle.
4.1. Meiosis and Ascospore Production
Ascosporegenesis is, by definition, dependant on a successful meiotic division cycle. There are a number of genes that have been identified in both A. nidulans and N. crassa that specifically ensure that prophase I, the first phase of meiosis, can begin. In A. nidulans, the deletion of either the tubB [130] or grrA [131] gene results in mutants that are able to produce asci-housing ascomata, but that cannot initiate meiosis. The similar phenotypes are particularly noteworthy given the different role each of these genes plays—while tubB encodes the structural α-tubulin protein, an important component in the microtubule assembly toolbox [130], grrA encodes a substrate adaptor protein that plays a role in the protein ubiquitylation and degradation pathway [131]. Similarly, deletion of either of the meiotic silencing genes, sad1 and sad2 of N. crassa, results in an almost identical phenotype, with mature fruiting bodies harbouring intact asci that are also unable to undergo meiosis [132,133].
Upon the completion of a successful cycle of meiosis, the developing ascospores must undergo further maturation, including partitioning and delineation within the ascus. In N. crassa, this process is at least partially regulated by asd-1, which encodes a rhamnogalacturonase that is expressed predominantly during mating [134]. In Δasd-1 mutants, the eight nuclei formed during meiosis remain diffuse and do not delineate into individual spores, leading to the sterility of the mutants [134].
The maturation process depends on the expression of the cAMP-PKA pathway as well as correct calcium signalling in F. graminearum. Given the importance of both these pathways in signal transduction, it is not surprising that the proteins associated with these pathways are essential for sexual reproduction. CPK1, a catalytic subunit of the PKA, ensures the transition of an immature spore into a single-celled ascospore harbouring only one nucleus [135]. Additionally, the deletion of mid1, a component of the HACS (discussed below), results in abnormal ascospores that are two-celled and septate, with fragile cell walls [136].
4.2. Ascospore Discharge
Fungal spores tend not to be motile and thus many diverse and elegant mechanisms of propagule dissemination have evolved across the Kingdom. A common mechanism of dissemination includes the forcible discharge of ascospores into their environment. This typically involves a build-up of turgor pressure, followed by the swift release of the ascospores [137].
As discussed earlier in this review, calcium is an essential mineral required for sexual reproduction in certain filamentous fungi. While LACS was discussed in terms of ascomatal formation, HACS is important for the forcible discharge of ascospores in F. graminearum [70,136,137]. This supports the multi-phase importance for calcium during sexual reproduction, where this element is required during the initiation of sexual development as well as in the final stages of sexual reproduction.
The HACS is made up of two ion channels, MID1, a mechanosensitive protein [136], and CCH1, an L-type protein [137]. Mutant F. graminearum strains of both these genes are defective in their ability to discharge ascospores [70,136,137], with the mutant phenotypes being partially rescued by the provision of exogenous calcium. This potentially activates the LACS, allowing for calcium transport via another pathway. Interestingly, despite this obvious phenotype in F. graminearum, the N. crassa mid1 homolog is dispensable for ascospore discharge and mid1 deletion strains display no other sexual defects [138]. This provides an elegant example of how protein similarity, at the sequence or structure level, is not always a good predictor of shared function.
Ascospore release in F. graminearum also relies on the expression of a kinase gene, kin1 [139]. This gene encodes a member of a kinase protein family that typically harbours proteins involved in both cell-polarity and microtubule transport [139,140]. The kinase localizes to the septal pores in the newly produced ascospores and subsequently to their germ tubes [139]. In knockout studies, Δkin1 strains lack the ability to release the spores, despite having produced fully matured ascomata and asci [139]. Furthermore, the absence of kin1 also interferes with the inhibition of ascospore germination prior to release. This results in the premature germination of the unreleased ascospores [139], similar to the phenotype previously discussed for Δamd1 strains of the same species.
It is worth noting that not all ascomycetes forcibly discharge their spores. For example, in some species, including Magnaporthe salvinii [141] and those belonging to the polyphyletic grouping of the ophiostomatoid fungi [142], the asci deliquesce before the spores are discharged, thus leaving them free within the ascoma. Typically, these spores are then exuded in a slimy matrix from a pore, sometimes found at the tips of the ascomatal necks. Such fungi consequently rely on a variety of dissemination means, other than forcible discharge. In species of Graphium, Dipodascus, and Ceratocystis, for example, these exudates are typically sticky and can be picked up by insects, which then act as dispersal agents [143].
5. Conclusions
Sexual reproduction in fungi provides a complex, diverse, and intriguing system to study tissue differentiation in eukaryotes. Many fungi provide simple, easy to use models, often with very well-characterized life cycles. Given that sexual reproduction relies upon the recognition and response to a variety of endogenous and exogenous cues, this system can be used to model protein–protein, metabolite–protein, and protein–DNA interactions. Furthermore, signal transduction events as well as fine-scale and global changes in the transcriptome and proteome can be tracked in response to environmental changes.
Despite the intensive research that has already been committed to understanding the genes that play a role in fungal sexual reproduction, there are many important and intriguing questions that remain to be answered. Even in the best studied model organisms, certain aspects of sexual reproduction remain uncharacterized. This is partly due to the fact that many of the genes involved in sexual reproduction show pleiotropic effects when modified. For example, if they play a role early in sexual reproduction, this can preclude opportunities to understand how they function in later stages of the process as well. Furthermore, determining the role of genes in a single species does not necessarily imply that they have similar functions in other species, regardless of how closely related the species or how similar the gene sequence. Future research will thus likely be focused on identifying the downstream targets of the MAT transcription factors in different species. This will further elucidate the genetic pathways that underlie the different sexual strategies and identify the genes that ensure opposite mating type nuclei can be recognized as such.
The growing availability of next generation sequencing methods is facilitating increasingly rapid progress in many aspects of biological research. Genomics has, for example, allowed for the identification of genes involved in sexual reproduction in species previously thought to be asexual. Likewise, transcriptomics has enabled the elucidation of genes and pathways that are specifically expressed during sexual reproduction. In the future, we are likely to witness an increase in research that combines these technologies with classical functional characterization. This will be true not only for model species, but also for those fungi that are less well-known.
Acknowledgments
We would like to thank Emma Steenkamp for her helpful review of this manuscript during its preparation. We would also like to thank Glenda Brits of the Department of Education Innovation at the University of Pretoria for producing the figures used in this review. We are also especially grateful to the anonymous reviewers for their careful reading of this manuscript and for their many insightful comments and suggestions that led to the significant improvement of this publication.
Author Contributions
Conceptualization, A.M.W. and B.D.W.; writing—original draft preparation, A.M.W., writing—review and editing, P.M.W., M.A.v.d.N., M.J.W. and B.D.W.; visualization, A.M.W., supervision, P.M.W., M.A.v.d.N., M.J.W. and B.D.W.
Funding
This research was funded by the National Research Foundation (NRF) of South Africa, grant number 116448.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hawksworth D.L., Luecking R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0052-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell M. The Fungi: 1, 2, 3 … 5.1 Million Species? Am. J. Bot. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 3.Hawksworth D. Global Species Numbers of Fungi: Are Tropical Studies and Molecular Approaches Contributing to a More Robust Estimate? Biodivers. Conserv. 2012;21:2425–2433. doi: 10.1007/s10531-012-0335-x. [DOI] [Google Scholar]
- 4.Billiard S., Lopez Villavicencio M., Hood M., Giraud T. Sex, Outcrossing and Mating Types: Unsolved Questions in Fungi and Beyond. J. Evol. Biol. 2012;25:1020–1038. doi: 10.1111/j.1420-9101.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 5.Ni M., Feretzaki M., Sun S., Wang X., Heitman J. Sex in Fungi. Annu. Rev. Genet. 2011;45:405–430. doi: 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James T.Y., Kauff F., Schoch C.L., Matheny P.B., Hofstetter V., Cox C.J., Celio G., Gueidan C., Fraker E., Miadlikowska J. Reconstructing the Early Evolution of Fungi using a Six-Gene Phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 7.Stajich J.E., Berbee M.L., Blackwell M., Hibbett D.S., James T.Y., Spatafora J.W., Taylor J.W. The Fungi. Curr. Biol. 2009;19:R840–R845. doi: 10.1016/j.cub.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyer P.S. Sexual Reproduction and the Significance of MAT in the Aspergilli. In: Heitman J., Kronstad J.W., Taylor J.W., Casselton L.A., editors. Sex in Fungi: Molecular Determination and Evolutionary Implications. American Society of Microbiology Press; Washington, DC, USA: 2007. pp. 123–142. [Google Scholar]
- 9.Dyer P., Inderbitzin P., Debuchy R. Mating-Type Structure, Function, Regulation and Evolution in the Pezizomycotina. In: Wendland J., editor. Growth, Differentiation and Sexuality. The Mycota. Volume 1. Springer; Cham, Switzerland: 2016. pp. 351–385. [Google Scholar]
- 10.Klix V., Nowrousian M., Ringelberg C., Loros J., Dunlap J., Pöggeler S. Functional Characterization of MAT1-1-Specific Mating-Type Genes in the Homothallic Ascomycete Sordaria macrospora provides New Insights into Essential and Nonessential Sexual Regulators. Eukaryot. Cell. 2010;9:894–905. doi: 10.1128/EC.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira A.V.-B., An Z., Metzenberg R.L., Glass N.L. Characterization of matA-2, matA-3 and ΔmatA Mating-Type Mutants of Neurospora crassa. Genetics. 1998;148:1069–1079. doi: 10.1093/genetics/148.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler G. The Evolution of MAT: The Ascomycetes. In: Heitman J.K., Taylor J.W., Casselton L.A., editors. Sex in Fungi: Molecular Determination and Evolutionary Implications. American Society of Microbiology Press; Washington, DC, USA: 2007. pp. 3–18. [Google Scholar]
- 13.Blakeslee A.F. Sexual Reproduction in the Mucorineae. Proc. Am. Acad. Arts Sci. 1904;40:205–319. doi: 10.2307/20021962. [DOI] [Google Scholar]
- 14.Lin X., Heitman J. Mechanisms of Homothallism in Fungi and Transitions between Heterothallism and Homothallism. In: Heitman J., Kronstad J.W., Taylor J.W., Casselton L.A., editors. Sex in Fungi: Molecular Determination and Evolutionary Implications. American Society of Microbiology Press; Washington, DC, USA: 2007. pp. 35–57. [Google Scholar]
- 15.Turgeon B.G., Yoder O. Proposed Nomenclature for Mating Type Genes of Filamentous Ascomycetes. Fungal Genet. Biol. 2000;31:1–5. doi: 10.1006/fgbi.2000.1227. [DOI] [PubMed] [Google Scholar]
- 16.Wilken P.M., Steenkamp E.T., Wingfield M.J., De Beer Z.W., Wingfield B.D. Which MAT gene? Pezizomycotina (Ascomycota) Mating-Type Gene Nomenclature Reconsidered. Fungal Biol. Rev. 2017;31:199–211. doi: 10.1016/j.fbr.2017.05.003. [DOI] [Google Scholar]
- 17.Colot H.V., Park G., Turner G.E., Ringelberg C., Crew C.M., Litvinkova L., Weiss R.L., Borkovich K.A., Dunlap J.C. A High-Throughput Gene Knockout Procedure for Neurospora reveals Functions for Multiple Transcription Factors. Proc. Natl. Acad. Sci. USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vienken K., Fischer R. The Zn(II)2Cys6 Putative Transcription Factor nosA controls Fruiting Body Formation in Aspergillus nidulans. Mol. Microbiol. 2006;61:544–554. doi: 10.1111/j.1365-2958.2006.05257.x. [DOI] [PubMed] [Google Scholar]
- 19.Vienken K., Scherer M., Fischer R. The Zn(II)2Cys6 Putative Aspergillus nidulans Transcription Factor RosA (Repressor of Sexual Development) Inhibits Sexual Development Under Low-Carbon Conditions and in Submersed Culture. Genetics. 2005;169:619–630. doi: 10.1534/genetics.104.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q., Choi G.H., Nuss D.L. Hypovirus-Responsive Transcription Factor Gene pro1 of the Chestnut Blight Fungus Cryphonectria parasitica is required for Female Fertility, Asexual Spore Development, and Stable Maintenance of Hypovirus Infection. Eukaryot. Cell. 2009;8:262–270. doi: 10.1128/EC.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier V., Tong L., Nguyen T.-S., Debuchy R., Silar P. PaPro1 and IDC4, Two Genes controlling Stationary Phase, Sexual Development and Cell Degeneration in Podospora anserina. J. Fungi. 2018;4:85. doi: 10.3390/jof4030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benkhali J.A., Coppin E., Brun S., Peraza-Reyes L., Martin T., Dixelius C., Lazar N., Van Tilbeurgh H., Debuchy R. A Network of HMG-Box Transcription Factors regulates Sexual Cycle in the Fungus Podospora anserina. Plos Genet. 2013;9:e1003642. doi: 10.1371/journal.pgen.1003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman P. HMG Domain Superfamily of DNA-bending Proteins: HMG, UBF, TCF, LEF, SOX, SRY and Related Proteins. e LS. 2001 doi: 10.1002/9780470015902.a0002325.pub2. [DOI] [Google Scholar]
- 24.Bazafkan H., Dattenböck C., Böhmdorfer S., Tisch D., Stappler E., Schmoll M. Mating Type-Dependent Partner Sensing as Mediated by VEL1 in Trichoderma reesei. Mol. Microbiol. 2015;96:1103–1118. doi: 10.1111/mmi.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seibel C., Tisch D., Kubicek C.P., Schmoll M. ENVOY is a Major Determinant in Regulation of Sexual Development in Hypocrea jecorina (Trichoderma reesei) Eukaryot. Cell. 2012;11:885–895. doi: 10.1128/EC.05321-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellanos F., Schmoll M., Martínez P., Tisch D., Kubicek C.P., Herrera-Estrella A., Esquivel-Naranjo E.U. Crucial Factors of the Light Perception Machinery and their Impact on Growth and Cellulase Gene Transcription in Trichoderma reesei. Fungal Genet. Biol. 2010;47:468–476. doi: 10.1016/j.fgb.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Schmoll M., Esquivel-Naranjo E.U., Herrera-Estrella A. Trichoderma in the Light of Day—Physiology and Development. Fungal Genet. Biol. 2010;47:909–916. doi: 10.1016/j.fgb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmoll M., Franchi L., Kubicek C.P. ENVOY, a PAS/LOV Domain Protein of Hypocrea jecorina (Anamorph Trichoderma reesei), modulates Cellulase Gene Transcription in Response to Light. Eukaryot. Cell. 2005;4:1998–2007. doi: 10.1128/EC.4.12.1998-2007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debuchy R., Coppin E. The Mating Types of Podospora anserina: Functional Analysis and Sequence of the Fertilization Domains. Mol. Gen. Genet. 1992;233:113–121. doi: 10.1007/BF00587568. [DOI] [PubMed] [Google Scholar]
- 30.Paoletti M., Seymour F.A., Alcocer M.J., Kaur N., Calvo A.M., Archer D.B., Dyer P.S. Mating Type and the Genetic Basis of Self-Fertility in the Model Fungus Aspergillus nidulans. Curr. Biol. 2007;17:1384–1389. doi: 10.1016/j.cub.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Staben C., Yanofsky C. Neurospora crassa a Mating-Type Region. Proc. Natl. Acad. Sci. USA. 1990;87:4917–4921. doi: 10.1073/pnas.87.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J., Lee T., Lee Y.W., Yun S.H., Turgeon B.G. Shifting Fungal Reproductive Mode by Manipulation of Mating Type Genes: Obligatory Heterothallism of Gibberella zeae. Mol. Microbiol. 2003;50:145–152. doi: 10.1046/j.1365-2958.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 33.Böhm J., Hoff B., O’Gorman C.M., Wolfers S., Klix V., Binger D., Zadra I., Kürnsteiner H., Pöggeler S., Dyer P.S. Sexual Reproduction and Mating-Type–Mediated Strain Development in the Penicillin-Producing Fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA. 2013;110:1476–1481. doi: 10.1073/pnas.1217943110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Böhm J., Dahlmann T.A., Gümüşer H., Kück U. A MAT1–2 Wild-Type Strain from Penicillium chrysogenum: Functional Mating-Type Locus Characterization, Genome Sequencing and Mating with an Industrial Penicillin-Producing Strain. Mol. Microbiol. 2015;95:859–874. doi: 10.1111/mmi.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pöggeler S., Nowrousian M., Ringelberg C., Loros J., Dunlap J., Kück U. Microarray and Real-Time PCR Analyses Reveal Mating Type-Dependent Gene Expression in a Homothallic Fungus. Mol. Genet. Genom. 2006;275:492–503. doi: 10.1007/s00438-006-0107-y. [DOI] [PubMed] [Google Scholar]
- 36.Kim H.-K., Cho E.J., Lee S., Lee Y.-S., Yun S.-H. Functional Analyses of Individual Mating-Type Transcripts at MAT Loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol. Lett. 2012;337:89–96. doi: 10.1111/1574-6968.12012. [DOI] [PubMed] [Google Scholar]
- 37.Arnaise S., Debuchy R., Picard M. What is a Bona Fide Mating-Type Gene? Internuclear Complementation of mat Mutants in Podospora anserina. Mol. Gen. Genet. 1997;256:169–178. doi: 10.1007/PL00008611. [DOI] [PubMed] [Google Scholar]
- 38.Doughan B., Rollins J.A. Characterization of MAT Gene Functions in the Life Cycle of Sclerotinia sclerotiorum Reveals a Lineage-Specific MAT Gene Functioning in Apothecium Morphogenesis. Fungal Biol. 2016;120:1105–1117. doi: 10.1016/j.funbio.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Rodenburg S.Y., Terhem R.B., Veloso J., Stassen J.H., van Kan J.A. Functional Analysis of Mating Type Genes and Transcriptome Analysis during Fruiting Body Development of Botrytis cinerea. mBio. 2018;9:e01939-17. doi: 10.1128/mBio.01939-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnaise S., Zickler D., Le Bilcot S., Poisier C., Debuchy R. Mutations in Mating-Type Genes of the Heterothallic Fungus Podospora anserina lead to Self-Fertility. Genetics. 2001;159:545–556. doi: 10.1093/genetics/159.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zickler D., Arnaise S., Coppin E., Debuchy R., Picard M. Altered Mating-Type Identity in the Fungus Podospora anserina leads to Selfish Nuclei, Uniparental Progeny, and Haploid Meiosis. Genetics. 1995;140:493–503. doi: 10.1093/genetics/140.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y., Amich J., Will C., Eagle C.E., Dyer P.S., Krappmann S. The Novel Aspergillus fumigatus MAT1-2-4 Mating-Type Gene is Required for Mating and Cleistothecia Formation. Fungal Genet. Biol. 2017;108:1–12. doi: 10.1016/j.fgb.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Braus G.H., Irniger S., Bayram Ö. Fungal Development and the COP9 Signalosome. Curr. Opin. Microbiol. 2010;13:672–676. doi: 10.1016/j.mib.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Busch S., Schwier E.U., Nahlik K., Bayram Ö., Helmstaedt K., Draht O.W., Krappmann S., Valerius O., Lipscomb W.N., Braus G.H. An Eight-Subunit COP9 Signalosome with an Intact JAMM Motif is required for Fungal Fruit Body Formation. Proc. Natl. Acad. Sci. USA. 2007;104:8089–8094. doi: 10.1073/pnas.0702108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busch S., Eckert S.E., Krappmann S., Braus G.H. The COP9 Signalosome is an Essential Regulator of Development in the Filamentous Fungus Aspergillus nidulans. Mol. Microbiol. 2003;49:717–730. doi: 10.1046/j.1365-2958.2003.03612.x. [DOI] [PubMed] [Google Scholar]
- 46.Teichert I., Nowrousian M., Pöggeler S., Kück U. The Filamentous Fungus Sordaria macrospora as a Genetic Model to Study Fruiting Body Development. Adv. Genet. 2014;87:199–244. doi: 10.1016/B978-0-12-800149-3.00004-4. [DOI] [PubMed] [Google Scholar]
- 47.Hwang J., Pallas D.C. STRIPAK Complexes: Structure, Biological Function, and Involvement in Human Diseases. Int. J. Biochem. Cell Biol. 2014;47:118–148. doi: 10.1016/j.biocel.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro P.S., Josué F., Wepf A., Wehr M.C., Rinner O., Kelly G., Tapon N., Gstaiger M. Combined Functional Genomic and Proteomic Approaches identify a PP2A Complex as a Negative Regulator of Hippo Signaling. Mol. Cell. 2010;39:521–534. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Bernhards Y., Pöggeler S. The Phocein Homologue SmMOB3 is Essential for Vegetative Cell Fusion and Sexual Development in the Filamentous Ascomycete Sordaria macrospora. Curr. Genet. 2011;57:133–149. doi: 10.1007/s00294-010-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloemendal S., Bernhards Y., Bartho K., Dettmann A., Voigt O., Teichert I., Seiler S., Wolters D.A., Pöggeler S., Kück U. A Homologue of the Human STRIPAK Complex controls Sexual Development in Fungi. Mol. Microbiol. 2012;84:310–323. doi: 10.1111/j.1365-2958.2012.08024.x. [DOI] [PubMed] [Google Scholar]
- 51.Pöggeler S., Kück U. A WD40 Repeat Protein regulates Fungal Cell Differentiation and can be replaced Functionally by the Mammalian Homologue Striatin. Eukaryot. Cell. 2004;3:232–240. doi: 10.1128/EC.3.1.232-240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han K.H., Lee D.B., Kim J.H., Kim M.S., Han K.Y., Kim W.S., Park Y.S., Kim H.B., Han D.M. Environmental Factors Affecting Development of Aspergillus nidulans. J. Microbiol. 2003;41:34–40. [Google Scholar]
- 53.Malagnac F., Lalucque H., Lepère G., Silar P. Two NADPH Oxidase Isoforms are required for Sexual Reproduction and Ascospore Germination in the Filamentous Fungus Podospora anserina. Fungal Genet. Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Johnston M. Feasting, Fasting and Fermenting: Glucose Sensing in Yeast and Other Cells. Trends Genet. 1999;15:29–33. doi: 10.1016/S0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- 55.Kraakman L., Lemaire K., Ma P., Teunissen A.W., Donaton M.C., Van Dijck P., Winderickx J., De Winde J.H., Thevelein J.M. A Saccharomyces cerevisiae G-Protein Coupled Receptor, Gpr1, is specifically Required for Glucose Activation of the cAMP Pathway during the Transition to Growth on Glucose. Mol. Microbiol. 1999;32:1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- 56.Welton R.M., Hoffman C.S. Glucose Monitoring in Fission Yeast via the gpa2 Gα, the git5 Gβ and the git3 Putative Glucose Receptor. Genetics. 2000;156:513–521. doi: 10.1093/genetics/156.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown N.A., dos Reis T.F., Ries L.N.A., Caldana C., Mah J.H., Yu J.H., Macdonald J.M., Goldman G.H. G-Protein Coupled Receptor-Mediated Nutrient Sensing and Developmental Control in Aspergillus nidulans. Mol. Microbiol. 2015;98:420–439. doi: 10.1111/mmi.13135. [DOI] [PubMed] [Google Scholar]
- 58.Han K.H., Seo J.A., Yu J.H. A Putative G-Protein Coupled Receptor Negatively Controls Sexual Development in Aspergillus nidulans. Mol. Microbiol. 2004;51:1333–1345. doi: 10.1111/j.1365-2958.2003.03940.x. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann B., Wanke C., LaPaglia S.K., Braus G.H. c-Jun and RACK1 Homologues Regulate a Control Point for Sexual Development in Aspergillus nidulans. Mol. Microbiol. 2000;37:28–41. doi: 10.1046/j.1365-2958.2000.01954.x. [DOI] [PubMed] [Google Scholar]
- 60.Kong Q., Wang L., Liu Z., Kwon N.J., Kim S.C., Yu J.H. Gβ-Like CpcB Plays a Crucial Role for Growth and Development of Aspergillus nidulans and Aspergillus fumigatus. PLoS ONE. 2013;8:e70355. doi: 10.1371/journal.pone.0070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eckert S.E., Hoffmann B., Wanke C., Braus G.H. Sexual Development of Aspergillus nidulans in Tryptophan Auxotrophic Strains. Arch. Microbiol. 1999;172:157–166. doi: 10.1007/s002030050755. [DOI] [PubMed] [Google Scholar]
- 62.Käfer E. The Anthranilate Synthetase Enzyme Complex and the Trifunctional trpC gene of Aspergillus. Can. J. Genet. Cytol. 1977;19:723–738. doi: 10.1139/g77-079. [DOI] [PubMed] [Google Scholar]
- 63.Serlupi-Crescenzi O., Kurtz M.B., Champe S.P. Developmental Defects Resulting from Arginine Auxotrophy in Aspergillus nidulans. Microbiology. 1983;129:3535–3544. doi: 10.1099/00221287-129-11-3535. [DOI] [PubMed] [Google Scholar]
- 64.Berridge M.J., Lipp P., Bootman M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 65.Sanders D., Pelloux J., Brownlee C., Harper J.F. Calcium at the Crossroads of Signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer M., Schnell N., Chattaway J., Davies P., Dixon G., Sanders D. The Saccharomyces cerevisiae cch1 Gene is Involved in Calcium Influx and Mating. FEBS Lett. 1997;419:259–262. doi: 10.1016/S0014-5793(97)01466-X. [DOI] [PubMed] [Google Scholar]
- 67.Iida H., Nakamura H., Ono T., Okumura M.S., Anraku Y. mid1, A Novel Saccharomyces cerevisiae Gene Encoding a Plasma Membrane Protein, is Required for Ca2+ Influx and Mating. Mol. Cell. Biol. 1994;14:8259–8271. doi: 10.1128/MCB.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller E.M., Mackin N.A., Erdman S.E., Cunningham K.W. FIG1p Facilitates Ca2+ Influx and Cell Fusion during Mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003;40:38461–38469. doi: 10.1074/jbc.M304089200. [DOI] [PubMed] [Google Scholar]
- 69.Cavinder B., Trail F. Role of fig1, a Component of the Low-Affinity Calcium Uptake System, in Growth and Sexual Development of Filamentous Fungi. Eukaryot. Cell. 2012;11:978–988. doi: 10.1128/EC.00007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim H.S., Kim J.E., Son H., Frailey D., Cirino R., Lee Y.W., Duncan R., Czymmek K.J., Kang S. Roles of Three Fusarium graminearum Membrane Ca2+ Channels in the Formation of Ca2+ Signatures, Growth, Development, Pathogenicity and Mycotoxin Production. Fungal Genet. Biol. 2018;111:30–46. doi: 10.1016/j.fgb.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Zhang S., Zheng H., Long N., Carbó N., Chen P., Aguilar P.S., Lu L. FigA, A Putative Homolog of Low-Affinity Calcium System Member Fig1 in Saccharomyces cerevisiae, is Involved in Growth and Asexual and Sexual Development in Aspergillus nidulans. Eukaryot. Cell. 2014;13:295–303. doi: 10.1128/EC.00257-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez-Romero J., Hedtke M., Kastner C., Müller S., Fischer R. Fungi, Hidden in Soil or Up in the Air: Light makes a Difference. Annu. Rev. Microbiol. 2010;64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- 73.He Q., Cheng P., Yang Y., Wang L., Gardner K.H., Liu Y. White Collar-1, A DNA Binding Transcription Factor and a Light Sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- 74.Ballario P., Talora C., Galli D., Linden H., Macino G. Roles in Dimerization and Blue Light Photoresponse of the PAS and LOV Domains of Neurospora crassa White Collar Proteins. Mol. Microbiol. 1998;29:719–729. doi: 10.1046/j.1365-2958.1998.00955.x. [DOI] [PubMed] [Google Scholar]
- 75.Linden H., Macino G. White Collar 2, A Partner in Blue-Light Signal Transduction, Controlling Expression of Light–Regulated Genes in Neurospora crassa. Embo J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballario P., Vittorioso P., Magrelli A., Talora C., Cabibbo A., Macino G. White Collar-1, A Central Regulator of Blue Light Responses in Neurospora, is a Zinc Finger Protein. EMBO J. 1996;15:1650–1657. doi: 10.1002/j.1460-2075.1996.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Innocenti F.D., Pohl U., Russo V. Photoinduction of Protoperithecia in Neurospora crassa by Blue Light. Photochem. Photobiol. 1983;37:49–51. doi: 10.1111/j.1751-1097.1983.tb04432.x. [DOI] [PubMed] [Google Scholar]
- 78.Harding R.W., Melles S. Genetic Analysis of Phototropism of Neurospora crassa Perithecial Beaks using White Collar and Albino Mutants. Plant Physiol. 1983;72:996–1000. doi: 10.1104/pp.72.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mooney J.L., Yager L.N. Light is Required for Conidiation in Aspergillus nidulans. Genes Dev. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- 80.Bayram Ö., Braus G.H. Coordination of Secondary Metabolism and Development in Fungi: The Velvet Family of Regulatory Proteins. FEMS Microbiol. Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 81.Bayram Ö., Krappmann S., Ni M., Bok J.W., Helmstaedt K., Valerius O., Braus-Stromeyer S., Kwon N.J., Keller N.P., Yu J.H. VelB/VeA/LaeA Complex Coordinates Light Signal with Fungal Development and Secondary Metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 82.Kim H.S., Han K.Y., Kim K.J., Han D.M., Jahng K.Y., Chae K.S. The veA Gene Activates Sexual Development in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:72–80. doi: 10.1016/S1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 83.Stinnett S.M., Espeso E.A., Cobeño L., Araújo-Bazán L., Calvo A.M. Aspergillus nidulans VeA Subcellular Localization is Dependent on the Importin α Carrier and on Light. Mol. Microbiol. 2007;63:242–255. doi: 10.1111/j.1365-2958.2006.05506.x. [DOI] [PubMed] [Google Scholar]
- 84.Ni M., Yu J.H. A Novel Regulator Couples Sporogenesis and Trehalose Biogenesis in Aspergillus nidulans. PLoS ONE. 2007;2:e970. doi: 10.1371/journal.pone.0000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blumenstein A., Vienken K., Tasler R., Purschwitz J., Veith D., Frankenberg-Dinkel N., Fischer R. The Aspergillus nidulans Phytochrome FphA Represses Sexual Development in Red Light. Curr. Biol. 2005;15:1833–1838. doi: 10.1016/j.cub.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 86.Purschwitz J., Müller S., Fischer R. Mapping the Interaction Sites of Aspergillus nidulans Phytochrome FphA with the Global Regulator VeA and the White Collar Protein LreB. Mol. Genet. Genom. 2009;281:35–42. doi: 10.1007/s00438-008-0390-x. [DOI] [PubMed] [Google Scholar]
- 87.Aguirre J., Ríos-Momberg M., Hewitt D., Hansberg W. Reactive Oxygen Species and Development in Microbial Eukaryotes. Trends Microbiol. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Takemoto D., Tanaka A., Scott B. NADPH Oxidases in Fungi: Diverse Roles of Reactive Oxygen Species in Fungal Cellular Differentiation. Fungal Genet. Biol. 2007;44:1065–1076. doi: 10.1016/j.fgb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Scherer M., Wei H., Liese R., Fischer R. Aspergillus nidulans Catalase-Peroxidase Gene (cpeA) is Transcriptionally Induced during Sexual Development through the Transcription Factor StuA. Eukaryot. Cell. 2002;1:725–735. doi: 10.1128/EC.1.5.725-735.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lara-Ortíz T., Riveros-Rosas H., Aguirre J. Reactive Oxygen Species generated by Microbial NADPH Oxidase NoxA regulate Sexual Development in Aspergillus nidulans. Mol. Microbiol. 2003;50:1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- 91.Cano-Domínguez N., Álvarez-Delfín K., Hansberg W., Aguirre J. NADPH Oxidases NOX-1 and NOX-2 require the Regulatory Subunit NOR-1 to control Cell Differentiation and Growth in Neurospora crassa. Eukaryot. Cell. 2008;7:1352–1361. doi: 10.1128/EC.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stowers L., Liberles S.D. State-Dependent Responses to Sex Pheromones in Mouse. Curr. Opin. Neurobiol. 2016;38:74–79. doi: 10.1016/j.conb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raabe M. Insect Reproduction: Regulation of Successive Steps. Adv. Insect Physiol. 1987;19:29–154. [Google Scholar]
- 94.Houck L.D. Pheromone Communication in Amphibians and Reptiles. Annu. Rev. Physiol. 2009;71:161–176. doi: 10.1146/annurev.physiol.010908.163134. [DOI] [PubMed] [Google Scholar]
- 95.Karlson P., Luscher M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- 96.Bölker M., Kahmann R. Sexual Pheromones and Mating Responses in Fungi. Plant Cell. 1993;5:1461. doi: 10.1105/tpc.5.10.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones S.K., Bennett R.J. Fungal Mating Pheromones: Choreographing the Dating Game. Fungal Genet. Biol. 2011;48:668–676. doi: 10.1016/j.fgb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang L., Baasiri R.A., Van Alfen N.K. Viral Repression of Fungal Pheromone Precursor Gene Expression. Mol. Cell. Biol. 1998;18:953–959. doi: 10.1128/MCB.18.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen W.-C., Bobrowicz P., Ebbole D.J. Isolation of Pheromone Precursor Genes of Magnaporthe grisea. Fungal Genet. Biol. 1999;27:253–263. doi: 10.1006/fgbi.1999.1151. [DOI] [PubMed] [Google Scholar]
- 100.Bobrowicz P., Pawlak R., Correa A., Bell-Pedersen D., Ebbole D.J. The Neurospora crassa Pheromone Precursor Genes are Regulated by the Mating Type Locus and the Circadian Clock. Mol. Microbiol. 2002;45:795–804. doi: 10.1046/j.1365-2958.2002.03052.x. [DOI] [PubMed] [Google Scholar]
- 101.Coppin E., de Renty C., Debuchy R. The Function of the Coding Sequences for the Putative Pheromone Precursors in Podospora anserina is Restricted to Fertilization. Eukaryot. Cell. 2004;4:407–420. doi: 10.1128/EC.4.2.407-420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin S.H., Wingfield B.D., Wingfield M.J., Steenkamp E.T. Causes and Consequences of Variability in Peptide Mating Pheromones of Ascomycete Fungi. Mol. Biol. Evol. 2011;28:1987–2003. doi: 10.1093/molbev/msr022. [DOI] [PubMed] [Google Scholar]
- 103.Wilson A.M., van der Nest M.A., Wilken P.M., Wingfield M.J., Wingfield B.D. Pheromone Expression reveals Putative Mechanism of Unisexuality in a Saprobic Ascomycete Fungus. PLoS ONE. 2018;13:e0192517. doi: 10.1371/journal.pone.0192517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J., Leslie J.F., Bowden R.L. Expression and Function of Sex Pheromones and Receptors in the Homothallic Ascomycete Gibberella zeae. Eukaryot. Cell. 2008;7:1211–1221. doi: 10.1128/EC.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pöggeler S. Two Pheromone Precursor Genes are Transcriptionally Expressed in the Homothallic Ascomycete Sordaria macrospora. Curr. Genet. 2000;37:403–411. doi: 10.1007/s002940000120. [DOI] [PubMed] [Google Scholar]
- 106.Kim H., Borkovich K.A. Pheromones are Essential for Male Fertility and Sufficient to Direct Chemotropic Polarized Growth of Trichogynes during Mating in Neurospora crassa. Eukaryot. Cell. 2006;5:544–554. doi: 10.1128/EC.5.3.544-554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim H., Metzenberg R.L., Nelson M.A. Multiple Functions of mfa-1, a Putative Pheromone Precursor Gene of Neurospora crassa. Eukaryot. Cell. 2002;1:987–999. doi: 10.1128/EC.1.6.987-999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turina M., Prodi A., Van Alfen N.K. Role of the mf1-1 Pheromone Precursor Gene of the Filamentous Ascomycete Cryphonectria parasitica. Fungal Genet. Biol. 2003;40:242–251. doi: 10.1016/S1087-1845(03)00084-7. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L., Churchill A., Kazmierczak P., Kim D.H., Van Alfen N. Hypovirulence-Associated Traits Induced by a Mycovirus of Cryphonectria parasitica are Mimicked by Targeted Inactivation of a Host Gene. Mol. Cell. Biol. 1993;13:7782–7792. doi: 10.1128/MCB.13.12.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmoll M., Seibel C., Tisch D., Dorrer M., Kubicek C.P. A Novel Class of Peptide Pheromone Precursors in Ascomycetous Fungi. Mol. Microbiol. 2010;77:1483–1501. doi: 10.1111/j.1365-2958.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xue C., Hsueh Y.P., Heitman J. Magnificent Seven: Roles of G Protein-Coupled Receptors in Extracellular Sensing in Fungi. FEMS Microbiol. Rev. 2008;32:1010–1032. doi: 10.1111/j.1574-6976.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim H., Borkovich K.A. A Pheromone Receptor Gene, pre-1, is Essential for Mating Type-Specific Directional Growth and Fusion of Trichogynes and Female Fertility in Neurospora crassa. Mol. Microbiol. 2004;52:1781–1798. doi: 10.1111/j.1365-2958.2004.04096.x. [DOI] [PubMed] [Google Scholar]
- 113.Kim H., Wright S.J., Park G., Ouyang S., Krystofova S., Borkovich K.A. Roles for Receptors, Pheromones, G Proteins and Mating Type Genes During Sexual Reproduction in Neurospora crassa. Genetics. 2012;190:1389–1404. doi: 10.1534/genetics.111.136358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pöggeler S., Kück U. Comparative Analysis of the Mating-Type Loci from Neurospora crassa and Sordaria macrospora: Identification of Novel Transcribed ORFs. Mol. Gen. Genet. 2000;263:292–301. doi: 10.1007/s004380051171. [DOI] [PubMed] [Google Scholar]
- 115.Pöggeler S., Kück U. Identification of Transcriptionally Expressed Pheromone Receptor Genes in Filamentous Ascomycetes. Gene. 2001;280:9–17. doi: 10.1016/S0378-1119(01)00786-7. [DOI] [PubMed] [Google Scholar]
- 116.Seo J.A., Han K.H., Yu J.H. The gprA and gprB Genes Encode Putative G Protein-Coupled Receptors Required for Self-Fertilization in Aspergillus nidulans. Mol. Microbiol. 2004;53:1611–1623. doi: 10.1111/j.1365-2958.2004.04232.x. [DOI] [PubMed] [Google Scholar]
- 117.Seo J.A., Han K.H., Yu J.H. Multiple Roles of a Heterotrimeric G-Protein γ-Subunit in Governing Growth and Development of Aspergillus nidulans. Genetics. 2005;171:81–89. doi: 10.1534/genetics.105.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dyer P.S., Paoletti M., Archer D.B. Genomics Reveals Sexual Secrets of Aspergillus. Microbiology. 2003;149:2301–2303. doi: 10.1099/mic.0.C0119-0. [DOI] [PubMed] [Google Scholar]
- 119.Coppin E., Debuchy R., Arnaise S., Picard M. Mating Types and Sexual Development in Filamentous Ascomycetes. Microbiol. Mol. Biol. Rev. 1997;61:411–428. doi: 10.1128/mmbr.61.4.411-428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Son H., Lee J., Lee Y.-W. A Novel Gene, gea1, is Required for Ascus Cell-Wall Development in the Ascomycete Fungus Fusarium graminearum. Microbiology. 2013;159:1077–1085. doi: 10.1099/mic.0.064287-0. [DOI] [PubMed] [Google Scholar]
- 121.Lee J., Park C., Kim J.C., Kim J.E., Lee Y.W. Identification and Functional Characterization of Genes Involved in the Sexual Reproduction of the Ascomycete Fungus Gibberella zeae. Biochem. Biophys. Res. Commun. 2010;401:48–52. doi: 10.1016/j.bbrc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 122.Cao S., He Y., Hao C., Xu Y., Zhang H., Wang C., Liu H., Xu J.-R. RNA Editing of the amd1 Gene is Important for Ascus Maturation and Ascospore Discharge in Fusarium graminearum. Sci. Rep. 2017;7:4617. doi: 10.1038/s41598-017-04960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng B., Haas H., Marzluf G.A. ASD4, A New GATA factor of Neurospora crassa, Displays Sequence-Specific DNA Binding and Functions in Ascus and Ascospore Development. Biochemistry. 2000;39:11065–11073. doi: 10.1021/bi000886j. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z., Lopez-Giraldez F., Lehr N., Farré M., Common R., Trail F., Townsend J.P. Global Gene Expression and Focused Knockout Analysis reveals Genes Associated with Fungal Fruiting Body Development in Neurospora crassa. Eukaryot. Cell. 2014;13:154–169. doi: 10.1128/EC.00248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee D.W., Pratt R.J., McLaughlin M., Aramayo R. An Argonaute-Like Protein is Required for Meiotic Silencing. Genetics. 2003;164:821–828. doi: 10.1093/genetics/164.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bayne E.H., White S.A., Kagansky A., Bijos D.A., Sanchez-Pulido L., Hoe K.L., Kim D.U., Park H.O., Ponting C.P., Rappsilber J. STC1: A Critical Link between RNAi and Chromatin Modification required for Heterochromatin Integrity. Cell. 2010;140:666–677. doi: 10.1016/j.cell.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berteaux-Lecellier V., Picard M., Thompson-Coffe C., Zickler D., Panvier-Adoutte A., Simonet J.M. A Nonmammalian Homolog of the PAF1 Gene (Zellweger syndrome) Discovered as a Gene Involved in Caryogamy in the Fungus Podospora anserina. Cell. 1995;81:1043–1051. doi: 10.1016/S0092-8674(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 128.Boisnard S., Zickler D., Picard M., Berteaux-Lecellier V. Overexpression of a Human and a Fungal ABC Transporter similarly Suppresses the Differentiation Defects of a Fungal Peroxisomal Mutant but Introduces Pleiotropic Cellular Effects. Mol. Microbiol. 2003;49:1287–1296. doi: 10.1046/j.1365-2958.2003.03630.x. [DOI] [PubMed] [Google Scholar]
- 129.Peraza Reyes L., Berteaux-Lecellier V. Peroxisomes and Sexual Development in Fungi. Front. Physiol. 2013;4:244. doi: 10.3389/fphys.2013.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kirk K., Morris N. The tubB α-Tubulin Gene is Essential for Sexual Development in Aspergillus nidulans. Genes Dev. 1991;5:2014–2023. doi: 10.1101/gad.5.11.2014. [DOI] [PubMed] [Google Scholar]
- 131.Krappmann S., Jung N., Medic B., Busch S., Prade R.A., Braus G.H. The Aspergillus nidulans F-Box Protein GrrA links SCF Activity to Meiosis. Mol. Microbiol. 2006;61:76–88. doi: 10.1111/j.1365-2958.2006.05215.x. [DOI] [PubMed] [Google Scholar]
- 132.Shiu P.K., Raju N.B., Zickler D., Metzenberg R.L. Meiotic Silencing by Unpaired DNA. Cell. 2001;107:905–916. doi: 10.1016/S0092-8674(01)00609-2. [DOI] [PubMed] [Google Scholar]
- 133.Shiu P.K., Zickler D., Raju N.B., Ruprich-Robert G., Metzenberg R.L. SAD-2 is Required for Meiotic Silencing by Unpaired DNA and Perinuclear Localization of SAD-1 RNA-Directed RNA Polymerase. Proc. Natl. Acad. Sci. USA. 2006;103:2243–2248. doi: 10.1073/pnas.0508896103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nelson M.A., Merino S.T., Metzenberg R.L. A Putative Rhamnogalacturonase Required for Sexual Development of Neurospora crassa. Genetics. 1997;146:531–540. doi: 10.1093/genetics/146.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hu S., Zhou X., Gu X., Cao S., Wang C., Xu J.R. The cAMP-PKA Pathway Regulates Growth, Sexual and Asexual Differentiation, and Pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2014;27:557–566. doi: 10.1094/MPMI-10-13-0306-R. [DOI] [PubMed] [Google Scholar]
- 136.Cavinder B., Hamam A., Lew R.R., Trail F. Mid1, a Mechanosensitive Calcium Ion Channel, affects Growth, Development, and Ascospore discharge in the Filamentous Fungus Gibberella zeae. Eukaryot. Cell. 2011;10:832–841. doi: 10.1128/EC.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hallen H.E., Trail F. The L-Type Calcium Ion Channel cch1 affects Ascospore Discharge and Mycelial Growth in the Filamentous Fungus Gibberella zeae (Anamorph Fusarium graminearum) Eukaryot. Cell. 2008;7:415–424. doi: 10.1128/EC.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lew R.R., Abbas Z., Anderca M.I., Free S.J. Phenotype of a Mechanosensitive Channel Mutant, mid-1, in a Filamentous Fungus, Neurospora crassa. Eukaryot. Cell. 2008;7:647–655. doi: 10.1128/EC.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo Y., Zhang H., Qi L., Zhang S., Zhou X., Zhang Y., Xu J.R. FgKin1 Kinase Localizes to the Septal Pore and plays a Role in Hyphal Growth, Ascospore Germination, Pathogenesis, and Localization of Tub1 β-Tubulins in Fusarium graminearum. New Phytol. 2014;204:943–954. doi: 10.1111/nph.12953. [DOI] [PubMed] [Google Scholar]
- 140.Tassan J.P., Le Goff X. An Overview of the KIN1/PAR-1/MARK Kinase Family. Biol. Cell. 2004;96:193–199. doi: 10.1016/j.biolcel.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 141.Krause R.A., Webster R. The Morphology, Taxonomy, and Sexuality of the Rice Stem Rot Fungus, Magnaporthe salvinii (Leptosphaeria salvinii) Mycologia. 1972;64:103–114. doi: 10.1080/00275514.1972.12019240. [DOI] [Google Scholar]
- 142.Spatafora J.W., Blackwell M. The Polyphyletic Origins of Ophiostomatoid Fungi. Mycol. Res. 1994;98:1–9. doi: 10.1016/S0953-7562(09)80327-4. [DOI] [Google Scholar]
- 143.Ingold C. The Stalked Spore-Drop. New Phytol. 1961;60:181–183. doi: 10.1111/j.1469-8137.1961.tb06250.x. [DOI] [Google Scholar]