Abstract
Despite the introduction of direct acting oral anticoagulants, warfarin remains the most commonly prescribed oral anticoagulant. However, warfarin therapy is plagued by the large inter- and intrapatient variability. The variability in dosing fueled research to identify clinical and genetic predictors and develop more accurate dosing algorithms. Observational studies have demonstrated the significant impact of single nucleotide polymorphisms in CYP2C9 and VKORC1 on warfarin dose in patients of European ancestry and African–Americans. This evidence supported the design and conduct of clinical trials to assess whether genotype-guided dosing results in improved anticoagulation control and outcomes. The trial results have shown discordance by race, with pharmacogenetic algorithms improving dose and anticoagulation control among European ancestry patients compared with African–American patients. Herein, we review the evidence from observational and interventional studies, highlight the need for inclusion of minority race groups and propose the need to develop race specific dosing algorithms.
Keywords: : pharmacogenetic dosing algorithms, race, warfarin
Warfarin is an oral anticoagulant that acts by inhibiting VKORC1, thus interfering with clotting factor activation [1,2]. The most common indications for warfarin include venous thromboembolism (VTE) and thromboembolic events related to atrial fibrillation (AF) or prosthetic heart valves [3–5]. Warfarin is still the most commonly prescribed anticoagulant for the prophylaxis and treatment of thromboembolism despite the gradual increase in the uptake of direct oral anticoagulants [6,7]. However, warfarin therapy is complicated by large inter-patient variability in dose requirements and large intrapatient variability in anticoagulation control [8]. The International Normalized Ratio (INR) is used to monitor the level of anticoagulation, with values between 2 to 3 considered optimal for most indications other than prosthetic heart valves (INR: 2.5–3.5) [9,10]. Doses vary by as much as 20-fold among patients initiating warfarin, often resulting in suboptimal response and leading either to an increased risk of thrombosis (INR <2) or an increased risk of bleeding (INR >4) [11–17].
Given the high rate of adverse outcomes, underutilization remains a major issue, with almost a third to half of the patients in need of anticoagulation not receiving warfarin [7,18,19]. To improve dose prediction and anticoagulation control and reduce the number of adverse events, investigations have identified clinical (e.g., age, comedications) [11,20–23] and genetic factors that explain a significant proportion of dose variability [24–28]. Although several genes influence warfarin dose [29–33], SNPs in the CYP2C9 (the principal enzyme that metabolizes warfarin) andVKORC1 (the target protein inhibited by warfarin) genes were determined to account for substantial dose variability [34–39].
In 2007, the US FDA revised its product label for the first time to include information on the pharmacogenomics of warfarin, describing the bleeding risk as well as percent dose reductions observed in patients of European ancestry (EA) in relation to the CYP2C9 and VKORC1 SNPs [40]. In 2011, the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines [28] refined these dosing recommendations based on the number of genetic variants present in a patient for the CYP2C9 and VKORC1 SNPs [41]. These changes were enabled by evolving evidence from large observational studies that supported the development of algorithms using genetic and clinical factors for the prediction of therapeutic warfarin dose. Since early 2000's, close to 105 such algorithms have been published [27,42], with the Gage [38] and the International Warfarin Pharmacogenetics Consortium (IWPC) [43] being the most widely used algorithms. A vast majority of these studies have been conducted in patients of European or Asian ancestry, with only a handful of studies evaluating other race/ethnicities such as patients of African descent [21,25,26,44–52].
Although race specific differences in allele frequencies are widely recognized, their differential influence on warfarin dose requirements across race groups is beginning to be appreciated. This is reflected in the updated warfarin dosing guidelines by CPIC (2017) which now include recommendations according to race [53].
Herein, we synthesize the evidence on pharmacogenetics (PGx) of warfarin dosing in EA and African–American (AA) patients from observational and interventional studies. We provide additional discussion of studies which recruited sizable African ancestry patients and developed either race-specific or race-stratified dosing algorithms.
Warfarin related genes & SNPs
To frame this discussion, we first present the allele frequencies for SNPs (Table 1) known to influence warfarin dose by race groups. CYP2C9 is the primary enzyme involved in the metabolism of warfarin and thus the polymorphic nature of the gene encoding this enzyme greatly affects the pharmacokinetics of warfarin, resulting in wide inter-patient variability [2]. The most widely studied CYP2C9 variants, *2 and *3, have very low minor allele frequencies (MAF) among AAs compared with the populations of European descent. AA-specific variants include *5, *6, *8 and * 11, which together were reported to explain as much of the variability in warfarin dose among AAs as the *2 and *3 variants do in the European populations [24,48,54]. Recently, a genome-wide association study conducted specifically among AAs identified a novel variant (rs12777823) that alone explained about 5% of the variability in warfarin dose. This SNP is more prevalent in AA (MAF-25%) compared with EA (MAF-15%) populations. Including this SNP in the model led to a 21% relative improvement in dose over the standard IWPC dosing algorithm [45].
Table 1. . Allele frequencies from the 1000 Genomes Project for genetic polymorphisms that affect warfarin dose.
| Gene (role) | SNP-id | MAF (%)† | |||
|---|---|---|---|---|---|
| African | European | Latino | Asian | ||
| CYP2C9 (principal enzyme involved in warfarin metabolism) | *2 (rs1799853) | 0.8 | 12.4 | 9.9 | 0.1 |
| *3 (rs1057910) | 0.2 | 7.3 | 3.7 | 3.4 | |
| *5 (rs28371686) | 1.7 | 0.0 | 0.1 | 0.0 | |
| *6 (rs9332131) | 0.8 | 0.0 | 0.0 | 0.0 | |
| *8 (rs7900194) | 5.3 | 0.2 | 0.1 | 0.0 | |
| *11 (rs28371685) | 2.4 | 0.2 | 0.1 | 0.0 | |
| 18786A>T (rs7089580) | 20.6 | 22.2 | 11.8 | 1.5 | |
| CYP2C | G>A (rs12777823) | 25.1 | 15.1 | 10.7 | 31.4 |
| VKORC1 (target protein inhibited by warfarin) | 1639G>A (rs9923231) | 5.4 | 38.8 | 41.1 | 88.5 |
| 1173C>T (rs9934438) | 5.4 | 38.8 | 41.2 | 88.5 | |
| 8191A>G (rs61162043) | 45.8 | 60.5 | 57.2 | 11.5 | |
| 6830T>C (rs17886199) | 5.5 | 0.1 | 0.1 | 0.0 | |
| 6853C>G (rs8050894) | 25.6 | 40.0 | 44.2 | 88.5 | |
| 2255C>T (rs2359612) | 17.9 | 38.8 | 42.5 | 88.5 | |
| 3730G>A (rs7294) | 45.4 | 36.6 | 40.2 | 11.2 | |
| CYP4F2 (enzyme involved in vitamin K1 metabolism) | *3 (V433M) (rs2108622) | 8.2 | 29.0 | 23.8 | 21.4 |
| GGCX (enzyme activates vitamin-K dependent proteins) | C>G (rs11676382) | 0.2 | 9.4 | 2.6 | 0.0 |
| (CAA) 16/17 (rs10654848) | 2.5 | 0.0 | 0.0 | 0.0 | |
| CALU (inhibits GGCX) | T>C (rs339097) | 14.4 | 0.0 | 1.2 | 1.4 |
| NQO1 (enzyme involved in vitamin K-dependent clotting factor synthesis) | *2 (rs1800566) | 17.5 | 21.1 | 33.3 | 41.9 |
| EPHX1 (enzyme hydrolyses epoxide intermediates, i.e., vitamin K epoxide) | 337T>C (rs1051740) | 14.1 | 30.4 | 32.0 | 48.2 |
| 357G>A (rs1131873) | 11.6 | 16.2 | 14.8 | 28.7 | |
†Minor allele frequencies were obtained from the 1000 Genomes Project using super population codes for African, European, Admixed American and east Asian populations.
MAF: Minor Allele Frequency.
Warfarin exerts its action by inhibiting the VKORC1 enzyme that is responsible for the activation of clotting factors. Although, several SNPs in the VKORC1 gene have been studied, including two common haplotypes (A and B), the rs9923231 (-1639G>A) SNP was demonstrated to explain the greatest variability in warfarin dose, similar to the haplotype groups tested, with differences in percent variation across racial groups largely explained by the differing allele frequencies [21,44,55]. As a result, this one SNP was included in the PGx algorithms evaluated by most studies.
The SNPs discussed so far result in lower warfarin dose requirements but the variant associated with the CYP4F2 gene, rs2108622 (*3), results in higher warfarin dose requirements, as the variant greatly reduces the enzyme concentration involved in the metabolism of vitamin K1 [30,47]. However, this SNP only explains ∼1% of the dose variability among EA patients and is not associated with dose among AAs [56]. Other genes that are either involved in the activation (GGCX or NQO1) or inhibition (CALU or EPHX1) of clotting factor synthesis have been shown to influence warfarin dose in some studies [31,52,57,58]. However, sufficient evidence does not exist to warrant their inclusion in dosing algorithms at present [53]. As such, we focus our discussion on racial differences in warfarin dosing in the context of SNPs related to the CYP2C9, CYP2C, VKORC1 and CYP4F2 genes.
Overview of warfarin dosing algorithm studies
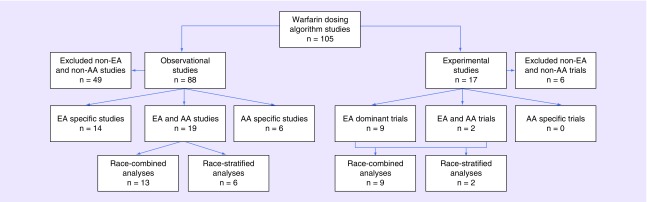
Figure 1 presents both observational and interventional studies that have been developing and evaluating warfarin dosing algorithms, focusing particularly on studies of European and African ancestry populations. We found a total of 105 articles that have either tested the performance of new or established dosing algorithms or implemented them in clinical trials to determine the efficacy or effectiveness of using them in routine clinical care. The majority of the studies are observational, comprising 84% (n = 88) of the total, with clinical trials making up the rest (n = 17, 16%). We excluded studies that did not involve European or African ancestry populations, which resulted in the removal of 49 (56%) observational studies and 6 (35%) interventional studies. The remaining observational (n = 39) and interventional (n = 11) studies are discussed below.
Figure 1. . Flowchart of warfarin pharmacogenetic dosing algorithm studies with a focus on those conducted in the European ancestry and African–American populations.
AA: African–American; EA: European ancestry.
Pharmacogenetic algorithms from observational studies
Of the 39 observational studies included in this review (Figure 1), only six have been conducted exclusively among AA populations, the remaining include only EA or combined EA and AA populations in the analysis. Although 49% of the studies listed include diverse populations, AA patients constitute a small fraction of the total population in many of these studies. The proportion of AAs is ∼10% in more than half of the studies included, with as low as 2% in the study by Gong et al. [59] and as high as 49% in the study by Limdi et al. [60]. Thus, the limited subgroup size resulted in adjustment for race rather than stratification by race in model development. Of the 19 studies including racially diverse populations, 13 studies combined the EA and AA data when evaluating the performance of the dosing algorithms. Of these, seven accounted for race in their models, the remaining six studies excluding race from their final models. Studies in the race-combined group that included >20% AA population had limited sample sizes and therefore used the combined AA and EA data to develop dosing algorithms [59,61–67]. Therefore these studies, including the study by Gage et al. adjusted for race [38,39,68–70].
Table 2 provides the details of the warfarin dosing algorithms, including the performance of the genetic and clinical predictors separately when available, the performance of the combined (clinical + genetic) dosing algorithms, and whether those studies also evaluated the performance of the Gage or IWPC algorithms. We focus our discussion on studies that included a racially diverse population and either adjusted or stratified for race. We initially discuss the studies that adjusted for race in their model development. The earliest dosing algorithm by Gage et al. included only the CYP2C9 variants and explained 39% of the variation in warfarin dose after adjusting for race [71]. The CYP2C9 variants (including *2, *3 and *5) alone explained 10% of the variability in dose in this diverse cohort of patients that included 29% AAs. With the discovery of VKORC1 in 2004, algorithms incorporated both CYP2C9 *2, *3 and the VKORC1 -1639G>A or -1173C>T variants. Limdi et al. evaluated the predictive ability of a single VKORC1 (-1639G>A) SNP versus VKORC1 haplotype and haplotype clusters in both AA and European–Americans [44]. The single VKORC1 SNP explained 5% of the variability in warfarin dose among AA and 19% among European–Americans, the latter finding being consistent with the report from Gage et al. [38]. Limdi et al. then extended these findings to include Asian patients, matching the performance of the single VKORC1 -1639G>A SNP with the haplotype in all three race groups (EA: 23%, AA: 4%, Asian: 29%) [21]. The equivalent performance of the VKORC1 -1639G>A SNP enabled the inclusion of a single SNP in the algorithm, improving the feasibility of PGx testing in a clinical care setting. Another commonly used race-adjusted algorithm, the IWPC algorithm [39], was found to have similar predictive performance as the Gage algorithm [38] although it incorporated different predictors, including enzyme inducers and excluding smoking status and VTE which were part of the Gage model.
Table 2. . Pharmacogenetic dosing algorithms and their performance in observational studies.
| Study (year) | n† | Treatment of race in the model | Clinical and genetic predictors | R2 explained (%) | Assessed prediction with the IWPC or Gage'08 algorithm | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Genetic | Clinical | Total | ||||||
| Kamali et al. (2004) | E: 119 | Restricted to EA | Age, CYP2C9*2, *3 | 6 | 17 | 20 | – | [24] |
| Hillman et al. (2004) | E: 453 | Restricted to EA | Age, BSA, valve replacement, diabetes, CYP2C9*2, *3 | – | – | 34 | – | [88] |
| Gage et al. (2004) | E: 261 | Adjusted for race | Age, gender, BSA, race, target INR, amiodarone, simvastatin, CYP2C9*2, *3 | 10 (includes CYP2C9*5) | – | 39 | – | [71] |
| A: 108 | ||||||||
| Voora et al. (2005) | E: 40 | Adjusted for race | Age, gender, race, weight, height, simvastatin, amiodarone, CYP2C9*2, *3 | – | – | 42 | – | [67] |
| A: 5 | ||||||||
| Sconce et al. (2006) | E: 297 | Restricted to EA | Age, height, CYP2C9*2, *3; VKORC1-1639A | – | – | 55 | – | [89] |
| Herman et al. (2006) | E: 165 | Restricted to EA | Age, BSA, CYP2C9*2, *3; VKORC1-1639A | – | – | 60 | – | [90] |
| Carlquist et al. (2006) | E: 213 | Restricted to EA | Age, gender, weight, CYP2C9*2, *3; VKORC1-1639A | 33 | 12 | 45 | – | [91] |
| Aquilante et al. (2006) | E: 319 | Unadjusted for race | Age, weight, smoking status, mean and goal INR, vitamin K intake, enzyme inhibitors and inducers, CYP2C9*2, *3, *5; VKORC1-1639A; Factor VII I/D, Factor X I/D | – | – | 51 | – | [66] |
| A: 25 | ||||||||
| Caldwell et al. (2007) | E: 570 | Restricted to EA | Age, gender, BSA, valve replacement, diabetes, CYP2C9*2, *3; VKORC1-6853C | – | – | 56 | – | [92] |
| Limdi et al. (2007) | E:249 | Stratified by race | Age, gender, BMI, drug interactions, average alcohol intake, average vitamin K intake, # comorbid conditions, CYP2C9 *2, *3, *5, *6, *10 or *11 | 6 | – | 19 | – | [60] |
| A:239 | 1 | – | ||||||
| 28 | – | |||||||
| Momary et al. (2007) | A: 115 | Restricted to AA | Age, BSA, CYP2C9*2, *3, *5 | – | – | 33 | – | [93] |
| Zhu et al. (2007) | E: 65 | Restricted to EA | Age, gender, weight, CYP2C9*2, *3; VKORC1-1639A | – | – | 61 | – | [94] |
| Gage et al. (2008) | E: 838 | Adjusted for race | Age, race, BSA, current smoking, target INR, VTE, amiodarone, CYP2C9*2, *3; VKORC1-1639A | – | 17 | 57 | 53 | [38] |
| A: 153 | 31 | |||||||
| Perini et al. (2008) | E: 196 | Unadjusted for race | Age, weight, valve replacement, VTE, amiodarone, simvastatin, CYP2C9*2, *3, *5, *11; VKORC1-1639A | – | – | 51 | – | [61] |
| A: 76 | ||||||||
| Limdi et al. (2008) | E: 302 | Stratified by race | Age, gender, BMI, follow-up clinic, income, smoking status, education, health insurance, drug interactions, alcohol intake, vitamin K intake, comorbid conditions, CYP2C9*2, *3, *5, *6, *11 and VKORC1-1173C>T | – | – | 42 | – | [25] |
| A: 273 | – | – | 36 | – | ||||
| Schelleman et al. (2008) | E: 147 | Stratified by race | E: Age, gender, BMI, # medications that potentiate warfarin, most # drinks on one occasion, h/o DVT, CYP2C9*2, *3 and VKORC1 -1173C>T | – | – | 43 | 42 | [75] |
| A: 112 | A: Age, BMI, # medications that potentiate warfarin, VKORC1 -1173C>T, Factor VII -401 G>T, APOE | – | – | 28 | 34 | |||
| Wu et al. (2008) | E: 33 | Adjusted for race | Age, race, gender, height, weight, smoking, amiodarone, sulfamethoxazole, CYP2C9*2, *3; VKORC1-1639A, 2255T | – | – | 59 | – | [62] |
| A: 31 | ||||||||
| Michaud et al. (2008) | E: 131 | Restricted to EA | Age, BSA, INR and Model 1: S:R-warfarin ratio at 14 h Model 2: dose-adjusted INR at day 4, CYP2C9*2, *3 VKORC1-1639A, 3730T |
– | – | 51 | – | [95] |
| Wadelius et al. (2009) | E: 1496 | Restricted to EA | Age, gender, drugs that increase INR, CYP2C9*2, *3; VKORC1-1639A | – | – | 59 | – | [96] |
| Klein et al. (2009) (IWPC) | E: 2233 | Adjusted for race | Age, race, height, weight, amiodarone, enzyme inducers, CYP2C9*2, *3; VKORC1-1639A | – | 27 | 45 | 47 | [43] |
| A: 353 | 26 | |||||||
| Cavallari et al. (2010) | A: 226 | Restricted to AA | Age, BSA, stroke/TIA, CYP2C9 *2, *3, *5, *6, *8, or *11, VKORC1 -1639G>A | 15 | – | 36 | – | [48] |
| Wells et al. (2010 ) | E: 232 | Restricted to EA | Age, BMI, height, exercise level, angiotensin II receptor antagonists, beta blockers, CYP2C9*2, *3; VKORC1-1639A; CYP4F2*3 | – | – | 58 | – | [97] |
| Lenzini et al. (2010) | E: 818 | Adjusted for race in the PGx model | Age, BSA, diabetes, stroke, amiodarone, fluvastatin, target INR, log INR, dose 2–4 days before INR measurement, CYP2C9*2, *3; VKORC1-1639A | – | 48 | 63 | – | [68] |
| A: 95 | ||||||||
| Zambon et al. (2011) | E: 274 | Restricted to EA | Age, BSA, CYP2C9*2, *3; VKORC1-1639A; CYP4F2*3 | – | – | 65 | 63 | [98] |
| Botton et al. (2011) | E: 279 | Restricted to EA | Age, weight, amiodarone, carbamazepine, beta blockers, amlodipine, diuretics, CYP2C9 *2, *3; VKORC1 haplotype based on -1639A, -1173T, -3730A; CYP4F2*3, F2 -494T | – | – | 63 | IWPC: 46 Gage: 42 |
[99] |
| Gong et al. (2011) | E:159 | Unadjusted for race | Age, weight, gender, amiodarone, CYP2C9*2, *3; VKORC1-1639A; CYP4F2*3 | – | – | 42 | – | [59] |
| A: 4 | ||||||||
| Perera et al. (2011) | A: 330 | Restricted to AA | Age, weight, DVT/PE, CYP2C9*2, *3, -18786T; VKORC1-1173T, -8191A | – | 22 | 40 | 26 | [49] |
| Avery et al. (2011) | E: 671 | Restricted to EA | Age, height, weight, amiodarone, CYP2C9*2, *3; VKORC1-1639A | – | – | 42 | – | [100] |
| Cini et al. (2012) | E: 55 | Restricted to EA | Age, height, weight, gender, smoking, dietary intake, VTE, diabetes, CYP2C9*2, *3; VKORC1 -1639A, 3730A | – | – | 44 | IWPC: 47 Gage: 54 |
[101] |
| Ramos et al. (2012) | E: 146 | Unadjusted for race | Age, PE, dose-adjusted INR at day 3, amiodarone, CYP2C9*2, *3, *5; VKORC1-1639A | 34 | – | 67 | 36 | [65] |
| A: 17 | ||||||||
| Horne et al. (2012) | E: 1115 | Unadjusted for race | Age, BSA, stroke, target INR, INR values, day of therapy, amiodarone, simvastatin, fluvastatin or enzyme inducers, CYP2C9*2, *3; VKORC1-1639A | – | 65 | 72 | 49 | [69] |
| A: 64 | ||||||||
| Ramirez et al. (2012) | E: 1022 | Stratified by race | Age, gender, BSA, smoking, atrial fibrillation vs. VTE, amiodarone, CYP2C9*2, *3, *6, *8; VKORC1 -1639A; CALU rs339097; CYP4F2*3 | – | 23 | 53 | 50 | [70] |
| A: 145 | – | 24 | 41 | 29 | ||||
| Shuen et al. (2012) | E: 264 | Unadjusted for race | Age, gender, target INR, CYP2C9*2, *3; VKORC1-1639A, VKORC1 Asp36Tyr | – | – | 54 | – | [63] |
| A: 52 | ||||||||
| Bress et al. (2012) | A: 260 | Restricted to AA | Age, BSA, hypertension, CYP2C9*2, *3, *5, *6, *8, *11; VKORC1 -1639A | – | 23 | 37 | – | [58] |
| Hernandez et al. (2014) | A: 349 | Restricted to AA | Age, weight, VTE, CYP2C9*2, *3, *5, *8, *11, 18786T, VKORC1 -1639A, -8191A; rs12777823 | – | – | 27 | IWPC Clinical: 16, Total: 15 |
[76] |
| Santos et al. (2015) | E: 574 | Adjusted for race | Age, gender, race, weight, height, amiodarone, enzyme inducers, CYP2C9*2, *3; VKORC1-1639A | – | – | 40 | – | [64] |
| A: 67 | ||||||||
| Limdi et al. (2015) | E: 762 | Stratified by race | Age, BSA, amiodarone, CKD, CYP2C9*2, *3, *5, *6, *11; VKORC1 -1639A, CYP4F2*3, rs12777823 | 35 | 16 | 54 | 51 | [46] |
| A: 595 | 10 | 23 | 34 | 29 | ||||
| Alzubiedi et al. (2016) | A: 163 | Restricted to AA | Age, height, weight, CHF, amiodarone, aspirin, paracetamol, enzyme inducers, CYP2C9*2, *3, *5; VKORC1 -1639A, CYP4F2*3, rs12777823 | – | – | 38 | 26 | [75] |
| Wiley et al. (2017) | E: 1928 | Four models related to race - unadjusted, adjusted, % ancestry and stratified | Age, BSA, smoking, amiodarone, enzyme inducers, CYP2C9*2, *3, *5, *6, *8, *11, VKORC1 -1639A; rs12777823 Other CYP2C9 and VKORC1 SNPs – either alone, combined or as haplotypes |
– | 20 | 56 | 45 | [73] |
| A: 253 | – | 22 | 40 | 20 | ||||
†Individuals of other race/ethnic groups are not listed in this table.
A: African descent; BMI: Body mass index; BSA: Body surface area; CHF: Congestive heart failure; CKD: Chronic kidney disease; DVT: deep vein thrombosis; E: European descent; INR: International normalized atio; IWPC: International Warfarin Pharmacogenetics Consortium; PE: Pulmonary embolism; VTE: Venous thromboembolism.
The Gage and IWPC algorithms include genetic and clinical predictors to guide dose initiation. Extending this work further, Lenzini et al., Ramos et al. and Horne et al. incorporated INR values and day(s) of therapy (Horne) or prior dose (Lenzini) to further guide warfarin dose [65,68,69]. These models demonstrate significantly better performance as they incorporate intermediate responses such as changes in INR to modify dose. Comparing the R2 values across all studies for the PGx dosing algorithms, the models by Horne et al. and Ramos et al. explained the greatest proportion of variation in warfarin dose, 72 and 67%, respectively [65,69]. The PGx algorithms by Horne et al. and Lenzini et al. were derived as dose-revision algorithms at days 6–11 and 4–5, respectively. The model by Ramos et al., although not a dose-revision algorithm, includes dose-adjusted INR at day 3 which requires the use of other methods to determine the dose for the first two days. INR and prior dose/day of therapy were influential in improving the R2 of these models. For example, the dose-adjusted INR at day 3 alone explained 19% of the variability in warfarin dose in the model by Ramos et al. [65]. However, Lenzini et al. demonstrated that inclusion of genetic information in their model reduced the R2 for INR compared with the R2 noted for INR in the clinical algorithm [68]. Thus, including SNPs together with dose and INR may improve our ability to predict dose-revisions. Yet, it is not clear whether genetic predictors would improve dose-revision if they are already accounted for in the dose-initiation algorithms [72]. Future studies are needed to test whether the use of PGx algorithms for both dose-initiation and dose-revision result in better percentage time in therapeutic range (PTTR) and clinical outcomes in patients.
Race-adjusted or race-combined models assume that the predictors have the same impact on dose across race groups. However, as noted in all the race-stratified analyses, the proportion of variability explained by the PGx algorithms in AA populations remains considerably lower compared with the EA populations. For instance, Gage et al. noted that the same PGx algorithm that explains 57% of the variability in EA only explained about 31% of the variability in AA patients [38]. The IWPC model reports a similar disparity (45% EA vs 26% AA) [43]. Limdi et al. recently evaluated the Gage algorithm and demonstrated that the PGx model explained 46, 51 and 29% of the variability, in the race-combined, EA and AA populations, with the genetic factors alone explaining 22, 34 and 7% of the variation, respectively [46].
Other studies evaluating the Gage or the IWPC algorithm by race have reported similar findings wherein the dose variability explained among AAs is considerably lower compared with the EA populations [70,73,74]. Thus, the three genetic variants most often included in the PGx algorithms are not good predictors of warfarin dose in the AA populations, with clinical algorithms even outperforming PGx algorithms in some studies. As a result, there was an impetus to study minority populations alone or stratified by race in diverse cohorts to discover novel variants or develop dosing algorithms that predict dose better among these populations.
We found six studies that evaluated race-stratified dosing algorithms, three of which were conducted by Limdi et al. [25,46,60], one by Schelleman et al. [74], one by Ramirez et al. [70] and the last by Wiley et al. [73]. The study by Schelleman et al. is the only one that presents two completely separate dosing algorithms for EA and AA patients, with different predictors included in the model for each. The genetic predictors included in the two models were especially different except for the VKORC1 -1173C>T variant. Only the EA algorithm included the CYP2C9*2, *3 variants whereas the AA algorithm included Factor VII-401 G>T and APOE instead. Their dosing model in EAs performed similarly to the Gage model (43 vs 42%), however, their AA model was only able to explain 28% of dose variability compared with 34% explained by the Gage model. This low R2 was attributed to likely unmeasured or unknown genetic or environmental factors, including the AA-specific CYP2C9 variants. The remaining race-stratified studies evaluate the same dosing algorithms with the same set of predictors, but modeled separately in each race group. Thus, the coefficients and the performance parameters obtained from these studies are different for the EA and AA populations. However, only the study by Limdi et al. presents the beta coefficient values for the two groups separately, showing how the same predictors have a differential effect between the two race groups [46]. For example, the presence of the VKORC1 -1639G>A variant required a significant dose reduction in both EA and AA groups. However, the dose reduction needed was significantly higher among EA than AA patients (29 vs 20%, p = 0.003). A race-combined algorithm would not achieve sufficient dose reduction among EA patients but at the same time result in a higher dose reduction among AA patients, thus failing to improve dose prediction in both race groups.
The earlier studies including AA patients modeled SNP genotypes as variant versus wild-type, most likely due to the low prevalence of these variants and/or the limited sample size of this subgroup. As a result, independent influences of each genotype (e.g., CYP2C9 *1/*2, *1/*3, etc.) were not assessed until 2012 when Ramirez et al. showed that CYP2C9*2 did not influence warfarin dose in AA patients using Vanderbilt's BioVU data repository [70]. Perera et al. also demonstrated the same finding in their genome-wide association study among AAs, reporting no association of the CYP2C9*2 variant with dose [45]. On the other hand, CYP2C9*2 is significantly associated with dose decrease among EA patients. This difference in the SNP effect could be multifactorial but still highlights the need for race-stratified algorithms that would include predictors significantly influencing warfarin dose. For instance, the inclusion of the rs12777823 SNP in our dosing algorithm predicted a 12% dose decrease in the AA patients [46]. The inclusion of AA-specific SNPs considerably improve the dose variability explained by the algorithm among AAs, as noted by the R2 values observed in some studies – for examole, 41% – Ramirez et al. [70], 40% – Perera et al. [49], 38% – Alzubiedi et al. [75] – among others. In addition, Cavallari et al. noted that the removal of the CYP2C9*5, *8 and *11 SNPs from their PGx model decreased the variability explained from 36 to 30%, showing that these AA-specific variants undoubtedly improve dose prediction among AAs [48]. Overall, we see an improvement in dose prediction with the inclusion of AA-specific SNPs, but these improvements still do not match or supersede the improvements noted in the EA populations. Inclusion of more SNPs in the model does not appear to improve the model performance substantially, as observed from the study by Wiley et al. [73]. Discovery of new genetic variants and/or clinical/environmental factors may further explain the variability, but would require larger studies with sample sizes that match EA populations. Another factor that may contribute to the variability is genetic ancestry which is a more accurate measure for self-reported race.
Very few studies have examined the effect of genetic ancestry on warfarin dose. Bress et al. and Hernandez et al. accounted for west African ancestry (WAA) but reported no significant association with model performance or improvement in warfarin dose prediction after inclusion in their PGx models [58,76]. However, Hernandez et al. reported an inverse association of WAA with warfarin dose that was predicted using the IWPC PGx algorithm among patients requiring substantially higher doses (≥60 mg/week). They suggested that the lack of AA-specific genetic variants in the IWPC model possibly diminished its warfarin dose predictability. On the other hand, Wiley et al., demonstrated that their haplotype model including percent African ancestry explained 42% of the variation in warfarin dose, the highest across all dosing algorithms among AAs in their study [73]. This is in contrast to the earlier findings where African ancestry did not remain significant after the inclusion of AA-specific SNPs in the PGx models. The adjustment for genetic ancestry, however, has been used in reports of race-combined models, improving upon the use of self-reported race. Accounting for genetic ancestry, both local and global, in race-specific models has the potential to further improve dose prediction and SNP parameter estimates by removing any underlying confounding due to admixture in these populations [77].
Sufficient evidence exists that indicates the need for AA-specific algorithms or the inclusion of AA-specific SNPs in the current warfarin dose prediction models. However, before we consider clinical implementation and utility of any of these algorithms, a more uniform or standardized dosing algorithm needs to be developed that maximizes dose prediction and minimizes adverse outcomes in AA populations.
Pharmacogenetic algorithms from interventional studies
Of the 11 interventional studies included in this review (Figure 1), not a single one has been conducted exclusively among AA patients whereas two trials were performed exclusively among EA populations. Except for the Genotype-guided Warfarin Therapy Trial (WARFPGX) [78] and the Clarification of Oral Anticoagulation through Genetics (COAG) trial [79] which included ∼27% AA patients in both studies, the seven remaining trials included at least 90% EA's with the European Pharmacogenetics of Anticoagulant Therapy (EU-PACT) [80] and the trial by Burmester et al. [81] including ∼98% EA patients in their trials. Since most of these trials only report the percent EA populations, it is hard to discern the race/ethnicities of the remaining trial population.
Table 3 provides the details of the warfarin dosing algorithms used in the interventional trials, the performance of the PGx algorithms and the clinical and genetic components separately if available. Finally, we have also included PTTR achieved by genotype-guided therapy compared with clinical or standard therapy in these trials. PTTR is an established measure of anticoagulation control which has been used as a surrogate marker of hemorrhage in almost all the clinical trials so far and thus provides a standard measure of comparison across all trials. Only the COAG trial provides a thorough comparison between the EA and AA populations, including but not limited to the model performances as well as PTTR [79]. Despite having a higher proportion of AAs, the WARFPGX trial was not powered to detect differences in their outcomes, let alone race-stratified ones [78]. The only other trial to compare PTTR between the two ancestry populations is the Genetics Informatics Trial (GIFT) [82]. The remaining studies present their findings for the race-combined analyses since they included a largely EA population.
Table 3. . Pharmacogenetic dosing algorithms and their performance in interventional studies.
| Study (year) | n‡ | Treatment of race in the model | Clinical and genetic predictors† | R2 explained (%) | PTTR (%) (genotype guided vs clinical/standard) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Genetic | Clinical | Total | ||||||
| Hillman et al. (2005) | E: 38 | Restricted to EA | Age, BSA, indication of therapy, diabetes, CYP2C9*2, *3 | – | – | 34 | 42, 42 | [83] |
| Anderson et al. (2007) (CoumaGen) | E: 189 | Restricted to EA | Age, gender, weight, amiodarone, CYP2C9*2, *3; VKORC1 -1639A | 32 | 15 | 47 | 70, 69 | [86] |
| McMillin et al. (2010) | E: 206 | Restricted to EA | Age, height, CYP2C9*2, *3; VKORC1 -1639A | 18 | – | – | 43, 45 | [102] |
| Burmester et al. (2011) | E: 225 | Restricted to EA | Age, gender, BSA, valve replacement, CYP2C9*2, *3; VKORC1 -1639A, CYP4F2*3 Age*Valve replacement as well in the clinical arm |
– | 35 | 65 | 29, 29 | [81] |
| Anderson et al. (2012) (CoumaGen II) | E: 2744 | Restricted to EA | Age, height, weight, race, amiodarone, enzyme inducers, CYP2C9*2, *3; VKORC1 -1639A | 37 | 13 | 49 | 71, 59 | [84] |
| Borgman et al. (2012) (PerMIT) | E: 24 | Restricted to EA | Age, gender, bodyweight, CYP2C9*2, *3; VKORC1 -1639A | – | – | – | 63, 55 | [85] |
| Jonas et al. (2013) (WARFPGX) | E: 79 | Adjusted for race | Age, race, BSA, smoking status, indication for warfarin, target INR, amiodarone, CYP2C9*2, *3; VKORC1 -1639A | – | – | – | 45, 49 | [78] |
| A: 30 | ||||||||
| Pirmohamed et al. (2013) (EU-PACT) | E: 447 | Unadjusted for race | Age, height, weight, amiodarone (BSA, log INR, target INR, dose given 2–4 days before INR measurement), CYP2C9*2, *3; VKORC1 -1639A | – | – | – | 67, 60 | [80] |
| A: 5 | ||||||||
| Kimmel et al. (2013) (COAG) | E: 675 | Adjusted for race | Age, race, smoking status, BSA, amiodarone, target INR, DVT/PE (diabetes, stroke, fluvastatin, log INR, dose given 2–4 days before INR measurement), CYP2C9*2, *3; VKORC1 -1639A |
– | 17 (51) | 52 (75) | 49, 46 | [79] |
| A: 275 | – | 33 (50) | 21 (40) | 35, 44 | ||||
| Pengo et al. (2015) | E: 180 | Restricted to EA | Age, BSA, CYP2C9*2, *3; VKORC1-1639A; CYP4F2*3 | – | – | 50 | 52, 53 | [87] |
| Gage et al. (2017) (GIFT) | E: 1454 | Adjusted for race | Age, race, BSA, current smoking, baseline and target INR, indication for warfarin, amiodarone, azoles and other antibiotics, CYP2C9*2, *3; VKORC1-1639A; CYP4F2*3 | – | – | – | 55, 51 | [82] |
| A: 102 | – | – | – | 51, 51 | ||||
†Parentheses include variables that were part of the dose revision algorithm, in addition to the variables listed in that cell that were part of the dose initiation algorithm.
‡Individuals of other race/ethnic groups are not listed in this table.
A: African descent; BSA: Body surface area; COAG: Clarification of Oral Anticoagulation through Genetics; DVT/PE: Deep vein thrombosis or pulmonary embolism; E: European descent; INR: International Normalized Ratio; PTTR: Percentage time in therapeutic range; COAG: Clarification of Optimal Anticoagulation through Genetics; EU-PACT: European Pharmacogenetics of Anticoagulant Therapy; GIFT: Genetics Informatics Trial of warfarin; PerMIT: Personalized Medicine Interface Tool for genotype-based warfarin dosing; WARFPGX: Genotype-guided warfarin therapy trial.
The trial by Hillman et al. is one of the first trials that was conducted to determine the feasibility of using a genotype-guided dosing algorithm in clinical practice [83]. It only included the CYP2C9 SNPs and reported warfarin dose, response and INR-related outcomes in addition to its feasibility measures. However, they found no significant differences in the two groups related to these outcomes, including PTTR. The remaining ten trials included both the CYP2C9 (*2 and *3) and the VKORC1 -1639A SNPs, with the GIFT and the trial by Burmester et al. including the CYP4F2*3 variant as well.
The genotype-guided dosing algorithms were compared with standard therapy in seven of 11 trials whereas the GIFT [82], COAG [79], WARFPGX [78] and Burmester [81] trials used clinically derived dosing algorithms for the control arm. The PTTR reported by most studies was not significantly different between the genotype-guided and the clinically-guided/standard therapy groups, except in the case of CoumaGen II [84], Personalized Medicine Interface Tool (PerMIT) [85] and the EU-PACT [80] trials. In the GIFT trial, PTTR was significantly different between the intervention and control arms, but only among the non-AA group (55 vs 51%, p = 0.003) [82]. Despite finding no difference in PTTR, Burmester et al., reported an improved predictive ability of the PGx algorithm compared with the clinically-guided dosing algorithm (65% vs. 35%) [81]. The CoumaGen trials (I and II) also reported the performance of their algorithms, with the PGx models (47 and 49%) performing better than the genetics only (32 and 37%) as well as the clinical models (15 and 13%) [84,86]. Similarly, the trial by Pengo et al. among Italian Caucasians also demonstrated that their PGx algorithm accounted for ∼50% of the variability in warfarin dose [87].
The EU-PACT [80] and COAG [79] trials published their findings in 2013 but reported very disparate results. Both employed dose-initiation as well as dose-revision algorithms for their genotype-guided dosing groups. However, these two trials differed in some fundamental ways. The EU-PACT trial included almost all EA patients (98.6%) compared with ∼73% in the COAG trial. COAG used a clinically guided dosing algorithm for the control arm whereas EU-PACT employed a standard dosing method. Furthermore, the PGx dosing algorithms in themselves were different, varying in the clinical predictors that were included during the dose-initiation and dose-revision algorithms. Finally, the EU-PACT trial used a modified IWPC algorithm to incorporate loading doses during days 1–3. Loading doses reduce the time taken to reach therapeutic INR, which may have contributed to a higher PTTR in the genotype-guided group. As such, the EU-PACT trial was able to report a significant difference in PTTR between the PGx and standard dose therapy groups (67 vs 60%; p < 0.001) while the COAG trial reported no benefit in the overall population (45 vs 45%; p = 0.91). However, significant differences were observed in the subgroup analysis by race wherein the PTTR was significantly higher among AAs (44 vs 35%; p = 0.01) but in the clinically-guided dosing arm. A possible explanation for this discrepancy is the use of the race-adjusted algorithm [38] which often resulted in the dispensation of doses that were significantly different (≥1 mg/day higher or lower) among the AA group (versus the non-AA group), both during dose-initiation (62 vs 42%; p = 0.002) and dose-revisions (50 vs 34%; p = 0.01). The results demonstrate that the PGx algorithm predicted dose more accurately among EAs compared with AAs during both dose-initiation (52 vs 21%; p = 0.001) and dose-revision (75 vs 40%; p < 0.001) stages. On the other hand, the clinical algorithm was better at dose prediction only among AA's and only during dose-initiation (33 vs 17%; p = 0.08).
The success of the EU-PACT trial can be attributed to the use of the IWPC algorithm that was developed and implemented among a largely EA population and which also included SNPs that are known to substantially influence warfarin dose in this race group. On the other hand, the COAG trial was designed to allow the examination of differences by race, but still used a PGx algorithm that was derived and validated in a mostly EA population. It did not include any AA-specific SNPs, and could not include the new CYP2C rs12777823 AA-specific variant, the results for which were not published until after the COAG trial was completed. As mentioned before, the use of the race-adjusted algorithm [38] assigned the same effect sizes for a predictor in both EA and AA. And, as the algorithm was derived mainly from EA, the parameter estimates were most likely weighted towards the EA population, resulting in less than optimal doses in the AA population. Thus, these findings highlight the need for adequate representation of minority populations, assessing race-specific SNPs and implementing race-specific algorithms to evaluate their efficacy and safety.
Conclusion & future perspective
The field of warfarin PGx has made tremendous strides in the last two decades. The discovery of genetic variants that explain a substantial proportion of the variability in warfarin dose among EA populations paved the way for the development of PGx algorithms that have improved dose prediction significantly in this race group. These dosing algorithms have undergone several iterations, each involving some variation in the clinical or genetic predictors included in the models. The CYP2C9*2, *3 and VKORC1 variants have been the most consistent genetic predictors across all models, with the AA-specific ones also including other CYP2C9 variants and more recently the rs12777823 SNP. The improvements in dose variability explained have been substantial, however, ∼40% still remains unexplained. Dose revision algorithms including INR, warfarin dose as well as genetic variants add to the variability explained but warrant further investigation to determine if the results are consistent across all populations and clinical settings. Interventional trials have established the role of genotype-guided dosing algorithms in improving anticoagulation control and are now moving towards the evaluation of safety outcomes. These trials show that implementation of the PGx algorithms in clinical care is feasible and would be beneficial at least in populations of European descent.
The focus should now shift to under-represented populations to enable similar discoveries and developments so that dose prediction in these populations can at least be brought to par with what has been achieved in the EA populations. This requires adequate representation of minority populations, especially in clinical trials, and the inclusion of race-specific SNPs if not the use of race-specific algorithms to better predict dose in these race-groups. However, as with the Gage or IWPC models that are considered established algorithms for the EA populations, there is a need to develop and validate an algorithm that can be considered a gold standard among the AA population, which best explains the variability in warfarin dose and can be used in routine practice across all care settings.
Studies in patients from Asian, Hispanic and Middle-Eastern populations are growing, however, we have not included these studies in the current review. As we show herein, the impact of genetic factors on warfarin dose varies among African vs. European populations, but it is also known to differ among other groups (e.g., Hispanic). The evaluation of these other race groups would be best served through a separate focused review.
Executive summary.
Warfarin pharmacogenetic dosing algorithms
The discovery of the CYP2C9 and VKORC1 polymorphisms resulted in the development and evaluation of dosing algorithms to better predict warfarin dose.
Most of the observational as well as interventional studies on warfarin dosing algorithms have been conducted among populations of European descent.
Observational studies among other race/ethnic groups such as individuals of African descent led to the discovery of novel and race-specific genetic variants that improved dose prediction in these racial groups.
Pharmacogenetic algorithms from observational studies
A major proportion of the evidence for pharmacogenetic (PGx) algorithms comes from observational studies. Of these, 39 included European ancestry (EA) or African–Americans (AA) populations but only half of them included both groups.
Among the racially diverse studies, only six performed a race-stratified analyses to evaluate the performance of these algorithms in EA and AA separately.
The differences in prediction identified in race-stratified analyses highlight the need for race-specific dosing algorithms.
Pharmacogenetic algorithms from interventional studies
Genotype-guided dosing has been evaluated in 17 studies, of which 11 were conducted in either EA or both EA and AA populations.
Two of the interventional studies also evaluated outcomes by race but only the Clarification of Oral Anticoagulation through Genetics trial presented the performance of the models in both race groups.
PGx algorithms improved dose prediction and percentage time in therapeutic range among patients of European descent but not among AA.
Future directions
Dosing algorithms including race-specific genetic variants need to be developed and validated in minority populations.
Similar to the Gage and International Warfarin Pharmacogenetics Consortium algorithms, a dosing algorithm needs to be developed for the AA populations, one that explains the greatest variability in warfarin dose and can serve as a gold-standard of therapy in this population.
Footnotes
Financial & competing interests disclosure
This work was supported in part by grants from the National Heart Lung and Blood Institute (RO1HL092173; 1K24HL133373), National Institute of General Medical Sciences (R01GM081488) and the NIH Clinical and Translational Science Award (CTSA) program (UL1TR000165). NA Limdi is on the advisory board of Admera. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Li T, Lange LA, Li X, et al. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J. Med. Genet. 2006;43(9):740–744. doi: 10.1136/jmg.2005.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl. 6):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 3.Tadros R, Shakib S. Warfarin – indications, risks and drug interactions. Aust. Fam. Physician. 2010;39(7):476–479. [PubMed] [Google Scholar]
- 4.Hon HH, Elmously A, Stehly CD, et al. Inappropriate preinjury warfarin use in trauma patients: a call for a safety initiative. J. Postgrad. Med. 2016;62(2):73–79. doi: 10.4103/0022-3859.175004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhovsek M, Motlagh B, Crowther MA, et al. Quality of anticoagulation and use of warfarin-interacting medications in long-term care: a chart review. BMC Geriatr. 2008;8:13. doi: 10.1186/1471-2318-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am. J. Med. 2015;128(12):1300–1305. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alalwan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am. J. Health Syst. Pharm. 2017;74(16):1237–1244. doi: 10.2146/ajhp160756. [DOI] [PubMed] [Google Scholar]
- 8.Wittkowsky AK. Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. Semin. Vasc. Med. 2003;3(3):221–230. doi: 10.1055/s-2003-44457. [DOI] [PubMed] [Google Scholar]
- 9.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Absher RK, Moore ME, Parker MH, Rivera-Miranda G, Perreault MM. Patient-specific factors predictive of warfarin dosage requirements. Ann. Pharmacother. 2002;36(10):1512–1517. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 12.Wittkowsky AK, Whitely KS, Devine EB, Nutescu E. Effect of age on international normalized ratio at the time of major bleeding in patients treated with warfarin. Pharmacotherapy. 2004;24(5):600–605. doi: 10.1592/phco.24.6.600.34735. [DOI] [PubMed] [Google Scholar]
- 13.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N. Engl. J. Med. 1996;335(8):540–546. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 14.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. New Engl. J. Med. 2003;349(11):1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 15.McGriff-Lee NJ, Csako G, Chen JT, et al. Search for predictors of nontherapeutic INR results with warfarin therapy. Ann. Pharmacother. 2005;39(12):1996–2002. doi: 10.1345/aph.1E381. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JA. Warfarin pharmacogenetics: a rising tide for its clinical value. Circulation. 2012;125(16):1964–1966. doi: 10.1161/CIRCULATIONAHA.112.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hylek EM. Vitamin K antagonists and time in the therapeutic range: implications, challenges, and strategies for improvement. J. Thromb. Thrombolysis. 2013;35(3):333–335. doi: 10.1007/s11239-013-0900-5. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Dahlberg KW. Physician's fear of anticoagulant therapy in nonvalvular atrial fibrillation. Am. J. Med. Sci. 2014;348(6):513–521. doi: 10.1097/MAJ.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316(20):2115–2125. doi: 10.1001/jama.2016.16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shendre A, Parmar GM, Dillon C, Beasley TM, Limdi NA. Influence of age on warfarin dose, anticoagulation control, and risk of hemorrhage. Pharmacotherapy. 2018;38(6):588–596. doi: 10.1002/phar.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limdi NA, Wadelius M, Cavallari L, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115(18):3827–3834. doi: 10.1182/blood-2009-12-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limdi NA, Wiener H, Goldstein JA, Acton RT, Beasley TM. Influence of CYP2C9 and VKORC1 on warfarin response during initiation of therapy. Blood Cells Mol. Dis. 2009;43(1):119–128. doi: 10.1016/j.bcmd.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanoski Ca PBJ, Pharmd. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest. 2002;121:19–23. doi: 10.1378/chest.121.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Kamali F, Khan TI, King BP, et al. Contribution of age, body size, and CYP2C9 genotype to anticoagulant response to warfarin. Clin. Pharmacol. Ther. 2004;75(3):204–212. doi: 10.1016/j.clpt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Limdi NA, Arnett DK, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 on warfarin dose, anticoagulation attainment and maintenance among European–Americans and African–Americans. Pharmacogenomics. 2008;9(5):511–526. doi: 10.2217/14622416.9.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavallari LH, Limdi NA. Warfarin pharmacogenomics. Curr. Opin. Mol. Ther. 2009;11(3):243–251. [PubMed] [Google Scholar]
- 27.Kaye JB, Schultz LE, Steiner HE, Kittles RA, Cavallari LH, Karnes JH. Warfarin pharmacogenomics in diverse populations. Pharmacotherapy. 2017;37(9):1150–1163. doi: 10.1002/phar.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohnke H, Sörlin K, Granath G, Wadelius M. Warfarin dose related to apolipoprotein E (APOE) genotype. Eur. J. Clin. Pharmacol. 2005;61(5–6):381–388. doi: 10.1007/s00228-005-0936-3. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008;111(8):4106–4112. doi: 10.1182/blood-2007-11-122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pautas E, Moreau C, Gouin-Thibault I, et al. Genetic factors (VKORC1, CYP2C9, EPHX1, and CYP4F2) are predictor variables for warfarin response in very elderly, frail inpatients. Clin. Pharmacol. Ther. 2010;87(1):57–64. doi: 10.1038/clpt.2009.178. [DOI] [PubMed] [Google Scholar]
- 32.Luxembourg B, Schneider K, Sittinger K, et al. Impact of pharmacokinetic (CYP2C9) and pharmacodynamic (VKORC1, F7, GGCX, CALU, EPHX1) gene variants on the initiation and maintenance phases of phenprocoumon therapy. Thromb. Haemost. 2011;105(1):169–180. doi: 10.1160/TH10-03-0194. [DOI] [PubMed] [Google Scholar]
- 33.Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum. Genet. 2007;121(1):23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flockhart DA, O'Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet. Med. 2008;10(2):139–150. doi: 10.1097/GIM.0b013e318163c35f. [DOI] [PubMed] [Google Scholar]
- 35.Mcclain MR, Palomaki GE, Piper M, Haddow JE. A rapid-ACCE review of CYP2C9 and VKORC1 alleles testing to inform warfarin dosing in adults at elevated risk for thrombotic events to avoid serious bleeding. Genet. Med. 2008;10(2):89–98. doi: 10.1097/GIM.0b013e31815bf924. [DOI] [PubMed] [Google Scholar]
- 36.Gage BF, Eby CS. Pharmacogenetics and anticoagulant therapy. J. Thromb. Thrombolysis. 2003;16(1–2):73–78. doi: 10.1023/B:THRO.0000014598.24114.62. [DOI] [PubMed] [Google Scholar]
- 37.Schelleman H, Limdi NA, Kimmel SE. Ethnic differences in warfarin maintenance dose requirement and its relationship with genetics. Pharmacogenomics. 2008;9(9):1331–1346. doi: 10.2217/14622416.9.9.1331. [DOI] [PubMed] [Google Scholar]
- 38.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warfarin product labeling. 2007. 2018(02/01/2018)
- 41.Warfarin product labeling. 2011. 2018(02/01/2018)
- 42.Verhoef TI, Redekop WK, Daly AK, Van Schie RM, De Boer A, Maitland-Van Der Zee AH. Pharmacogenetic-guided dosing of coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br. J. Clin. Pharmacol. 2014;77(4):626–641. doi: 10.1111/bcp.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Warfarin Pharmacogenetics Consortium. Klein TE, Altman RB, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limdi NA, Beasley TM, Crowley MR, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African–Americans and European–Americans. Pharmacogenomics. 2008;9(10):1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera MA, Cavallari LH, Limdi NA, et al. Genetic variants associated with warfarin dose in African–American individuals: a genome-wide association study. Lancet. 2013;382(9894):790–796. doi: 10.1016/S0140-6736(13)60681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limdi NA, Brown TM, Yan Q, et al. Race influences warfarin dose changes associated with genetic factors. Blood. 2015;126(4):539–545. doi: 10.1182/blood-2015-02-627042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shendre A, Brown TM, Liu N, et al. Race-specific influence of CYP4F2 on dose and risk of hemorrhage among warfarin users. Pharmacotherapy. 2016;36(3):263–272. doi: 10.1002/phar.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavallari LH, Langaee TY, Momary KM, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87(4):459–464. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 49.Perera MA, Gamazon E, Cavallari LH, et al. The missing association: sequencing-based discovery of novel SNPs in VKORC1 and CYP2C9 that affect warfarin dose in African Americans. Clin. Pharmacol. Ther. 2011;89(3):408–415. doi: 10.1038/clpt.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavallari LH, Butler C, Langaee TY, et al. Association of apolipoprotein E genotype with duration of time to achieve a stable warfarin dose in African–American patients. Pharmacotherapy. 2011;31(8):785–792. doi: 10.1592/phco.31.8.785. [DOI] [PubMed] [Google Scholar]
- 51.Cavallari LH, Momary KM, Patel SR, Shapiro NL, Nutescu E, Viana MA. Pharmacogenomics of warfarin dose requirements in Hispanics. Blood Cells Mol. Dis. 2011;46(2):147–150. doi: 10.1016/j.bcmd.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Cavallari LH, Perera M, Wadelius M, et al. Association of the GGCX (CAA)16/17 repeat polymorphism with higher warfarin dose requirements in African Americans. Pharmacogenet. Genomics. 2012;22(2):152–158. doi: 10.1097/FPC.0b013e32834f288f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson JA, Caudle KE, Gong L, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin. Pharmacol. Ther. 2017;102(3):397–404. doi: 10.1002/cpt.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper GM, Johnson JA, Langaee TY, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112(4):1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352(22):2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 56.Cavallari LH, Perera MA. The future of warfarin pharmacogenetics in under-represented minority groups. Future Cardiol. 2012;8(4):563–576. doi: 10.2217/fca.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voora D, Koboldt DC, King CR, et al. A polymorphism in the VKORC1 regulator calumenin predicts higher warfarin dose requirements in African Americans. Clin. Pharmacol. Ther. 2010;87(4):445–451. doi: 10.1038/clpt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bress A, Patel SR, Perera MA, Campbell RT, Kittles RA, Cavallari LH. Effect of NQO1 and CYP4F2 genotypes on warfarin dose requirements in Hispanic–Americans and African–Americans. Pharmacogenomics. 2012;13(16):1925–1935. doi: 10.2217/pgs.12.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong IY, Schwarz UI, Crown N, et al. Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation. PLoS ONE. 2011;6(11):e27808. doi: 10.1371/journal.pone.0027808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Limdi N, Goldstein J, Blaisdell J, Beasley T, Rivers C, Acton R. Influence of CYP2C9 genotype on warfarin dose among African American and European Americans. Per. Med. 2007;4(2):157–169. doi: 10.2217/17410541.4.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perini JA, Struchiner CJ, Silva-Assuncao E, et al. Pharmacogenetics of warfarin: development of a dosing algorithm for brazilian patients. Clin. Pharmacol. Ther. 2008;84(6):722–728. doi: 10.1038/clpt.2008.166. [DOI] [PubMed] [Google Scholar]
- 62.Wu AH, Wang P, Smith A, et al. Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics. 2008;9(2):169–178. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]
- 63.Shuen AY, Wong BY, Fu L, Selby R, Cole DE. Evaluation of the warfarin-resistance polymorphism, VKORC1 Asp36Tyr, and its effect on dosage algorithms in a genetically heterogeneous anticoagulant clinic. Clin. Biochem. 2012;45(6):397–401. doi: 10.1016/j.clinbiochem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Santos PC, Marcatto LR, Duarte NE, et al. Development of a pharmacogenetic-based warfarin dosing algorithm and its performance in Brazilian patients: highlighting the importance of population-specific calibration. Pharmacogenomics. 2015;16(8):865–876. doi: 10.2217/pgs.15.48. [DOI] [PubMed] [Google Scholar]
- 65.Ramos AS, Seip RL, Rivera-Miranda G, et al. Development of a pharmacogenetic-guided warfarin dosing algorithm for Puerto Rican patients. Pharmacogenomics. 2012;13(16):1937–1950. doi: 10.2217/pgs.12.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aquilante CL, Langaee TY, Lopez LM, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin. Pharmacol. Ther. 2006;79(4):291–302. doi: 10.1016/j.clpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Voora D, Eby C, Linder MW, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thromb. Haemost. 2005;93(4):700–705. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 68.Lenzini P, Wadelius M, Kimmel S, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin. Pharmacol. Ther. 2010;87(5):572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horne BD, Lenzini PA, Wadelius M, et al. Pharmacogenetic warfarin dose refinements remain significantly influenced by genetic factors after one week of therapy. Thromb. Haemost. 2012;107(2):232–240. doi: 10.1160/TH11-06-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramirez AH, Shi Y, Schildcrout JS, et al. Predicting warfarin dosage in European–Americans and African–Americans using DNA samples linked to an electronic health record. Pharmacogenomics. 2012;13(4):407–418. doi: 10.2217/pgs.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb. Haemost. 2004;91(01):87–94. doi: 10.1160/TH03-06-0379. [DOI] [PubMed] [Google Scholar]
- 72.Lenzini PA, Grice GR, Milligan PE, et al. Laboratory and clinical outcomes of pharmacogenetic vs. clinical protocols for warfarin initiation in orthopedic patients. J. Thromb. Haemost. 2008;6(10):1655–1662. doi: 10.1111/j.1538-7836.2008.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiley LK, Vanhouten JP, Samuels DC, et al. Strategies for equitable pharmacogenomic-guided warfarin dosing among European and African American individuals in a clinical population. Pac. Symp. Biocomput. 2017;22:545–556. doi: 10.1142/9789813207813_0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schelleman H, Chen J, Chen Z, et al. Dosing algorithms to predict warfarin maintenance dose in Caucasians and African Americans. Clin. Pharmacol. Ther. 2008;84(3):332–339. doi: 10.1038/clpt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alzubiedi S, Saleh MI. Pharmacogenetic-guided warfarin dosing algorithm in African–Americans. J. Cardiovasc. Pharmacol. 2016;67(1):86–92. doi: 10.1097/FJC.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez W, Gamazon ER, Aquino-Michaels K, et al. Ethnicity-specific pharmacogenetics: the case of warfarin in African Americans. Pharmacogenomics J. 2014;14(3):223–228. doi: 10.1038/tpj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shriner D, Adeyemo A, Rotimi CN. Joint ancestry and association testing in admixed individuals. PLoS Comput. Biol. 2011;7(12):e1002325. doi: 10.1371/journal.pcbi.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jonas DE, Evans JP, McLeod HL, et al. Impact of genotype-guided dosing on anticoagulation visits for adults starting warfarin: a randomized controlled trial. Pharmacogenomics. 2013;14(13):1593–1603. doi: 10.2217/pgs.13.145. [DOI] [PubMed] [Google Scholar]
- 79.Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med. 2013;369(24):2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pirmohamed M, Burnside G, Eriksson N, et al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med. 2013;369(24):2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 81.Burmester JK, Berg RL, Yale SH, et al. A randomized controlled trial of genotype-based Coumadin initiation. Genet. Med. 2011;13(6):509–518. doi: 10.1097/GIM.0b013e31820ad77d. [DOI] [PubMed] [Google Scholar]
- 82.Gage BF, Bass AR, Lin H, et al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT randomized clinical trial. JAMA. 2017;318(12):1115–1124. doi: 10.1001/jama.2017.11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hillman MA, Wilke RA, Yale SH, et al. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin. Med. Res. 2005;3(3):137–145. doi: 10.3121/cmr.3.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anderson JL, Horne BD, Stevens SM, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II) Circulation. 2012;125(16):1997–2005. doi: 10.1161/CIRCULATIONAHA.111.070920. [DOI] [PubMed] [Google Scholar]
- 85.Borgman MP, Pendleton RC, McMillin GA, et al. Prospective pilot trial of PerMIT versus standard anticoagulation service management of patients initiating oral anticoagulation. Thromb. Haemost. 2012;108(3):561–569. doi: 10.1160/TH12-03-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 87.Pengo V, Zambon CF, Fogar P, et al. A randomized trial of pharmacogenetic warfarin dosing in naive patients with non-valvular atrial fibrillation. PLoS ONE. 2015;10(12):e0145318. doi: 10.1371/journal.pone.0145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hillman MA, Wilke RA, Caldwell MD, Berg RL, Glurich I, Burmester JK. Relative impact of covariates in prescribing warfarin according to CYP2C9 genotype. Pharmacogenetics. 2004;14(8):539–547. doi: 10.1097/01.fpc.0000114760.08559.dc. [DOI] [PubMed] [Google Scholar]
- 89.Sconce EA, Kamali F. Appraisal of current vitamin K dosing algorithms for the reversal of over-anticoagulation with warfarin: the need for a more tailored dosing regimen. Eur. J. Haematol. 2006;77(6):457–462. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2957.x. [DOI] [PubMed] [Google Scholar]
- 90.Herman D, Peternel P, Stegnar M, Breskvar K, Dolzan V. The influence of sequence variations in factor VII, gamma-glutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb. Haemost. 2006;95(5):782–787. [PubMed] [Google Scholar]
- 91.Carlquist JF, Horne BD, Muhlestein JB, et al. Genotypes of the cytochrome P450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J. Thromb. Thrombolysis. 2006;22(3):191–197. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 92.Caldwell MD, Berg RL, Kai QZ, et al. Evaluation of genetic factors for warfarin dose prediction. Clin. Med. Res. 2007;5(1):8–16. doi: 10.3121/cmr.2007.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Momary KM, Shapiro NL, Viana MA, Nutescu EA, Helgason CM, Cavallari LH. Factors influencing warfarin dose requirements in African–Americans. Pharmacogenomics. 2007;8(11):1535–1544. doi: 10.2217/14622416.8.11.1535. [DOI] [PubMed] [Google Scholar]
- 94.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (-1639 G>A) and CYP2C9 genotypes. Clin. Chem. 2007;53(7):1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 95.Michaud V, Vanier MC, Brouillette D, et al. Combination of phenotype assessments and CYP2C9–VKORC1 polymorphisms in the determination of warfarin dose requirements in heavily medicated patients. Clin. Pharmacol. Ther. 2008;83(5):740–748. doi: 10.1038/sj.clpt.6100434. [DOI] [PubMed] [Google Scholar]
- 96.Wadelius M, Chen LY, Lindh JD, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113(4):784–792. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wells PS, Majeed H, Kassem S, et al. A regression model to predict warfarin dose from clinical variables and polymorphisms in CYP2C9, CYP4F2, and VKORC1: derivation in a sample with predominantly a history of venous thromboembolism. Thromb. Res. 2010;125(6):e259–e264. doi: 10.1016/j.thromres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 98.Zambon CF, Pengo V, Padrini R, et al. VKORC1, CYP2C9 and CYP4F2 genetic-based algorithm for warfarin dosing: an Italian retrospective study. Pharmacogenomics. 2011;12(1):15–25. doi: 10.2217/pgs.10.162. [DOI] [PubMed] [Google Scholar]
- 99.Botton MR, Bandinelli E, Rohde LE, Amon LC, Hutz MH. Influence of genetic, biological and pharmacological factors on warfarin dose in a southern Brazilian population of European ancestry. Br. J. Clin. Pharmacol. 2011;72(3):442–450. doi: 10.1111/j.1365-2125.2011.03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avery PJ, Jorgensen A, Hamberg AK, et al. A proposal for an individualized pharmacogenetics-based warfarin initiation dose regimen for patients commencing anticoagulation therapy. Clin. Pharmacol. Ther. 2011;90(5):701–706. doi: 10.1038/clpt.2011.186. [DOI] [PubMed] [Google Scholar]
- 101.Cini M, Legnani C, Cosmi B, et al. A new warfarin dosing algorithm including VKORC1 3730 G >A polymorphism: comparison with results obtained by other published algorithms. Eur. J. Clin. Pharmacol. 2012;68(8):1167–1174. doi: 10.1007/s00228-012-1226-5. [DOI] [PubMed] [Google Scholar]
- 102.Mcmillin GA, Melis R, Wilson A, et al. Gene-based warfarin dosing compared with standard of care practices in an orthopedic surgery population: a prospective, parallel cohort study. Ther. Drug Monit. 2010;32(3):338–345. doi: 10.1097/FTD.0b013e3181d925bb. [DOI] [PubMed] [Google Scholar]