Abstract
Aim:
A sensitive and selective LC–MS/MS method was validated for quantitation of ivermectin (IVM) in plasma.
Method:
The IVM was extracted from plasma using solid-phase extraction with C-18 cartridges. Separation of analytes was achieved on an ACE C18 column with isocratic elution using 0.1% acetic acid and methanol: acetonitrile (1:1, v/v) as mobile phase. The IVM was quantitated using electrospray ionization operating in negative multiple reaction monitoring mode.
Results:
The MS/MS response was linear over the concentration range from 0.1–1000 ng/ml. The method for human plasma was validated as per US FDA guidelines. The LC–MS/MS method is sensitive, reproducible, has easy sample preparation and is suitable for IVM quantitation in clinical samples.
Keywords: : abamectin, ivermectin, LC–MS/MS, pharmacokinetics
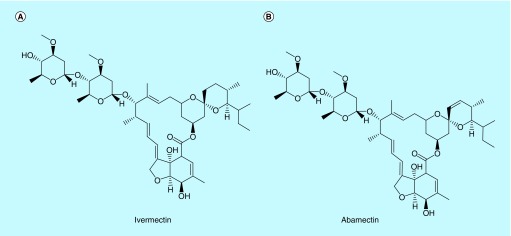
Ivermectin (IVM), named as 22, 23-dihydro-avermectin B1 (Figure 1), is a mixture of two semisynthetic analogs produced by the bacteria Streptomycetes avermetilis [1–3], which is well known for its excellent potency and wide spectrum against nematode, microfilaria and parasites in invertebrates (mosquitoes) and mammals (mouse, cattle, sheep, dog, monkey and human) [4]. Ivermectin and avermectin are approved for the control and treatment of human and animal onchocerciasis, lymphatic flariasis (LF), strongyloidiasis and scabies [5–8]. Wuchereria bancrofti (and less commonly Brugia malayi or B. timori) infects an estimated 120 million people in the tropics with another 1 billion at risk [9]. The WHO has targeted LF for global elimination as a public health problem by 2020. The LF is treated with triple drug therapy including diethylcarbamazine combined with albendazole and IVM.
Figure 1. . The chemical structures of (A) ivermectinand (B) abamectin (internal standard).
A number of IVM formulations are available for human administration including, topical (cream and lotion) and oral formulations (tablet). When oral administration for systemic treatment is not feasible, often a veterinary product is administered either subcutaneously or intravenously [10–14]. Moreover, the optimal dose and systemic concentration of IVM required for the successful eradiation of disseminated strongyloidiasis is not known. Therefore, it is necessary to evaluate the relation between pharmacokinetics and drug response to IVM. Besides formulation, animal species and route of administration may substantially affect IVM plasma drug concentration [15–17]. IVM is metabolized by CYP3A4 and it is also a substrate and potent inducer of P-gp, which illustrates a theoretical possibility of interaction of IVM with CYP3A4 and P-gp substrates [18,19]. At the same time, clinical pharmacokinetic studies of IVM-based therapy and plasma concentration data are lacking for some special groups including patients over 65 years, pregnant, lactating and HIV-infected individuals, among others [4]. There is a need to establish safe and effective IVM dosing in special populations such as pregnant individuals, infants and children and the elderly. Moreover, the optimal drug plasma concentration required for anthelmintic activity has not been determined [10,20], indicating the need for a simple and sensitive method for the determination of IVM concentration in human plasma.
Meanwhile, preclinical studies in different species, such as mouse and monkey are necessary to assess the treatment efficacy and safety of IVM on animals and fully understand the drug's pharmacokinetic properties and to understand species’ specific differences in drug disposition and metabolism [21]. IVM's use has been reported in a variety of animals [22]. As part of ongoing studies to assess alternative dosing strategies for IVM, we evaluated the plasma concentration of IVM in mice and monkeys following oral IVM therapy. These species were chosen as they provided a way to establish the IVM plasma: cerebrospinal fluid (CSF) ratio in an animal model. To facilitate these studies, it is necessary to develop a simple and highly sensitive assay that can utilize small sample volumes with a high throughput that can accurately measure trace levels of IVM in plasma samples.
Various techniques have been utilized for the separation, detection and quantification of IVM from soil, water, vegetables and biological samples. The HPLC-fluorescence methods (HPLC-FL) are most commonly utilized for quantitation of IVM [23–26]. Several methods include gas chromatography-mass spectrometry (GC-MS) [27]. However, both GC–MS and HPLC-FL-based methods require sample derivatization, which is labor intensive, time-consuming and expensive. Although LC–MS/MS methods have been successfully applied to the determination of IVM (Table 1) but limitations still preclude their implementation [28–37]. A number of LC/MS/MS methods for the quantitation of IVM have been described, including in bovine muscle (LLOQ; 0.9 μg/kg) [28], animal plasma (LLOQ; 1 ng/ml) [29], vegetables (LLOQ; 0.53 ng/ml) [36] and milk [30,37]. Huang et al. reported a method for the quantitation of IVM in edible oils (LLOQ; 0.3 μg/kg) [31]. Krogh et al. reported a method for the quantitation of IVM in water, sediment and soil (LLOQ 0.5–2.5 ng/kg) [32]. Ortiz et al. reported a method for the quantitation of IVM in tissues of dung (LLOQ 0.1 ng/g) [33]. Hence, the aim of this study was to develop a simple and sensitive LC–MS/MS method with a LLOQ of 0.1 ng/ml for the absolute quantification of IVM from human, mouse and monkey plasma that is more suited for pharmacokinetic studies of either human or animal species. We have successfully validated a LC–MS/MS method for IVM quantitation in human plasma with a LLOQ of 0.1 ng/ml. The performance of the LC–MS/MS method was compared with a previously validated HPLC-FL method by re-analyzing previously assayed clinical samples. This method has been applied to mouse and monkey plasma with acceptable precision and minimal bias.
Table 1. . Summarizes methods of ivermectin quantitated in various biological and inorganic matrices by liquid chromatography–tandem mass spectrometry.
| Matrix | Matrix volume | LLOQ | Analysis run time (minutes) | Sample pretreatment | Ref. |
|---|---|---|---|---|---|
| Bovine muscle | 5 gm | 0.9 μg/kg | 30 min | Protein precipitation | [28] |
| Animal plasma | 500 μl | 1.0 ng/ml | 6 min | Protein precipitation | [29] |
| Milk | 5 gm | 0.2 μg/kg | NA | Protein precipitation followed by liquid–liquid extraction | [30] |
| Edible oils | 5 gm | 5.0 ng/ml | 5 min | Liquid–liquid extraction | [31] |
| Water and soil and sediment | 1 gm | 2.5 ng/ml and 0.5 ng/ml | 11 min | Solid phase extraction and liquid–liquid extraction | [32] |
| Postmortem and tissues | 10 mg– 1 gm | 0.1–10 ng/gm | 15 min | Solid-phase extraction | [33] |
| Animal tissue | 2.5 gm | NA | – | Solid-phase extraction | [34] |
| Plant-based matrices | 5 g | 0.53 ng/gm | – | Solid-phase extraction | [36] |
| Human, monkey and mouse plasma | 100 μl | 0.1 ng/ml | 9 min | Solid-phase extraction | Current method |
NA: Not applicable.
Materials & methods
Chemicals & materials
Pharmaceutical grade IVM (purity; ≥90%), and abamectin (ABA, purity; ≥97.1%) (internal standard, IS) were purchased from Sigma-Aldrich (MO, USA). LC-MS-grade methanol (MeOH), acetonitrile (MeCN), sodium acetate and acetic acid (AA), were obtained from Fisher Scientific (NJ, USA). Centrifuge tube filters were purchased from Corning Co. (NY, USA). Agilent bond Elute C18, 50 mg per 1 ml cartridges were purchased from Agilent (DE, USA). A water purification system Barnstead GenPure, Thermo Scientific (NA, USA) was used for generating ultrapure water. All other reagents used were purchased from standard suppliers and of analytical grade or higher.
Liquid chromatographic & mass spectrometric conditions
The instrumentation consisted of a Shimadzu 8060 LC–MS/MS system (Shimadzu Scientific Instruments, MD, USA), a binary pump system (LC-30 AD), column oven (CTO-30AS) and an auto-sampler (SIL-30AC). The mass spectrometer was operated in negative electrospray ionization mode using a dual ion source. The MS/MS system was operated at unit resolution in the multiple reaction monitoring (MRM) mode. The analytical column was an ACE C18 (50 × 3 mm, 3 μ, Advanced Chromatography Technologies, Ltd) column equipped with a C18 guard column (Phenomenex, Inc., CA, USA).
The mobile phase was 0.1% AA in water (mobile phase A) and MeOH/MeCN (1:1; mobile phase B; 20:80 v/v), delivered at 0.3 ml/min with a column oven temperature maintained at 40°C. An isocratic elution over 9.0 min was utilized for chromatographic separation. The injection volume was 10 μl.
Quantification of all analytes was achieved in negative MRM mode. The nebulizer gas flow was set at 2.0 l/min, the heating gas and the drying gas at 10 l/min. The interface temperature was 375°C, desolvation line temperature of 250°C and heat block temperature of 400°C with an interface voltage of 4000V were utilized. The MRM precursor ion→product ion transitions for IVM and IS were m/z 873.50→567.25 and 871.50→565.35 respectively. LabSolutions LCMS Ver.5.6 (Shimadzu Scientific, Inc.) was used for data acquisition.
Preparation of stock, calibration standards & quality control samples
Stock solutions of 1 mg/ml of IVM and IS were prepared in 50:50 MeOH:H2O. Two separate stock solutions were prepared for IVM for the preparation of calibration curve standards (CCs) and quality control samples (QCs). Then, the stocks were diluted with methanol to make working standard solutions, which were further diluted to prepare the working solutions of QCs and CCs. The CCs were prepared by spiking 20 μl of working standard solution into 200 μl of plasma (10x high) to obtain a concentration range of 0.1–1000 ng/ml for IVM. The final CCs concentrations were 0.1, 0.2, 1, 5, 10, 50, 500, 1000 ng/ml in matrix. The QCs at the LLOQ, 0.1 ng/ml, low quality control (LQC, 0.5 ng/ml), middle quality control (MQC, 200 ng/ml) and high quality control (HQC, 750 ng/ml) were prepared respectively in five replicates.
Sample preparation
Agilent bond Elute C18 SPE cartridges, (50 mg 1 ml cartridge, Agilent Technologies, DE, USA) were used for the plasma sample preparation of mouse, monkey and human samples. Briefly, into a 2.5 ml polypropylene tube, a total of 200 μl of plasma were added and spiked with 10 μl of IS working solution (ABA 1000 ng/ml). The sample was vortexed for 10 s, diluted with 400 μl of 1% 10 mM sodium acetate buffer and vortexed again. Subsequently, 200 μl of MeOH was added to the sample. The entire sample was vortexed for 5 min, followed by centrifugation at 3,000 × g for 5 min and the supernatant loaded onto SPE cartridges that had been activated with1 ml of MeOH followed by 1 ml of HPLC grade water. Loaded cartridges were washed with 2 ml of 15% MeOH and eluted with 2 ml of isopropyl alcohol. All eluates were evaporated under a gentle stream of nitrogen at 50°C temperature and reconstituted in 100 μl of MeOH/MeCN: 0.1% AA (80:20). A total of 10 μl of the reconstituted sample were injected into LC–MS/MS for analysis.
HPLC-fluorescence assay partial validation
The partial validation and plasma sample preparation were performed for HPLC-FL assay by following Kitzman's method [23]. Quantitation of IVM was performed using Shimadzu Shimadzu, HPLC system equipped with a binary pump system (LC-10 AD), column oven (CTO-10A), an auto-sampler (SIL-10AD), degasser (DGU-114A) and fluorescence detector (RF-10AXL) with a data processing software LabSolutions (Shimadzu Scientific, Inc.). The separation was performed on a Ultrasphere 5 ODS column, 250 × 4.6 mm, 5 μm (HiChrom, UK) with a mobile phase composed of tetrahydrofuran–acetonitrile–water (40:38:22 v/v/v). The fluorescence detector was set at excitation and emission wavelength of 365 and 475 nm, respectively. The retention times of IVM and moxidectin (IS) were 24.5 and 12.5 min, respectively. The assay for ivermectin in human plasma is linear over the concentration range of 0.2–400 ng/ml.
Liquid chromatography–tandem mass spectrometry assay validation
The LC–MS/MS method was validated according to the guidance for industry: bioanalytical method validation of USFDA, 2018 [38,39].
Selectivity & specificity
Selectivity and specificity processed as per extraction procedure were carried out by analyzing the six different blank mouse, monkey or human plasma samples spiked with analytes and IS for the assessment of potential interferences with endogenous substances. Each calibration curve consisted of eight nonzero concentrations, a blank sample and a zero sample (blank + IS). The results were fitted using linear regression analysis with the use of weighting factor (1/x2). Acceptance criteria required the calibration curve to have a correlation coefficient (r2) of 0.998 or better for all analytes.
Sensitivity
The signal-to-noise ratio of the analyte response in the calibration standards was used to calculate the sensitivity of the method. The signal-to-noise ratio was required to be greater than three for the LOD and ten for the LLOQ respectively.
Accuracy & precision
Accuracy and precision (intra- and interday) were evaluated for three consecutive days by analyzing five replicates of QC samples at four different concentrations for IVM (0.1, 0.5, 200, and 750 ng/ml) in mouse, monkey or human plasma. All the QCs were required to be ± 15% standard deviation (SD) from the nominal values with a precision of ± 15% relative standard deviation (RSD), except for LLOQ, where the limit was ± 20% of SD to meet acceptance criteria.
Recovery & matrix effect
Extraction recoveries were determined by comparing the peak area of IVM at three different QC levels (LQC, MQC and HQC) in extracted samples with pure authentic standards for IVM in reconstitution solvent. The recovery of the IS was determined utilizing the same methodology.
The matrix effect was evaluated at each QC level and for mouse, monkey or human plasma. After extraction of blank plasma, the dry extract was spiked with IVM and IS and the mean peak area of the spiked analytes in blank matrix was compared with QCs prepared in the reconstitution solvent.
Dilution integrity
Dilution integrity was investigated to ensure that samples could be diluted with blank plasma without affecting the result. The IVM spiked human, mouse or monkey plasma samples prepared at 1500 and 3750 ng/ml concentrations were diluted with pooled human, mouse or monkey plasma at dilution factors of two and five in five replicates and analysed. The five replicates were required to meet both precision (≤15%) and accuracy (100 ± 15%) criteria similar to the QCs samples.
Carryover
To assess carry-over, two zero samples were injected following an HQC sample of IVM. The first zero sample was required to be <20% of the response of a processed LLOQ sample of IVM to meet acceptance criteria.
Stability
The stability of IVM in plasma samples following three freeze–thaw cycles (room temperature to -80°C to room temperature), bench-top storage (20°C for 8 h) and auto-sampler (4°C for 36 h) stability was calculated by determining concentrations at LQC, MQC and HQC (n = 3). The long-term sample storage (-80°C for 30 days) and re-analysis of clinical samples after 1 year (-80°C for 1 year) were evaluated.
Application of the method for human samples analysis
For comparison of our newly developed LC–MS/MS method with a previously validated HPLC-FL method, we re-analyzed randomly selected samples from a clinical trial previously reported [40].
Statistics
The agreement between the LC–MS/MS and HPLC-FL values were assessed using linear regression and the method of Bland and Altman [41]. The Wilcoxon signed rank test was used for comparison between concentrations measured by two assays using the JMP software version 13 (NC, USA).
Results & discussion
Mass spectrometric, chromatographic & extraction conditions optimization
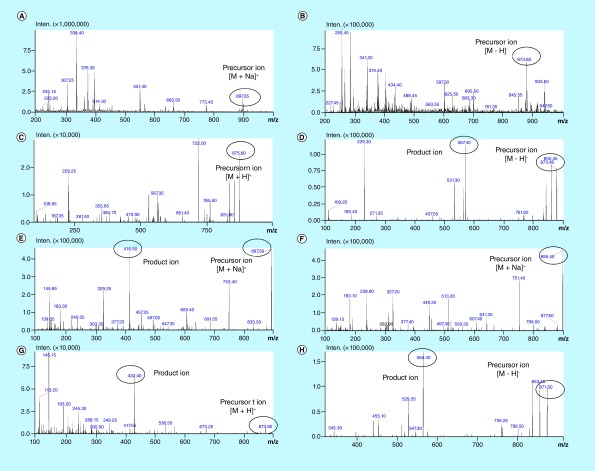
The MS/MS conditions were initially optimized by injecting standards at concentration of 1 μg/ml and tuning the cone and collision energies using mobile phase A containing 0.1% AA in water and mobile phase B containing MeOH:MeCN (50:50, v/v). The product ion spectra for IVM and IS were monitored in the both positive and negative ionization with ESI. The product ion spectra of [M−H]− or [M+H]+ or [M+Na]+ ions were observed when monitoring positive or negative ions formed from these compounds (Figure 2). In positive ion mode, a primary peak at m/z 897 [M+Na]+ rather than at an anticipated m/z 875 [M+H]+ was observed for IVM, similar to results shown for IS. The parent ions selected were [M−H]− ions when monitoring negative ions, and either [M+H]+ or [M+Na]+ ions when collecting data in positive ion mode. The ESI source in positive mode with [M+Na]+ was more sensitive but [M−H]− was selected for quantifying IVM because it could offer a more consistent signal intensity than [M+Na]+. The calibration curves using the Na adduct ions were nonlinear with a strong positive curvature that was not reproducible. The product ion spectra [M−H]− obtained are 873.50 >567.25 and 871.50 >565.35, consisted [M − H − 306 (oleandrose)]− for IVM and ABA (Figure 2). The major fragment of IVM and IS in negative ESI mode, is the loss of the oleandrose portion (loss of 306 Da) to leave the macrocyclic lactone ion, which is used as the primary ion for quantitation. The loss of 82 Da, the neutral pyranose fragment or loss of 110 Da, the dimethylpyranose fragment is consistent with previous observations [29,34,35].
Figure 2. . Mass spectra of ivermectin and abamectin spectra.
(A) Q1spectra of ivermectin (IVM) in positive electrospray ionization (ESI) mode, [M+ Na]+ (897.5). (B) Q1spectra of IVM in negative ESI mode shows, prominent parent peak at [M− H]− (873.45). (C) Q1spectra of IVM in positive ESI mode [M+ H]+ (875.5). (D) MS/MS product ion spectra of IVM in negative ESI mode [M− H]− (873.45). (E) MS/MS product ion spectra of IVM in positive ESI mode [M+ Na]+ (897.5). (F) MS/MS product ion spectra of abamectinin positive ESI mode [M+ Na]+ (895.5). (G) MS/MS product ion spectra of IVM in positive ESI mode [M+ H]− (873.5). (E) MS/MS product ion spectra of IVM in negative ESI mode[M− H]+ (871.5).
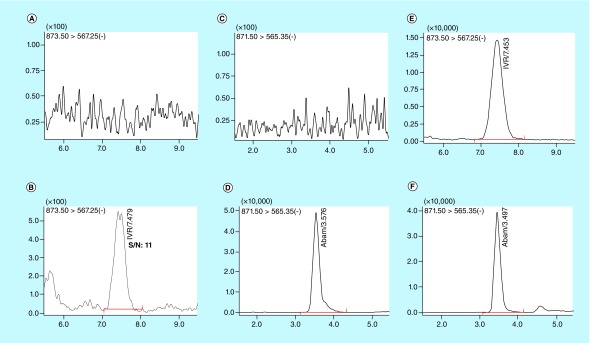
Different chromatographic conditions such as different mobile phases (i.e., MeCN, MeOH and water), analytical columns (C8, C18 and C18 PFP) and additives (i.e., formic acid, AA, ammonium acetate and formate) were tested for achieving an increased sensitivity, improved peak resolution and shorter chromatographic times for IVM and IS. The IVM gave a good electrospray response in the negative ionization mode when using the mobile phase 0.1% (v/v) acetic acid in water (mobile phase A) and MeOH:MeCN (50:50, v/v; mobile phase B). Isocratic elution (A:B, 20:80 v/v) was performed to obtain complete chromatographic resolution and best peak shape for both IVM and the IS, on ACE C18 (50 × 3 mm, 3 μ) column equipped with a C18 guard column. The retention times for IVM and IS were 7.5 and 3.5 min, respectively. These retention times were recorded at a flow rate of 0.3 ml/min with 40°C column oven temperature and the total chromatographic run time was 10 min. The reproducibility (%CV) in the measurement of retention time for 100 injections was 0.5%. Typical mass chromatograms from human blank plasma showed no interference of endogenous compounds with the analysis of IVM and IS (Figure 3).
Figure 3. . Representative multiple reaction monitoring ion-chromatograms.
(A) Blank human plasma using the conditions for ivermectin (IVM) detection, (B) Plasma spiked with IVM at Lower limit of quantification (rt. 7.5 min, 0.1 ng/ml), (C) Blank human plasma using the conditions for abamectin (ABA) detection, (D) Plasma spiked with ABA (rt. 3.5 min, 1000 ng/ml), (E) Plasma from clinical study sample (48 hr time point) showing IVM, (F) Plasma afrom clinical study spiked with ABA.
Our aim was to develop a selective, sensitive and simple method to determine IVM in human monkey and mouse plasma. Optimization of the extraction method from plasma, the variables involved in the procedure such as extraction methods and extraction solvents were investigated. We tested a protein precipitation method with MeOH and MeCN, but the sensitivity was not high enough and distorted peaks. Subsequently, we tested solid phase extraction (SPE) with different SPE cartridges namely C8 and C18 and the extraction recovery of our final method was greater than that of simple protein precipitation (data not shown). In addition, the SPE method was preferred over the protein precipitation and liquid–liquid extraction for analyzing the in-vivo samples because there was less matrix effect (data not shown). The bond elute C18, 1 cc cartridge resulted in high extraction efficiency (∼85%) of IVM. The method was optimized with 200 μl of plasma sample. Desorption conditions both the desorbing solvent and its volume were investigated to ensure the effective elution of analytes from the sorbent. The best recoveries were observed for both analytes when isopropyl alcohol was used as the elution solvent. After the expected LOQ values for IVM were achieved, the method was validated according to the FDA guidance.
Structurally similar ABA was used as the IS for IVM. Both compounds had chromatographic separation to avoid cross talk and a similar ionization response in ESI mode.
Assay validation
Specificity & selectivity
The specificity of the method was evaluated by analyzing human plasma samples from six different sources, pooled mouse plasma or pooled monkey plasma to investigate potential interferences at the retention time of all analytes and IS. No coeluting peaks >20% of the LLOQ or >5% of the area of IS were observed. The representative UPLC, blank human plasma chromatogram, samples spiked at LLOQ (0.1 ng/ml) concentration is shown in Figure 3. The retention time for IVM and IS were 7.5 and 3.5 min, respectively.
Calibration curve & linearity
The method was linear over a concentration range from 0.1–1000 ng/ml for IVM. The calibration model was selected based on the analysis of the data by linear regression with or without intercepts (y = mx + c and y = mx) and weighting factors (1/y2). The lowest concentration with relative standard deviation (RSD) <20% was taken as LLOQ and was found to be 0.1 ng/ml.
Carryover
After the high standard (1000 ng/ml), blank samples were injected, IVM showed no significant peak in blank samples (<20% of the LLOQ) indicating no significant carry-over effect.
Accuracy & precision
The interday and intraday accuracy and precision results for the detection of IVM in mouse, monkey and human plasma at four different concentrations are presented in Table 2. The % RSD of precision values ranged from 1.70 to 15.26%. The bias ranged from -12.96 to 12.40% and was within the acceptance limits.
Table 2. . Intra- and interday precision (%RSD) and accuracy (% bias) for ivermectin in human, monkey and mouse plasma.
| Level | Nominal concentration (ng/ml) | Intraday | Interday | ||
|---|---|---|---|---|---|
| % Bias | RSD (%) | % Bias | RSD (%) | ||
| Human plasma | |||||
| LLOQ | 0.1 | 6.59 | 5.59 | 3.33 | 12.25 |
| LQC | 0.5 | 10.60 | 7.76 | 12.40 | 6.92 |
| MQC | 200 | 0.64 | 2.31 | 0.98 | 11.60 |
| HQC | 750 | -7.87 | 8.15 | -7.95 | 5.64 |
| Monkey plasma | |||||
| LLOQ | 0.1 | -6.93 | 18.97 | 1.33 | 15.26 |
| LQC | 0.5 | 5.46 | 1.82 | -6.53 | 14.55 |
| MQC | 200 | -4.81 | 3.62 | 2.02 | 8.89 |
| HQC | 750 | -12.96 | 2.79 | -12.32 | 2.69 |
| Mouse plasma | |||||
| LLOQ | 0.1 | -6.51 | 10.35 | -8.67 | 13.73 |
| LQC | 0.5 | 10.92 | 8.52 | 8.80 | 7.08 |
| MQC | 200 | -2.21 | 1.70 | 2.02 | 7.33 |
| HQC | 750 | -6.14 | 6.46 | -3.88 | 9.48 |
HQC: High quality control; LQC: Lower quality control; MQC: Middle quality control; RSD: Relative standard deviation.
Recovery & matrix effect
The absolute mean recoveries are shown in Table 3 for IVM. Extraction recovery was >80% in all the three matrices. The mean recovery of IS was 46.2 ± 4.6. The matrix effect for both analytes at LQC, MQC and HQC concentrations levels in plasma were <± 15% (90.3–107.8%). Thus, no significant matrix effect was observed.
Table 3. . Mean extraction recoveries of the ivermectin from human, monkey and mouse plasma.
| Matrix | % Extraction recoveries (mean ± SD; n = 5) | ||
|---|---|---|---|
| LQC | MQC | HQC | |
| Human plasma | 83.9 ± 8.7 | 86.4 ± 4.1 | 86.4 ± 4.1 |
| Monkey plasma | 81.5 ± 8.3 | 84 ± 4.1 | 91.8 ± 5.4 |
| Mouse plasma | 80.2 ± 7.7 | 81.3 ± 7.2 | 84.8 ± 10.2 |
HQC: High quality control; LQC: Lower quality control; MQC: Middle quality control; SD: Standard deviation.
Dilution integrity
The precision for dilution integrity of 1:2 and 1:5 dilutions were within the acceptance limits of ± 15% for precision (CV) and 85.0–115.0% for accuracy.
Stability
Stability data for IVM are presented in Table 4. The IVM was stable in human plasma and found to be within ± 15% of the actual concentration at all QC concentrations.
Table 4. . Mean stability recoveries of the Ivermectin at different storage conditions in human plasma.
| Analyte | % Stability recoveries (mean ± SD) | |||
|---|---|---|---|---|
| Freeze-thaw (-80 ± 5°C after 3 cycle) | Long-term (-80 ± 5°C, 30 day) | Auto-sampler (4°C, 24 h) | Bench-top (20°C, 4 h) | |
| LQC | 112.9 ± 10.9 | 102.5 ± 3.1 | 92.5 ± 7.5 | 106.3 ± 8.8 |
| MQC | 92.5 ± 2.2 | 107.6 ± 0.8 | 103.4 ± 3.7 | 110.9 ± 2.3 |
| HQC | 108.5 ± 2.0 | 100.5 ± 7.3 | 104.5 ± 2.5 | 103.4 ± 9.0 |
HQC: High quality control; LQC: Lower quality control; MQC: Middle quality control; SD: Standard deviation.
Application of the liquid chromatography–tandem mass spectrometry & high-performance liquid chromatography-fluorescence method for clinical samples analysis
We compared IVM plasma concentrations measured by HPLC-FL and LC–MS/MS assay in samples from four patients receiving treatment of diethylcarbamazine-citrate (DEC-citrate) 6 mg/kg + ABZ 400 mg + IVM 200 μg/kg as part of a clinical trial. The study protocol and related documents were approved by institutional review boards in Cleveland, USA (University Hospitals Cleveland Medical Center IRB #03-16-09) and in Côte d'Ivoire (Comité National d'Ethique et de la Recherche, CNER, N/Ref:022/MSLS/CNER-kp) and the study registered at clinicaltrials.gov (NCT02845713). All participants provided written informed consent before they were enrolled in the study.
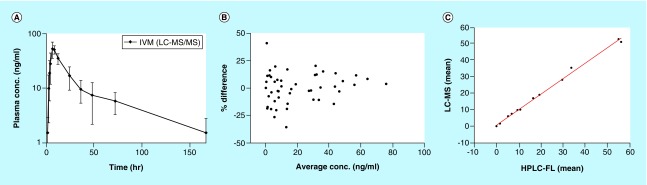
We randomly selected 52 clinical samples (from four volunteers,∼8% of total study samples) using our validated LC–MS/MS method for re-analysis (incurred sample analysis) [41,42]. The samples were previously analysed one year earlier using HPLC-FL method. The concentration time curve for IVM samples are shown (Figure 4A). We also evaluated the average concentration of IVM determined via HPLC-FL and LC–MS/MS assay and percent difference between the tests calculated and displayed using a Bland and Altman plot (Figure 4B). The percentage difference in concentration at all-time points (1 –168 h) were within ± 20% of the average value for 94% of the samples, except for three time points (Figure 4B) [41,42]. In addition, there was good linear correlation (r2 = 0.9956, with slope of 0.6824) obtained between concentrations for the two different assays (Figure 4C). The reanalyzed plasma IVM mean concentrations using LC–MS/MS were found to be within ± 15% of the initially reported value (Table 5). Thus, no significant differences were observed between the two assays. These results also reinforce the fact that additional drugs in actual patient samples such as albendazole or diethylcarbamazine do not cause any interference or alteration of results as there was good agreement between both assays. The Wilcoxon signed rank test showed no significant difference between concentrations measured by two assays. These analyses indicate that the two methods yield very similar results.
Figure 4. . Plasma concentration and assay performance plots.
(A) Plasma concentration-time profile of IVM in human volunteers (mean ± standard deviation, n = 4) determined by LC–MS/MS. (B) Bland and Altman plot showing relation between average concentrations and percent difference (zero is line of no difference and ± 20% was difference limit). (C) Correlation between the measurements between LC–MS/MS and HPLC-FL assay.
IVM: Ivermectin.
Table 5. . Human plasma concentration of ivermectin via Liquid chromatography–tandem mass spectrometry and high-performance liquid chromatography-fluorescence method in clinical study samples. Each value represent mean + standard deviation (n = 4).
| Time (h) | LC–MS/MS after long-term storage (-80 ± 5°C; 1 year) | HPLC-FL | ||
|---|---|---|---|---|
| Mean concentration (ng/ml) | SD | Mean concentration (ng/ml) | SD | |
| 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 1.5 | 1.4 | 1.4 | 1.4 |
| 2 | 9.9 | 7.6 | 10.7 | 8.1 |
| 3 | 19.0 | 13.1 | 19.3 | 14.4 |
| 4 | 28.1 | 16.2 | 29.5 | 16.0 |
| 6 | 52.6 | 17.2 | 54.7 | 16.8 |
| 8 | 50.9 | 8.8 | 56.0 | 9.1 |
| 12 | 35.3 | 7.7 | 33.6 | 4.8 |
| 24 | 17.0 | 7.7 | 16.4 | 7.4 |
| 36 | 9.6 | 4.3 | 9.4 | 4.1 |
| 48 | 7.4 | 5.2 | 6.8 | 3.2 |
| 72 | 5.9 | 2.5 | 5.2 | 2.1 |
| 168 | 1.5 | 1.3 | 1.6 | 1.4 |
HPLC-FL: High-performance liquid chromatography-fluorescence; SD: Standard deviation.
Conclusion
A LC–MS/MS method for the determination of IVM concentrations in mouse, monkey and human plasma was developed and validated over the concentration range of 0.1–1000 ng/ml. This method offers significant advantages in terms of: simplicity of analysis as it does not require derivitaztion; absence of carry over; absence of matrix effect and validation over a large dynamic range (calibrators from 0.1–1000 ng/ml). The established LLOQ of 0.1 ng/ml was sufficiently low for pharmacokinetic studies and only required 200 μl of plasma. This method was successfully applied for re-analysis of clinical samples. The reanalyzed plasma IVM mean concentrations were found to be within ± 15% of the initially reported value and found stable for 1 year at least. Therefore, the current LC–MS/MS method provides a valuable tool to improve the efficacy and safety of IVM therapy. This method will therefore be useful for evaluating the disposition of IVM and relation of plasma concentration to effect in future preclinical and clinical studies.
Future perspective
Ivermectin is approved for the control and treatment of human and animal onchocerciasis, LF, strongyloidiasis and scabies. The WHO has targeted LF as a public health problem to be globally eliminated by 2020. These efforts have been based on a mass drug administration approach utilizing antifilarial drug regimens including diethylcarbamazine combined with albendazole and IVM. Efficacy rates of IVM are directly correlated with patient adherence rates to antifilarial medications. Routine analysis of IVM within biological samples will continue to provide clinicians and researchers an objective measure to assess the efficacy of these antifilarial treatments. Our method provides a robust, sensitive and specific approach for the bioanalysis of IVM. Additional agents to further improve treatment of LF and additional studies focusing on the development of analogues (i.e., abamectin, dorametin, eprinomecting, emamectin and moxidectin) should be a priority.
Summary points.
Background
We developed a novel and sensitive LC–MS/MS method for ivermectin (IVM) in plasma.
Methods
The IVM was extracted from plasma using solid-phase extraction and quantitated using electrospray ionization operating in negative mode.
Results & conclusion
The method was linear over the concentration range from 0.1–1000 ng/ml. The LC–MS/MS method is sensitive, reproducible, has easy sample preparation and is suitable for IVM quantitation in clinical samples.
Footnotes
Financial & competing interests disclosure
The work was supported in part by the Bill and Melinda Gates Foundation, the University of Nebraska Medical Center and by the Fred & Pamela Buffett Cancer Center Support Grant from the National Cancer Institute under award number P30 CA036727. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. C Edi received partial support from Case Western Reserve and support from the Wellcome Trust under the grant 110430/Z/15/Z. We also acknowledge the helpful discussions with RJ Classon at Shimadzu Scientific Instruments, MD, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995;59(2):139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- 2.Moreno L, Dominguez P, Farias C, et al. Ivermectin pharmacokinetics, metabolism and tissue/egg residue profiles in laying hens. J. Agric. Food. Chem. 2015;63(47):10327–10332. doi: 10.1021/acs.jafc.5b04632. [DOI] [PubMed] [Google Scholar]
- 3.Laffont CM, Toutain P-L, Alvinerie M, Bousquet-Mélou A. Intestinal secretion is a major route for parent ivermectin elimination in the rat. Drug Metab. Dispos. 2002;30(6):626–630. doi: 10.1124/dmd.30.6.626. [DOI] [PubMed] [Google Scholar]
- 4.Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar. J. 2017;16(1):161. doi: 10.1186/s12936-017-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merck & Co.; NJ, USA: Stromectol, TGA-Australia approved package insert. [Google Scholar]
- 6.Kose LP, Gülçin İ, Özdemir H, Atasever A, Alwasel SH, Supuran CT. The effects of some avermectins on bovine carbonic anhydrase enzyme. J. Enzyme Inhib. Med. Chem. 2016;31(5):773–778. doi: 10.3109/14756366.2015.1064406. [DOI] [PubMed] [Google Scholar]
- 7.Cuneyt C, İlhami G. The toxicological effects of some avermectins on goat liver carbonic anhydrase enzyme. J. Biochem. Mol. Toxicol. 2018;32(1):e22010. doi: 10.1002/jbt.22010. [DOI] [PubMed] [Google Scholar]
- 8.Köksal Z, Kalın R, Gülçin İ, Özdemir H, Atasever A. Impact of some avermectins on lactoperoxidase in bovine milk. Int. J. Food Prop. 2016;19(6):1207–1216. [Google Scholar]
- 9.Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376(9747):1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 10.Lifschitz A, Virkel G, Pis A, et al. Ivermectin disposition kinetics after subcutaneous and intramuscular administration of an oil-based formulation to cattle. Vet. Parasitol. 1999;86(3):203–215. doi: 10.1016/s0304-4017(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 11.Mckellar QA, Benchaoui HA. Avermectins and milbemycins. J. Vet. Pharmacol. Ther. 1996;19(5):331–351. doi: 10.1111/j.1365-2885.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 12.Turner SA, MacLean JD, Fleckenstein L, Greenaway C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am. J. Trop. Med. Hyg. 2005;73(5):911–914. [PubMed] [Google Scholar]
- 13.Thomsen EK, Sanuku N, Baea M, et al. Efficacy, safety and pharmacokinetics of coadministered diethylcarbamazine, albendazole and Ivermectin for treatment of bancroftian filariasis. Clin. Infect. Dis. 2016;62(3):334–341. doi: 10.1093/cid/civ882. [DOI] [PubMed] [Google Scholar]; • Efficacy, safety and pharmacokinetics of combination drug therapy including ivermectin (IVM).
- 14.Leung V, Al-Rawahi GN, Grant J, Fleckenstein L, Bowie W. Failure of subcutaneous ivermectin in treating strongyloides hyperinfection. Am J. Trop. Med. Hyg. 2008;79(6):853–855. [PubMed] [Google Scholar]
- 15.Pérez R, Godoy C, Palma C, et al. Plasma profiles of ivermectin in horses following oral or intramuscular administration. J. Vet. Med. A. 2003;50(6):297–302. doi: 10.1046/j.1439-0442.2003.00531.x. [DOI] [PubMed] [Google Scholar]
- 16.Chiodini PL, Reid AJC, Wiselka MJ, Firmin R, Foweraker J. Parenteral ivermectin in strongyloides hyperinfection. Lancet. 2000;355(9197):43–44. doi: 10.1016/s0140-6736(99)02744-0. [DOI] [PubMed] [Google Scholar]
- 17.Marty FM, Lowry CM, Rodriguez M, et al. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clin. Infect. Dis. 2005;41(1):e5–e8. doi: 10.1086/430827. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Z, Andrew NW, Arison BH, Luffer-Atlas D, Wang RW. Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica. 1998;28(3):313–321. doi: 10.1080/004982598239597. [DOI] [PubMed] [Google Scholar]
- 19.Lespine A, Ménez C, Bourguinat C, Prichard RK. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: prospects for reversing transport-dependent anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2012;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokbulut C, Bilgili A, Hanedan B, Aksit D, Aksoy AM, Turgut C. Breed-related plasma disposition of ivermectin following subcutaneous administration in kilis and damascus goats. Res. Vet. Sci. 2009;87(3):445–448. doi: 10.1016/j.rvsc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Ricart Arbona RJ, Lipman NS, Riedel ER, Wolf FR. Treatment and eradication of murine fur mites: I. toxicologic evaluation of ivermectin-compounded feed. J. Am. Assoc. Lab. Anim. Sci. 2010;49(5):564–570. [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett DC, Cheng KM. Ivermectin residues in squab. Poult. Sci. 2012;91(11):2808–2811. doi: 10.3382/ps.2012-02529. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman D, Wei S-Y, Fleckenstein L. Liquid chromatographic assay of ivermectin in human plasma for application to clinical pharmacokinetic studies. J. Pharm. Biomed. Anal. 2006;40(4):1013–1020. doi: 10.1016/j.jpba.2005.08.026. [DOI] [PubMed] [Google Scholar]; • HPLC-FL assay of IVM in human plasma.
- 24.Macedo F, Marsico ET, Conte-Júnior CA, De Resende MF, Brasil TF, Pereira Netto AD. Development and validation of a method for the determination of low-ppb levels of macrocyclic lactones in butter, using HPLC-fluorescence. Food Chem. 2015;179(Supplement C):239–245. doi: 10.1016/j.foodchem.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Åsbakk K, Bendiksen HR, Oksanen A. Ivermectin in reindeer feces: determination by HPLC. J. Agric. Food. Chem. 1999;47(3):999–1003. doi: 10.1021/jf980919q. [DOI] [PubMed] [Google Scholar]
- 26.Payne LD, Hicks MB, Wehner TA. Determination of abamectin and/or ivermectin in cattle feces at low parts per billion levels using HPLC with fluorescence detection. J. Agric. Food. Chem. 1995;43(5):1233–1237. [Google Scholar]
- 27.Sanbonsuge A, Takase T, Shiho D-I, Takagai Y. Gas chromatography-mass spectrometric determination of ivermectin following trimethylsilylation with application to residue analysis in biological meat tissue samples. Anal. Methods. 2011;3(9):2160–2164. [Google Scholar]
- 28.Rübensam G, Barreto F, Hoff RB, Pizzolato TM. Determination of avermectin and milbemycin residues in bovine muscle by liquid chromatography-tandem mass spectrometry and fluorescence detection using solvent extraction and low temperature cleanup. Food Control. 2013;29(1):55–60. [Google Scholar]
- 29.Croubels S, De Baere S, Cherlet M, De Backer P. Determination of ivermectin B1a in animal plasma by liquid chromatography combined with electrospray ionization mass spectrometry. J. Mass Spectrom. 2002;37(8):840–847. doi: 10.1002/jms.343. [DOI] [PubMed] [Google Scholar]; • LC–MS/MS assay of IVM in animal plasma.
- 30.Dahiya M, Dubey N, Singh P, Singh GN. Development and validation of LC–MS/MS method to determine the residue of veterinary drugs ivermectin, doramectin and moxidectin in milk. Indian J. Chem. 2013;52:1313–1317. [Google Scholar]
- 31.Huang J-X, Lu D-H, Wan K, Wang F-H. Low temperature purification method for the determination of abamectin and ivermectin in edible oils by liquid chromatography–tandem mass spectrometry. Chin. Chem. Lett. 2014;25(4):635–639. [Google Scholar]
- 32.Krogh KA, Björklund E, Loeffler D, Fink G, Halling-Sørensen B, Ternes TA. Development of an analytical method to determine avermectins in water, sediments and soils using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2008;1211(1):60–69. doi: 10.1016/j.chroma.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz AJ, Cortez V, Azzouz A, Verdú JR. Isolation and determination of ivermectin in postmortem and in vivo tissues of dung beetles using a continuous solidphase extraction method followed by LC-ESI+-MS/MS. PLoS ONE. 2017;12(2):e0172202. doi: 10.1371/journal.pone.0172202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howells L, Sauer MJ. Multiresidue analysis of avermectins and moxidectin by ion-trap LC-MS. Analyst. 2001;126(2):155–160. doi: 10.1039/b008305o. [DOI] [PubMed] [Google Scholar]
- 35.Lehner AF, Petzinger E, Stewart J, et al. ESI+ MS/MS confirmation of canine ivermectin toxicity. J. Mass Spectrom. 2009;44(1):111–119. doi: 10.1002/jms.1477. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Li X, Zhang L, et al. Mesoporous alumina as a solid-phase extraction adsorbent for the determination of abamectin and ivermectin in vegetables by liquid chromatography-tandem mass spectrometry. Anal. Methods. 2014;6(13):4734–4741. [Google Scholar]
- 37.Cheng C, Liu L-C. On-line solid-phase extraction coupled liquid chromatography-ESI-ion trap-mass spectrometry for analysis of abamectin and ivermectin residues in milk. Anal. Methods. 2014;6(5):1581–1589. [Google Scholar]
- 38.US FDA. Center for Drug Evaluation and Research, US Department of Health and Human Services; MD, USA: 2013. Guidance for industry – bioanalytical method validation.http://academy.gmp-compliance.org/guidemgr/files/UCM368107.PDF [Google Scholar]
- 39.US FDA. Center for Drug Evaluation and Research, US Department for Health and Human Services, Center for Veterinary Medicine; MD, USA: 2018. Guidance for industry-bioanalytical method validation.https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf [Google Scholar]
- 40.Chhonker YS, Edi C, Murry DJ. LC–MS/MS method for simultaneous determination of diethylcarbamazine, albendazole and albendazole metabolites in human plasma: application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2018;151:84–90. doi: 10.1016/j.jpba.2017.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 42.Fluhler E, Vazvaei F, Singhal P, et al. Repeat analysis and incurred sample reanalysis: recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J. 2014;16(6):1167–1174. doi: 10.1208/s12248-014-9644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]