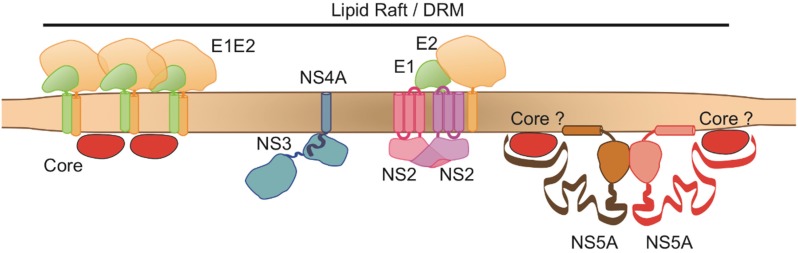
Figure 6.
Model of the interactions of HCV proteins and their localization at the proposed viral assembly platform. Our experiments characterized associations of HCV proteins with two different DRM subdomains: a stable domain (Brown region) associated with NS2, NS3 (NS4A), AR3A-E2, and A4-E1, and a less stable domain (light brown region) accommodating NS5A, core, and AR5A-E1E2. One of the first steps in the process is the recruitment by NS2 of glycoproteins (E1, recognized by A4, and E2, recognized by AR3A) to stable, raft-like structures through direct protein–protein interactions. NS2 simultaneously recruits NS3-4A to these same structures through an indirect DRM-driven interaction. NS5A as well as core associate with NS2 through unstable, possibly outlying, regions of DRMs.