Abstract
Based on our miRNA expression signatures, we focused on miR-150-5p (the guide strand) and miR-150-3p (the passenger strand) to investigate their functional significance in lung adenocarcinoma (LUAD). Downregulation of miR-150 duplex was confirmed in LUAD clinical specimens. In vitro assays revealed that ectopic expression of miR-150-5p and miR-150-3p inhibited cancer cell malignancy. We performed genome-wide gene expression analyses and in silico database searches to identify their oncogenic targets in LUAD cells. A total of 41 and 26 genes were identified as miR-150-5p and miR-150-3p targets, respectively, and they were closely involved in LUAD pathogenesis. Among the targets, we investigated the oncogenic roles of tensin 4 (TNS4) because high expression of TNS4 was strongly related to poorer prognosis of LUAD patients (disease-free survival: p = 0.0213 and overall survival: p = 0.0003). Expression of TNS4 was directly regulated by miR-150-3p in LUAD cells. Aberrant expression of TNS4 was detected in LUAD clinical specimens and its aberrant expression increased the aggressiveness of LUAD cells. Furthermore, we identified genes downstream from TNS4 that were associated with critical regulators of genomic stability. Our approach (discovery of anti-tumor miRNAs and their target RNAs for LUAD) will contribute to the elucidation of molecular networks involved in the malignant transformation of LUAD.
Keywords: MicroRNA, miR-150-5p, miR-150-3p, lung adenocarcinoma, TNS4
1. Introduction
Lung cancer accounts for the largest number of cancer-related deaths in the world and its morbidity increases annually [1]. Lung adenocarcinoma (LUAD) is the most common histological subtype of non-small cell lung cancer (NSCLC) and this type of cancer is the leading cause of cancer-related deaths [2]. The survival rate of lung cancer has increased due to effective treatment strategies, including molecularly-targeted drugs and immune checkpoint inhibitors [3]. However, the efficacy of treatments for patients with distant metastases is limited, thereby resulting in poor prognosis [2]. In LUAD, metastasis occurs even if the primary tumor is small [4,5]. Therefore, the prognosis of advanced LUAD patients is poor, with average 5-year survival rates below 20% [6]. Therefore, additional research to identify novel biomarkers for earlier detection and to develop effective targeted molecular therapies for LUAD is indispensable.
A vast number of studies have revealed that an extremely large number of non-coding RNAs are transcribed from the human genome and actually functional in various cellular processes. Among these non-coding RNAs, microRNAs (miRNAs) are endogenous single-stranded RNA molecules (19–22 nucleotides in length) that function as fine-tuners of RNA expression [7,8,9,10,11]. Importantly, a single miRNA can control a large number of RNA transcripts in normal and disease cells [7,8,9,10,11]. Recent studies showed that aberrant expression of miRNAs closely contributes to the pathogenesis of human diseases, including cancers via disruption of RNA networks [8,9,10,11,12,13].
We have been analyzing novel anti-tumor miRNA-mediated oncogenic targets and pathways that contribute to lung tumorigenesis [14,15,16,17,18,19]. Interestingly, our miRNA studies revealed that some passenger strands of miRNAs (e.g., miR-144-5p and miR-145-3p) derived from miRNA-duplex actually acted as anti-tumor miRNAs in lung squamous cell carcinoma [15,19]. More recently, we demonstrated that two miRNA species derived from miR-145-duplex (miR-145-5p and miR-145-3p) acted as anti-tumor miRNAs in LUAD via targeting several oncogenes [17].
Initially, it was thought that two types of miRNA were derived from double-stranded pre-miRNAs: guide strand miRNAs that control the target genes, and passenger strand miRNAs that lacked function and were degraded [20]. Our studies have altered the conventional understanding of miRNA biogenesis and have shown the importance of exploring passenger strands of miRNAs in cancer cells.
RNA sequencing-based miRNA expression signatures contribute to a new development of molecular pathogenesis of human cancers. miRNA expression signatures in human cancers revealed that two miRNAs derived from miR-150-duplex—miR-150-5p (the guide strand) and miR-150-3p (the passenger strand)—are frequently downregulated in several types of cancers [21,22,23]. Reports of miR-150-5p in cancers are scattered across public databases, but there are few reports of miR-150-3p. We have analyzed the anti-tumor activity of miR-150-3p in esophageal and head and neck squamous cell carcinomas [22,23]. With regard to LUAD, no such reports are available, and this is an important facet of the functional analysis of miR-150-3p and the search for target genes.
The aim of this study was to investigate the anti-tumor roles of both strands of miR-150-duplex and to identify their targets with close associations with LUAD tumorigenesis. The Cancer Genome Atlas (TCGA) revealed that low expression of miR-150-5p and miR-150-3p predicted poor prognosis. Ectopic expression of two miRNAs significantly attenuated the malignant phenotypes of cancer cells. Moreover, we identified several oncogenic targets by miR-150-3p regulation in LUAD cells.
2. Results
2.1. miR-150-5p and miR-150-3p were Downregulated in Lung Adenocarcinoma (LUAD) Specimens and Cell Lines
To explore whether miR-150-5p and miR-150-3p are downregulated in LUAD, expression levels of miR-150-5p and miR-150-3p in clinical specimens were measured. The characteristics of the patients are recorded in Table 1.
Table 1.
Characteristics of lung cancer and non-cancerous cases.
| A. Characteristics of the Lung Cancer Cases | ||
| Lung Cancer Patients | n | (%) |
| Total number | 18 | |
| Median age (range) | 73.5 (59–86) | |
| Gender | ||
| Male | 9 | 50.0 |
| Female | 9 | 50.0 |
| Pathological stage: | ||
| IA | 1 | 5.6 |
| IB | 4 | 22.2 |
| IIA | 8 | 44.4 |
| IIB | 1 | 5.6 |
| IIIA | 4 | 22.2 |
| IIIB | 0 | 0.0 |
| B. Characteristics of the Non-Cancerous Cases | ||
| Non-Cancerous Tissues | n | (%) |
| Total number | 28 | |
| Median age (range) | 71 (50–88) | |
| Gender: | ||
| Male | 25 | 89.3 |
| Female | 3 | 10.7 |
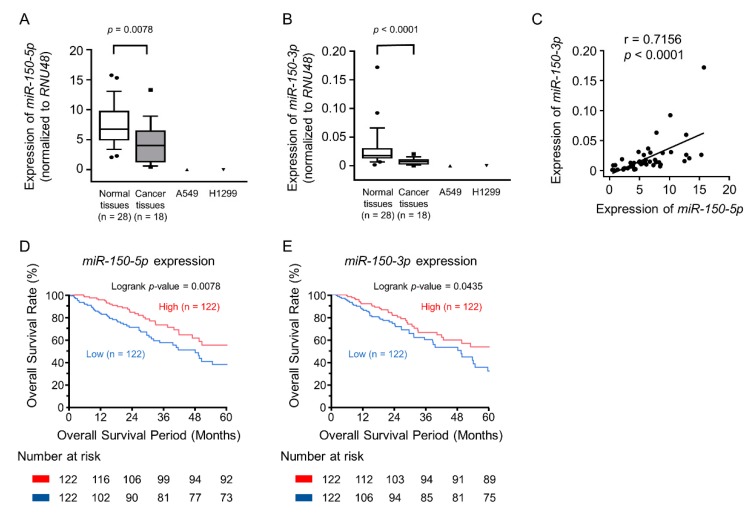
We found that expression of the miR-150 duplex was significantly decreased in LUAD specimens and LUAD cell lines relative to non-cancerous tissues (p = 0.0078 and p < 0.0001, respectively, Figure 1A,B). There were positive correlations between the expression levels of miR-150-5p and miR-150-3p by Spearman’s rank test (r = 0.7156 and p < 0.0001, Figure 1C). According to the Kaplan–Meier overall survival curves using TCGA database, we found that the low expression levels of miR-150-5p and miR-150-3p were associated with poor prognosis in LUAD patients (overall survival, p = 0.0078 and p = 0.0435, respectively, Figure 1D,E).
Figure 1.
The clinical significance of miR-150-5p and miR-150-3p expression in lung adenocarcinoma (LUAD). (A,B) Downregulation of miR-150-5p and miR-150-3p expression in clinical specimens of LUAD and two cell lines (A549 and H1299). Expression of RNU48 was used as an internal control. (C) Expression of two miRNAs derived from miR-150-duplex were positive correlation by Spearman’s rank test. (D,E) The Kaplan–Meier overall survival curve analyses of patients with LUAD by The Cancer Genome Atlas (TCGA) database. Patients were divided into two groups according to miRNA expression and analyzed.
2.2. Overexpression of miR-150-5p and miR-150-3p Inhibits Cancer Cell Aggressiveness
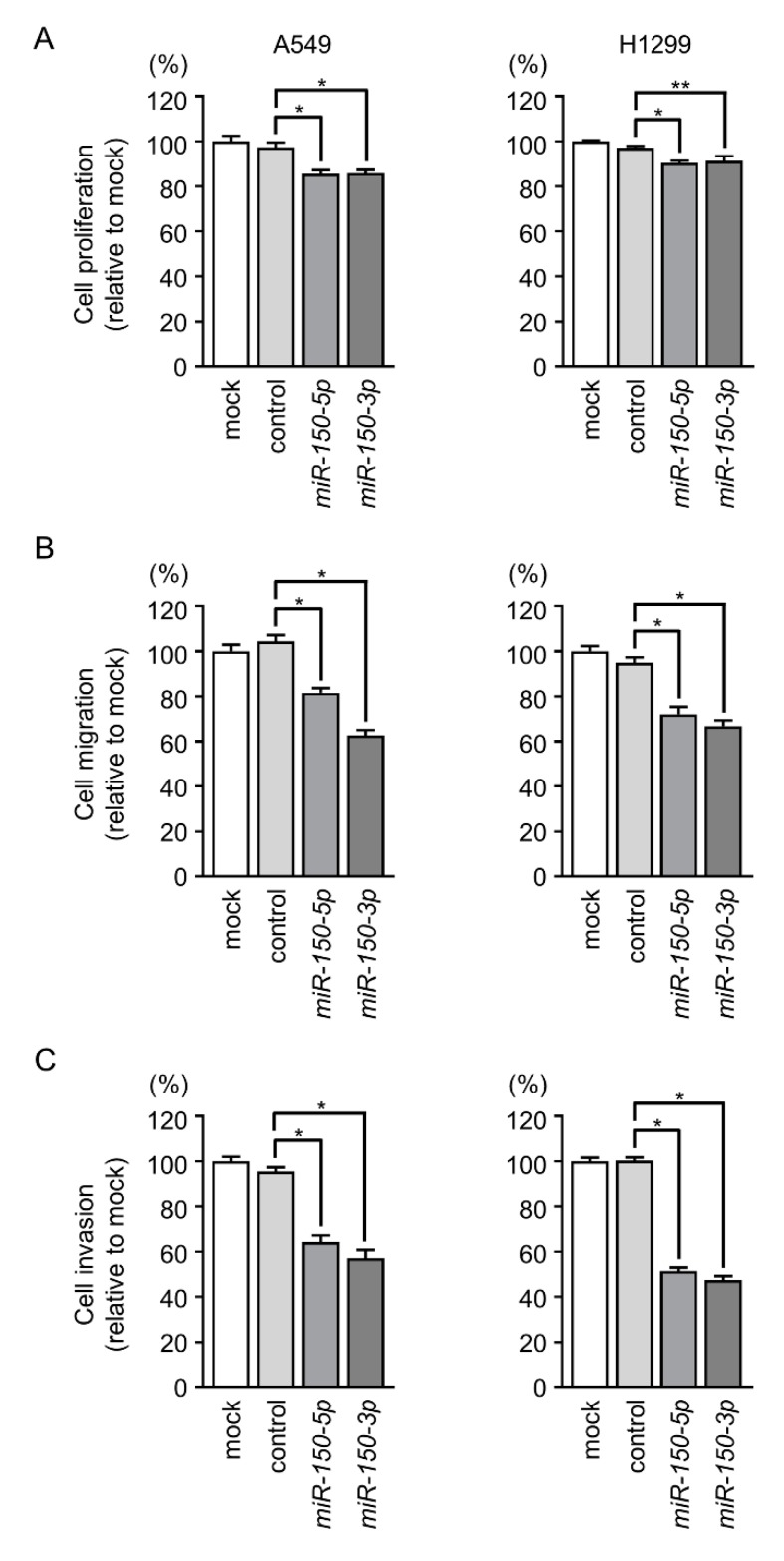
To investigate the biological functions of miR-150-5p and miR-150-3p in LUAD, we performed gain-of-function assays using miRNA transfection into LUAD cell lines (A549 and H1299). Cell proliferation assays showed that miR-150-5p- and miR-150-3p- transfected LUAD cells had reduced cell growth compared with mock- or miR-control-transfected LUAD cells (Figure 2A). Also, cell migratory and invasive abilities were markedly decreased in the LUAD cells transfected with miR-150-5p and miR-150-3p (Figure 2B,C).
Figure 2.
Functional assays of miR-150-5p and miR-150-3p in LUAD cells (A549 and H1299). (A–C) Cell proliferation, migration, and invasive activities were significantly blocked by ectopic expression of miR-150-5p or miR-150-3p. * p < 0.01, ** p < 0.05.
Furthermore, we performed functional analysis by reducing the concentration of miR-150-3p (1 nM and 0.1 nM) transfection into LUAD cells. Our data showed that antitumor functions (inhibition of cancer cell proliferation, migration, and invasion) were observed at 1 nM concentration, although there were no antitumor functions at 0.1 nM concentration on A549 and H1299 cells (Figure S1).
2.3. Incorporation of miR-150-5p and miR-150-3p into the RNA-induced silencing complex (RISC) in LUAD Cells
We next performed immunoprecipitation with antibodies targeting Ago2, which plays a pivotal role in the uptake of miRNAs into the RISC (Figure S2A). After transfection of A549 cells with miR-150-5p and immunoprecipitation by anti-Ago2 antibodies, miR-150-5p levels in the immunoprecipitates were significantly higher than those of mock- or miR-control-transfected cells and those of miR-150-3p-transfected cells (p < 0.01; Figure S2B–D). Similarly, after miR-150-3p transfection (10 nM, 1 nM, and 0.1 nM), substantial amounts of miR-150-3p were detected in Ago2 immunoprecipitates (p < 0.01; Figure S2B–D).
2.4. Candidate Target Genes of miR-150-5p and miR-150-3p Regulation in LUAD: Clinical Significance of TNS4, SFXN1, SKA3, and SPOCK1 Expression
Our selection strategy of miR-150-5p- and miR-150-3p-regulated oncogenic targets is shown in Figure S3A,B. A total of 41 and 26 oncogenic targets regulated by miR-150-5p and miR-150-3p were identified in LUAD cells (Table 2 and Table 3).
Table 2.
Candidate target genes regulated by miR-150-5p.
| Entrez Gene | Gene Symbol | Gene Name | Total Sites | A549 miR-150-5p Transfectant FC (log2) | GSE19188 FC (log2) | TCGA OncoLnc p-Value |
|---|---|---|---|---|---|---|
| 4751 | NEK2 | NIMA-related kinase 2 | 1 | −0.565 | 3.323 | <0.0001 |
| 64065 | PERP | PERP, TP53 apoptosis effector | 3 | −3.012 | 1.835 | <0.0001 |
| 5122 | PCSK1 | Proprotein convertase subtilisin/kexin type 1 | 1 | −0.680 | 2.532 | 0.0006 |
| 84985 | FAM83A | Family with sequence similarity 83, member A | 1 | −0.717 | 3.188 | 0.0010 |
| 1033 | CDKN3 | Cyclin-dependent kinase inhibitor 3 | 2 | −0.582 | 2.889 | 0.0011 |
| 6241 | RRM2 | Ribonucleotide reductase M2 | 3 | −2.433 | 3.000 | 0.0013 |
| 79801 | SHCBP1 | SHC SH2-domain binding protein 1 | 1 | −1.118 | 1.841 | 0.0015 |
| 29127 | RACGAP1 | Rac GTPase activating protein 1 | 2 | −0.545 | 1.677 | 0.0019 |
| 24137 | KIF4A | Kinesin family member 4A | 1 | −0.933 | 3.309 | 0.0030 |
| 6695 | SPOCK1 | Pparc/osteonectin, cwcv and kazal-like domains proteoglycan (testican) 1 | 1 | −0.764 | 1.696 | 0.0031 |
| 9837 | GINS1 | GINS complex subunit 1 (Psf1 homolog) | 2 | −0.749 | 2.991 | 0.0076 |
| 339761 | CYP27C1 | Cytochrome P450, family 27, subfamily C, polypeptide 1 | 4 | −2.360 | 1.706 | 0.0126 |
| 10331 | B3GNT3 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 3 | 2 | −0.939 | 1.608 | 0.0129 |
| 79962 | DNAJC22 | DnaJ (Hsp40) homolog, subfamily C, member 22 | 5 | −0.908 | 2.031 | 0.0201 |
| 10635 | RAD51AP1 | RAD51-associated protein 1 | 1 | −0.611 | 2.470 | 0.0228 |
| 10797 | MTHFD2 | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2, methenyltetrahydrofolate cyclohydrolase | 1 | −1.905 | 1.887 | 0.0236 |
| 9699 | RIMS2 | Regulating synaptic membrane exocytosis 2 | 2 | −0.697 | 1.976 | 0.0265 |
| 1058 | CENPA | Centromere protein A | 1 | −1.019 | 3.488 | 0.0365 |
| 23657 | SLC7A11 | Solute carrier family 7 (anionic amino acid transporter light chain, xc- system), member 11 | 3 | −1.093 | 2.014 | 0.0474 |
| 3755 | KCNG1 | Potassium voltage-gated channel, subfamily G, member 1 | 5 | −1.069 | 1.887 | 0.0557 |
| 1825 | DSC3 | Desmocollin 3 | 1 | −1.247 | 2.488 | 0.0667 |
| 388228 | SBK1 | SH3 domain binding kinase 1 | 1 | −0.658 | 1.745 | 0.0939 |
| 8038 | ADAM12 | ADAM metallopeptidase domain 12 | 2 | −0.668 | 2.753 | 0.1097 |
| 84733 | CBX2 | Chromobox homolog 2 | 1 | −0.591 | 1.988 | 0.1116 |
| 130827 | TMEM182 | Transmembrane protein 182 | 1 | −0.690 | 1.568 | 0.1189 |
| 92312 | MEX3A | mex-3 RNA binding family member A | 3 | −0.584 | 1.986 | 0.1271 |
| 4323 | MMP14 | Matrix metallopeptidase 14 | 3 | −0.621 | 1.872 | 0.1296 |
| 4151 | MB | myoglobin | 3 | −1.119 | 1.614 | 0.1346 |
| 57167 | SALL4 | Sal-like 4 (Drosophila) | 1 | −0.582 | 2.836 | 0.2382 |
| 256714 | MAP7D2 | MAP7 domain containing 2 | 1 | −1.449 | 2.007 | 0.2798 |
| 6273 | S100A2 | S100 calcium binding protein A2 | 1 | −0.643 | 2.513 | 0.3132 |
| 200844 | C3orf67 | Chromosome 3 open reading frame 67 | 1 | −0.514 | 1.584 | 0.3482 |
| 1690 | COCH | Cochlin | 2 | −0.592 | 3.406 | 0.3696 |
| 10447 | FAM3C | Family with sequence similarity 3, member C | 1 | −1.570 | 1.536 | 0.3847 |
| 147920 | IGFL2 | IGF-like family member 2 | 1 | −0.719 | 2.569 | 0.4596 |
| 547 | KIF1A | Kinesin family member 1A | 4 | −0.733 | 2.518 | 0.6354 |
| 9066 | SYT7 | Synaptotagmin VII | 2 | −0.921 | 1.730 | 0.7141 |
| 55220 | KLHDC8A | Kelch domain containing 8A | 4 | −1.168 | 1.709 | 0.7484 |
| 440590 | ZYG11A | Zyg-11 family member A, cell cycle regulator | 2 | −1.619 | 1.826 | 0.7530 |
| 3141 | HLCS | Holocarboxylase synthetase | 1 | −0.897 | 1.791 | 0.8947 |
| 9547 | CXCL14 | Chemokine (C-X-C motif) ligand 14 | 2 | −0.750 | 1.800 | 0.9229 |
Table 3.
Candidate target genes regulated by miR-150-3p.
| Entrez Gene | Gene Symbol | Gene Name | Total Sites | A549 miR-150-3p Transfectant FC (log2) | GSE19188 FC (log2) | TCGA OncoLnc p-Value |
|---|---|---|---|---|---|---|
| 84951 | TNS4 | Tensin 4 | 1 | −1.318 | 2.560 | 0.0003 |
| 94081 | SFXN1 | Sideroflexin 1 | 1 | −1.307 | 1.404 | 0.0020 |
| 221150 | SKA3 | Spindle and kinetochore-associated complex subunit 3 | 1 | −1.006 | 2.015 | 0.0027 |
| 6695 | SPOCK1 | Sparc/osteonectin, cwcv and kazal-like domains proteoglycan (testican) 1 | 3 | −2.040 | 1.696 | 0.0031 |
| 89874 | SLC25A21 | Solute carrier family 25 (mitochondrial oxoadipate carrier), member 21 | 2 | −2.557 | 1.358 | 0.0149 |
| 94032 | CAMK2N2 | Calcium/calmodulin-dependent protein kinase II inhibitor 2 | 1 | −1.303 | 1.538 | 0.0208 |
| 5738 | PTGFRN | Prostaglandin F2 receptor inhibitor | 2 | −1.750 | 1.237 | 0.0291 |
| 23105 | FSTL4 | Follistatin-like 4 | 1 | −1.602 | 1.485 | 0.0589 |
| 130574 | LYPD6 | LY6/PLAUR domain containing 6 | 1 | −1.004 | 1.917 | 0.0591 |
| 150223 | YDJC | YdjC homolog (bacterial) | 1 | −1.592 | 1.088 | 0.0699 |
| 3174 | HNF4G | Hepatocyte nuclear factor 4, gamma | 1 | −1.827 | 1.709 | 0.0717 |
| 6857 | SYT1 | Synaptotagmin I | 1 | −2.264 | 2.058 | 0.0735 |
| 5522 | PPP2R2C | Protein phosphatase 2, regulatory subunit B, gamma | 2 | −1.004 | 2.491 | 0.1067 |
| 8038 | ADAM12 | ADAM metallopeptidase domain 12 | 2 | −1.555 | 2.753 | 0.1097 |
| 84216 | TMEM117 | Transmembrane protein 117 | 1 | −3.167 | 1.049 | 0.1894 |
| 55753 | OGDHL | Oxoglutarate dehydrogenase-like | 1 | −1.896 | 1.776 | 0.3167 |
| 79944 | L2HGDH | L-2-hydroxyglutarate dehydrogenase | 1 | −1.921 | 1.730 | 0.3221 |
| 79776 | ZFHX4 | Zinc finger homeobox 4 | 1 | −2.113 | 1.248 | 0.3578 |
| 4647 | MYO7A | Myosin VIIA | 1 | −1.193 | 1.047 | 0.4080 |
| 23321 | TRIM2 | Tripartite motif containing 2 | 2 | −1.550 | 1.755 | 0.4560 |
| 401474 | SAMD12 | Sterile alpha motif domain containing 12 | 1 | −1.950 | 1.027 | 0.4853 |
| 145282 | MIPOL1 | Mirror-image polydactyly 1 | 1 | −1.401 | 1.108 | 0.5861 |
| 8821 | INPP4B | Inositol polyphosphate-4-phosphatase, type II, 105kDa | 3 | −1.412 | 1.414 | 0.6077 |
| 80310 | PDGFD | Platelet-derived growth factor D | 1 | −1.555 | 1.142 | 0.6859 |
| 9802 | DAZAP2 | DAZ-associated protein 2 | 2 | −1.439 | 1.144 | 0.8241 |
| 85439 | STON2 | Stonin 2 | 1 | −1.371 | 1.079 | 0.9773 |
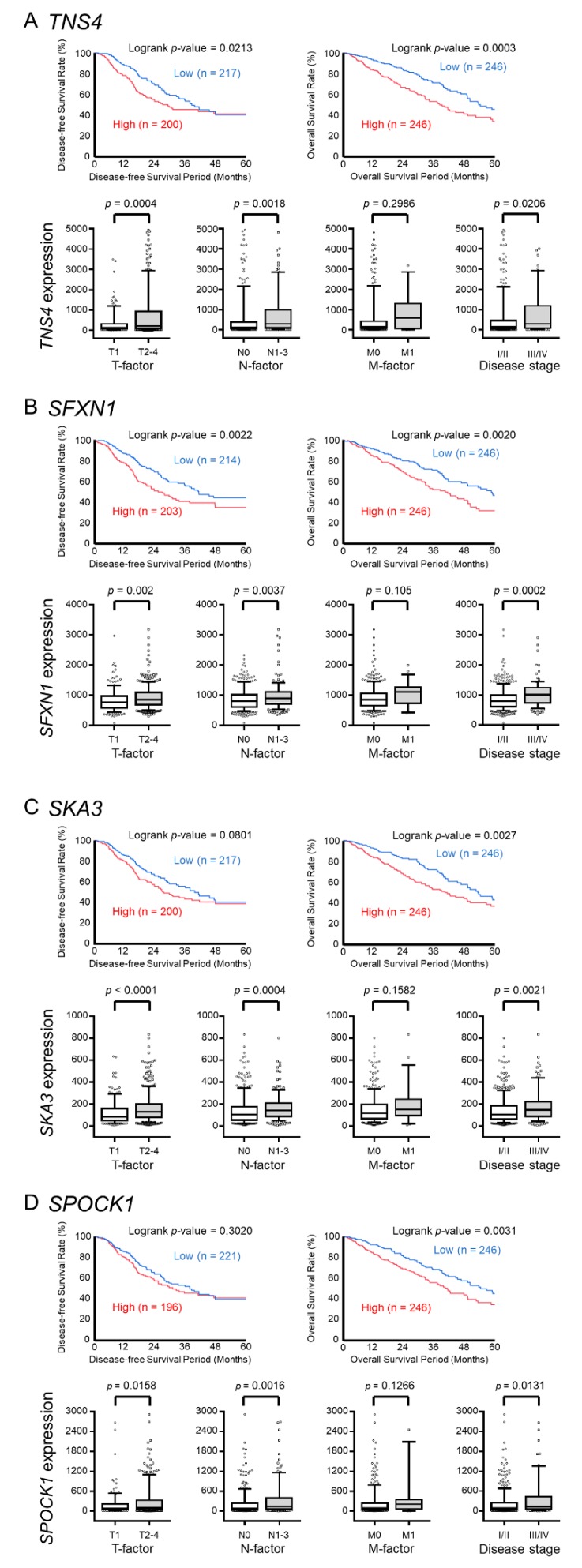
In this study, we focused on miR-150-3p, which is the passenger strand of the miR-150 duplex and had pronounced anti-tumor function. We examined the relation between the pathogenesis of LUAD and these targets using TCGA database and found four genes (TNS4, SFXN1, SKA3, SPOCK1) that were strongly associated with patient outcomes (5-year overall survival, p < 0.01, Figure 3A–D). Finally, we focused on TNS4, the expression of which was strongly associated with poor prognosis of LUAD patients (disease-free survival: p = 0.0213 and overall survival: p = 0.0003) among the four genes and validated the effect on LUAD cells.
Figure 3.
The relationship between the expression levels of four genes (TNS4, SFXN1, SKA3, and SPOCK1) and clinical significance based on The Cancer Genome Atlas (TCGA) database. (A–D) The Kaplan–Meier disease-free survival curves and overall survival curves, T factor, N factor, M factor, and disease stage of the high- and low-expression groups for four genes (TNS4, SFXN1, SKA3, and SPOCK1).
2.5. miR-150-3p Directly Regulated TNS4 in A549 Cells
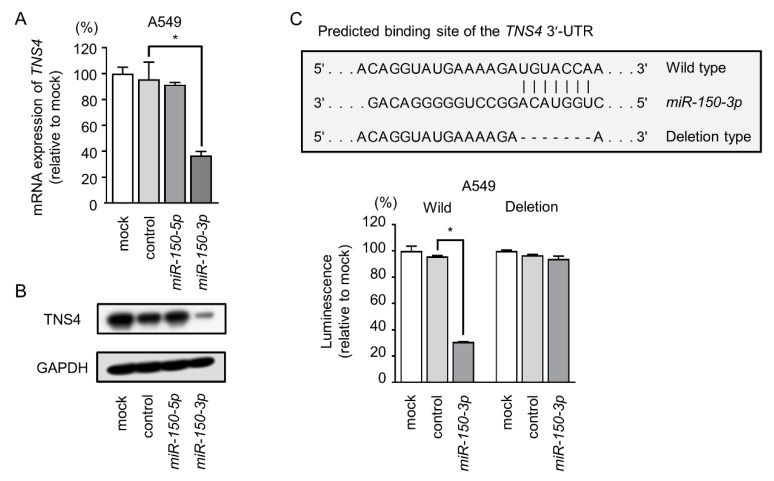
In cells transfected with miR-150-3p, the levels of TNS4 mRNA and TNS4 protein were significantly lower than mock- or miR-control-transfected cells (Figure 4A,B). Furthermore, the expression of TNS4 mRNA and TNS4 protein was suppressed at the diluted concentration of miR-150-3p precursor (1 nM and 0.1 nM) (Figure S4A,B).
Figure 4.
TNS4 was directly controlled by miR-150-3p in LUAD cells. (A,B) TNS4 mRNA and protein expression was reduced by miR-150-3p ectopic expression (48 h after transfection). GUSB was used as an expression control. GAPDH was used as a loading control. * p < 0.01. (C) Dual luciferase reporter assays using vectors encoding the wild-type TNS4 3′-UTR sequence containing one putative miR-150-3p target site (wild) and 3′-UTR sequences with deletions of the target site (deletion). Normalized data were calculated as the ratio of Renilla/firefly luciferase activities. * p < 0.01. UTR: untranslated region.
In order to confirm the binding site of miR-150-3p, the nucleotide sequences of 3’UTR (UTR: untranslated region) of TNS4 in A549 cells was examined independently. Our data showed that several variants of the 3’UTR of TNS4 existed in A549 cells (Figure S5). As a result of sequencing analyses, one putative binding site of the miR-150-3p was found in 3’UTR of TNS4 (Figure S5). Based on our sequence data, we used luciferase reporter assays with vectors carrying either the wild-type or deletion-type 3’-UTR of TNS4 (Figure 4C). We observed significantly reduced luminescence intensities after transfection with miR-150-3p and the wild-type 3’-UTR of TNS4 (Figure 4C). Transfection with the deletion-type vector did not reduced luminescence intensities in A549 cells (Figure 4C). Thus, miR-150-3p directly bound to TNS4 in the 3’-UTR. Although TargetScanHuman database predicted putative binding sites of miR-150-5p in 3’UTR of TNS4, our sequencing data could not confirm the sequences.
2.6. Expression of TNS4 Protein in Clinical LUAD Specimens
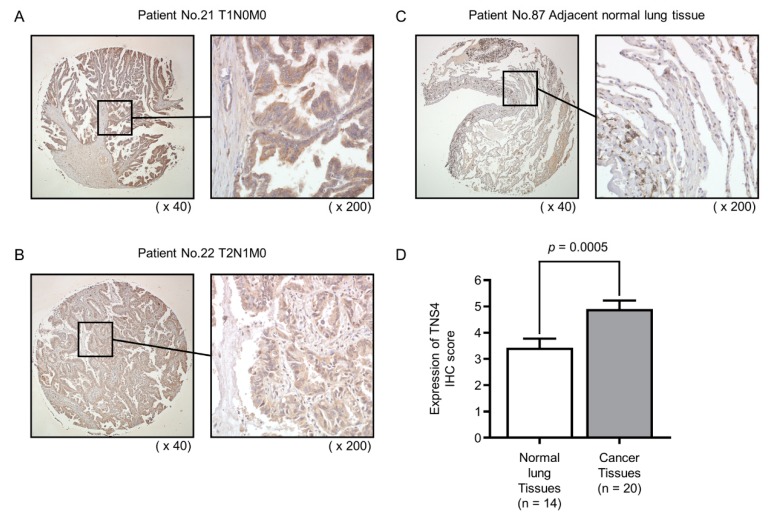
Analysis using a tissue microarray was performed to examine TNS4 expression at the protein level. We validated the expression of TNS4 by using immunohistochemical staining. In this study, we stained 20 LUAD specimens and 14 non-cancerous specimens. Clinical information on the tissue microarray is shown in the Table S2. Compared with non-cancerous tissues, TNS4 proteins were highly expressed in LUAD specimens (Figure 5).
Figure 5.
Immunohistochemical staining of TNS4 protein in clinical LUAD specimens. (A,B) The overexpression of TNS4 was observed in the cytoplasm of cancer cells. (C) TNS4 was weakly stained or not detected in normal lung specimens. (D) Comparison of immunohistochemical staining of TNS4 in LUAD specimens and normal lung specimens. LUAD specimens showed higher expression of TNS4 than normal lung specimens.
2.7. TNS4 Silencing Suppresses the Aggressiveness of LUAD Cells
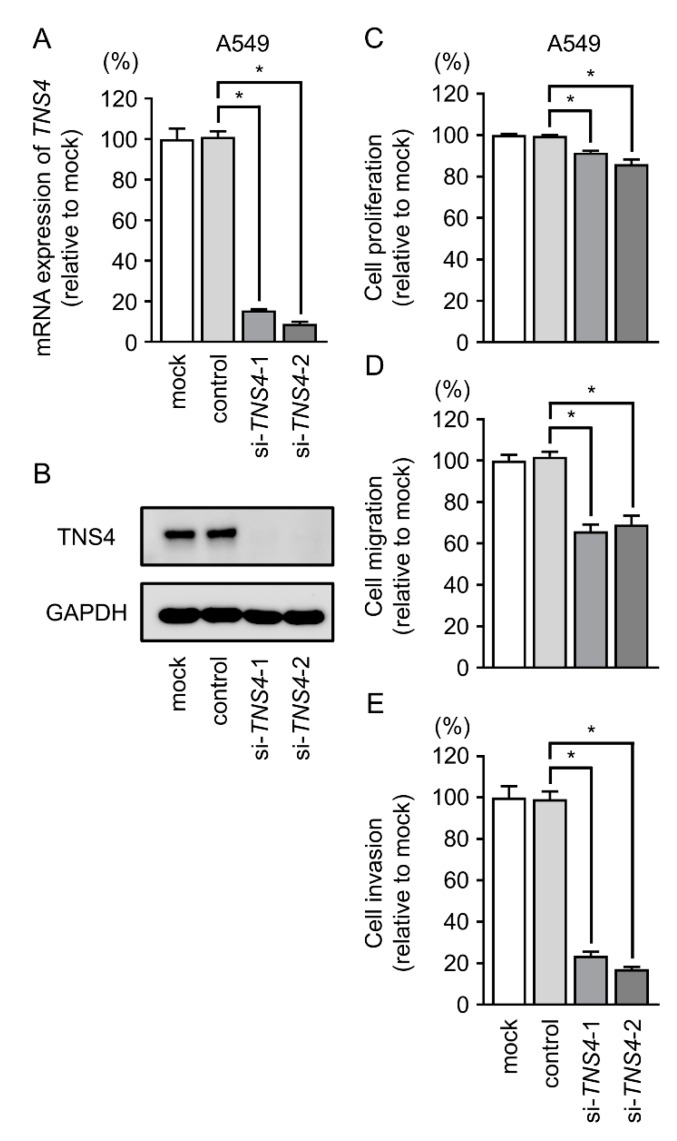
To confirm the effect of TNS4 on LUAD cells, we used si-TNS4 to knock down its expression in A549 cells. RT-PCR and Western blotting showed that expression levels of both TNS4 mRNA and TNS4 protein were markedly reduced by both si-TNS4-1 and si-TNS4-2 (Figure 6A,B). In functional assays, cell proliferation, migration, and invasive abilities were significantly suppressed by si-TNS4 transfection in LUAD cells (Figure 6C–E).
Figure 6.
Knockdown studies of TNS4/TNS4 using si-TNS4 in LUAD cells (A549 and H1299). (A,B) TNS4 mRNA and protein expression 48 h after transfection of si-TNS4-1 or si-TNS4-2 in LUAD cell lines. (C–E) Cell proliferation, migration, and invasive activities were significantly blocked by si-TNS4 transfection into LUAD cell lines. * p < 0.01.
2.8. Gain-of-Function Studies by TNS4 Expression Vector
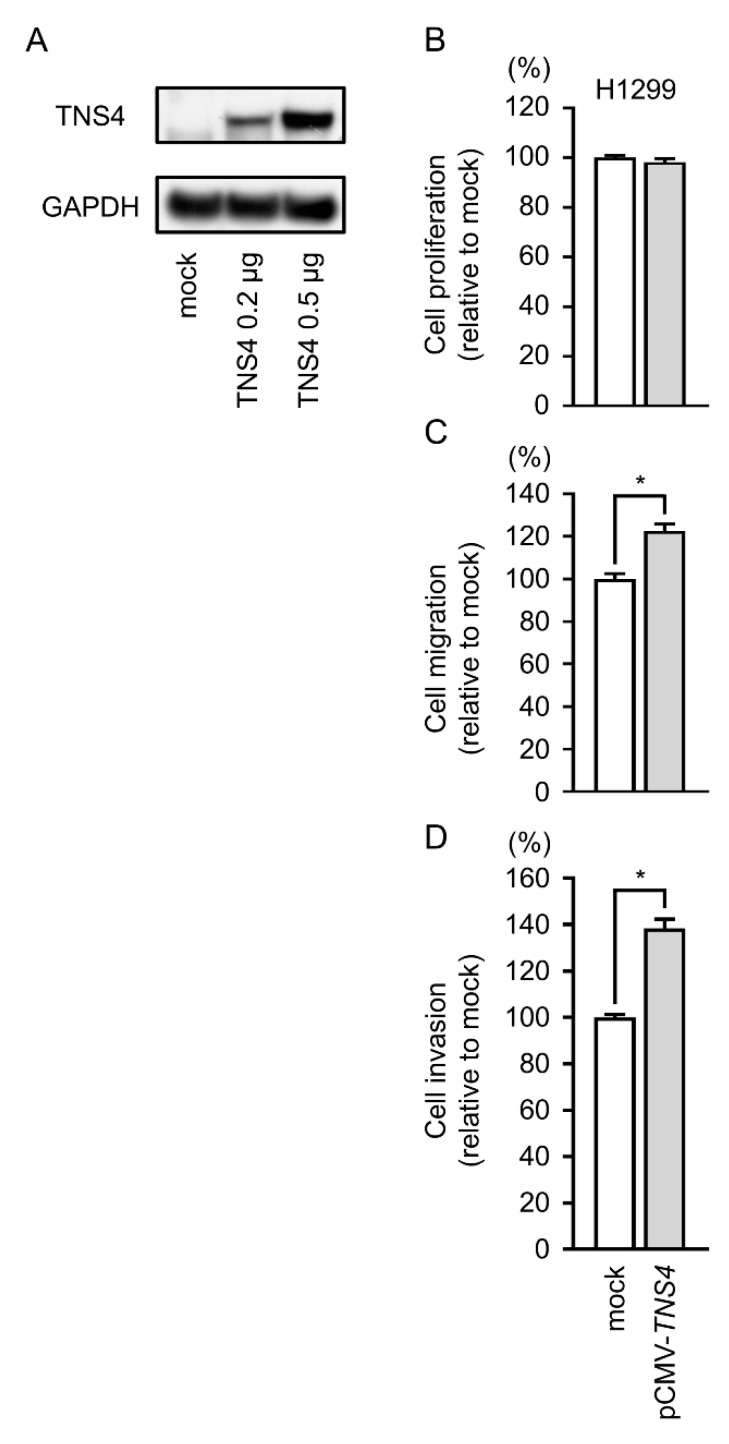
To explore whether TNS4 promoted cell proliferation, migration, and invasive abilities, we transfected pCMV-TNS4 into H1299 cells and performed functional assays. Western blotting indicated that overexpression of TNS4 protein was observed in pCMV-TNS4 vector-transfected cells (Figure 7A). Furthermore, cell migration and invasion were significantly enhanced in pCMV-TNS4 vector-transfected cells (Figure 7B–D).
Figure 7.
The effects of TNS4 overexpression by H1299 cells. (A) Expression levels of TNS4 protein 48 h after transfection with pCMV-TNS4 vector into H1299 cells. (B) Cell proliferation was evaluated by XTT assays. (C) Cell migration was determined by wound healing assay 24 h after forward transfection with the pCMV-TNS4 vector. (D) Cell invasion was determined by Matrigel assay 24 h after forward transfection with the pCMV-TNS4 vector. These assays showed that migratory and invasive activities were increased in pCMV-TNS4 vector-transfected cell lines. * p < 0.01.
2.9. Downstream Genes Affected by the Silencing of TNS4 in LUAD Cells
We performed genome-wide gene expression analysis in LUAD cells transfected with si-TNS4 and in silico analysis to investigate the downstream genes regulated by TNS4. A total of 1521 downregulated genes were identified using gene expression analysis in LUAD cells transfected with si-TNS4. Among them, we found high expression of genes in the NSCLC clinical expression profiles from the GEO database (GEO accession no: GSE 19188). A total of 88 genes were identified as TNS4-modulated genes (Table 4 and Figure S6).
Table 4.
Candidate downstream genes modulated by TNS4 in lung adenocarcinoma.
| Entrez Gene | Gene Symbol | Gene Name | GSE19188 FC (log2) | A549 si-TNS4-2 Transfectant FC (log2) | TCGA OncoLnc p-Value |
|---|---|---|---|---|---|
| 114904 | C1QTNF6 | C1q and tumor necrosis factor related protein 6 | 2.046 | −1.159 | <0.0001 |
| 983 | CDK1 | Cyclin-dependent kinase 1 | 2.400 | −1.119 | 0.0003 |
| 10615 | SPAG5 | Sperm-associated antigen 5 | 2.196 | −1.598 | 0.0003 |
| 84951 | TNS4 | Tensin 4 | 2.560 | −2.690 | 0.0003 |
| 4288 | MKI67 | Marker of proliferation Ki-67 | 2.835 | −1.475 | 0.0004 |
| 8208 | CHAF1B | Chromatin assembly factor 1, subunit B (p60) | 1.723 | −1.112 | 0.0005 |
| 9824 | ARHGAP11A | Rho GTPase activating protein 11A | 1.638 | −1.263 | 0.0007 |
| 9055 | PRC1 | Protein regulator of cytokinesis 1 | 2.540 | −1.120 | 0.0007 |
| 171177 | RHOV | Ras homolog family member V | 2.330 | −1.890 | 0.0008 |
| 1033 | CDKN3 | Cyclin-dependent kinase inhibitor 3 | 2.889 | −1.594 | 0.0011 |
| 6241 | RRM2 | Ribonucleotide reductase M2 | 3.000 | −1.356 | 0.0013 |
| 57405 | SPC25 | SPC25, NDC80 kinetochore complex component | 2.417 | −1.456 | 0.0014 |
| 5318 | PKP2 | Plakophilin 2 | 1.584 | −1.108 | 0.0016 |
| 701 | BUB1B | BUB1 mitotic checkpoint serine/threonine kinase B | 2.669 | −1.278 | 0.0017 |
| 4085 | MAD2L1 | MAD2 mitotic arrest deficient-like 1 (yeast) | 2.768 | −1.726 | 0.0018 |
| 81624 | DIAPH3 | Diaphanous-related formin 3 | 1.926 | −1.016 | 0.0022 |
| 3832 | KIF11 | Kinesin family member 11 | 2.479 | −1.007 | 0.0022 |
| 79019 | CENPM | Centromere protein M | 2.391 | −1.057 | 0.0023 |
| 55635 | DEPDC1 | DEP domain containing 1 | 3.443 | −1.282 | 0.0024 |
| 147841 | SPC24 | SPC24, NDC80 kinetochore complex component | 2.179 | −1.490 | 0.0031 |
| 195828 | ZNF367 | Zinc finger protein 367 | 1.583 | −1.454 | 0.0033 |
| 1063 | CENPF | Centromere protein F, 350/400kDa | 2.985 | −1.129 | 0.0048 |
| 83540 | NUF2 | NUF2, NDC80 kinetochore complex component | 3.442 | −1.069 | 0.0048 |
| 29089 | UBE2T | Ubiquitin-conjugating enzyme E2T | 3.317 | −1.092 | 0.0051 |
| 7348 | UPK1B | Uroplakin 1B | 1.603 | −1.179 | 0.0059 |
| 11130 | ZWINT | ZW10 interacting kinetochore protein | 2.184 | −1.188 | 0.0064 |
| 11169 | WDHD1 | WD repeat and HMG-box DNA binding protein 1 | 2.094 | −1.151 | 0.0065 |
| 55215 | FANCI | Fanconi anemia, complementation group I | 2.298 | −1.563 | 0.0073 |
| 4176 | MCM7 | Minichromosome maintenance complex component 7 | 1.555 | −1.164 | 0.0076 |
| 10403 | NDC80 | NDC80 kinetochore complex component | 2.493 | −1.053 | 0.0076 |
| 699 | BUB1 | BUB1 mitotic checkpoint serine/threonine kinase | 3.206 | −1.180 | 0.0081 |
| 79075 | DSCC1 | DNA replication and sister chromatid cohesion 1 | 1.704 | −1.187 | 0.0088 |
| 51514 | DTL | Denticleless E3 ubiquitin protein ligase homolog (Drosophila) | 2.098 | −1.187 | 0.0091 |
| 8914 | TIMELESS | Timeless circadian clock | 1.650 | −1.447 | 0.0093 |
| 79733 | E2F8 | E2F transcription factor 8 | 2.879 | −2.313 | 0.0114 |
| 4605 | MYBL2 | v-Myb avian myeloblastosis viral oncogene homolog-like 2 | 3.003 | −1.063 | 0.0122 |
| 5427 | POLE2 | Polymerase (DNA directed), epsilon 2, accessory subunit | 1.600 | −1.074 | 0.0122 |
| 10331 | B3GNT3 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 3 | 1.608 | −1.005 | 0.0129 |
| 7083 | TK1 | Thymidine kinase 1, soluble | 2.086 | −1.399 | 0.0131 |
| 6790 | AURKA | Aurora kinase A | 2.542 | −1.018 | 0.0132 |
| 79623 | GALNT14 | Polypeptide N-acetylgalactosaminyltransferase 14 | 2.572 | −1.075 | 0.0140 |
| 64151 | NCAPG | Non-SMC condensin I complex, subunit G | 2.833 | −2.675 | 0.0147 |
| 8318 | CDC45 | Cell division cycle 45 | 3.829 | −1.605 | 0.0159 |
| 55165 | CEP55 | Centrosomal protein 55kDa | 2.875 | −1.077 | 0.0203 |
| 54478 | FAM64A | Family with sequence similarity 64, member A | 2.713 | −1.697 | 0.0219 |
| 79172 | CENPO | Centromere protein O | 1.620 | −1.048 | 0.0248 |
| 2491 | CENPI | Centromere protein I | 2.088 | −1.685 | 0.0258 |
| 1356 | CP | Ceruloplasmin (ferroxidase) | 1.604 | −2.134 | 0.0300 |
| 2244 | FGB | Fibrinogen beta chain | 1.887 | −3.066 | 0.0305 |
| 29128 | UHRF1 | Ubiquitin-like with PHD and ring finger domains 1 | 2.576 | −1.442 | 0.0312 |
| 1058 | CENPA | Centromere protein A | 3.488 | −1.338 | 0.0365 |
| 2877 | GPX2 | Glutathione peroxidase 2 (gastrointestinal) | 3.579 | −1.038 | 0.0446 |
| 5888 | RAD51 | RAD51 recombinase | 2.085 | −1.344 | 0.0478 |
| 8438 | RAD54L | RAD54-like (S. cerevisiae) | 2.920 | −1.013 | 0.0509 |
| 1789 | DNMT3B | DNA (cytosine-5-)-methyltransferase 3 beta | 1.606 | −1.029 | 0.0607 |
| 5984 | RFC4 | Replication factor C (activator 1) 4, 37kDa | 2.004 | −1.166 | 0.0938 |
| 91057 | CCDC34 | Coiled-coil domain containing 34 | 1.995 | −1.985 | 0.1035 |
| 202915 | TMEM184A | Transmembrane protein 184A | 1.940 | −1.983 | 0.1067 |
| 8038 | ADAM12 | ADAM metallopeptidase domain 12 | 2.753 | −1.464 | 0.1097 |
| 51557 | LGSN | Lengsin, lens protein with glutamine synthetase domain | 1.721 | −1.008 | 0.1253 |
| 4151 | MB | Myoglobin | 1.614 | −2.307 | 0.1346 |
| 201299 | RDM1 | RAD52 motif containing 1 | 1.503 | −1.596 | 0.1583 |
| 5080 | PAX6 | Paired box 6 | 1.602 | −1.692 | 0.1649 |
| 114907 | FBXO32 | F-box protein 32 | 1.990 | −1.044 | 0.1654 |
| 286151 | FBXO43 | F-box protein 43 | 1.569 | −1.906 | 0.1729 |
| 10293 | TRAIP | TRAF interacting protein | 2.362 | −1.114 | 0.1742 |
| 83990 | BRIP1 | BRCA1 interacting protein C-terminal helicase 1 | 2.051 | −1.439 | 0.1829 |
| 349136 | WDR86 | WD repeat domain 86 | 1.653 | −1.306 | 0.1904 |
| 1870 | E2F2 | E2F transcription factor 2 | 1.704 | −1.075 | 0.1993 |
| 3007 | HIST1H1D | Histone cluster 1, H1d | 1.568 | −1.265 | 0.2918 |
| 6676 | SPAG4 | Sperm-associated antigen 4 | 1.552 | −1.003 | 0.3275 |
| 200844 | C3orf67 | Chromosome 3 open reading frame 67 | 1.584 | −1.436 | 0.3482 |
| 57016 | AKR1B10 | Aldo-keto reductase family 1, member B10 (aldose reductase) | 3.628 | −2.490 | 0.3591 |
| 1719 | DHFR | Dihydrofolate reductase | 1.538 | −1.033 | 0.4901 |
| 8581 | LY6D | Lymphocyte antigen 6 complex, locus D | 2.171 | −1.384 | 0.5123 |
| 6518 | SLC2A5 | Solute carrier family 2 (facilitated glucose/fructose transporter), member 5 | 1.986 | −1.018 | 0.5410 |
| 10535 | RNASEH2A | Ribonuclease H2, subunit A | 1.751 | −1.020 | 0.5517 |
| 10018 | BCL2L11 | BCL2-like 11 (apoptosis facilitator) | 1.592 | −1.310 | 0.5755 |
| 25837 | RAB26 | RAB26, member RAS oncogene family | 2.112 | −1.420 | 0.5804 |
| 57834 | CYP4F11 | Cytochrome P450, family 4, subfamily F, polypeptide 11 | 1.795 | −1.032 | 0.6899 |
| 100133941 | CD24 | CD24 molecule | 2.092 | −1.777 | 0.7765 |
| 56521 | DNAJC12 | DnaJ (Hsp40) homolog, subfamily C, member 12 | 2.208 | −1.687 | 0.7922 |
| 3141 | HLCS | Holocarboxylase synthetase | 1.791 | −1.020 | 0.8947 |
| 222962 | SLC29A4 | Solute carrier family 29 (equilibrative nucleoside transporter), member 4 | 1.617 | −1.100 | 0.9072 |
| 1645 | AKR1C1 | Aldo-keto reductase family 1, member C1 | 2.257 | –1.546 | 0.9583 |
| 152404 | IGSF11 | Immunoglobulin superfamily, member 11 | 1.590 | –1.572 | 0.9790 |
| 85285 | KRTAP4-1 | Keratin-associated protein 4-1 | 2.215 | –1.029 | no data |
| 25859 | PART1 | Prostate androgen-regulated transcript 1 (non-protein coding) | 1.915 | –1.383 | no data |
3. Discussion
According to the current concept of miRNA biogenesis, miRNA passenger strands are degraded and have no cellular functions. In contrast to this concept, our miRNA signatures based on RNA sequencing revealed that some passenger strands are aberrantly expressed in cancer tissues [10,16,21,24,25,26,27]. Importantly, functional assays showed that some passenger strands of miRNAs (e.g., miR-144-5p, miR-145-3p, miR-139-3p, miR-199-3p, miR-223-3p, and miR-455-5p) actually acted as anti-tumor miRNAs by controlling cancer-related genes [17,25,28,29,30,31,32,33]. In general theory, passenger strand of miRNAs derived from miRNA-duplex have degraded in cytoplasm and have no function. In fact, the expression of passenger strand of miRNA is overwhelmingly lower than that of guide strand. Is the passenger strand of miRNA actually functional in vivo? This is an important issue in miRNA research. Expression levels of miR-150-3p were lower (100 ×) than miR-150-5p in LUAD cells. Our in vitro functional assays showed that antitumor effects are observed even if the transfection concentration of mature miR-150-3p is lowered (1 nM and 0.1 nM). Elucidation of functions of passenger strand of miRNA in vivo is an important biological theme. The involvement of passenger strands of miRNAs in cancer pathogenesis is an attractive proposal for cancer research. Identification of novel molecules controlled by miRNA (the passenger strand of miRNA duplex) will contribute to the understanding of the oncogenic networks of LUAD.
In previous studies, tumor suppressive function of miR-150-5p (the guide strand) was reported in several cancers [18,22,23]. In contrast to this, very few reports have investigated the functional significance of miR-150-3p (the passenger strand) in cancer cells and its controlled cancer-related genes. We have revealed anti-tumor function of miR-150-3p in esophageal, head and neck, and lung squamous cell carcinomas [21,22,23]. Moreover, we revealed miR-150-3p targets oncogenes involved in the focal adhesion pathways (e.g., SPOCK1, TNC, ITGA3, and ITGA6) [21,22,23]. Importantly, these genes were overexpressed in cancer tissues and their high expression was significantly correlated with poor prognosis [21,22,23].
In the present study, we finally identified four oncogenes (TNS4, SFXN1, SKA3, and SPOCK1) regulated by miR-150-3p in LUAD cells. Expression of these genes were significantly associated with LUAD pathogenesis. It is interesting to note that knockdown of SPOCK1 significantly attenuated cancer cell migration and invasive abilities [21,22,23]. Another target gene, SKA3, was overexpressed in renal cell carcinoma and its aberrant expression was associated with cancer cell malignant phenotypes [32]. These data showed that target genes by miR-150-3p regulation closely contributed to LUAD pathogenesis and tumorigenesis. Detailed analysis of miR-150-3p target genes is indispensable for elucidating the molecular mechanism of LUAD.
We further investigated the oncogenic roles of TNS4 (tensin 4) because high expression of TNS4 was strongly associated with poor prognosis of LUAD patients (disease-free survival: p = 0.0213 and overall survival: p = 0.0003). TNS4 (alias CTEN) is a member of the tensin family, which includes TNS1–TNS4. TNS4 contains SH2 (Src homology 2) and PTB (phosphotyrosine binding) domains at the C-terminal region, both of which are shared with other tensin family members [34]. Interestingly, these domains are essential to bind integrin β1, c-Cbl, β-catenin, and Eepidermal growth factor (EGF) receptor [35,36]. Previous study showed that c-Cbl is an E3 ubiquitin protein ligase and induced EGFR ubiquitination. TNS4 bound to c-Cbl reduced EGFR ubiquitination and degradation [36]. As a result, overexpression of TNS4 stabilized EGFR and enhanced its oncogenic signaling in cancer cells [36].
Aberrant expression of TNS4 was reported in cancers of the breast, colon, pancreas, and lung, and its expression was associated with poorer prognosis of these patients [34,37,38,39]. In hepatocellular carcinoma, EGF-induced extracellular signal-regulated kinase (ERK)1/2 activation enhanced TNS4 expression and these events enhanced the epithelial–mesenchymal transition (EMT) phenotype [40]. In lung cancer, TNS4 was upregulated by EGF-mediated STAT3 activation and its aberrant expression increased cancer cell invasiveness [41]. Our present data confirmed the oncogenic features of TNS4 in LUAD cells. These finding indicate that expression of TNS4 might be a good prognostic indicator and a promising therapeutic target for LUAD.
Finally, to investigate TNS4-modulated oncogenes in LUAD cells, we applied genome-wide gene expression analyses using knockdown of TNS4. A total of 88 genes were identified as putative TNS4-modulated targets in LUAD cells. Aberrant expression of nine genes (C1QTNF6, TNS4, CDK1, SPAG5, MKI67, CHAF1B, ARHGAP11A, PRC1, RHOV, p < 0.001) was closely associated with poor prognosis of patients with LUAD. Cyclin-dependent kinases (CDKs) are critical regulators of cell cycle progression and related to cancer aggressiveness. CDK1 is essential for cycle progression during the G2/M transition and mitosis. High expression of CDK1 is associated with poor prognosis in LUAD [42]. SPAG5 is a microtubule-associated protein and is involved in regulating cell cycle progression [43]. Overexpression of SPAG5 was observed in NSCLC and promoted cell proliferation and invasion through activation of the Akt signaling pathway [44]. PRC1, which acts as an organizing anti-parallel microtubule in the central spindle in cytokinesis, is required for tumorigenesis driven by oncogenic K-RAS and loss of p53 in a mouse model for NSCLC [45]. Thus, the data revealed that many of the genes controlled by anti-tumor miR-150-3p and by TNS are closely involved in the pathogenesis of cancer. The elucidation of the novel targets controlled by anti-tumor miRNAs will accelerate comprehensive understanding of oncogenic networks of LUAD.
4. Materials and Methods
4.1. Human LUAD Specimens and Cell Lines
In this study, 18 LUAD clinical samples and 28 normal lung samples were obtained from the patients who underwent lung surgery at Kagoshima University Hospital from 2010 to 2013. Table 1 presents the clinical characteristics of these patients. The LUAD samples were staged according to the Association for the Study of Lung Cancer TNM classification, seventh edition.
We used the two LUAD cell lines: A549 and H1299, purchased from the American Type Culture Collection (Manassas, VA, USA).
We obtained informed consent from all of the patients. The present study was approved by the Bioethics Committee of Kagoshima University (Kagoshima, Japan; approval no. 26-164).
4.2. RNA Extraction and Quantitative Real-Time PCR
We carried out RNA extraction from formalin-fixed, paraffin-embedded specimens and cell lines and quantitative real-time reverse transcription-PCR (qRT-PCR) as previously described [18,46,47,48]. The TaqMan probes and primers were listed in Table S1.
4.3. Transfection of miRNAs, siRNAs, and Plasmid Vectors into LUAD Cells
Transfection protocol of miRNA or siRNA species into cancer cells was described in our previous studies [18,46,47,48]. The reagents used in this study are listed in Table S1.
4.4. Incorporation of miR-150-5p or miR-150-3p into the RISC by Ago2 Immunoprecipitation
A549 cells were transfected with 10 nM miRNAs by reverse transfection. After 72 h, immunoprecipitation was performed using a microRNA Isolation Kit, Human Ago2 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) as described previously [47,48]. Expression levels of miR-150-5p and miR-150-3p were analyzed by qRT-PCR. MiRNA data were normalized to expressions of miR-16-5p, miR-21-5p, and miR-26a, which were not affected by miR-150-5p and miR-150-3p.
4.5. Cell Proliferation, Migration, and Invasion Assays
The procedures for assessing cell proliferation, migration and invasion were described previously [18,46,47,48].
4.6. Identification of Putative Target Genes Regulated by miR-150-5p and miR-150-3p in LUAD Cells
We identified putative target genes possessing sequences binding to miR-150-5p and miR-150-3p from the TargetScanHuman database (http://www.targetscan.org/vert_72/). GEO databases GSE19188 and GSE93290 was used for assessment of the association between target genes and the expression of NSCLC clinical specimens. Our strategy for identification of miR-150-5p and miR-150-3p target genes is outlined in Figure S3A,B.
4.7. Plasmid Construction and Dual Luciferase Reporter Assay
The following two sequences were cloned into the psiCHECK-2 vector (C8021; Promega, Madison, WI, USA): the wild-type sequence of the 3’ untranslated regions (UTRs) of TNS4, or the deletion type, which lacked the miR-150-3p target sites from TNS4. The procedures for transfection and dual luciferase reporter assays were provided in previous studies [47,48].
4.8. Clinical Database Analysis of LUAD
We investigated the clinical significance of miRNAs and their target genes with TCGA (https://tcga-data.nci.nih.gov/tcga/) in LUAD. Gene expression and clinical data were obtained from cBioPortal (http://www.cbioportal.org/) and OncoLnc (http://www.oncolnc.org/) (data downloaded on 28 September 2018) [46,47,48,49].
4.9. Western Blotting and Immunohistochemistry
The procedures for Western blotting and immunohistochemistry were described previously [18,46,47,48]. A tissue microarray was bought from US Biomax (catalog no: BC04002a; Derwood, MD, USA). Primary antibodies were shown in Table S1.
4.10. Statistical Analysis
To assess the significance of differences between two groups, we used Mann–Whitney U tests. Differences between multiple groups were assessed by one-way ANOVA and Tukey tests for post-hoc analysis. Tests utilized GraphPad Prism7 (GraphPad Software, La Jolla, CA, USA) and JMP Pro 14 (SAS Institute Inc., Cary, NC, USA).
5. Conclusions
Our results showed that the expression of both strands of the pre-miR-150 duplex was significantly downregulated in LUAD clinical specimens and the miR-150 duplex acted as an anti-tumor miRNA in LUAD cells. Involvement of the passenger strand of miRNA in LUAD oncogenesis suggests new possible mechanisms of pathogenesis. A total of 26 genes regulated by miR-150-3p were closely associated with LUAD pathogenesis. Among these targets, aberrant expression of TNS4 enhanced cancer aggressiveness, suggesting that TNS4 could be a promising therapeutic target for LUAD.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/5/601/s1. Figure S1: Functional assays of miR-150-3p (0.1 nM, 1 nM, 10 nM) in LUAD cells (A549 and H1299), Figure S2: Both strands of miR-150-5p and miR-150-3p were incorporated into the RISC, Figure S3: The strategy for identification of genes regulated by miR-150-5p and miR-150-3p, Figure S4: TNS4 was directly suppressed at the diluted concentration of miR-150-3p precursor (1 nM and 0.1 nM), Figure S5: The nucleotide sequences of 3’UTR of TNS4 in A549 cells, Figure S6: The strategy for identification of TNS4-modulated genes, Table S1: Reagent used in this study, Table S2: Immunohistochemical status and characteristics of the lung cancer and non-cancerous cases.
Author Contributions
Conceptualization, S.M., N.S. and H.I.; methodology, N.S.; validation, A.U., H.S. and T.K.; formal analysis, S.M. and T.K.; investigation, N.S., S.M. and K.M.; resources, N.S., K.M., Y.Y., N.K., T.S. and H.I.; writing—original draft preparation, S.M. and N.S.; writing—review and editing, N.S., K.M., Y.Y., A.U., H.S., T.K. and T.S.; visualization, S.M. and T.K.; supervision, N.S.; funding acquisition, N.S., N.K., T.K. and H.I.
Funding
This research was funded by KAKENHI grants, 17K09660 and 18K09338.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2018 doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Cheng T.Y., Cramb S.M., Baade P.D., Youlden D.R., Nwogu C., Reid M.E. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2016;11:1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 4.Heyneman L.E., Herndon J.E., Goodman P.C., Patz E.F., Jr. Stage distribution in patients with a small (< or = 3 cm) primary nonsmall cell lung carcinoma. Implication for lung carcinoma screening. Cancer. 2001;92:3051–3055. doi: 10.1002/1097-0142(20011215)92:12<3051::aid-cncr10106>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Lin P.Y., Chang Y.C., Chen H.Y., Chen C.H., Tsui H.C., Yang P.C. Tumor size matters differently in pulmonary adenocarcinoma and squamous cell carcinoma. Lung Cancer. 2010;67:296–300. doi: 10.1016/j.lungcan.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto Y., Kurozumi A., Enokida H., Ichikawa T., Seki N. Functional significance of aberrantly expressed microRNAs in prostate cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2015;22:242–252. doi: 10.1111/iju.12700. [DOI] [PubMed] [Google Scholar]
- 9.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koshizuka K., Hanazawa T., Fukumoto I., Kikkawa N., Okamoto Y., Seki N. The microRNA signatures: Aberrantly expressed microRNAs in head and neck squamous cell carcinoma. J. Hum. Genet. 2017;62:3–13. doi: 10.1038/jhg.2016.105. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno K., Mataki H., Seki N., Kumamoto T., Kamikawaji K., Inoue H. MicroRNAs in non-small cell lung cancer and idiopathic pulmonary fibrosis. J. Hum. Genet. 2017;62:57–65. doi: 10.1038/jhg.2016.98. [DOI] [PubMed] [Google Scholar]
- 12.Gulyaeva L.F., Kushlinskiy N.E. Regulatory mechanisms of microRNA expression. J. Transl. Med. 2016;14:143. doi: 10.1186/s12967-016-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramassone A., Pagotto S., Veronese A., Visone R. Epigenetics and MicroRNAs in Cancer. Int. J. Mol. Sci. 2018;19:459. doi: 10.3390/ijms19020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumamoto T., Seki N., Mataki H., Mizuno K., Kamikawaji K., Samukawa T., Koshizuka K., Goto Y., Inoue H. Regulation of TPD52 by antitumor microRNA-218 suppresses cancer cell migration and invasion in lung squamous cell carcinoma. Int. J. Oncol. 2016;49:1870–1880. doi: 10.3892/ijo.2016.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mataki H., Seki N., Mizuno K., Nohata N., Kamikawaji K., Kumamoto T., Koshizuka K., Goto Y., Inoue H. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7:72084–72098. doi: 10.18632/oncotarget.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno K., Mataki H., Arai T., Okato A., Kamikawaji K., Kumamoto T., Hiraki T., Hatanaka K., Inoue H., Seki N. The microRNA expression signature of small cell lung cancer: Tumor suppressors of miR-27a-5p and miR-34b-3p and their targeted oncogenes. J. Hum. Genet. 2017;62:671–678. doi: 10.1038/jhg.2017.27. [DOI] [PubMed] [Google Scholar]
- 17.Misono S., Seki N., Mizuno K., Yamada Y., Uchida A., Arai T., Kumamoto T., Sanada H., Suetsugu T., Inoue H. Dual strands of the miR-145 duplex (miR-145-5p and miR-145-3p) regulate oncogenes in lung adenocarcinoma pathogenesis. J. Hum. Genet. 2018;63:1015–1028. doi: 10.1038/s10038-018-0497-9. [DOI] [PubMed] [Google Scholar]
- 18.Suetsugu T., Koshizuka K., Seki N., Mizuno K., Okato A., Arai T., Misono S., Uchida A., Kumamoto T., Inoue H. Downregulation of matrix metalloproteinase 14 by the antitumor miRNA, miR-150-5p, inhibits the aggressiveness of lung squamous cell carcinoma cells. Int. J. Oncol. 2018;52:913–924. doi: 10.3892/ijo.2017.4232. [DOI] [PubMed] [Google Scholar]
- 19.Uchida A., Seki N., Mizuno K., Misono S., Yamada Y., Kikkawa N., Sanada H., Kumamoto T., Suetsugu T., Inoue H. Involvement of dual-strand of the miR-144 duplex and their targets in the pathogenesis of lung squamous cell carcinoma. Cancer Sci. 2018 doi: 10.1111/cas.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matranga C., Tomari Y., Shin C., Bartel D.P., Zamore P.D. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Koshizuka K., Nohata N., Hanazawa T., Kikkawa N., Arai T., Okato A., Fukumoto I., Katada K., Okamoto Y., Seki N. Deep sequencing-based microRNA expression signatures in head and neck squamous cell carcinoma: Dual strands of pre-miR-150 as antitumor miRNAs. Oncotarget. 2017;8:30288–30304. doi: 10.18632/oncotarget.16327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osako Y., Seki N., Koshizuka K., Okato A., Idichi T., Arai T., Omoto I., Sasaki K., Uchikado Y., Kita Y., et al. Regulation of SPOCK1 by dual strands of pre-miR-150 inhibit cancer cell migration and invasion in esophageal squamous cell carcinoma. J. Hum. Genet. 2017;62:935–944. doi: 10.1038/jhg.2017.69. [DOI] [PubMed] [Google Scholar]
- 23.Koshizuka K., Hanazawa T., Kikkawa N., Katada K., Okato A., Arai T., Idichi T., Osako Y., Okamoto Y., Seki N. Antitumor miR-150-5p and miR-150-3p inhibit cancer cell aggressiveness by targeting SPOCK1 in head and neck squamous cell carcinoma. AurisNasusLarynx. 2018;45:854–865. doi: 10.1016/j.anl.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Goto Y., Kurozumi A., Nohata N., Kojima S., Matsushita R., Yoshino H., Yamazaki K., Ishida Y., Ichikawa T., Naya Y., et al. The microRNA signature of patients with sunitinib failure: Regulation of UHRF1 pathways by microRNA-101 in renal cell carcinoma. Oncotarget. 2016;7:59070–59086. doi: 10.18632/oncotarget.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goto Y., Kurozumi A., Arai T., Nohata N., Kojima S., Okato A., Kato M., Yamazaki K., Ishida Y., Naya Y., et al. Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br. J. Cancer. 2017;117:409–420. doi: 10.1038/bjc.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yonemori K., Seki N., Idichi T., Kurahara H., Osako Y., Koshizuka K., Arai T., Okato A., Kita Y., Arigami T., et al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: Anti-tumour functions of the microRNA-216 cluster. Oncotarget. 2017;8:70097–70115. doi: 10.18632/oncotarget.19591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toda H., Kurozumi S., Kijima Y., Idichi T., Shinden Y., Yamada Y., Arai T., Maemura K., Fujii T., Horiguchi J., et al. Molecular pathogenesis of triple-negative breast cancer based on microRNA expression signatures: Antitumor miR-204-5p targets AP1S3. J. Hum. Genet. 2018;63:1197–1210. doi: 10.1038/s10038-018-0510-3. [DOI] [PubMed] [Google Scholar]
- 28.Yonemori M., Seki N., Yoshino H., Matsushita R., Miyamoto K., Nakagawa M., Enokida H. Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016;107:1233–1242. doi: 10.1111/cas.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshizuka K., Hanazawa T., Kikkawa N., Arai T., Okato A., Kurozumi A., Kato M., Katada K., Okamoto Y., Seki N. Regulation of ITGA3 by the anti-tumor miR-199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017;108:1681–1692. doi: 10.1111/cas.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugawara S., Yamada Y., Arai T., Okato A., Idichi T., Kato M., Koshizuka K., Ichikawa T., Seki N. Dual strands of the miR-223 duplex (miR-223-5p and miR-223-3p) inhibit cancer cell aggressiveness: Targeted genes are involved in bladder cancer pathogenesis. J. Hum. Genet. 2018;63:657–668. doi: 10.1038/s10038-018-0437-8. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y., Arai T., Kojima S., Sugawara S., Kato M., Okato A., Yamazaki K., Naya Y., Ichikawa T., Seki N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 2018;109:2919–2936. doi: 10.1111/cas.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y., Arai T., Kojima S., Sugawara S., Kato M., Okato A., Yamazaki K., Naya Y., Ichikawa T., Seki N. Anti-tumor roles of both strands of the miR-455 duplex: Their targets SKA1 and SKA3 are involved in the pathogenesis of renal cell carcinoma. Oncotarget. 2018;9:26638–26658. doi: 10.18632/oncotarget.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada Y., Koshizuka K., Hanazawa T., Kikkawa N., Okato A., Idichi T., Arai T., Sugawara S., Katada K., Okamoto Y., et al. Passenger strand of miR-145-3p acts as a tumor-suppressor by targeting MYO1B in head and neck squamous cell carcinoma. Int. J. Oncol. 2018;52:166–178. doi: 10.3892/ijo.2017.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo S.H. C-terminal tensin-like (CTEN): A promising biomarker and target for cancer. Int. J. Biochem. Cell Biol. 2014;51:150–154. doi: 10.1016/j.biocel.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz M., Amit I., Citri A., Shay T., Carvalho S., Lavi S., Milanezi F., Lyass L., Amariglio N., Jacob-Hirsch J., et al. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat. Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1622. [DOI] [PubMed] [Google Scholar]
- 36.Hong S.Y., Shih Y.P., Li T., Carraway K.L., 3rd, Lo S.H. CTEN prolongs signaling by EGFR through reducing its ligand-induced degradation. Cancer Res. 2013;73:5266–5276. doi: 10.1158/0008-5472.CAN-12-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y.C., Chen N.T., Shih Y.P., Dong Y., Lo S.H. Up-regulation of C-terminal tensin-like molecule promotes the tumorigenicity of colon cancer through beta-catenin. Cancer Res. 2009;69:4563–4566. doi: 10.1158/0008-5472.CAN-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albasri A., Al-Ghamdi S., Fadhil W., Aleskandarany M., Liao Y.C., Jackson D., Lobo D.N., Lo S.H., Kumari R., Durrant L., et al. Cten signals through integrin-linked kinase (ILK) and may promote metastasis in colorectal cancer. Oncogene. 2011;30:2997–3002. doi: 10.1038/onc.2011.26. [DOI] [PubMed] [Google Scholar]
- 39.Al-Ghamdi S., Cachat J., Albasri A., Ahmed M., Jackson D., Zaitoun A., Guppy N., Otto W.R., Alison M.R., Kindle K.B., et al. C-terminal tensin-like gene functions as an oncogene and promotes cell motility in pancreatic cancer. Pancreas. 2013;42:135–140. doi: 10.1097/MPA.0b013e3182557ceb. [DOI] [PubMed] [Google Scholar]
- 40.Chan L.K., Chiu Y.T., Sze K.M., Ng I.O. Tensin4 is up-regulated by EGF-induced ERK1/2 activity and promotes cell proliferation and migration in hepatocellular carcinoma. Oncotarget. 2015;6:20964–20976. doi: 10.18632/oncotarget.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett D.T., Reece T.B., Foley L.S., Sjoberg A., Meng X., Fullerton D.A., Weyant M.J. C-terminal tensin-like protein mediates invasion of human lung cancer cells and is regulated by signal transducer and activator of transcription 3. J. Thorac. Cardiovasc. Surg. 2015;149:369–375. doi: 10.1016/j.jtcvs.2014.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y.X., Zhu T., Zou T., Zhuo W., Chen Y.X., Huang M.S., Zheng W., Wang C.J., Li X., Mao X.Y., et al. Prognostic and predictive values of CDK1 and MAD2L1 in lung adenocarcinoma. Oncotarget. 2016;7:85235–85243. doi: 10.18632/oncotarget.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber J., Harborth J., Schnabel J., Weber K., Hatzfeld M. The mitotic-spindle-associated protein astrin is essential for progression through mitosis. J. Cell Sci. 2002;115:4053–4059. doi: 10.1242/jcs.00088. [DOI] [PubMed] [Google Scholar]
- 44.Song L., Dai Z., Zhang S., Zhang H., Liu C., Ma X., Liu D., Zan Y., Yin X. MicroRNA-1179 suppresses cell growth and invasion by targeting sperm-associated antigen 5-mediated Akt signaling in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018;504:164–170. doi: 10.1016/j.bbrc.2018.08.149. [DOI] [PubMed] [Google Scholar]
- 45.Hanselmann S., Wolter P., Malkmus J., Gaubatz S. The microtubule-associated protein PRC1 is a potential therapeutic target for lung cancer. Oncotarget. 2018;9:4985–4997. doi: 10.18632/oncotarget.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamikawaji K., Seki N., Watanabe M., Mataki H., Kumamoto T., Takagi K., Mizuno K., Inoue H. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J. Hum. Genet. 2016;61:985–993. doi: 10.1038/jhg.2016.99. [DOI] [PubMed] [Google Scholar]
- 47.Arai T., Kojima S., Yamada Y., Sugawara S., Kato M., Yamazaki K., Naya Y., Ichikawa T., Seki N. Pirin: A potential novel therapeutic target for castration-resistant prostate cancer regulated by miR-455-5p. Mol. Oncol. 2018 doi: 10.1002/1878-0261.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada Y., Sugawara S., Arai T., Kojima S., Kato M., Okato A., Yamazaki K., Naya Y., Ichikawa T., Seki N. Molecular pathogenesis of renal cell carcinoma: Impact of the anti-tumor miR-29 family on gene regulation. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2018;25:953–965. doi: 10.1111/iju.13783. [DOI] [PubMed] [Google Scholar]
- 49.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.