Abstract
Background:
Tramadol and codeine are metabolized by CYP2D6 and are subject to drug–gene and drug–drug interactions.
Methods:
This interim analysis examined prescribing behavior and efficacy in 102 individuals prescribed tramadol or codeine while receiving pharmaco-genotyping as part of the INGENIOUS trial (NCT02297126).
Results:
Within 60 days of receiving tramadol or codeine, clinicians more frequently prescribed an alternative opioid in ultrarapid and poor metabolizers (odds ratio: 19.0; 95% CI: 2.8–160.4) as compared with normal or indeterminate metabolizers (p = 0.01). After adjusting the CYP2D6 activity score for drug–drug interactions, uncontrolled pain was reported more frequently in individuals with reduced CYP2D6 activity (odds ratio: 0.50; 95% CI: 0.25–0.94).
Conclusion:
Phenoconversion for drug–drug and drug–gene interactions is an important consideration in pharmacogenomic implementation; drug–drug interactions may obscure the potential benefits of genotyping.
Keywords: : adverse side effects, CYP2D6, IGNITE, INGENIOUS, pharmacogenetics; opioids, pharmacogenomics, phenoconversion
Tramadol and codeine are widely used drugs in the treatment of mild to moderately severe pain. However, the analgesic effect of tramadol and codeine is highly variable. Both are prodrugs that are converted into active metabolites by cytochrome P450 2D6 (CYP2D6) [1]. The CYP2D6 gene is highly polymorphic, with over 100 allelic variants (www.pharmvar.org/gene/CYP2D6). A well-known activity score system facilitates conversion of CYP2D6 genotype information into numeric scores associated with ultrarapid (UM), normal, reduced and poor metabolizer (PM) phenotypes [2]. It follows that CYP2D6 genetic variations and drug–drug interactions (DDIs) are important determinants of the variable effects of tramadol and codeine. Indeed, based on an individual's CYP2D6 metabolic activity, dosage and usage guidelines for both drugs have been promulgated by the Dutch Pharmacogenomic Working Group (DPWG) and the Clinical Pharmacogenomics Implementation Consortium (CPIC) [3–5]. For example, PM of CYP2D6 are expected to have reduced efficacy of codeine and tramadol, placing them at increased risk for insufficient pain relief. Conversely, ultrarapid metabolizers may be at higher risk of toxicity. The CPIC and DPWG recommend avoidance of codeine and tramadol in individuals with PM and UM metabolism phenotypes.
Pharmacogenomic implementation efforts have sought to test the utility and collective evidence supporting genotype-guided recommendations in the clinical environment [6–9]. However, complexities specific to opioids exist because these drugs are prescribed for heterogeneous indications including multiple severities of acute and chronic pain [10]. As the deleterious consequences of the opioid epidemic are beginning to be understood [11], medical societies have called for caution in the prescription of opioids [12,13]. Nonetheless, a consistent segment of the population remains unsatisfied with pain control [14–17]. Perhaps as a result of increased guidance or scrutiny, opioid prescribing has actually plateaued since 2010 in many clinical specialties [18]. The use of pharmacogenomic-guided opioid therapy may assist prescribers in determining which patients will respond as expected to tramadol and codeine, thereby reducing adverse events and maximizing efficacy [19–21]. The genotyping may further serve to reinforce or validate a patient's complaint of uncontrolled pain.
Given the prolific usage of opioids, integration of algorithms into the electronic health record (EHR) has proven essential to ensure clinicians are able to access genotype-guided dosing recommendations expediently; however, these EHR algorithms may not simultaneously account for DDIs [22]. The advantages of pheno-converting a drug–gene based activity score to one that incorporates both drug–gene and DDIs have garnered increasing recognition [2,23–28]. Phenoconversion of CYP2D6 activity, often driven by nongenetic factors like concomitant medication use, can have a significant impact on the interpretation of pragmatic pharmacogenomic clinical trial outcomes since these trials may include patients taking inhibitors [28]. The CPIC currently provides guidance on the appropriate degree of reduction in activity score for strong and moderate inhibitors [4].
The ongoing Indiana Genomics Implementation: An Opportunity for the Underserved (INGENIOUS) trial (NCT02297126) is a member program within the Implementing Genomics in Practice (IGNITE) Network [29–31]. A description of the trial and the clinical support has been previously reported [6] and is summarized in the methods. As a pragmatic trial, INGENIOUS builds upon pharmacogenomic discoveries that have been culled from tightly controlled clinical efficacy trials, testing the clinical effectiveness of these relationships in a routine practice setting. We present an interim analysis of tramadol and codeine prescribing behavior and efficacy from the intervention arm of the INGENIOUS trial, comparing subjects (who have received genotyping) based on their predicted metabolizer status. We hypothesized that subjects with actionable genotypes (having drug–gene or drug–drug interactions) would be more likely to undergo changes in prescribing behavior as compared to those with nonactionable genotypes. Additionally, we tested whether the presence of uncontrolled pain would correspond to an individual's metabolism activity score and whether the presence of DDIs would affect both prescribing behavior and efficacy. We test these hypotheses in the ensuing analysis.
Subjects & methods
INGENIOUS trial
The INGENIOUS trial (NCT02297126) enrolls eligible subjects who receive a new prescription of one of 27 common medications ( Supplementary Table 1 ). Tramadol and codeine are included among the 27 drugs that precipitate enrollment. Subjects undergo either standard care (the control arm) or pharmaco-genotyping (intervention arm) [6]. Both arms are followed for 1 year after enrollment; outcomes include composite healthcare costs and adverse event incidence. Within 7 days of enrollment, genotype-guided dosing recommendations in alignment with the DPWG or CPIC guidelines are sent to the prescriber and are uploaded as a searchable document within the EHR. An adjudication committee reviews genotypes and medication lists for participants to ensure prescribers receive actionable results (including drug–drug interactions) and to identify incidental findings [6]. The healthcare provider may elect to continue the current therapy or may alter drug selection or dosage based on the pharmacogenomic test results and recommendations. Additional input from clinical pharmacology consultants is available upon request [32].
New prescriptions were identified through an electronic algorithm, and subjects are recruited from among patients of providers in two large health systems (Indiana University Health and Eskenazi Health) spread across the state of Indiana [33,34]. INGENIOUS has broad inclusion criteria, and clinicians can choose whether or not to follow its recommendations. According to the Pragmatic Explanatory Continuum Indicator Summary 2 (PRECIS-2) tool [35], INGENIOUS is highly pragmatic, minimizing study-related measures that would impact usual care.
Subjects
Subjects were included in this interim analysis if they met the following criteria: they were enrolled in the intervention arm of the INGENIOUS trial prior to 1 September 2017, gave informed consent, had a blood or saliva specimen, and they received at least one dose of tramadol or codeine as a primary or secondary medication that precipitated the delivery of pharmaco-genotyping results. The indications for tramadol or codeine included both acute and chronic pain. A total of 176 subjects met these criteria. A primary precipitant enrollment was defined as a new prescription of tramadol or codeine prompting entry into the trial (N = 172). A secondary precipitant medication enrollment refers to an individual enrolled after a new prescription of a nonopioid (e.g., clopidogrel), but who then received a new prescription of tramadol or codeine within 1 year (N = 4). One subject received tramadol as a primary precipitating medication and acetaminophen with codeine as a secondary precipitating medication and was analyzed based on receiving tramadol. All subjects included in the present analysis were from the intervention arm; our comparisons are made between groups of genotyped subjects.
Subjects were excluded from the analysis if: the patient never received a tramadol or codeine prescription, it could be confirmed that the patient never filled or took the trigger medication despite a prescription, the precipitating medication was for a nonpain indication such as cough, the patient's sample failed genotyping, the patient had a positive urine drug screen (UDS) during follow-up or the patient received a simultaneous and concurrent prescription of a second opioid (e.g., tramadol and oxycodone were prescribed on the same day). The latter exclusion was used because it was not possible to separate the effects of the two drugs.
Genotyping
The DNA was extracted from whole blood or saliva using the Qiagen EZ1 XL (MD, USA) according to manufacturer's recommendations. Genotyping of CYP2D6 was performed by using PCR and Taqman® (ThermoFisher, MA, USA) allele discrimination in a custom designed microarray for the following variants: CYP2D6*2 (c.2850C>T and c.4180G>C), CYP2D6*3 (c.2549delA), CYP2D6*4 (c.1846G>A and c.100C>T), CYP2D6*6 (c.1707delT), CYP2D6*7 (c.2935A>C), CYP2D6*9 (c.2615_2617delAAG), CYP2D6*10 (c.100C>T and c.4180G>C), CYP2D6*17 (c.1023C>T), CYP2D6*29 (c.3183G>A), CYP2D6*41 (c.2988G>A). Copy number (e.g, 0, 1, 2, 3 or more) of CYP2D6 was performed using the ΔΔ Ct method (ThermoFisher). Subjects were reported as an indeterminate phenotype (i.e., metabolizer status), if a duplication/multiplication was observed and it was not possible to determine which allele was duplicated/multiplicated.
Prescribing behavior outcomes
The INGENIOUS trial does not have prespecified outcomes to measure prescribing behavior with regard to tramadol and codeine. The outcomes below were adjudicated retrospectively by manual search of each participant's Indiana University Health or Eskenazi Health system EHR data by two clinically trained investigators. All available follow-up data were utilized. As an interim analysis, all included subjects were followed for a minimum of 90 days postopioid prescription. The maximum follow-up was 1 year for those who had completed the INGENIOUS study. Comparisons were made between individuals with actionable (ultrarapid, intermediate/reduced or poor) and nonactionable (normal/extensive, indeterminate) metabolizer status. The rationale for these comparisons lies in the provider recommendations that the INGENIOUS trial generates in the EHR, wherein no change in dose or drug selection is recommended for either normal or indeterminate metabolizers, but changes are recommended for ultrarapid, poor and intermediate/reduced metabolizers. An additional comparison was made using a logistic regression model to account for the strength of recommendation. Normal and indeterminate results recommended no change to standard therapy; intermediate/reduced metabolizer results contained moderate recommendations regarding dosage or monitoring and UM or PM results contained strong recommendations for alternative drug selection.
Outcomes assessed included: an alternative opioid prescribed during follow-up periods of 30, 60 and 90 days, and 1 year, purposeful discontinuation of tramadol or codeine by a provider prior to the expiration or conclusion of the prescription, a change in dose during follow-up, a refill of tramadol or codeine during follow-up and an nonsteroidal anti-inflammatory drugs (NSAIDs) prescribed or noted in the EHR during follow-up. Alternative opioids included hydrocodone, oxycodone, morphine, hydromorphone, and fentanyl. Alternative opioids administered for preprocedural sedation were excluded. For example, a single dose of intravenous fentanyl for a colonoscopy was not counted toward an alternative opioid outcome.
Efficacy & adverse event measures
All efficacy and adverse event outcomes of tramadol and codeine usage were determined retrospectively using the EHR. The main efficacy outcome was the presence of patient reported uncontrolled pain after the initial opioid prescription. To ascertain efficacy, two investigators reviewed each patient's EHR data and all relevant documentation to determine the presence of uncontrolled pain as documented by a provider. Discrete scores on a ‘pain scale’ of 8, 9 or 10 were also deemed to represent uncontrolled pain.
Additionally, two investigators screened relevant EHR entries to determine if a participant experienced the toxicity side effects of constipation or sedation. Reports of constipation or sedation that were not related or unlikely to have been related to the precipitating medication were excluded.
Activity scores & drug–drug interactions
For each CYP2D6 allele variant, a value relative to the fully functional CYP2D6*1 reference allele was assigned to construct a CYP2D6 activity score, ranging from 0 for poor to 3 for UM. The allele based contributions to the activity score were: score of 0: *3, *4, *4xN, *5, *6, *7, *16, *36, *40, *42, *56B, score of 0.5: *9, *10, *17, *29, *41, *45, *46, score of 1: *1, *2, *35, *43, *45xN and score of 2: *1xN, *2xN, *35xN [2]. Normal metabolizers (activity score of 2) served as the comparator group for adverse event analyses. Indeterminate metabolizers (with *1/*4/xN or *2/*4/xN genotypes) had unpredictable activity scores and were excluded from the efficacy and toxicity analyses. Comparisons were made using a logistic regression model between activity and efficacy or toxicity outcomes.
Medication lists were screened to identify concomitant DDIs with CYP2D6 moderate and strong inhibitors. According to CPIC recommendations, activity scores were adjusted to 0 with concomitant use of the strong inhibitors paroxetine, quinidine, terbinafine, thioridazine, propafenone, fluoxetine or bupropion. For the moderate inhibitors, duloxetine, fluvoxamine, mirabegron, cinacalcet and cimetidine, activity scores based on genotype were divided in half and rounded down to the nearest 0.5 increment [4].
Data analysis
All data were integrated and analyzed using SAS callable SUDAAN 9.0 (RTI International, Research Triangle Park, NC, USA) and R. Logistic regression was used to determine the significance of prescribing behavior, efficacy and adverse events when appropriate. Sensitivity analyses were performed for indeterminate metabolizers and individuals prescribed opioids for nonpain indications. For the cohort, odds ratios (OR) with 95% CI were calculated with nonactionable genotypes as the reference comparator for prescribing behavior and normal metabolizers as the reference group for efficacy and adverse events. Covariates such as age, race, gender, ethnicity and health system were adjusted in each logistic regression. Due to the small sample size, some comparisons produced cell sizes that violated the law of large numbers required for logistic regression, and the Fisher's Exact Test was conducted to determine if there were significant differences (p < 0.05).
Results
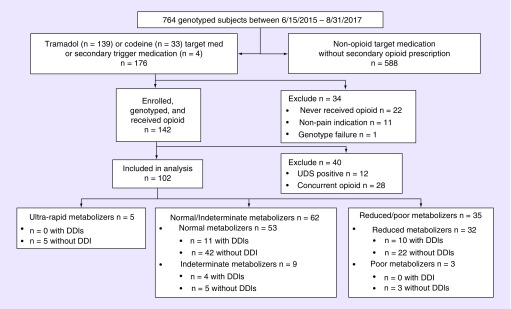
Of 764 subjects enrolled in the INGENIOUS trial intervention arm, 102 met inclusion criteria for this analysis based on a new prescription of tramadol or codeine. Table 1 describes the demographic information of the subjects included in the analysis. The mean age was 51.5 years, and 64.7% of subjects were female. The majority of individuals were enrolled following outpatient or emergency department prescriptions of either opioid. A number of exclusion criteria were employed (Figure 1). Patients, who had a genotype failure, neither filled nor took the precipitating medication or received the medication for a nonanalgesic purpose (e.g., codeine for cough) were excluded. Patients, who had a positive UDS during follow-up, were removed from the analysis because a positive test for illicit substances skewed prescribing behavior by prompting clinicians to discontinue the opioid and to avoid prescribing future alternate opioids. Dual opioid prescriptions were common as 28 of 142 (20%) enrolled individuals received a second new opioid prescription on the same day as their initial tramadol or codeine prescription. Common rationales for this prescribing behavior included: instructions to take different medications according to pain severity and using one medication as a maintenance dose and another as breakthrough therapy. Participants, who received planned concurrent opioid analgesia, were excluded from the analysis because the effects of each drug could not be differentiated. Supplementary Table 2 includes demographic information for all individuals who were enrolled, genotyped and received the precipitating opioid (N = 142). No significant difference was noted between those included (N = 102) and those excluded (N = 40) from the final analysis.
Table 1. . Demographics.
| Number of participants | N = 102 |
|---|---|
| Gender, N (%) | |
| – Female | 66 (64.7%) |
| – Male | 36 (35.3%) |
| Age, mean (range) | 51.5 years (21–95 years) |
| – Ethnicity, N (%) | |
| – Hispanic or Latino | 2 (2%) |
| – Not Hispanic or Latino | 95 (93%) |
| – Prefer not to answer | 5 (5%) |
| Race, N (%) | |
| – Asian | 1 (1.0%) |
| – Black or African–American | 34 (33.3%) |
| – White | 65 (63.7%) |
| – Other or unreported | 2 (2.0%) |
| Patient location | |
| – Emergency department | 2 (2%) |
| – Inpatient | 6 (6%) |
| – Outpatient | 94 (92%) |
| Indication: | |
| – Pain | 102 (100%) |
Figure 1. . Inclusion and exclusion criteria for the 102 participants in the analysis.
All participants were enrolled prior to 31 August 2017. Primary trigger medications are medications prompting enrollment. Secondary trigger medication indicates a prescription for tramadol or codeine within 1 year of a subject's trial enrollment for a nonopioid medication.
DDI: Drug–drug interaction with a CYP2D6 inhibitor; UDS: Urine drug screen.
Table 2 describes the distribution of predicted phenotypes in the participants included in the final analysis. A genotype was considered actionable if the laboratory result report contained a recommendation for a change in dose or agent. Reports for UM and PMs contained strong recommendations for alternate agent selection or dose change, whereas reports for reduced metabolizers contained moderate recommendations for a dose change. A portion of the cohort had DDIs at the time of enrollment (24.5%). The trial's adjudication committee alerts prescribers to the presence of drug–drug–gene interactions upon the initial prescription. Most participants (65.7%) concurrently used prescribed or over-the-counter NSAIDs either at the time of enrollment or during the follow-up period. No significant difference in NSAID use was identified between groups stratified by metabolizer status (Table 2).
Table 2. . Clinical characteristics.
| Category | N (%) |
|---|---|
| Metabolizer status inferred from genotype | |
| – Ultrarapid | 5 (4.9%) |
| – Normal | 53 (52.0%) |
| – Indeterminate | 9 (8.8%) |
| – Reduced/intermediate | 32 (31.3%) |
| – Poor | 3 (2.9%) |
| Genotype status | |
| – Actionable | 40 (39.2%) |
| – Nonactionable | 62 (60.8%) |
| Drug–drug interactions | |
| – Total | 25 (24.5%) |
| – Fluoxetine† | 6 (5.9%) |
| – Buproprion† | 5 (4.9%) |
| – Paroxetine | 5 (4.9%) |
| – Duloxetine† | 10 (9.8%) |
| – Mirabegron | 1 (1.0%) |
| NSAID use | |
| – Total | 67 (65.7%) |
| – Ultrarapid or poor | 5 (62.5%) |
| – Reduced | 22 (68.8%) |
| – Normal/indeterminate | 40 (64.5%) |
†Two drug–drug interactions involved multiple CYP2D6 inhibitors including buproprion with either fluoxetine or duloxetine.
NSAID: Nonsteroidal anti-inflammatory drug.
A significant association between genotype actionability and prescribing behavior was identified (Table 3). Individuals were grouped according to genotype-based recommendations. Within 30 days of an initial tramadol or codeine prescription, individuals with an actionable genotype were prescribed an alternate opioid with greater frequency than those with a nonactionable genotype (p = 0.04). Analogous observations were made at 60 and 90 days, but not with up to 1 year of follow-up. The frequency of a provider-initiated discontinuation of a precipitating medication was also measured. Only prescriptions that were stopped by a provider prior to expiration or completion of the prescription duration were counted. Participants with actionable genotypes were more likely than those with nonactionable genotypes to have their tramadol or codeine actively stopped (p = 0.002). No significant difference in opioid dose change was associated with genotype (p = 0.79). However, normal and indeterminate metabolizers were more likely to be provided a refill by their providers in the available follow-up period of up to 1 year (p = 0.01).
Table 3. . Logistic regression association between prescribing behavior and genotype.
| Variable | (A) Poor/ultrarapid N = 8 (%) | (B) Reduced N = 32 (%) | (C) Normal/indeterminate N = 62 (%) | OR (95% CI) (A) v (C) | OR (95% CI) (B) v (C) | p-value |
|---|---|---|---|---|---|---|
| Alternate opioid prescribed by 30 days (Y) | 3 (38) | 3 (9) | 3 (5) | 15.1 (1.8–161.3) | 2.3 (0.4–13.7) | 0.04 |
| Alternate opioid prescribed by 60 days (Y) | 4 (50) | 4 (12) | 3 (5) | 19.0 (2.8– 160.4) | 2.9 (0.6– 16.2) | 0.01 |
| Alternate opioid prescribed by 90 days (Y) | 4 (50) | 8 (25) | 7 (11) | 9.5 (1.7– 57.3) | 2.6 (0.8– 8.8) | 0.03 |
| Alternative opioid prescribed during follow-up (Y)† | 5 (63) | 11 (34) | 17 (27) | 4.6 (0.9–25.9) | 1.5 (0.6– 4.0) | 0.16 |
| Discontinuation of tramadol or codeine during follow-up (Y)† | 7 (88) | 12 (38) | 15 (24) | 24.7 (3.6– 503.1) | 1.9 (0.7– 5.1) | 0.002 |
| Dose change (Y)† | 1 (13) | 4 (13) | 13 (21) | 0.6 (0.03– 4.6) | 0.7 (0.2– 2.2) | 0.79 |
| Refill prescribed (Y)† | 0 (0) | 14 (44) | 30 (48) | 0 (NA)‡ | 0.9 (0.3– 2.2) | 0.01 |
†All available follow-up (up to 1 year) was used to determine the outcome.
‡95% CI not available due to sample size.
OR: Odds ratio; Y: Yes or positive outcome.
All p-value calculations are based on a multivariate logistic regression model.
Similar associations were identified after metabolizer status was adjusted for DDIs (Table 4). After accounting for DDIs, 18 participants were reclassified as PMs. Individuals with actionable metabolizer phenotypes were more likely to be prescribed an alternate opiate at 30, 60 days or have their precipitating medication discontinued during follow-up. Subjects with actionable genotypes also had a reduced refill frequency (p = 0.046). No statistically significant differences for alternate opioid prescriptions within 90 days or during all follow-up were found between actionable and nonactionable genotypes across the entire drug–gene and drug–drug interaction (DGI + DDI) logistic regression model. Two sensitivity analyses for prescribing behavior were performed by either including individuals with nonpain indications (N = 11) or excluding indeterminate metabolizers (N = 9). Similar results were found in both sensitivity analyses. When including nonpain opioid indications, individuals with actionable genotypes were more likely to have their tramadol or codeine discontinued (p = 0.03) or an alternate opioid prescribed in the first 30 days (p = 0.04). When removing indeterminate metabolizers from the analysis, the actionable individuals were still more likely to have their opioid discontinued (p = 0.02) or an alternate opioid prescribed (p = 0.004).
Table 4. . Logistic regression association between prescribing behavior and genotype adjusted for drug–drug interactions.
| Variable | (A) Poor/ultrarapid N = 25 (%) | (B) Reduced N = 30 (%) | (C) Normal/indeterminate N = 47 (%) | OR (95% CI) (A) v (C) | OR (95% CI) (B) v (C) | p-value |
|---|---|---|---|---|---|---|
| Alternate opioid prescribed by 30 days (Y) | 6 (24) | 0 (0) | 3 (6) | 4.6 (NA)† | 0 (NA)† | 0.01 |
| Alternate opioid prescribed by 60 days (Y) | 7 (28) | 1 (3) | 3 (6) | 4.9 (1.2–26.1) | 0.6 (0.03–5.0) | 0.003 |
| Alternate opioid prescribed by 90 days (Y) | 7 (28) | 6 (20) | 6 (13) | 3.4 (0.9–13.4) | 1.8 (0.4–3.3) | 0.19 |
| Alternative opioid prescribed during follow-up (Y)‡ | 13 (52) | 8 (27) | 12 (26) | 3.6 (1.3–11.20 | 1.3 (0.4–4.1) | 0.054 |
| Discontinuation of tramadol or codeine during follow-up (Y)‡ | 13 (52) | 11 (37) | 10 (21) | 5.0 (1.7–16.7) | 2.8 (0.9–0.1) | 0.01 |
| Dose change (Y)‡ | 3 (12) | 4 (13) | 11 (23) | 0.5 (0.1–1.8) | 0.7 (0.2–2.6) | 0.58 |
| Refill prescribed (Y)‡ | 6 (24) | 14 (47) | 23 (49) | 0.3 (0.1–0.9) | 1.1 (0.4–3.1) | 0.046 |
†95% CI not available due to sample size.
‡All available follow-up (up to 1 year) was used to determine the outcome.
OR: Odds ratio; Y: Yes or positive outcome.
All p-value calculations are based on a multivariate logistic regression model.
Associations between metabolizer status and efficacy were examined (Table 5). The incidence of uncontrolled pain reported in the EHR during follow-up was 22.7%. Individuals were assigned an activity score according to two models: genotype alone in a drug–gene interaction model (DGI) or genotype adjusted for DDIs in the combined DGI + DDI model. Indeterminate metabolizers in whom an activity score could not be assigned were excluded. According to the DGI model, no significant association between activity score and uncontrolled pain was found. In the DGI + DDI model, a significant association with reported pain was identified. For every 1 unit increase in activity score, the odds ratio of reporting uncontrolled pain was 0.50 (95% CI: 0.25–0.94). Among individuals with a DDI-adjusted activity score of 0.5 or less, 40.7% complained of uncontrolled pain. Among individuals with activity score of 1 or greater, only 15.7% relayed similar complaints. The incidence of adverse events such as constipation and sedation was examined according to both models, but reporting of these adverse events in the EHR was low. No significant association between activity score and either constipation or sedation was identified.
Table 5. . Association of adverse events and inefficacy with according to activity score scale.
| Variable | Drug–gene model OR (95% CI) per unit activity score | p-value | DGI + DDI model OR (95% CI) per unit activity score | p-value |
|---|---|---|---|---|
| Uncontrolled pain (Y) | 0.929 (0.44–1.93) | 0.84 | 0.50 (0.25–0.94) | 0.03 |
DGI + DDI model: Drug–gene interaction and drug–drug interaction logistic regression model; OR: Odds ratio; Y: Yes or positive outcome.
The apparent acceptance rate by clinicians of the pharmacogenomic-guided dose recommendations was 81.8% (45 of 55) in subjects with actionable phenotypes (as evidenced by dose changes, discontinuation or alternate opioid prescriptions). The high acceptance rate precluded a statistical comparison of uncontrolled pain, stratified according to acceptance and nonacceptance. In summary, small but important differences were noted when clinical information on DDIs is added to genotype information.
Discussion
In this study, we present an interim analysis of the prescribing behavior and efficacy associated with tramadol and codeine use, as gleaned from the INGENIOUS trial. The two major findings of our study are: genotype and metabolizer status are associated with differential prescribing behavior, and genotype alone was insufficient to predict opioid efficacy (pain control) without consideration of concomitant drug–drug interactions. At the onset, the INGENIOUS trial did not contain prespecified outcomes related to pain control for codeine or tramadol. Although the INGENIOUS investigators contacted each provider with regard to participants with drug–gene and drug–drug interactions, prescribers are free to adjust dosing as they see fit. Thus, the cause and effect relationship between genotyping and prescribing behavior cannot be completely ascertained. Specifically, we cannot distinguish whether the provider changed a prescription based on the genotyping test or whether the genotype simply reflects the natural history of efficacy and toxicity, which would have prompted alterations in prescriber behavior.
This pragmatic study builds upon a strong foundation of pharmacokinetic and clinical efficacy studies. Several preceding investigations have illustrated the relationships between CYP2D6 genotype, tramadol pharmacokinetics and tramadol efficacy [36–39]. These collective data ultimately culminated in DPWG guidelines recommending genotype-guided tramadol therapy [40]. Controlled studies of codeine that relate metabolizer status to metabolite concentrations and efficacy [41–43], as well as several high profile case studies [44,45], have led to the curation of CPIC guidelines for pharmacogenomic-guided dosing of codeine [4]. Corroborating our results, a prior study revealed that implementation of pharmacogenomics in a large health system impacted prescribing behavior of codeine for children with sickle cell disease [8].
When comparing those with and without actionable genotypes, our analysis revealed that healthcare providers displayed different prescribing behavior. Ultrarapid, poor and intermediate/reduced metabolizers were more likely to receive an alternate opioid or to have their prescription discontinued as compared with normal or indeterminate metabolizers. These same individuals were less likely to receive a refill than normal metabolizers were. Most practitioners never documented their rationale in the clinical notes for dose changes, medication discontinuations or refills. Therefore, during retrospective adjudication, it was not possible to determine whether prescribing behavior was a surrogate for undocumented adverse events or inefficacy. Further, adverse events were infrequently recorded and almost never coded as a diagnosis. Similarly, pain control was not a defined end point and it is possible that inefficacy was under-reported. After adjusting activity score for DDIs, uncontrolled pain was found more frequently reported in individuals with reduced CYP2D6 activity. This finding was obscured when only the drug–gene model was implemented and speaks to the importance of phenoconversion for DDIs. A future aim is to conduct a full analysis of all participants when the INGENIOUS trial follow-up concludes, including a comparison between those who were genotyped and those who were randomized to the control arm and those who were randomized to the intervention arm but were not genotyped because they could not be reached or declined to provide informed consent. This interim analysis based on a manual EHR search will help to inform a more comprehensive bioinformatics approach to evaluate tramadol and codeine outcomes.
There are significant limitations in this investigation. Foremost among these limitations is the small sample size and the decision to forego an intention-to-treat comparison. In INGENIOUS, 176 individuals were consented in the intervention arm after a tramadol or codeine trigger medication, but only 102 were included in this study's final analysis. The pragmatic nature of INGENIOUS allowed for broad inclusion criteria. The EHR's automated enrollment request was prompted by a prescription, but upon chart review there was evidence to confirm that 22 of the 176 patients, who consented to genotyping, never filled their prescription or the prescription was canceled immediately. These circumstances were eliminated after one of the two health systems adopted a new EHR. The trial protocol adapted, enrolling subjects only after they filled their prescription. A second limitation is that efficacy is not independent of prescribing and may be impacted by changes in prescribing behavior. In order to disentangle this inter-relationship, the sequence of events matters. In most cases, the genotyping data were provided 7 days after the precipitating opioid prescription, suggesting that the second prescribing action taken during follow-up may have been in response to inefficacy or toxicity. If the change was in response to inefficacy or toxicity, rather than providers accepting our initial recommendations, this would also support the value of implementing pharmacogenetic genotyping to improve therapy outcomes.
Subjects with positive UDS tests and concurrent opioid therapy were excluded from the analysis. An opioid prescription was universally discontinued in individuals who had a positive UDS, and the patients were referred to a pain management service. Because the outcomes we measured included pain control and prescribing behavior, we also excluded the planned use of concurrent opioid therapy. Given the small sample size, response to UDS tests and concurrent opioid therapy may have masked any associations with genotype or drug–drug interactions. However, these considerations also become important in the planning of future trials, which test the impact of pharmacogenomic-guided dosing on pain control or opioid adverse events. Two deaths were noted during the follow-up period but were determined to be unrelated to tramadol or codeine use.
The opioid epidemic has been associated with an increase in adverse drug effects [11,46], but recognition of this crisis has also heightened awareness of the variability in response to these medications [18,47]. State laws continue to evolve surrounding the prescribing of opioids. Beginning July 2017, a new Indiana state law (SEA 226) required additional documentation if providers intended to prescribe an opioid for more than 7 days duration in any adult patient receiving a first-time prescription [48]. It is likely that moving forward, this legislation will have a greater impact on prescribing behavior and will need to be considered in future trial protocol development.
Future perspective
This investigation supports the findings of prior studies showing a relationship between genotype, prescribing behavior and opioid efficacy. In the USA, a major challenge to the advancement of pharmacogenomics is the level of evidence supporting payer reimbursement. Due to their greater applicability to the real-world setting, it is suggested that results from pragmatic trials have a capacity to inform policy-making processes [49,50]. The understanding of a patient's pharmacogenomic response profile furnishes a healthcare provider with vital information that will guide therapeutic decisions [51,52]. Incorporation of genotype biomarkers has the potential to improve the utility and efficacy of current strategies and to guide the development of new approaches for pain management [52,53]. However, knowledge of pharmacogenomic results alone is insufficient to accurately predict metabolizer status phenotypes. Clinicians must remain vigilant for DDIs in order to select appropriate drugs and dosages. Increased sophistication of computer decision support tools may aid clinicians in reconciling pharmacogenomics and DDIs, but until these tools are broadly implemented, continued awareness is required. In addition, since many patients see healthcare providers from multiple healthcare systems, pharmacogenomic results must be shared across healthcare systems, or a system is needed for the patients to provide the pharmacogenomic information at the provider encounters. This interim study lays the foundation for future investigations, which examine EHR data for adverse events and clinical outcomes within the INGENIOUS trial. The IGNITE Network and the INGENIOUS trial continue to contribute to the clinical evidence required to support pharmacogenomic implementation.
Summary points.
The INGENIOUS trial is a pragmatic implementation trial and part of the IGNITE network, which aims to test the real world impact of pharmacogenomic testing outside of a tightly controlled environment.
We tested whether providers would change prescribing behavior with regard to opioids for subjects with actionable genotypes as compared with those with nonactionable genotypes and whether efficacy measures such as uncontrolled pain would correspond to an individual's metabolism activity score, with and without consideration of drug–drug interactions.
CYP2D6 genotype and metabolizer status were associated with differential prescribing behavior by clinicians.
Genotype alone was insufficient to predict opioid efficacy without including phenoconversion for concomitant drug–drug interactions.
This interim study lays the foundation for future investigations, which examine electronic health record data for adverse events and clinical outcomes of pharmacogenomic implementation.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/pgs-2018-0205
Financial & competing interests disclosure
The INGENIOUS trial (NCT02297126) is sponsored by an NIH/NHGRI U01-grant (HG007762). Y Zang, Z Desta, MB Rosenman, AM Holmes, KD Levy, VM Pratt, BT Gufford, PR Dexter and TC Skaar are supported by the NIH-U01 HG007762. MT Eadon was supported by an NIH/NIDDK award (K08DK107864). VM Pratt is also supported by the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative. BT Gufford was also supported by the Indiana Clinical and Translational Sciences Institute Young Investigator Award (NIH-UL1 TR001108). This project was also supported by an NIH/NIGMS award entitled the Indiana University Comprehensive Training in Clinical Pharmacology (T32GM008425), which provided stipend support for CR Fulton. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical disclosure
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Author contributions
CR Fulton, MT Eadon, Y Zang, Z Desta and MB Rosenman performed analysis of data and manuscript preparation. AM Holmes, BS Decker, TC Skaar, PR Dexter, KD Levy and JT Callaghan conducted the trial. VM Pratt and BT Gufford reviewed and edited final manuscript. All authors provided approval of the final version.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 2017;19(1):69–76. doi: 10.1038/gim.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008;83(2):234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]; •• Formed the basis of how we assigned activity scores based on CYP2D6 genotype.
- 3.Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, editors. Medical Genetics Summaries. National Center for Biotechnology Information; MD, USA: 2012. [PubMed] [Google Scholar]
- 4.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin. Pharmacol. Ther. 2014;95(4):376–382. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Details the Clinical Pharmacogenomics Implementation Consortium guidelines for CYP2D6 and codeine therapy.
- 5.Smith DM, Weitzel KW, Cavallari LH, Elsey AR, Schmidt SO. Clinical application of pharmacogenetics in pain management. Per. Med. 2018;15(2):117–126. doi: 10.2217/pme-2017-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eadon MT, Desta Z, Levy KD, et al. Implementation of a pharmacogenomics consult service to support the INGENIOUS trial. Clin. Pharmacol. Ther. 2016;100(1):63–66. doi: 10.1002/cpt.347. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the trial design and clinical support within the INGENIOUS study.
- 7.Bell GC, Crews KR, Wilkinson MR, et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J. Am. Med. Inform. Assoc. 2014;21(e1):e93–e99. doi: 10.1136/amiajnl-2013-001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gammal RS, Crews KR, Haidar CE, et al. Pharmacogenetics for safe codeine use in sickle cell disease. Pediatrics. 2016;138(1):e20153479. doi: 10.1542/peds.2015-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danahey K, Borden BA, Furner B, et al. Simplifying the use of pharmacogenomics in clinical practice: building the genomic prescribing system. J. Biomed. Inform. 2017;75:110–121. doi: 10.1016/j.jbi.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Reinecke H, Weber C, Lange K, Simon M, Stein C, Sorgatz H. Analgesic efficacy of opioids in chronic pain: recent meta-analyses. Br. J. Pharmacol. 2015;172(2):324–333. doi: 10.1111/bph.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm. Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 12.Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann. Intern. Med. 2011;155(5):325–328. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franklin GM American Academy Of Neurology. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83(14):1277–1284. doi: 10.1212/WNL.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 14.Watkins EA, Wollan PC, Melton LJ, 3rd, Yawn BP. A population in pain: report from the Olmsted County health study. Pain Med. 2008;9(2):166–174. doi: 10.1111/j.1526-4637.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 15.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J. Pain. 2015;16(8):769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J. Pain. 2019;20(2):146–160. doi: 10.1016/j.jpain.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy J, Roll JM, Schraudner T, Murphy S, Mcpherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J. Pain. 2014;15(10):979–984. doi: 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. Am. J. Prev. Med. 2015;49(3):409–413. doi: 10.1016/j.amepre.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressler LG, Bell GC, Ruch KD, Retamal JD, Krug PB, Paulus RA. Implementing a personalized medicine program in a community health system. Pharmacogenomics. 2018;19(17):1345–1356. doi: 10.2217/pgs-2018-0130. [DOI] [PubMed] [Google Scholar]
- 20.Dong OM, Li A, Suzuki O, et al. Projected impact of a multigene pharmacogenetic test to optimize medication prescribing in cardiovascular patients. Pharmacogenomics. 2018 doi: 10.2217/pgs-2018-0049. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiro AM, Margarit C, LL A. What is the future of pharmacogenomics in pain management? Pharmacogenomics. 2017;18(2):101–103. doi: 10.2217/pgs-2016-0173. [DOI] [PubMed] [Google Scholar]
- 22.Rosenman MB, Decker B, Levy KD, Holmes AM, Pratt VM, Eadon MT. Lessons learned when introducing pharmacogenomic panel testing into clinical practice. Value Health. 2017;20(1):54–59. doi: 10.1016/j.jval.2016.08.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blagec K, Kuch W, Samwald M. The Importance of gene-drug–drug-interactions in pharmacogenomics decision support: an analysis based on Austrian claims data. Stud. Health Technol. Inform. 2017;236:121–127. [PubMed] [Google Scholar]
- 24.Gaedigk A, Dinh JC, Jeong H, Prasad B, Leeder JS. Ten years’ experience with the CYP2D6 activity score: a perspective on future investigations to improve clinical predictions for precision therapeutics. J. Pers. Med. 2018;8(2) doi: 10.3390/jpm8020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knisely MR, Carpenter JS, Draucker CB, et al. CYP2D6 drug–gene and drug–drug–gene interactions among patients prescribed pharmacogenetically actionable opioids. Appl. Nurs. Res. 2017;38:107–110. doi: 10.1016/j.apnr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storelli F, Matthey A, Lenglet S, Thomas A, Desmeules J, Daali Y. Impact of CYP2D6 functional allelic variations on phenoconversion and drug–drug interactions. Clin. Pharmacol. Ther. 2017 doi: 10.1002/cpt.889. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Bahar MA, Setiawan D, Hak E, Wilffert B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics. 2017;18(7):701–739. doi: 10.2217/pgs-2017-0194. [DOI] [PubMed] [Google Scholar]; • Reviews the interacting sites within the IGNITE network.
- 28.Shah RR, Smith RL. Addressing phenoconversion: the Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015;79(2):222–240. doi: 10.1111/bcp.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med. Genomics. 2017;10(1):35. doi: 10.1186/s12920-017-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpi S, Bult CJ, Chisholm RL, et al. Research directions in the clinical implementation of pharmacogenomics: an overview of US programs and projects. Clin. Pharmacol. Ther. 2018;103(5):778–786. doi: 10.1002/cpt.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlando LA, Sperber NR, Voils C, et al. Developing a common framework for evaluating the implementation of genomic medicine interventions in clinical care: the IGNITE Network's Common Measures Working Group. Genet. Med. 2017;20(6):655–663. doi: 10.1038/gim.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierson RC, Gufford BT, Desta Z, Eadon MT. Clinical and educational impact of pharmacogenomics testing: a case series from the INGENIOUS trial. Pharmacogenomics. 2017;18(9):835–841. doi: 10.2217/pgs-2017-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter JS, Rosenman MB, Knisely MR, Decker BS, Levy KD, Flockhart DA. Pharmacogenomically actionable medications in a safety net health care system. SAGE Open Med. 2016;4:2050312115624333. doi: 10.1177/2050312115624333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy KD, Decker BS, Carpenter JS, et al. Prerequisites to implementing a pharmacogenomics program in a large healthcare system. Clin. Pharmacol. Ther. 2014;96(3):307–309. doi: 10.1038/clpt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford I, Norrie J. Pragmatic trials. N. Engl. J. Med. 2016;375(5):454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]; • Includes the Dutch Pharmacogenomic Working Group recommendations for many drugs including tramadol.
- 36.Enggaard TP, Poulsen L, Arendt-Nielsen L, Brosen K, Ossig J, Sindrup SH. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth. Analg. 2006;102(1):146–150. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- 37.Stamer UM, Lehnen K, Hothker F, et al. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. 2003;105(1-2):231–238. doi: 10.1016/s0304-3959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 38.Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in healthy subjects – the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur. J. Clin. Pharmacol. 2005;61(4):257–266. doi: 10.1007/s00228-005-0920-y. [DOI] [PubMed] [Google Scholar]
- 39.Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin. Pharmacol. Ther. 2007;82(1):41–47. doi: 10.1038/sj.clpt.6100152. [DOI] [PubMed] [Google Scholar]
- 40.Swen JJ, Nijenhuis M, De Boer A, et al. Pharmacogenetics: from bench to byte – an update of guidelines. Clin. Pharmacol. Ther. 2011;89(5):662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 41.Madadi P, Ross CJ, Hayden MR, et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case–control study. Clin. Pharmacol. Ther. 2009;85(1):31–35. doi: 10.1038/clpt.2008.157. [DOI] [PubMed] [Google Scholar]
- 42.Williams DG, Patel A, Howard RF. Pharmacogenetics of codeine metabolism in an urban population of children and its implications for analgesic reliability. Br. J. Anaesth. 2002;89(6):839–845. doi: 10.1093/bja/aef284. [DOI] [PubMed] [Google Scholar]
- 43.Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7(4):257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- 44.Gasche Y, Daali Y, Fathi M, et al. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N. Engl. J. Med. 2004;351(27):2827–2831. doi: 10.1056/NEJMoa041888. [DOI] [PubMed] [Google Scholar]
- 45.Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368(9536):704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- 46.Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15(11):1911–1929. doi: 10.1111/pme.12480. [DOI] [PubMed] [Google Scholar]
- 47.Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis Rheum. 2005;52(1):312–321. doi: 10.1002/art.20784. [DOI] [PubMed] [Google Scholar]
- 48.Merritt J RJ, Charbonneau E. Senate bill 226. 2017. https://legiscan.com/IN/bill/SB0226/2017
- 49.Moller HJ. Effectiveness studies: advantages and disadvantages. Dialogues Clin. Neurosci. 2011;13(2):199–207. doi: 10.31887/DCNS.2011.13.2/hmoeller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Califf RM, Sugarman J. Exploring the ethical and regulatory issues in pragmatic clinical trials. Clin. Trials. 2015;12(5):436–441. doi: 10.1177/1740774515598334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drewes AM, Jensen RD, Nielsen LM, et al. Differences between opioids: pharmacological, experimental, clinical and economical perspectives. Br. J. Clin. Pharmacol. 2013;75(1):60–78. doi: 10.1111/j.1365-2125.2012.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA. Applications of CYP 450 testing in the clinical setting. Mol. Diagn. Ther. 2013;17(3):165–184. doi: 10.1007/s40291-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang KL, Weitzel K, Schmidt S. Pharmacogenetics: using genetic information to guide drug therapy. Am. Fam. Physician. 2015;92(7):588–594. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.