Abstract
N-Myc downstream-regulated gene 2 (NDRG2) was characterized as a tumor suppressor, inducing anti-metastatic and anti-proliferative effects in several tumor cells. However, NDRG2 functions on anticancer drug sensitivity, and its molecular mechanisms are yet to be fully investigated. In this study, we investigated the mechanism of NDRG2-induced sensitization to As2O3 in the U937 cell line, which is one of the most frequently used cells in the field of resistance to As2O3. NDRG2-overexpressing U937 cells (U937-NDRG2) showed a higher sensitivity to As2O3 than mock control U937 cell (U937-Mock). The higher sensitivity to As2O3 in U937-NDRG2 was associated with Mcl-1 degradation through glycogen synthase kinase 3β (GSK3β) activation. Inhibitory phosphorylation of GSK3β was significantly reduced in U937-NDRG2, and the reduction was diminished by okadaic acid, a protein phosphatase inhibitor. NDRG2 mediated the interaction between GSK3β and protein phosphatase 2A (PP2A), inducing dephosphorylation of GSK3β at S9 by PP2A. Although the C-terminal deletion mutant of NDRG2 (ΔC NDRG2), which could not interact with PP2A, interacted with GSK3β, the mutant failed to dephosphorylate GSK3β at S9 and increased sensitivity to As2O3. Our findings suggest that NDRG2 is a kind of adaptor protein mediating the interaction between GSK3β and PP2A, inducing GSK3β activation through dephosphorylation at S9 by PP2A, which increases sensitivity to As2O3 in U937 cells.
Keywords: PP2A–NDRG2–GSK3β complex, myeloid leukemia, U937, Mcl-1, apoptosis, arsenic trioxide
1. Introduction
N-Myc downstream-regulated gene 2 (NDRG2) plays an important role in tumor suppression in several cancers or cancer cell lines [1,2]. NDRG2 expression is positively correlated with tumor differentiation but negatively correlated with metastasis and TNM classification of malignant tumors (TNM) stage [3,4,5,6]. Signaling pathways inducing epithelial–mesenchymal transition (EMT), including Wnt and phosphoinositide 3-kinase (PI3K), were regulated by glycogen synthase kinase 3β (GSK3β) [7,8]. NDRG2 expression was positively correlated with GSK3β activation [9,10], and various NDRG2-mediated GSK3β regulation mechanisms were suggested [11,12]. NDRG2 recruits PP2A to dephosphorylate phosphatase and tensin homolog (PTEN), which inhibits protein kinase B (AKT) activity through dephosphorylation. Active AKT induces phosphorylation of GSK3β at S9, an inactive form of GSK3β. Therefore, the PTEN–NDRG2–PP2A complex might induce GSK3β activation through inhibition of AKT activity, which inhibits tumor metastasis.
As2O3 is an effective anticancer drug for acute promyelocytic leukemia (APL) patients [13,14,15]. However, the drug’s clinical application is limited due to low sensitivity in other types of leukemia and its side effects [16,17,18]. It is known that Mcl-1 contributes to protect cells from apoptosis in APL cells [19]. The PI3K/AKT/mTOR pathway is known to promote cell survival through translational control of Mcl-1 in acute myeloid leukemia (AML) [20]. Mcl-1 has a short half-life due to rapid degradation after phosphorylation by GSK3β which is negatively regulated by active AKT [21]. Actually, Mcl-1 protein levels were decreased in As2O3-treated NB4 cells and As2O3-sensitive APL, and the decrease was dependent on GSK3β activation [22]. Therefore, strategies for AKT inhibition, GSK3β activation, and degradation of Mcl-1 could be important points to improve the sensitivity to As2O3 in APL.
In this study, we used U937 cells, which do not express NDRG2, to investigate whether NDRG2 affects cancer drug sensitivity, because the acute myeloid leukemia cell line, U937, is relatively resistant to As2O3 [23,24]. We found that NDRG2-overexpressing U937 (U937-NDRG2) cells were more sensitive to As2O3 compared to parental U937 (U937-Mock) cells. Although NDRG2 negatively regulates AKT activity through the PTEN–NDRG2–PP2A complex, the sensitivity of U937-NDRG2 cells to As2O3 does not mean that the complex inhibits the PI3K/AKT pathway, since the U937 cell line possesses a PTEN frameshift mutant [25]. In this study, we investigated and identified a novel mechanism of NDRG2-mediated GSK3β activation, which enhanced the sensitivity of U937 to As2O3 through Mcl-1 degradation.
2. Materials and Methods
2.1. Cell Culture, Reagents, and Short Hairpin RNAs (shRNAs)
Immortalized embryonic kidney cell lines, 293T/17 and HEK293, were purchased from American Type Culture Collection, ATCC (Manassas, VA, USA). The U937, U937-Mock, and U937-NDRG2 cell lines [26] were cultured in Roswell Park Memorial Institute (RPMI) -1640 medium containing 10% fetal bovine serum (FBS) (ATCC, Manassas, VA, USA), HEPES (Lonza, Basel, Swiss), β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA), and penicillin and streptomycin (Lonza, Basel, Swiss). The 293T/17 and HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% FBS, penicillin, and streptomycin at 37 °C in 5% CO2. In some experiments, the cells were treated with chemicals As2O3, MG132, 2’,7’-dichlorofluorescin diacetate (DCFH-DA), okadaic acid, SB216763 (Sigma-Aldrich, MO, USA), and z-VAD-fmk (ENZO, Seoul, Korea). The shMcl-1 clones (TRCN0000005514 and TRCN0000005516) were purchased from Sigma-Aldrich.
2.2. Western Blotting
The cell lysates were prepared in ProNATM CETi Lysis Buffer (TransLab, Daejeon, Korea). The protein extracts were concentrated by the Bradford protein assay (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. Equal amounts of proteins were separated by SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. Membrane blocking was performed using 5% skimmed milk (in Tris Buffered Saline with Tween 20) for 1 h at room temperature (RT), and the membrane was incubated with the primary antibody at 4 °C overnight. The horseradish peroxidase (HRP)-conjugated antibodies were then treated at RT for 1 h. The HRP signal was activated using Clarity™ ECL Western Blotting Substrate (Bio-Rad, Hercules, CA, USA). Band intensity was calculated using the Image J software (1.50g version, NIH, Bethesda, MD, USA). B-cell lymphoma 2 (Bcl-2), B-cell lymphoma–extra large (Bcl-xL), Bcl-2-associated X protein (Bax), and NDRG2 antibodies were purchased from Santa Cruz Biotechnology (TX, USA). Antibodies for PP2Ac, phosphorylated (p)-GSK3β (Ser9), GSK3β, Mcl-1, caspase-3, caspase-9, poly-ADP ribose polymerase (PARP), p-AKT (Thr308), p-AKT (Ser473), AKT, GST, and mouse anti-rabbit IgG-HRP were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-α-tubulin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-mouse Ig HRP was purchased from eBioscience (San Diego, CA, USA), and hemagglutinin (HA) antibody was purchased from abm (abm®, Vancouver, Canada).
2.3. Co-Immunoprecipitation and Glutathion S-Tranferase Tag(GST) Pull-Down Assay
After transfecting relevant vectors into HEK293 cells, or treatment of U937 cells with As2O3, the cells were harvested and lysed with buffer containing 0.5% NP40, 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, a protease inhibitor cocktail, and a phosphatase inhibitor cocktail. For GST pull-down assays, the cellular supernatants were incubated with 30 μL of 50% glutathione sepharose 4B slurry (GE Healthcare, Philadelphia, PA, USA) at 4 °C overnight. After incubation, the beads were washed three times using buffer containing 0.5% NP40, 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, and 1 mM ethylenediaminetetraacetic acid (EDTA) for 10 min. For co-immunoprecipitation, the cellular supernatants were incubated with 2 μg Flag antibody at 4 °C overnight, after which they were incubated with 20 μL of Protein G Plus/Protein A agarose beads (Millipore, Darmstadt, Germany) for an additional 2 h at 4 °C. The beads were washed three times, using the aforementioned buffer, for 10 min. All washed beads were incubated with protein sample buffer and boiled for 5 min. Twenty micrograms of protein from the cellular supernatants was used as whole-cell lysate (WCL) for downstream evaluation.
2.4. PP2Ac Activity Assay
PP2A activity was assessed using the Human/Mouse/Rat Active PP2A DuoSet IC Activity Assay (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The day before cell harvest, 96-well microplates were treated with PP2A-capturing antibody. The wells were then washed using buffer (0.05% Tween-20 in phosphate-buffered saline, pH 7.2–7.4) and blocked using 1% bovine serum albumin (BSA) and 0.05% NaN3 in PBS, pH 7.2–7.4. The conditioned cells were lysed with lysis buffer #8 (50 mM HEPES, 0.1 mM ethylene glycol tetraacetic acid (EGTA), 0.1 mM EDTA, 120 mM NaCl, 0.5% NP40 alternative (pH 7.5), and a protease cocktail). Protein samples (240 μg) were added to the coated 96-well microplates. The wells containing captured PP2A were treated with Ser/Thr phosphatase substrates, malachite green reagent A, and malachite green reagent B. Absorbance at 590 nm was measured using a Bio-Rad microplate reader (Bio-Rad, Hercules, CA, USA).
2.5. Lentivirus Production and Infection
For Mcl-1 knockdown, validated shRNAs against the gene were purchased from Sigma-Aldrich, and viral production and infection of cell lines were performed according to the ViraPowerTM Lentiviral Expression System protocol (Invitrogen, Carlsbad, CA, USA). The supernatant was used to infect U937 cells in a 12-well plate, and the infected cell population was selected by incubating with 5 μg/mL puromycin overnight.
2.6. Flow Cytometry
For apoptosis analysis, the cells were stained with 1× Annexin V binding buffer containing Annexin V-fluorescein isothiocyanate (BD Bioscience, Franklin Lakes, NJ, USA) and propidium iodide (PI; Sigma-Aldrich). For mitochondrial potential analysis, the cells were stained with 250 nM Mitotracker CMXRos (Invitrogen, Carlsbad, CA, USA) for 30 min at 37 °C. Fluorescence was measured with a FACSVerse™ flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA) and analyzed using FlowJo V10 software (FlowJo, Ashland, OR, USA).
2.7. Statistics
Data were acquired from three independent experiments and analyzed using the unpaired Student’s t-test. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. NDRG2 Expression Sensitizes U937 Cells to As2O3
To determine whether NDRG2 expression affects sensitivity to As2O3 in U937 cells, three NDRG2-overexpressing cell lines (U937-NDRG2) were established. When treated with 2 μM As2O3, higher apoptotic rates were observed in the U937-NDRG2 cells compared with U937-Mock cells (Figure 1A). U937-NDRG2 clone 1 was used in all experiments below. The increased apoptotic rate in the U937-NDRG2 cells also tended to show time or dose dependency (Figure 1B), and could be inhibited by a pan-caspase inhibitor, zVad-fmk (Figure 1C). Additionally, cleaved levels of caspase-3 and PARP after As2O3 treatment were higher in the U937-NDRG2 than in the U937-Mock cells (Figure 1D). These results suggest that NDRG2 increases the sensitivity of U937 to As2O3-induced apoptosis in a caspase-dependent manner.
Figure 1.
N-Myc downstream-regulated gene 2 (NDRG2) overexpression sensitized U937 cells to As2O3 in a caspase-dependent manner. (A) U937-Mock and three U937-NDRG2 lines were incubated with As2O3 (2 μM, 24 h). The cells were stained with Annexin V/propidium iodide (PI) and analyzed by flow cytometry. (B) The cells were incubated with As2O3 at the indicated time and concentration, and apoptotic cell population was validated with Annexin V/PI staining. (C) Here, 2 μM As2O3 was treated in the presence or absence of zVad-fmk (Pan-caspase inhibitor, 50 μM). The apoptotic population was validated with Annexin V/PI staining. (D) U937-Mock and U937-NDRG2 cells treated with As2O3 at the indicated dose. The cleavages (Cf) of Caspase3 and PARP were analyzed by immunoblotting. * is non-specific band. ** p < 0.01, *** p < 0.005 determined from t-tests. Data are presented as means ± standard error of the mean (SEM).
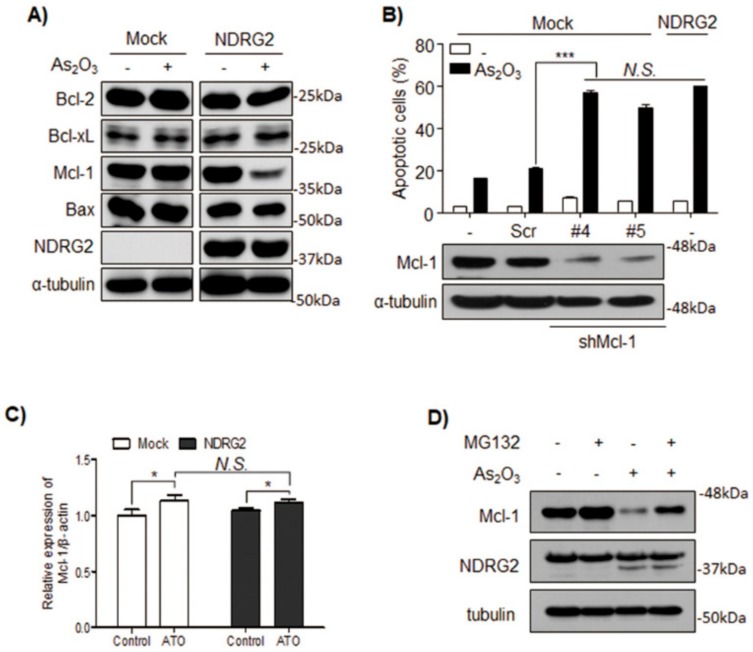
3.2. The Sensitivity of U937-NDRG2 to As2O3 Is Determined by Mcl-1 Degradation
Among Bcl-2 family proteins, Mcl-1, an anti-apoptotic protein, was significantly decreased in As2O3-treated U937-NDRG2 cells (Figure 2A). For validating whether the decrease of Mcl-1 was critical for the observed increased sensitivity to As2O3, Mcl-1 expression was silenced in the U937-Mock cells using shMcl-1. These cells showed higher sensitivity to As2O3, similar to the U937-NDRG2 cells (Figure 2B). Reduced Mcl-1 protein was not regulated at the transcriptional level (Figure 2C), but rather due to decreased protein stability, which could be offset by a proteasome complex inhibitor, MG132 (Figure 2D). Taken together, these results indicate that NDRG2 overexpression increases the sensitivity of the cells to As2O3 by reducing Mcl-1 protein stability.
Figure 2.
The sensitivity of U937-NDRG2 to As2O3 is mediated through Mcl-1 degradation. (A) Expression levels of pro-apoptotic Bcl-2 family protein (Bax) and pro-survival Bcl-2 family protein were analyzed by immunoblotting. (B) U937-Mock was infected by a lentivirus expressing scr or short hairpin Mcl-1 (shMcl-1) (clone #4 and #5). Successful knockdown of Mcl-1 was confirmed by immunoblotting, and apoptotic rate was analyzed using flow cytometry. (C) Mcl-1 messenger RNA (mRNA) expression in U937-Mock and U937-NDRG2 in the presence or absence of As2O3 was validated by qRT-PCR. (D) Cells were treated with As2O3 in the presence or absence of MG132, and then protein level of Mcl-1 was confirmed by immunoblotting. N.S.: Non-significant. * p < 0.05, *** p < 0.005 determined from t-test. Data are presented as means ± SEM.
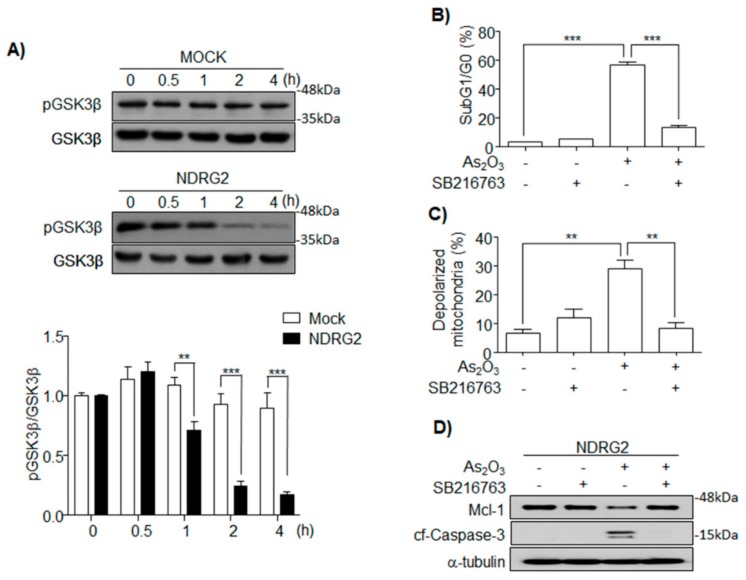
3.3. As2O3 Induces Mcl-1 Degradation through GSK3β Activation in U937-NDRG2
Active GSK3β phosphorylates Mcl-1, which is then degraded by the ubiquitin proteasome system [21]. In this study, we found that GSK3β was rapidly activated in U937-NDRG2 cells (Figure 3A). To confirm whether active GSK3β was associated with the sensitivity of U937-NDRG2 to As2O3, the U937-NDRG2 cells were co-treated with As2O3 and SB216763, a GSK3β inhibitor. The inhibition of GSK3β completely blocked As2O3-induced apoptosis and depolarization of mitochondria in these cells (Figure 3B,C). Additionally, As2O3-induced Mcl-1 degradation and caspase-3 cleavage were completely inhibited by SB216763 (Figure 3D). These results suggest that As2O3-induced GSK3β activation in the NDRG2 overexpressed condition increases the sensitivity of the cells to As2O3 through Mcl-1 degradation.
Figure 3.
As2O3-induced glycogen synthase kinase 3β (GSK3β) activation regulates Mcl-1 degradation and apoptosis in U937-NDRG2 cells. (A) Kinetics of phosphorylated GSK3β (Serine 9) in U937-Mock and U937-NDRG2 was checked after treatment with 2 μM As2O3 at the indicated time. Band intensity of phosphorylated GSK3β to total GSK3β was quantified using Image J program and presented graphically. U937-NDRG2 was treated with As2O3 in the presence or absence of a GSK3β inhibitor, SB216763 (10 μM). Cell death was determined using PI staining (B), and mitochondrial depolarization rate was done through Mitotracker CMXRos staining (C). The level of the indicated proteins was analyzed using immunoblotting (D). ** p < 0.01, *** p < 0.005 determined from t-test. Data are presented as means ± SEM.
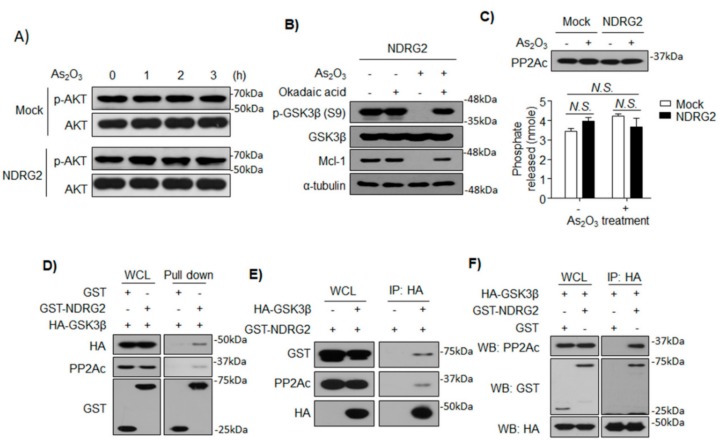
3.4. NDRG2 Mediates the Interaction between GSK3β and PP2A
Although a report suggested that NDRG2 mediates PP2A–PTEN complex formation, which inhibits the PI3K/AKT pathway through PTEN activation by PP2A [11], the phosphorylation on T308 of AKT regulated by PTEN was not changed in U937-Mock and U937-NDRG2 cells (Figure 4A). To determine whether PP2A could activate GSK3β, the U937-NDRG2 cells were treated with As2O3 in the presence or absence of okadaic acid, a broad PP2A inhibitor. PP2A inhibition rescued the inhibitory phosphorylation of GSK3β, which blocked the degradation of Mcl-1 (Figure 4B). NDRG2 might increase PP2A expression levels or its activity in the context of As2O3 treatment; however, neither were affected in the U937-NDRG2 cells (Figure 4C). To discern whether interaction among GSK3β, NDRG2, and PP2A was associated with the increased sensitivity to As2O3, we firstly performed GST pull-down assays with cell lysate from HEK293 cells transiently expressing GST or GST–NDRG2, and HA–GSK3β. GST–NDRG2 interacted with endogenous PP2Ac and exogenous HA–GSK3β, while GST did not interact with them (Figure 4D). Inversely, mock vector or HA–GSK3β was co-transfected with GST–NDRG2, and immunoprecipitation was performed with an anti-HA antibody. HA–GSK3β successfully interacted with exogenous GST–NDRG2 and endogenous PP2Ac (Figure 4E). To investigate whether NDRG2 plays a role as a bridge mediating the interaction between GSK3β and PP2A, the interaction between HA–GSK3β and endogenous PP2A was validated in the presence or absence of GST–NDRG2. HA–GSK3β was precipitated with endogenous PP2A, but only in the presence of GST–NDRG2 (Figure 4F). These results suggest that NDRG2 mediates the interaction between GSK3β and PP2Ac.
Figure 4.
GSK3β activation observed in U937-NDRG2 is mediated by phosphatase, PP2A, not by its upstream kinase AKT. (A) The phosphorylation of AKT on Thr308 was determined by immunoblotting using cell lysates of As2O3-treated U937-Mock and U937-NDRG2 cells at the indicated time points. (B) U937-NDRG2 cells were treated with As2O3 in the presence or absence of a phosphatase inhibitor, okadaic acid (20 nM). Protein levels of phosphorylated GSK3β and Mcl-1 were analyzed by immunoblotting. (C) PP2A activity acquired from U937-Mock or U937-NDRG2 treated with As2O3 was analyzed with Human/Mouse/Rat Total PP2A DuoSet IC ELISA. For the quantification of PP2Ac protein level, total lysate was subjected to immunoblotting. N.S. no significance determined from t-test. Data are presented as means ± SEM. (D) GST or GST–NDRG2 with HA–GSK3β was co-transfected in HEK293. Protein lysates acquired from the cells were subjected to GST pull-down assay. Whole-cell lysate (WCL) was used for loading control. (E) Mock or HA–GSK3β with GST–NDRG2 was co-expressed in HEK293. Protein lysates acquired from the cells were subjected to immunoprecipitation with HA antibody and then interaction was confirmed by immunoblotting. (F) Protein lysates from HEK293 expressing HA–GSK3β with GST or GST–NDRG2 was immunoprecipitated by HA antibody. Interactions among the indicated proteins were analyzed by immunoblotting.
3.5. The Formation of GSK3β/NDRG2/PP2A Complex Determines the Sensitivity of U937 to As2O3
A previous study reported that PP2Ac interacts with NDRG2 through its C-terminal domain [11]. Therefore, we established a U937 cell line expressing the C-terminal deletion mutant of NDRG2 (U937-ΔC NDRG2). Although ΔC NDRG2 could not interact with PP2A, it could still maintain its interaction with GSK3β (Figure 5A). As2O3-induced GSK3β activation and Mcl-1 degradation was not observed in the U937-ΔC NDRG2 cells (Figure 5B). Furthermore, the higher sensitivity to As2O3 shown in U937-NDRG2 cells was abolished in the U937-ΔC NDRG2 cells (Figure 5C). Therefore, the formation of GSK3β/NDRG2/PP2A complex was necessary for GSK3β activation and subsequent Mcl-1 degradation in U937-NDRG2 treated with As2O3.
Figure 5.
NDRG2 interacts with PP2Ac through its C-terminal, and NDRG2 ΔC is incapable of sensitizing U937-NDRG2 to As2O3. (A) NDRG2 wild type (WT) or NDRG2 ΔC was co-expressed with HA–GSK3β in HEK293. Prepared protein lysates were subjected to GST pull-down assay and the interaction was confirmed by immunoblotting. (B) U937-NDRG2 WT and U937-NDRG2 ΔC cells were treated with As2O3. Expressions of the indicated proteins were analyzed by immunoblotting. (C) U937-Mock, U937-NDRG2, and U937-NDRG2 ΔC were incubated with 2 μM As2O3 for 24 h. The apoptotic rate was analyzed by Annexin V/PI staining. ** p < 0.01 determined using t-test. Data are presented as means ± SEM.
4. Discussion
NDRG2, as a tumor suppressor, mainly suppresses cancer development and progression. It was proposed that, in clinical investigations, NDRG2 is positively correlated with survival rate and disease-free survival (DFS) probability, and negatively correlated with lymph node metastasis and TNM stage [4,5,6]. In this study, we investigated the molecular mechanism of NDRG2 function, as a kind of tumor suppressive gene, to overcome the low chemosensitivity of tumor cells.
As2O3 is approved by the Food and Drug Administration (FDA) to treat primary or relapsed acute promyelocytic leukemia (APL), a subtype of acute myeloid leukemia (AML) [27]. The therapeutic potential of As2O3 is not restricted to APL cells, and its application can induce apoptosis in non-APL acute myeloid leukemia cells, chronic myeloid leukemia cells, and other solid tumors in vitro [28,29,30]. To investigate NDRG2 function associated with drug sensitivity, the U937 cell line was used, because the cell line does not express NDRG2 and it is a representative one showing very low sensitivity to As2O3. We established NDRG2-overexpressing U937 (U937-NDRG2) cell lines, and the cells showed higher sensitivity to As2O3 compared with U937-Mock cells (Figure 1). The higher sensitivity was due to Mcl-1 degradation (Figure 2). Actually, the downregulation of Mcl-1 through GSK3β activation contributed to As2O3-induced apoptosis in acute myeloid leukemia [22]. The primary kinase regulating Mcl-1 stability is GSK3β, which phosphorylates Mcl-1 at S155, S159, and T163 [31,32]. The phosphorylated Mcl-1 is ubiquitinated by E3 ligases, F-box/WD repeat-containing protein 1A (β-TrCP), Mcl-1 ubiquitin ligase (Mule), or F-box/WD repeat-containing protein 7 (FBW7), and undergoes proteasome-dependent degradation [32,33,34]. Effective GSK3β activation and Mcl-1 degradation were induced in As2O3-treated U937-NDRG2 cells, and the inhibition of GSK3β using a specific inhibitor, SB216763, effectively decreased the sensitivity of the cells to As2O3, as well as Mcl-1 degradation (Figure 3). Mcl-1 is known as a crucial component in As2O3-induced apoptosis through GSK3β activation in acute myeloid leukemia [22,35].
As an upstream kinase of GSK3β, AKT is directly associated with the phosphorylation of GSK3β on Ser9, and its oncogenic mutations driving over-activation of PI3K/AKT pathway tend to result in excessive inactivation of GSK3β in various cancer cell lines [36]. Recently, NDRG2 was shown to inhibit PI3K/AKT signaling by activating PTEN through the recruitment of PP2A [11]. Furthermore, NDRG2-deficient mice showed inhibition of GSK3β through activated PI3K/AKT signaling [12]. In our study, although we observed GSK3β activation and Mcl-1 degradation in U937-NDRG2 treated with As2O3, these conditions did not reduce phosphorylation of T308 in AKT (Figure 4A). Therefore, this result suggested that the PI3K/AKT signaling regulated by PTEN/NDRG2/PP2A was not involved in the sensitivity of U937-NDRG2 to As2O3. Furthermore, since PTEN is mutated in the U937 cell line [37], the mechanism involving the inhibition of AKT by PTEN followed by GSK3β activation could be ruled out. A report suggested that PP2A directly dephosphorylates GSK3β through the relay of DNAJ homolog subfamily B member 6 (DNAJB6) [38]. DNAJB6 binds HSPA8 (heat-shock cognate protein, HSC70) and causes dephosphorylation of GSK3β at Ser9 by recruiting protein phosphatase PP2A. In this study, we hypothesize that NDRG2 acts as a bridge connecting GSK3β and PP2A, so that PP2A dephosphorylates the inhibitory phosphorylation of GSK3β. As shown in Figure 4, NDRG2 protein interacts with GSK3β and PP2A. Moreover, GSK3β and PP2A could not interact in the absence of NDRG2 expression. Although C-terminal-deleted NDRG2, which cannot bind to PP2A, interacts with GSK3β, GSK3β activation, Mcl-1 degradation, and higher sensitivity to As2O3 were suppressed in U937-ΔC NDRG2 cells (Figure 5). Although the NDRG2-mediated complex formation, GSK3β/NDRG2/PP2A, could not activate PP2A directly, it might be possible that As2O3 activates PP2A [39] and then accelerates activation of GSK3β through the complex.
Finally, our findings suggest a new regulatory AKT-independent pathway on GSK3β activation, in which NDRG2 positively regulates GSK3β by forming the GSK3β/NDRG2/PP2Acomplex, leading to PP2A-mediated removal of inhibitory phosphate on GSK3β (Figure 6). Thus, the GSK3β/NDRG2/PP2A complex is necessary for the GSK3β-mediated Mcl-1 degradation pathway, which consequently leads to enhanced sensitivity of the U937 cells to As2O3.
Figure 6.
Diagrammatic representation of the proposed apoptotic pathway that mediates the higher sensitivity to As2O3 in NDRG2-expressing U937 cells.
Acknowledgments
Thanks to Core Facility, Gyeongsang National University, that allowed the use of analytical equioments, flow cytometry and microplate reader.
Author Contributions
Conceptualization, S.P., H.-T.H. and K.D.K.; methodology, J.S.L., K.W.L. and M.K.; validation, S.-S.O., J.-W.J., and D.H.K.; formal analysis, S.P., M.K. and H.-T.H.; investigation, S.P. and H.-T.H.; resources, Y.Y.C., J.S.L., C.H., and J.Y.; data curation, Y.Y.C., C.H. and J.Y.; writing—original draft preparation, S.P., H.-T.H. and K.D.K.; writing—review and editing, K.D.K.; supervision, K.D.K.; project administration, K.D.K.; funding acquisition, K.D.K.
Funding
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science (NRF-015R1A2A2A01005083 and NRF-2018R1D1A1B07042569) and by next-generation biogreen21 (SSAC, PJ01107002), Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hu W., Yang Y., Fan C., Ma Z., Deng C., Li T., Lv J., Yao W., Gao J. Clinical and pathological significance of N-Myc downstream-regulated gene 2 (NDRG2) in diverse human cancers. Apoptosis. 2016;21:675–682. doi: 10.1007/s10495-016-1244-3. [DOI] [PubMed] [Google Scholar]
- 2.Hu W., Fan C., Jiang P., Ma Z., Yan X., Di S., Jiang S., Li T., Cheng Y., Yang Y. Emerging role of N-myc downstream-regulated gene 2 (NDRG2) in cancer. Oncotarget. 2016;7:209–223. doi: 10.18632/oncotarget.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee D.C., Kang Y.K., Kim W.H., Jang Y.J., Kim D.J., Park I.Y., Sohn B.H., Sohn H.A., Lee H.G., Lim J.S., et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–4220. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 4.Choi S.C., Yoon S.R., Park Y.P., Song E.Y., Kim J.W., Kim W.H., Yang Y., Lim J.S., Lee H.G. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death. Exp. Mol. Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.J., Yoon S.Y., Kim J.T., Song E.Y., Lee H.G., Son H.J., Kim S.Y., Cho D., Choi I., Kim J.H.C. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598–605. doi: 10.1093/carcin/bgp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh S.S., Kim D., Kim D.H., Chang H.H., Sohn K.C., Kim K.H., Jung S.H., Lee B.K., Kim J.H., Kim K.D. NDRG2 correlated with favorable recurrence-free survival inhibits metastasis of mouse breast cancer cells via attenuation of active TGF-beta production. Carcinogenesis. 2012;33:1882–1888. doi: 10.1093/carcin/bgs211. [DOI] [PubMed] [Google Scholar]
- 7.Manoukian A.S., Woodgett J.R. Role of glycogen synthase kinase-3 in cancer: Regulation by Wnts and other signaling pathways. Adv. Cancer Res. 2002;84:203–229. doi: 10.1016/s0065-230x(02)84007-6. [DOI] [PubMed] [Google Scholar]
- 8.Hardt S.E., Sadoshima J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Cir. Res. 2002;90:1055–1063. doi: 10.1161/01.RES.0000018952.70505.F1. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.T., Kim J.W., Kang Y.H., Kim K.D., Lee S.J., Choi S.C., Kim K.S., Chae S.K., Lim J.S., Lee H.G. NDRG2 and PRA1 interact and synergistically inhibit T-cell factor/beta-catenin signaling. FEBS Lett. 2012;586:3962–3968. doi: 10.1016/j.febslet.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.J., Kang H.B., Yim H.S., Kim J.H., Kim J.W. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncol. Rep. 2013;30:1890–1898. doi: 10.3892/or.2013.2642. [DOI] [PubMed] [Google Scholar]
- 11.Nakahata S., Ichikawa T., Maneesaay P., Saito Y., Nagai K., Tamura T., Manachai N., Yamakawa N., Hamasaki M., Kitabayashi I., et al. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat. Commun. 2014;5:3393. doi: 10.1038/ncomms4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichikawa T., Nakahata S., Tamura T., Manachai N., Morishita K. The loss of NDRG2 expression improves depressive behavior through increased phosphorylation of GSK3beta. Cell Signal. 2015;27:2087–2098. doi: 10.1016/j.cellsig.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Soignet S.L., Maslak P., Wang Z.G., Jhanwar S., Calleja E., Dardashti L.J., Corso D., DeBlasio A., Gabrilove J., Scheinberg D.A., et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. New Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 14.Niu C., Yan H., Yu T., Sun H.P., Liu J.X., Li X.S., Wu W., Zhang F.Q., Chen Y., Zhou L., et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: Remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 15.Mathews V., George B., Lakshmi K.M., Viswabandya A., Bajel A., Balasubramanian P., Shaji R.V., Srivastava V.M., Srivastava A., Chandy M. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F., van Besien K. Clinical activity of arsenic trioxide in Burkitt-like lymphoma. Leukemia. 2003;17:271–272. doi: 10.1038/sj.leu.2402735. [DOI] [PubMed] [Google Scholar]
- 17.Akao Y., Yamada H., Nakagawa Y. Arsenic-induced apoptosis in malignant cells in vitro. Leuk. Lymphoma. 2000;37:53–63. doi: 10.3109/10428190009057628. [DOI] [PubMed] [Google Scholar]
- 18.Rust D.M., Soignet S.L. Risk/benefit profile of arsenic trioxide. Oncologist. 2001;6(Suppl 2):29–32. doi: 10.1634/theoncologist.6-suppl_2-29. [DOI] [PubMed] [Google Scholar]
- 19.Mills J.R., Hippo Y., Robert F., Chen S.M., Malina A., Lin C.J., Trojahn U., Wendel H.G., Charest A., Bronson R.T., et al. mTORC1 promotes survival through translational control of Mcl-1. Proc. Natl. Acad. Sci. USA. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martelli A.M., Evangelisti C., Chiarini F., Grimaldi C., Manzoli L., McCubrey J.A. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Exp. Opin. Investig. Drugs. 2009;18:1333–1349. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 21.Thomas L.W., Lam C., Edwards S.W. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., Xia L., Gabrilove J., Waxman S., Jing Y. Downregulation of Mcl-1 through GSK-3beta activation contributes to arsenic trioxide-induced apoptosis in acute myeloid leukemia cells. Leukemia. 2013;27:315–324. doi: 10.1038/leu.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jing Y., Dai J., Chalmers-Redman R.M., Tatton W.G., Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 24.Iwama K., Nakajo S., Aiuchi T., Nakaya K. Apoptosis induced by arsenic trioxide in leukemia U937 cells is dependent on activation of p38, inactivation of ERK and the Ca2+-dependent production of superoxide. Int. J. Cancer. 2001;92:518–526. doi: 10.1002/ijc.1220. [DOI] [PubMed] [Google Scholar]
- 25.Dahia P.L., Aguiar R.C., Alberta J., Kum J.B., Caron S., Sill H., Marsh D.J., Ritz J., Freedman A., Stiles C., et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanismsin haematological malignancies. Hum. Mol. Genet. 1999;8:185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- 26.Choi S.C., Kim K.D., Kim J.T., Oh S.S., Yoon S.Y., Song E.Y., Lee H.G., Choe Y.K., Choi I., Lim J.S., et al. NDRG2 is one of novel intrinsic factors for regulation of IL-10 production in human myeloid cell. Biochem. Biophys. Res. Commun. 2010;396:684–690. doi: 10.1016/j.bbrc.2010.04.162. [DOI] [PubMed] [Google Scholar]
- 27.Lengfelder E., Hofmann W.K., Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2012;26:433–442. doi: 10.1038/leu.2011.245. [DOI] [PubMed] [Google Scholar]
- 28.Kchour G., Tarhini M., Kooshyar M.M., El Hajj H., Wattel E., Mahmoudi M., Hatoum H., Rahimi H., Maleki M., Rafatpanah H., et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL) Blood. 2009;113:6528–6532. doi: 10.1182/blood-2009-03-211821. [DOI] [PubMed] [Google Scholar]
- 29.Uslu R., Sanli U.A., Sezgin C., Karabulut B., Terzioglu E., Omay S.B., Goker E. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin. Cancer Res. 2000;6:4957–4964. [PubMed] [Google Scholar]
- 30.Rousselot P., Larghero J., Arnulf B., Poupon J., Royer B., Tibi A., Madelaine-Chambrin I., Cimerman P., Chevret S., Hermine O., et al. A clinical and pharmacological study of arsenic trioxide in advanced multiple myeloma patients. Leukemia. 2004;18:1518–1521. doi: 10.1038/sj.leu.2403424. [DOI] [PubMed] [Google Scholar]
- 31.Maurer U., Charvet C., Wagman A.S., Dejardin E., Green D.R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Ding Q., He X., Hsu J.M., Xia W., Chen C.T., Li L.Y., Lee D.F., Liu J.C., Zhong Q., Wang X., et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong Q., Gao W., Du F., Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Inuzuka H., Shaik S., Onoyama I., Gao D., Tseng A., Maser R.S., Zhai B., Wan L., Gutierrez A., Lau A.W., et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen P., Zhan W., Wang B., You P., Jin Q., Hou D., Wang X., You R., Zou H., Chen Y., et al. Homoharringtonine potentiates the antileukemic activity of arsenic trioxide against acute myeloid leukemia cells. Exp. Cell Res. 2019;376:114–123. doi: 10.1016/j.yexcr.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 36.McCubrey J.A., Steelman L.S., Bertrand F.E., Davis N.M., Sokolosky M., Abrams S.L., Montalto G., D’Assoro A.B., Libra M., Nicoletti F., et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggerholm A., Gronbaek K., Guldberg P., Hokland P. Mutational analysis of the tumour suppressor gene MMAC1/PTEN in malignant myeloid disorders. Eur. J. Haematol. 2000;65:109–113. doi: 10.1034/j.1600-0609.2000.90181.x. [DOI] [PubMed] [Google Scholar]
- 38.Mitra A., Menezes M.E., Pannell L.K., Mulekar M.S., Honkanen R.E., Shevde L.A., Samant R.S. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene. 2012;31:4472–4483. doi: 10.1038/onc.2011.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang Y.W., Chen M.W., Chiu C.F., Hong C.C., Cheng C.C., Hsiao M., Chen C.A., Wei L.H., Su J.L. Arsenic trioxide inhibits CXCR4-mediated metastasis by interfering miR-520h/PP2A/NF-kappaB signaling in cervical cancer. Ann. Surg. Oncol. 2014;21:S687–S695. doi: 10.1245/s10434-014-3812-5. [DOI] [PubMed] [Google Scholar]