Abstract
Lateral roots play an important role in water and nutrient uptake largely by increasing the root surface area. In an effort to characterize lateral root development in maize (Zea mays), we have isolated from Mutator (Mu) transposon stocks and characterized two nonallelic monogenic recessive mutants: slr1 and slr2 (short lateral roots1 and 2), which display short lateral roots as a result of impaired root cell elongation. The defects in both mutants act specifically during early postembryonic root development, affecting only the lateral roots emerging from the embryonic primary and seminal roots but not from the postembryonic nodal roots. These mutations have no major influence on the aboveground performance of the affected plants. The double mutant slr1; slr2 displays a strikingly different phenotype than the single mutants. The defect in slr1; slr2 does not only influence lateral root specific cell elongation, but also leads to disarranged cellular patterns in the primary and seminal roots. However, the phase-specific nature of the single mutants is retained in the double mutant, indicating that the two loci cooperate in the wild type to maintain the lateral root specificity during a short time of early root development.
The root system of maize (Zea mays) consists of embryonic and postembryonic roots, which can be distinguished spatially and temporally during development. The embryonic root system of maize emerges a few days after germination and consists of the primary root and a variable number of seminal roots formed at the scutellar node. Postembryonic roots include shoot-borne crown roots that form from consecutive underground nodes, brace roots that emerge late in development from aboveground nodes of the shoot, and lateral roots that develop on all root types (Feix et al., 2000). Crown and brace roots represent the major backbone of the complex root system in mature maize. They contribute to root lodging resistance and together with their lateral roots are responsible for most of the water uptake (McCully and Canny, 1988). Lateral roots are highly branched, thus increasing enormously the absorbing surface of the root system. A common feature of all maize root types is the longitudinal zoning in the growing parts including the root cap, the apical meristem, the elongation zone, and the differentiation or maturation zone (Ishikawa and Evans, 1995). In maize, the primordia of lateral roots are formed in the differentiation zone of preexisting roots by dedifferentiation of non-meristematic pericycle cells (Esau, 1965) and sometimes endodermal cells (Bell and McCully, 1970). Dedifferentiation of pericycle cells is also observed in lateral roots leading to secondary and higher order structures of lateral roots. Accurately predicting which pericycle cells will undergo dedifferentiation and form lateral root primordia is difficult because frequency and position of lateral root initiation depends on various environmental conditions (Charlton, 1996).
A detailed analysis of Arabidopsis lateral root development revealed that after the initiation of a lateral root primordium and the formation of the lateral root meristem (Laskowski et al., 1995), a sequence of events (Malamy and Benfey, 1997) including emergence, elongation, and root hair formation leads to mature lateral roots.
As lateral roots elongate, some epidermal cells called trichoblasts develop further into root hairs. Root hairs are always found in the proximal elongation zone and maturation region (Schiefelbein and Benfey, 1991); therefore, a certain degree of cell elongation seems to be the prerequisite for root hair development.
Genetic analysis has led to the isolation of a considerable number of Arabidopsis mutants displaying an aberrant root phenotype (summarized in Scheres and Wolkenfelt, 1998) including various lateral root system mutants. Three types of lateral root mutants have been identified in Arabidopsis: first, mutants with excessive lateral root formation, like the allelic mutants rty (King et al., 1995), sur1 (Boerjan et al., 1995), and alf1 (Celenza et al., 1995); second, mutants affected in lateral root initiation like alf3 (Celenza et al., 1995); and third, mutants initially forming normal lateral root primordia which later abandon cell division or elongation as seen in alf4 (Celenza et al., 1995) or the rml mutants (Cheng et al., 1995).
Based on the analysis of the alf mutants, Celenza et al. (1995) postulated a model for lateral root formation in Arabidospsis. In this model, the first step is lateral root initiation by auxin: Pericycle cells are induced to undergo mitosis and form lateral root primordia. Auxin is presumed to be transported from other parts of the plant to induce pericycle cells to form a primordium (Charlton, 1996). The ALF1 gene product is believed to modulate the level of free, active auxin and therefore the number of lateral roots. ALF4 is directly involved in lateral root initiation by sensing or responding to indole-3-acetic acid (IAA), implied by the IAA resistant phenotype of the alf4 mutant. In a second step the primordia require the ALF3 gene product, which locally elevates the level of IAA necessary for cell division and subsequent maintenance of the lateral root meristem leading to lateral root elongation.
In maize, only a few mutants with relevance to root formation have been isolated. The mutant rtcs is completely devoid of all shoot-borne roots including seminal, crown, and brace roots. The mutation acts before the initiation of these root types (Hetz et al., 1996). The recessive mutant rt1 forms few or no crown and brace roots, whereas the primary root and the seminal roots are not affected (Jenkins, 1930). The dominant mutant Asr1 is defective in the formation of seminal roots, whereas other root types are not affected (De Miranda, 1980). In the recessive mutant des21, seminal roots and root hairs are missing and the shoot phenotype is also altered (Gavazzi et al., 1993). The recessive mutants rth1-3 are affected in root hair elongation (Wen and Schnable, 1994) and the mutant agt1 displays an altered gravitropic response (Doyle, 1978).
So far, only one maize mutant has been isolated that is specifically affected in lateral root formation (Hochholdinger and Feix, 1998). The mutant lrt1 (lateral rootless1) is completely deficient in primordia formation of lateral roots at the primary, seminal, and crown roots forming at the coleoptilar node, thus affecting all early postembryonic roots. The lateral root formation on crown roots from the second node upwards is completely normal, indicating the presence of at least two root-type or phase-specific pathways of lateral root initiation in maize.
In this paper, we report the isolation of two new lateral root mutants of maize affected in lateral root elongation. Slr1 and slr2 were obtained from segregating transposon-tagged F2 families induced by the Mutator (Mu) transposon system. It is interesting that the defect of lateral root elongation is restricted to the lateral roots emerging from embryonic roots. The double mutant slr1; slr2 indicates synergistic effects of these two loci affecting not only the formation of lateral roots but also the development of all early root types, including embryonic primary and seminal roots and postembryonic crown roots from the coleoptilar node. The transient nature of the phenotype conditioned by single and double mutants suggests that the slr1 and slr2 genes are involved in root-specific development only in a limited developmental phase.
RESULTS
Isolation and Phenotypic Characterization of Maize Mutants Displaying Short Lateral Roots
Screening of about 2,000 segregating F2 families of Mu-tagged maize lines at the seedling level for aberrant root phenotypes led to the isolation of two families with seedlings exhibiting short lateral roots (Fig. 1, A and C). The two nonallelic mutants, classified as monogenic recessive by their 3:1 segregation ratio (Tables I and IIslr1 and slr2 (short lateral roots1 and 2), both showed the same aberrant root phenotype. Close-up inspection of the two mutants revealed that the very short lateral roots occurring at the emerging primary and seminal roots curved in many cases to random directions (Fig. 1B). The number of lateral roots was unchanged in the mutants (Table III). Both slr1 and slr2 displayed normal primary, seminal, and crown roots (Fig. 1, A and C). The rootstock of mature mutant plants looked very similar to the highly branched root system of WT plants (Fig. 1D).
Figure 1.
Phenotypic properties of slr1 and slr2. A, Phenotypes of 7-d-old wild-type (WT), slr1, and slr2 seedlings (from left to right; same order in B, C, E, and F). B, Close view of lateral roots on the primary root. C, Root system of 12-d-old seedlings. D, Root system of WT (left) and slr2 (right) after 90 d. E, Aboveground phenotypes after 32 d. The pale-green color of slr1 was not completely restored yet in these hydroponically grown plants. F, Aboveground phenotypes after 90 d. Bars indicate 2 cm (A), 1 mm (B), or 1 cm (C).
Table I.
Genetic analysis of slr1 and slr2
| Genetic Treatment | WT | Mutant | Observed Segregation | Expected Segregation | χ2 |
|---|---|---|---|---|---|
| Segregation of slr1 | |||||
| Initial F2 population | 12 | 3 | 4.0:1 | 3:1 | 0.20 |
| Selfed heterozygous WT (7)a | 90 | 22 | 4.1:1 | 3:1 | 1.72 |
| Selfed homozygous WT (8) | 121 | 0 | – | – | – |
| Outcross of slr1 (A632 × slr1) | |||||
| F2 from the cross (3) | 108 | 13 | 8.3:1b | 3:1 | 32.28 |
| Outcross of slr1 (B73 × slr1) | |||||
| F2 from the cross (6) | 156 | 19 | 8.2:1b | 3:1 | 18.67 |
| Segregation of slr2 | |||||
| Initial F2 population | 8 | 7 | 1.1:1 | 3:1 | 3.76 |
| Selfed heterozygous WT (7) | 196 | 74 | 2.6:1 | 3:1 | 0.84 |
| Selfed homozygous WT (7) | 166 | 0 | – | – | – |
| Selfed mutant (5) | 2 | 117 | – | – | – |
| Outcross of slr2 (slr2 × SWS2000) | |||||
| F2 from the cross (13) | 586 | 183 | 3.2:1 | 3:1 | 0.60 |
No. of analyzed plants in parentheses.
These segregation ratios might be indicative for 15:1 as a result of statistic analysis in A632 (χ2 = 4.0941, p = 0.01) and B73 (χ2 = 6.4192, p = 0.01) genetic backgrounds.
Table II.
Genetic analysis of slr1 and slr2
| Segregation of slr1; slr2 Double Mutant | WT | slr1 | slr2 | slr1; slr2 | Expected Segregation | χ2 |
|---|---|---|---|---|---|---|
| F2 from the cross of slr2 × slr1 (7)a | 208 | 39 | 40 | 12 | 9:3:3:1 | 21.60 |
No. of analyzed plants in parentheses.
Table III.
Characteristic properties of slr1 and slr2
| Observed Phenotypes | WT | slr1 | slr2 |
|---|---|---|---|
| Lateral root | |||
| Length (mm)a | 8.7 ± 4.6 (n = 75) | 1.8 ± 0.8 (n = 75) | 1.9 ± 1.1 (n = 35) |
| Frequency (cm−1)a | 11.0 ± 1.8 (n = 24) | 11.6 ± 1.4 (n = 17) | 9.6 ± 1.6 (n = 9) |
| Root hair formation | Normal | Fewb | Fewb |
| Color of first three leaves | Green | Pale greenc | Green |
| Pollen formation | Normal | Reduced | Normal |
Values are mean ± sd.
Measured 2 to 3 cm below the kernel in 12-d-old plants.
Only exceptionally elongated lateral roots have root hairs on the basal parts.
Caused by a closely linked locus.
The mutations slr1 and slr2 always followed statistically the Mendelian 3:1 segregation when heterozygous plants were selfed (Table I). However, the segregation ratio of slr1 showing short lateral roots increased to 8:1 after the outcrosses in the inbred lines A632 and B73, and statistically deviated from the Mendelian rule. Later outcrosses of slr2 were therefore performed with the local South German inbred line SWS2000 in which selfed heterozygous plants segregated according to the expected 3:1 Mendelian ratio.
Although slr1 and slr2 plants were slightly smaller than WT plants 1 month after germination (Fig. 1E), no difference in plant size was observed in mature plants (Fig. 1F). The development of slr1 and slr2 plants was comparable to WT plants regarding time of flowering and number of leaves. Slr1 plants were frequently male sterile (Table III). Application of auxin, auxin action inhibitor 4-chlorophenoxyisobutyric acid, ethylene, ethylene action inhibitor silverthiosulfate, and ethylene biosynthesis inhibitor aminoethoxyvinyl-Gly to germinating slr1 and slr2 had no effect on the length of the lateral roots. Hormone treatments were performed according to standard procedures described earlier (Hetz et al., 1996; Hochholdinger and Feix, 1998).
A difference in the shoot system between slr1 and slr2 was the transient pale-green color on the first two or three leaves of slr1 (Fig. 1A; Table III) caused by a closely linked gene.
Cell Elongation of Lateral Roots Is Affected to the Same Extent in slr1 and slr2
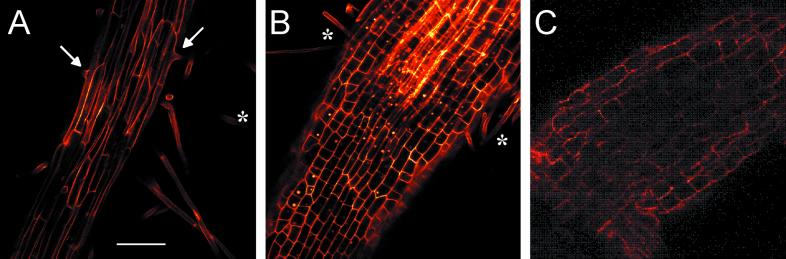
After showing through microscopic analyses that lateral root primordia formed and penetrated the cortex of the primary root in slr1 and slr2 as observed in WT seedlings, the elongation of lateral roots was studied. Scanning of the lateral roots with a confocal laser scanning microscope revealed striking differences in cell length between WT and the two mutants. Typical examples for these observations are shown in Figure 2. Well-elongated cells (106.6 ± 0.1 μm; mean ± se, and n = 68 from 12 roots) were observed in WT (Fig. 2A), whereas cells in slr1 (46.9 ± 1.7 μm; n = 74 from 13 roots) and slr2 (50.2 ± 2.1; n = 50 from 7 roots) were much shorter (Fig. 2, B and C). However, the organization of the lateral root tips was unchanged in the mutants compared with WT (data not shown). Root hairs were rarely observed on mutant lateral roots (Table III), whereas they were always observed on the WT lateral roots (Fig. 2A, arrows; root hairs marked with asterisks in Fig. 2B were from the primary root, not from lateral roots). Root hairs on primary roots were well developed both in WT and in the two mutants. Applying ethylene precursor (10 μm 1-aminocyclopropane-1-carboxylic acid) did not increase the number of root hairs on the lateral roots of either WT or slr1 under our growth condition.
Figure 2.
Confocal laser scanning analysis of lateral roots in WT, slr1, and slr2. Propidium iodide-stained lateral roots from the middle part of the primary root of 8-d-old maize seedlings were analyzed and cortical cells from the basal part of the lateral roots were focused. Optical sections inside the lateral roots were scrutinized at various levels, and images obtained from the basal part showing striking differences are presented in WT (A), slr1 (B), and slr2 (C). Bar indicates 200 μm. Arrows and asterisks indicate root hairs on lateral root and primary root, respectively.
The Defects of slr1 and slr2 Act Transiently and Affect Only the Lateral Root Development from Embryonic Roots
Analysis of the formation of short lateral roots at different developmental phases of all root types revealed a high specificity regarding root type and developmental stage. To observe the postembryonic crown roots, plants were grown under hydroponic conditions that allow an in vivo observation of their fragile and complex root structures during development. Although plants grown in a hydroponic culture system displayed a slightly different appearance in their root system compared with soil-grown plants, this did not disturb the analysis of short lateral roots. The observed short lateral roots became visible on the primary root as in the WT about 7 d after germination. Short lateral roots formed only for about the first 2 weeks at the growing primary root (see upper part of slr2 primary root in Fig. 3C). In the case of slr1, normal growth resumed earlier (Fig. 3A). At later stages of development, continuous lateral root growth was seen on the elongating primary root (see lower parts of slr1 and slr2 primary root in Fig. 3, C and A, respectively). However, these lateral roots do not reach the length of the lateral roots on the WT primary root. The same transient deficiency of lateral root elongation was observed at the lateral-seminal roots of slr1 and slr2 (data not shown).
Figure 3.
Impairment of lateral root elongation in the mutants is transient and specific to the embryonic roots. A, Lateral roots formed at the primary root of WT (left) and slr1 (right) after 25 d. B, Lateral roots formed at the crown root of WT (left) and slr1 (right) after 25 d. C, Lateral roots formed at the primary root of WT (left) and slr2 (right) after 27 d. D, Lateral roots formed at the crown root of WT (left) and slr2 (right) after 27 d. Plants were grown in paper rolls for 12 d and then incubated further in a hydroponic culture system. Bars indicate 2 cm.
Crown root development of WT, slr1, and slr2 plants was observed at several stages of plant development until maturity (Fig. 1A, 7 d after germination; Fig. 1C, 12 d after germination; Fig. 3, 25 and 27 d after germination; and Fig. 1D, 90 d after germination). A visual difference in the crown root performance was observed between WT and mutants at none of these stages. None of the crown roots analyzed in any of the single mutants at the various stages of development showed significantly shorter lateral roots as had been observed in the embryonic root system.
Lateral Root Specificity of slr1 and slr2 Is Released in the Double Mutant slr1; slr2
To study possible interaction between the two loci affected in slr1 and slr2, double mutants were isolated from segregating F2 families of crosses between slr1 and slr2 (Table II). Isolated double mutants were not uniform in appearance at early seedling stages and showed a varying degree of disturbances of the complete early root system (Fig. 4A). This is different from the highly specific defects of the single mutants slr1 and slr2 leading only to an abnormal elongation of lateral roots on primary and seminal roots.
Figure 4.
Phenotypic properties of the double-mutant slr1; slr2. A, Examples of 7-d-old double mutants. B and E, Close inspection of the primary root of a 12-d-old double mutant. D, The root system of a 25-d-old double mutant still displaying the defects in the early root system, but having late-developing crown roots with normal lateral roots. Coleoptilar node of WT (C) and double mutant (F), respectively. G, Aboveground phenotype of WT (left) and double mutant (right) after 32 d. H. Mature plants of WT (left) and double mutant (right) after 90 d. Arrows in C and F indicate the position of cross section for Figure 5, E and F. Bars represent 1 cm (A) or 1 mm (B–F).
Despite their variability in phenotypic appearance, all double mutants showed a decreased length of primary, seminal, and early crown roots and a reduction in the overall size of the seedlings (Fig. 4A; Table IV). The diameter of the primary and early crown roots and of the first internode was found to be slightly increased in the double mutant (Table IV; Fig. 4, C and F).
Table IV.
Phenotypes of 12-d-old slr1; slr2 double mutant
| Observed Phenotypes | WT (n = 24) | slr1 (n = 9) | slr2 (n = 9) | slr1; slr2 (n = 11) |
|---|---|---|---|---|
| Primary root | ||||
| Length (cm) | 12.8 ± 1.5 | 12.2 ± 1.1 | 11.8 ± 2.2 | 6.7 ± 3.8 |
| Diameter (mm) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.2 |
| First internode | ||||
| Length (cm) | 4.5 ± 0.6 | 3.8 ± 0.5 | 4.1 ± 0.2 | 2.8 ± 0.8 |
| Diameter (mm) | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 3.3 ± 0.7 |
| Leaves | ||||
| Nos. | 3 | 3 | 3 | 2 |
| First (length [cm]) | 5.2 ± 0.7 | 4.7 ± 0.7 | 4.6 ± 0.3 | 2.9 ± 1.0 |
| Second (length [cm]) | 11.7 ± 1.8 | 9.3 ± 2.1 | 9.6 ± 1.6 | 4.8 ± 2.3 |
Values are mean ± sd.
Furthermore, the lateral roots did not elongate any more from the primary root of the double mutant and only formed bulges at their sites of growth (Fig. 4B). These bulges extended up to 2 mm to the tip of the primary root (Fig. 4E), a region that is normally free of lateral roots.
The defects of the early root system of the double mutant were not restored later in development, even when crown roots were developing without defects in their length and capacity to form lateral roots (Fig. 4D). Therefore, the growth rate of the double mutant seedlings (Fig. 4G) was restored in time by the formation of crown roots without differences in size and flowering time between the double mutant and WT after maturation (Fig. 4H).
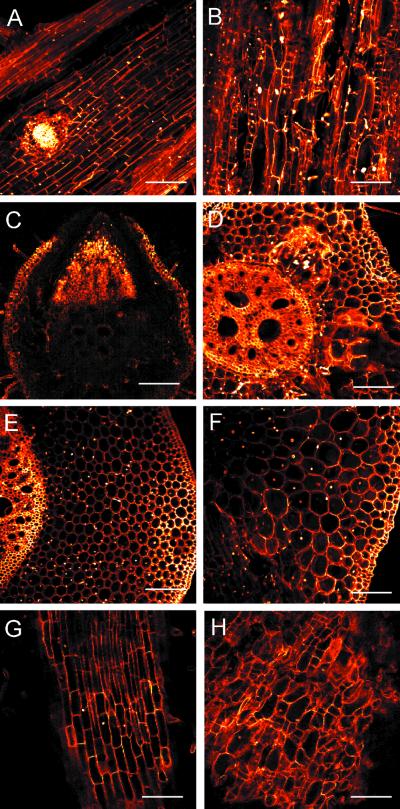
Longitudinal sections of the primary root of double mutants revealed a disorganized cellular pattern showing cell files with very small rectangular cells beside cell files with very long cells as well as cell files with a mixture of these cell types (Fig. 5B). WT and the single mutants slr1 and slr2 showed well-elongated, regularly arranged cells in this region (Fig. 5A). Cross sections of primary roots of the double mutant showed radially expanded cortical cells, but normal cellular organization (Fig. 5D). Similar characteristics of an unchanged radial pattern (Fig. 5F) with about a 30% increase in diameter (Table IV) caused by the expansion of cortical cells were also observed in the coleoptilar node (Fig. 5F). Epidermal cells remained unchanged even in the radially expanded organs (Fig. 5, C–F). The early crown roots from the coleoptilar node were shorter and thicker (Fig. 4F) than those of WT seedlings (Fig. 4C), and their longitudinal sections displayed the same disorganized patterns (Fig. 5H) as observed in the primary root (Fig. 5B).
Figure 5.
Confocal laser scanning analysis of various organs in a 12-d-old double mutant. Longitudinal sections of a WT (A) and a double mutant (B) primary root. Cross sections of a WT (C) and a double mutant (D) primary root. Cross sections of a WT (E) and a double mutant (F) coleoptilar node. Longitudinal section of a young crown root from the coleoptilar node of a WT (G) and a double mutant (H). The positions of cross sections of the coleoptilar node were marked by arrows in Figure 4, C and F. Bars indicate 100 μm (C–F) or 200 μm (A, B, G, and H).
Observation of embryos revealed no difference between the regular patterns of WT and double mutant root structures, including the primary root and the lateral-seminal root primordia at the scutellar node. Therefore, the defective cellular patterns of the affected roots in the double mutant were caused by irregular cell proliferation of the initial cells of the roots after germination.
DISCUSSION
This paper describes the isolation and characterization of the two nonallelic monogenic recessive mutants, slr1 and slr2, specifically affected in lateral root elongation. This defect leads to a reduced length of the lateral roots at the embryonic primary and seminal roots. Histological analysis of the affected lateral roots revealed that cortical cell elongation is impaired in slr1 and slr2. They are the first examples of mutants from monocotyledonous plants specifically affected in lateral root elongation. Both mutants display their defects during early postembryonic root development, indicating the possible involvement of signals with specificity for this particular developmental window. However, treatment with auxin, ethylene, and their inhibitors did not restore the mutant phenotypes.
The lateral root formation at the postembryonic crown and brace roots is not affected in the mutants allowing a normal development of these plants without differences in their aboveground size compared with their WT siblings. This is in contrast with the maize lateral root initiation mutant lrt1 that shows a drastically reduced aboveground phenotype (Hochholdinger and Feix, 1998). The difference could be explained by the fact that slr1 and slr2 are not so severely impaired in lateral root formation as lrt1. Lateral roots at the embryonic roots and the crown roots from the coleoptilar node are completely missing in lrt1, whereas only their elongation is affected in slr1 and slr2.
A common feature of all three mutants is the predominant or exclusive impairment of lateral root formation from embryonic, but not from postembryonic, roots. Therefore, different mechanisms for tissue specificity regarding lateral root formation might operate in embryonic and postembryonic roots. Such differences in embryonic and postembryonic lateral root formation may be unique to monocotyledonous plants because dicots like Arabidopsis normally do not form a postembryonic shoot-borne root system (Malamy and Benfey, 1997) if not induced by external stimuli. The maize root mutant rtcs has another tissue specificity to all the embryonic and postembryonic nodal tissues (Hetz et al., 1996).
The analyzed slr1 seedlings often exhibited a pale-green leaf color as a result of the action of another gene tightly linked to slr1 that is indicated by segregation data of the double mutant (data not shown). The deviation of the segregation ratios of the slr1 phenotype from the Mendelian rule after outcrossing in two different inbred lines (Table I) may be caused by one of the following factors: phenotypic variability of lateral roots in these backgrounds, Mu suppresion, an unstable Mu insertion, or the presence of a modifier of this gene in these genetic backgrounds. The latter hypothesis is supported by the fact that for both inbred lines a 15:1 segregation ratio is statistically supported for P = 0.01. The non-Mendelian segregation of the double mutant slr1; slr2 could also be explained by one of the factors that causes the deviation of the slr1 segregation from the 3:1 ratio. A further deviation from Mendelian inheritance was observed in the selfed homozygous slr2 population producing two WT plants that may again indicate the rare occurrence of reversions in the Mu-tagged populations, or pollen contamination.
In slr1 and slr2, the reduced lateral root elongation is unlikely to be the result of a nutrient deficiency because the mutations were not rescued under hydroponic conditions with an optimized maize nutrient solution (Marschner, 1995). However, this does not exclude the possibility that a regulator of response to nutrients in lateral roots like the Arabidopsis gene ANR1 is affected (Zhang and Forde, 1998). The MADS box gene ANR1, which is supposed to be a transcription factor controlling a group of genes, is nitrate inducible and specifically affects lateral but not primary root elongation.
No mutants with comparable defects like slr1 and slr2 are described so far in Arabidopsis, despite the availability of a large collection of root-specific mutants (Scheres and Wolkenfelt, 1998). The mutants alf3 (Celenza et al., 1995), rml1, and rml2 (Cheng et al., 1995) also show shorter lateral roots similar to slr1 and slr2 a few days after germination. However, the reduced lateral root length in alf3, rml1, and rml2 is caused by different mechanisms as compared with the mutants slr1 and slr2. Lateral root meristems are active in slr1 and slr2. However, they are proliferating new cells that are reduced in their final elongation capacity leading to shortened lateral roots. In contrast, rml1 and rml2 abandon lateral root growth by terminal differentiation of apical cells in the lateral roots (Cheng et al., 1995) and alf3 stops lateral root growth by death of these apical cells (Celenza et al., 1995). The cortical cells in the lateral roots of slr1 and slr2 always retain the rectangular shape that is typical for cells near the meristem and never gain the elongated form of WT cortical cells in the distal elongation zone (Ishikawa and Evans, 1995).
Work with the Arabidopsis mutants scr and shr has shown many morphological and developmental similarities between primary and lateral root formation (Scheres et al., 1995; Di Laurenzio et al., 1996), suggesting a common regulatory mechanism in both root types. The analysis of the mutants slr1 and slr2 indicates the existence of additional independent pathways for the formation of primary and lateral roots or different sensitivities toward signals controlling their formation. This difference is not surprising considering the origin of the primary root from meristematic embryonic tissue and that of lateral roots from already differentiated pericycle cells (Laskowski et al., 1995).
In contrast to the single mutants slr1 and slr2 with their highly specific effects on lateral root elongation derived from embryonic roots, the double mutant slr1; slr2 affects the complete early root system, leading to a longitudinal cellular disorganization of primary, seminal, and crown roots. Lateral root formation of the affected embryonic roots is reduced to the production of rudimentary, undifferentiated primordial structures that do not penetrate the cortex cell layers of the affected roots. These bulges appear already about 2 mm from the root tip, implying a disturbance of the regular longitudinal root zoning in slr1; slr2. Lateral roots normally are formed in the maturation or differentiation zone located 3 to 4 cm from the root tip when the root has reached its final differentiation status (Esau, 1965).
The idea of longitudinal alteration is supported by the histological analysis of slr1; slr2, characteristically with shorter and thicker primary, lateral-seminal, and crown roots from the coleoptilar node. Microscopy of the affected roots revealed a disturbed longitudinal pattern consisting of cell files with very small rectangular cells beside cell files with very long cells as well as cell files with a mixture of longitudinal and rectangular cells. The coleoptilar node of slr1; slr2 showed an increased diameter caused by enlarged cortical cells. However, the radial pattern was not changed by the double mutation.
These disturbances occur only in the embryonic and early postembryonic roots. Crown root formation from higher nodes and their lateral root formation is completely normal in the double mutant. It is interesting that the severe root phenotype in the double mutant slr1; slr2 does not lead to a reduced aboveground size of these plants at later developmental stages, whereas the mutant lrt1 displaying similar severe root defects showed a reduced aboveground size (Hochholdinger and Feix, 1998). The mechanism by which the double mutant slr1; slr2 compensates for these dramatic deficiencies in the early root system later in development remains unknown.
The double mutant slr1; slr2 reveals the particular situation of two loci which, if mutated, lead to a phenotype that is very different from those of the single mutants. In contrast to slr1 and slr2, which are specifically affected in lateral root formation at the embryonic roots, the double mutant shows a much more severe phenotype as described above. This indicates the synergistic cooperation of these two genes in programming lateral root specificity during early root development.
This could be accommodated by the cooperative influence of both affected genes on the action or on the perception of a signal. Lateral roots at embryonic roots may be more sensitive to the signal than those at postembryonic roots. The fact that the double mutant also affects crown root formation at the coleoptilar node, which is not affected in the single mutants, is an indication of the cooperative influence of both genes on the special developmental control of crown roots forming at the coleoptilar node.
The phenotype of slr1; slr2 supports the previously suggested definition of the early postembryonic phase as a developmental phase in maize (Hochholdinger and Feix, 1998). All embryonic as well as all early postembryonic roots are affected by the combined defects in the genes slr1 and slr2, whereas root development starting with crown root formation from the second node onward is completely normal in the double mutant. This suggests the beginning of a different developmental stage.
The restriction of the defects of slr1 and slr2 on the early postembryonic phase is also supported by the double mutant. Although the embryonic primary and seminal roots are impaired in the double mutant, the defect becomes apparent only postembryonically, when the different initial cells in the root tip region start proliferating new root cells.
The major impact of the cooperative action of the genes slr1 and slr2 appears in their influence on tissue specificity regarding lateral root formation as shown above. The cooperative action of the two genes in the developmental phasing is unlikely, because the timing of the developmental phase is the same in the single mutants and in their double mutant.
However, despite drastic differences in root performance between the single and double mutants, caution must be exercised concerning the interpretation of these phenotypes at the current stage of analysis. At the moment, only one allele of each mutant is available and the genetic status of these mutants (i.e. whether they are null alleles) is not yet known. At this stage of the analysis, however, the newly isolated mutants slr1 and slr2 will be valuable for the further study of lateral root formation in maize and may help to further understand the mechanisms involved in the complex root formation of monocotyledonous plants.
MATERIALS AND METHODS
Plant Material
The Mu-tagged F2 seeds of maize (Zea mays) for the initial mutant screening and the inbred line SWS2000 were provided by Dr. Michael Schwall (Südwestdeutsche Saatzucht, Rastatt, Germany).
Genetic Analysis and Growth Conditions
Selfings, outcrosses, and crosses for allelism tests and double-mutant isolation were performed in a phytochamber under a 12-h-light (28°C)/12-h-dark (18°C) cycle at 70% relative humidity. Propagation of heterozygous WT plants was performed on a local field in the summer of 1998.
For better observation of the young root system of maize, caryopses were grown in paper rolls as described earlier (Hetz et al., 1996). Kernels were surface sterilized with 6% (w/v) sodium hypochlorite, washed three times with distilled water, and arranged in a row on six sheets of connected paper towels (approximately 1 m × 13 cm). The paper was rolled up and longitudinally set into a 1-L beaker with the kernel row 3 cm from the top. The beaker was one-half filled with distilled water. Maize plants were transferred from paper rolls to soil for seed propagation in the phytochamber as described above. A hydroponic culture with maize nutrient solution (Marschner, 1995) was used for in vivo observation of old root systems to overcome the mechanical breakdown of roots removed from soil. Air was continuously supplied to the growth medium by a recycling pump during the entire incubation period.
Microscopic Analysis
Stereomicroscopy was carried out with a dissecting microscope set (Wild, Heerbrugg, Germany) with intact seedlings, using a camera system (M4-P, Leica, Leitz, Canada). The use of a yellow light source with this microscope gave the roots an orange appearence.
Confocal laser scanning microscopy (see Hepler and Gunning, 1998) was carried out with a rhodamine filter (Leica TCS 4D) using propidium iodide as a fluorescent dye (Oparka and Read, 1994). Intact lateral roots of maize or free-hand cuttings of primary roots or of coleoptilar nodes were stained with propidium iodide (5 μg mL−1) in 5 mm potassium phosphate buffer or distilled water for 30 min, washed three times with 50 mL of distilled water, mounted in 75% (v/v) glycerol, and observed. The obtained images were processed by computer.
ACKNOWLEDGMENTS
We thank Dr. Michael Schwall for providing the Mu-tagged F2 seeds, Dr. Dietrich Klein (University of Hohenheim, Germany) for assistance in growing and selfing plants, Nicholas M. Krohn of this institute for his excellent field management and careful reading of this manuscript, and Hannelore Jonas of this institute for excellent technical assistance.
Footnotes
W.J.P. was supported by a combined program of the Deutsche Forschungsgemeinschaft and the Korea Science and Engineering Foundation.
LITERATURE CITED
- Bell JK, McCully MG. A histological study of lateral root initiation and development in Zea mays. Protoplasma. 1970;70:179–205. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, van Onckelen H, van Montagu M. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Charlton WA. Lateral root initiation in plant roots. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant Roots: The Hidden Half. Ed 2. New York: Marcel Dekker Co.; 1996. pp. 107–128. [Google Scholar]
- Cheng JC, Seeley KA, Sung ZA. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda LT. Inheritance and linkage of root characteristic from Pueblo maize. Maize Genet Newsl. 1980;54:18–19. [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Doyle GG. An ageotropic primary root mutant. Maize Genet Newsl. 1978;52:77. [Google Scholar]
- Esau K. Plant Anatomy. New York: John Wiley and Sons, Inc.; 1965. [Google Scholar]
- Feix G, Hochholdinger F, Park WJ. Maize root system and genetic analysis of its formation. In: Waisel Y, Eshel A, Kafkafi U, editors. , Plant Roots: The Hidden Half. Ed 3. New York: Marcel Dekker Co; 2000. (in press) [Google Scholar]
- Gavazzi G, Dolfini S, Galbiati M, Heletjaris T, Landoni M, Pelucchi N, Todesco G. Mutants affecting germination and early seedling development in maize. Maydica. 1993;38:265–274. [Google Scholar]
- Hepler PK, Gunning BES. Confocal fluorescence microscopy of plant cells. Protoplasma. 1998;201:121–157. [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G. Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J. 1996;10:845–857. [Google Scholar]
- Hochholdinger F, Feix G. Early post-embryonic root formation is specifically affected in the maize mutant lrt1. Plant J. 1998;16:247–255. doi: 10.1046/j.1365-313x.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Specialized zones of development in roots. Plant Physiol. 1995;109:725–727. doi: 10.1104/pp.109.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MT. Heritable characters of maize XXXIV-rootless. J Hered. 1930;21:79–80. [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum C, Sussex IA. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. Ed 2. London: Academic Press Limited; 1995. [Google Scholar]
- McCully ME, Canny MJ. Pathways and processes of water and nutrient movements in roots. Plant Soil. 1988;111:159–170. [Google Scholar]
- Oparka KJ, Read ND. The use of fluorescent probes for studies of living plant cells. In: Harris N, Oparka KJ, editors. Plant Cell Biology: A Practical Approach. New York: Oxford University Press; 1994. pp. 27–50. [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. Mutations affecting the radial organisation of the Arabidopsis primary root and root meristem initials. Development. 1995;121:53–62. [Google Scholar]
- Scheres B, Wolkenfelt H. The Arabidopsis root as a model to study plant development. Plant Physiol Biochem. 1998;36:21–32. [Google Scholar]
- Schiefelbein JW, Benfey PN. The development of plant roots: new approaches to underground problems. Plant Cell. 1991;3:1147–1154. doi: 10.1105/tpc.3.11.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen T-J, Schnable PS. Analyses of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. Am J Bot. 1994;81:833–842. [Google Scholar]
- Zhang H, Forde B. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]