Abstract
Aim:
To comprehensively interrogate CYP2D6 by integrating genotyping, copy number analysis and novel strategies to identify CYP2D6*36 and characterize CYP2D6 duplications.
Methods:
Genotyping of 16 CYP2D6 alleles, multiplex ligation-dependent probe amplification (MLPA) and CYP2D6*36 and duplication allele-specific genotyping were performed on 427 African–American, Asian, Caucasian, Hispanic, and Ashkenazi Jewish individuals.
Results:
A novel PCR strategy determined that almost half of all CYP2D6*10 (100C>T) alleles are actually *36 (isolated or in tandem with *10) and all identified duplication alleles were characterized. Integrated results from all testing platforms enabled the refinement of genotype frequencies across all studied populations.
Conclusion:
The polymorphic CYP2D6 gene requires comprehensive interrogation to characterize allelic variation across ethnicities, which was enabled in this study by integrating multiplexed genotyping, MLPA copy number analysis, novel PCR strategies and duplication allele-specific genotyping.
Keywords: : copy number variation, CYP2D6, CYP2D6*36, genotyping, MLPA, pharmacogenetics
The CYP2D6 enzyme is involved in the hepatic metabolism of approximately 25% of commonly prescribed drugs, including antidepressants, antipsychotics, antiarrhythmics, opioids and β-blockers [1]. Variant CYP2D6 star (*) alleles encode enzymes with increased, decreased or no activity, which can contribute to interindividual variability in drug response and adverse reaction risk. Multiple techniques have been employed to interrogate CYP2D6 genotype and copy number, including long-range PCR and targeted genotyping, allele-specific PCR, quantitative real-time PCR, Sanger sequencing and long-read single molecule real-time (SMRT) sequencing [2–5]. The CYP2D6 gene is highly polymorphic with over a hundred star (*) alleles currently cataloged by the Pharmacogene Variation (PharmVar) Consortium (https://www.pharmvar.org/gene/CYP2D6) [6], which include single nucleotide variants, small insertions/deletions, gene conversions, copy number variants (e.g., CYP2D6*5, *36×2) and ‘tandem’ allele structural rearrangements (e.g., CYP2D6*36+*10). However, the highly homologous neighboring CYP2D7 and CYP2D8 pseudogenes make interrogating CYP2D6 technically challenging, which ultimately can lead to inconsistencies in results between genotyping platforms and potential inaccuracies in CYP2D6 metabolizer phenotype prediction [7,8].
The decreased function CYP2D6*10 haplotype is prevalent in populations of Asian ancestry; however, it can also be indicative of an undetected *36 or tandem *36+*10 allele due to shared variants that are commonly included in CYP2D6 genotyping platforms (i.e., 100C>T; 1661G>C; 4180G>C) [9–11]. The CYP2D6*36 allele differs from *10 by the presence of an exon 9 conversion that is not typically interrogated by common genotyping platforms [9]. The CYP2D6*10 allele is included in several genotyping platforms (Affymetrix DMET, Luminex xTAG, LifeTech TaqMan laboratory-developed assay, Agena Bioscience iPLEX ADME PGx Pro, Agena Bioscience CYP2D6 and Autogenomics CYP2D6); however, of these assays only the Agena Bioscience CYP2D6 panel directly interrogates CYP2D6*36 [9]. A PCR-based assay to detect CYP2D6*36 and *36 duplications (*36×N) has been reported [10]; however, this assay cannot identify an exon 9 conversion in the context of a tandem *36+*10 allele. Long-range PCR can also be utilized to infer the presence of CYP2D6*36+*10, as the tandem allele produces an approximately 10 kb long ‘fragment D’ encompassing the *36 gene copy and a CYP2D7-derived exon 9 and downstream region that make this amplicon 1.7 kb longer than a ‘fragment D’ generated from a ‘typical’ duplication (e.g., CYP2D6*2×2 or a *10×2) [12].
Another challenge with CYP2D6 genotyping is specifically interrogating duplicated alleles, as accurate prediction of CYP2D6 metabolizer status necessitates direct analysis of the gene copy (or copies) when an increased copy number is detected, particularly when identified concurrently with normal activity and no function alleles in compound heterozygosity (e.g., CYP2D6*1/*4, Dup) [2,13]. The CYP2D6 alleles that have been reported as duplications are listed within the structural variation document on the PharmVar website (https://www.pharmvar.org/gene/CYP2D6), and their activity scores are determined by multiplying the score of the specific star (*) allele by the number of copies (e.g., activity score of *41×2 is one [0.5 × 2]) [14]. The activity score system assigns values to specific alleles as a reflection of their activity, and the sum of the values of both alleles provides the activity score of a diplotype, which ultimately facilitates the translation of CYP2D6 diplotype to phenotype [15].
To address these technical challenges, we developed a rapid PCR strategy that can distinguish CYP2D6*36 and/or *36+*10 alleles from the related *10, as well as a duplication allele-specific genotyping application of a commercially available CYP2D6 genotyping assay. These were validated by orthogonal Sanger sequencing and tested across a large multiethnic cohort from the New York City metropolitan area (USA).
Materials & methods
Subjects
Peripheral blood samples from healthy adult donors who self-reported their racial and ethnic background (African–American [AA], Asian, Caucasian or Hispanic) and gave informed consent for the use of their DNA for research were obtained from the New York Blood Center (NY, USA) with Institutional Review Board approval as previously described [16]. In addition, blood samples were obtained with informed consent from unrelated, healthy Ashkenazi Jewish (AJ) individuals from the greater New York City metropolitan area as previously described [17–19]. All personal identifiers were removed, and isolated DNA samples were tested anonymously. Genomic DNA was isolated using the Puregene® DNA Purification kit (Qiagen, CA, USA) according to the manufacturer's instructions.
Genotyping
The CYP2D6 allele designations refer to those defined by the PharmVar Consortium (https://www.pharmvar.org). Genotyping of 16 variant CYP2D6 alleles (*2 - *11, *14, *15, *17, *29, *35, *41) and the gene duplication was performed using the xTAG CYP2D6 Kit v3 (Luminex Corporation, TX, USA) according to the manufacturer's instructions and as previously described [20]. Notably, the wild-type *1 allele was assigned in the absence of other detectable variant alleles.
Copy number analysis
CYP2D6 copy number at exons 1, 4, 6 and the 3′ downstream region was interrogated by multiplex ligation-dependent probe amplification (MLPA) using the SALSA MLPA P128-B1 Cytochrome P450 kit (MRC-Holland, Amsterdam, The Netherlands) according to the manufacturer's instructions and as previously described [21]. Of note, interrogating multiple loci across the CYP2D6 gene is necessary to detect copy number signatures that are indicative of structurally rearranged and tandem alleles (e.g., *36+10, *68+*4, etc.), in addition to the standard full gene deletion and duplication alleles. Raw data was analyzed using GeneMarker v1.90 software (SoftGenetics, PA, USA) and copy number was determined based on the following criteria: peak ratio ≥0.25 and <0.75 for one copy, ≥0.75 and <1.25 for two copies, ≥1.25 and <1.7 for three copies, ≥1.7 and <2.2 for four copies and ≥2.2 for more than four copies.
Distinguishing CYP2D6*36 alleles from *10
To distinguish the CYP2D6*36 exon 9 conversion among the samples that genotyped as the related *10 (i.e., 100C>T; 1661G>C; 4180G>C), a novel PCR strategy was developed (Figure 1). A set of primers were designed that amplify a 1222 base pair (bp) product when an exon 9 conversion occurs in either an ‘upstream’ or ‘downstream’ CYP2D6 copy (Table 1). This reaction was duplexed with a CYP2D6 internal control 262 bp amplicon. In addition, previously reported primers were used to concurrently amplify a 597 bp product when an exon 9 conversion occurs only in a ‘downstream’ CYP2D6 copy, which was duplexed with an internal control 860 bp amplicon [10]. The combined results of these two PCR assays can therefore identify and determine if an exon 9 conversion is located in an ‘upstream’ or ‘downstream’ CYP2D6 copy.
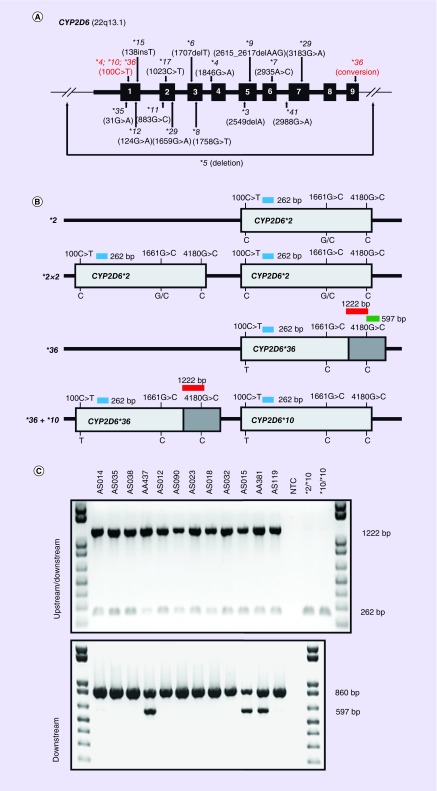
Figure 1. . CYP2D6 gene structure, targeted genotyping, and *36 allele identification.
(A) Gene diagram of CYP2D6 highlighting the location of variant star (*) alleles that are commonly included in targeted genotyping assays, including the deletion allele (*5), 100C>T and the exon 9 conversion. Variant positions are denoted by their common nucleotide nomenclature from M33388.1 GenBank reference sequence. (B) Illustration of the CYP2D6*36 PCR assay highlighting the ability to distinguish *36 and *36+*10 among individuals who were genotyped as *10 carriers (i.e., 100C>T; 1661G>C; 4180G>C). Dark gray bars represent the 3′ downstream conversion regions (C) (Top panel) Upstream/downstream duplex PCR results indicating the presence of an exon 9 conversion in all 12 tested samples (1222 bp product; red bar in the bottom panel). All samples also amplified the 262 bp CYP2D6 control product, including the *36-negative controls. (Bottom panel) Downstream duplex PCR results indicating the presence of a downstream exon 9 conversion in samples AA437, AS015 and AA381 (597 bp product; green bar in panel B). All samples amplified the 860 bp CYP3A7 control fragment. The combined upstream/downstream and downstream PCR results enable the detection of CYP2D6*36 and *36+*10 carriers.
bp: Base pair; NTC: No template control.
Table 1. . Primers and assay conditions for CYP2D6 PCR analysis.
| Primer sequence (5′–3′) | Amplicon size | Annealing temperature | Ref. | |
|---|---|---|---|---|
| CYP2D6 exon 9 | Exon 9 conversion | |||
| Upstream/downstream exon 9 conversion primers | ||||
| GTGAGCCCATCTGGGAAACA | ||||
| AAGCTGACGACACGAGAGTG | – | 1222 bp | ||
| GATGACCGTAGTCCGAGCTG | 262 bp | 262 bp | 62°C | |
| CCCACACTGAGCTTACAGCA | ||||
| Downstream exon 9 conversion primers | ||||
| AGCCACTCTCGTGTCGTCAGCTT | [10] | |||
| CGACTGAGCCCTGGGAGGTAGGTAG | – | 597 bp | [10] | |
| CACCTCTGCTAAGGGAAACAGGCC | 860 bp | 860 bp | 68°C | [10] |
| GCCAGCCTGAACATCCTTTTTGCTA | [10] | |||
| Upstream/downstream copy primers | ||||
| GAACCTCTGGAGCAGCCCATACCC | [2] | |||
| ACTGAGCCCTGGGAGGTAGGTAG | ∼5 kb | 68°C | [12] | |
| Downstream copy primers (‘Fragment D’ Fwd; ‘Fragment F’ Rev) | ||||
| CCAGAAGGCTTTGCAGGCTTCAG | [12] | |||
| CAGGCATGAGCTAAGGCACCCAGA | ∼8.1 kb | 63°C | [12] | |
| Upstream copy primers (‘Fragment D’) | ||||
| CCAGAAGGCTTTGCAGGCTTCAG | [12] | |||
| CGGCAGTGGTCAGCTAATGAC | ∼8.6 kb or 10.2 kb | 63°C | [12] | |
| Exon 8/9 Sanger sequencing primers | ||||
| CCGTCTAGTGGGGAGACAAA | ||||
| GTGGGGTAAGCAGGAATGAG | 842 bp | 60°C | ||
bp: Base pair; kb: Kilobase.
To validate the CYP2D6*36 PCR assay, long-range PCR of both ‘downstream’ and ‘upstream’ CYP2D6 gene copies (when present) were performed and amplicons subjected to Sanger sequencing. Long-range PCR of the ‘downstream’ copy was performed in 20 μl containing approximately 40 ng of DNA, 1× SequalPrep™ Reaction buffer (Invitrogen, CA, USA), 0.5 μM of forward and reverse primers (Table 1) and 1.8 units of SequalPrep™ Polymerase. Amplification consisted of an initial denaturation step at 94°C for 2 min followed by ten amplification cycles (94°C for 10 s, 63°C for 30 s and 68°C for 13 min), another 20 amplification cycles (94°C for 10 s, 63°C for 30 s and 68°C for 13 min + 20 s/cycle) and a final extension at 72°C for 5 min. A second long-range PCR that amplified all gene copies, that is, one or more duplicated ‘upstream’ gene copies as well as the ‘downstream’ gene copy was also employed. This reaction amplified a 5.0 kb fragment as above but with annealing and extension at 68°C, and an extension time of 6 min given the shorter amplicon length. Amplicons were subjected to Sanger sequencing with primers targeting CYP2D6 exon 9, and results were analyzed using FinchTV (Geospiza, WA, USA).
CYP2D6 duplication allele-specific genotyping
Duplicated copies of CYP2D6 were directly interrogated by the long-range PCR that specifically amplified ‘upstream’ copies of the gene [2,12]. As previously described, these primers specifically amplified an 8.6 kb fragment (also known as ‘fragment D’ [12]) that encompasses the entire ‘upstream’ CYP2D6 copy (or 10.2 kb in the presence of the *36 allele), allowing for star (*) allele determination of duplicated copies when present [2]. The PCR conditions for the ‘upstream’ long-range PCR were identical to the ‘downstream’ copy amplification detailed above. Both ‘upstream’ and ‘downstream’ amplicons were used as templates for a 5.0 kb nested PCR as detailed above, which subsequently were subjected to shrimp alkaline phosphatase (SAP)/exonuclease digestion and multiplexed allele-specific primer extension genotyping using the allele-specific primer extension and bead hybridization protocol of the xTAG CYP2D6 Kit v3 (Luminex Molecular Diagnostics, Toronto, ON, Canada).
Results
CYP2D6 genotyping & copy number analysis in a multiethnic cohort
Multiplexed targeted genotyping and MLPA analysis of 427 AA (n = 80), Asian (n = 87), Caucasian (n = 83), Hispanic (n = 81) and AJ (n = 96) individuals identified the frequencies of 16 variant CYP2D6 alleles and the copy number of exons 1, 4, 6 and 3′ downstream region (Supplementary Table 1). However, the absence of any genotyping probes for the CYP2D6*36 exon 9 conversion and the inability to definitively discriminate duplicated alleles by targeted genotyping or MLPA prompted the development of additional assays to further refine CYP2D6 allele frequencies in our multiethnic cohort.
Detection of CYP2D6*36 alleles
To distinguish the CYP2D6*36 allele series from the identified *10 carriers (100C>T; 1661G>C; 4180G>C), our PCR strategy was applied to all subjects that carried *10 based on Luminex genotyping. As illustrated in Figure 1, in the presence of an exon 9 conversion, both 1222 and 262 bp fragments amplified using the upstream/downstream CYP2D6*36 primer pair (Figure 1A). If no upstream or downstream exon 9 conversion was present, only the 262 bp control fragment amplified. Similarly, if the exon 9 conversion occurs in a downstream copy, both the 597 bp fragment and the 860 bp control fragment amplified using the downstream-specific CYP2D6*36 primers (Figure 1B). If no downstream exon 9 conversion was present, only the 860 bp control fragment amplified. The integrated results of this PCR strategy refined 43 of 94 (45.7%) detected *10 alleles into *36 (n = 3; 3.2%), *36+*10 (n = 30; 31.9%) and *36×N+*10 (n = 10; 10.6%), the majority of which being derived from the Asian cohort.
These results were further supported by MLPA, which indicated a 3′ downstream copy number loss across most carriers (see Discussion) as well as copy number gains at exons 1, 4 and 6 among the tandem allele carriers (Table 2). The results of the CYP2D6*36 PCR assay were also confirmed by long-range PCR and bidirectional Sanger sequencing of both upstream and downstream copies on 24 randomly selected Asian samples that were initially genotyped as *10 (data not shown).
Table 2. . Representative CYP2D6*10 cases that were revised by exon 9 conversion PCR and MLPA analysis.
| Original diplotype | Exon 9 conversion PCR | MLPA (copy number†) | Revised diplotype | ||||
|---|---|---|---|---|---|---|---|
| Upstream/downstream | Downstream | Exon 1 | Exon 4 | Exon 6 | Downstream | ||
| *1/*10 | + | ─ | 3 | 3 | 3 | 2 | *1/*36+*10 |
| *1/*10 | + | ─ | 4 | 4 | 4 | 2 | *1/*36×2+*10 |
| *2/*10 | + | ─ | 3 | 3 | 3 | 2 | *2/*36+*10 |
| *2/*10 | + | + | 2 | 2 | 2 | 2‡ | *2/*36 |
| *4/*10 | + | ─ | 3 | 3 | 3 | 2 | *4/*36+*10 |
| *5/*10 | + | ─ | 2 | 2 | 2 | 1 | *5/*36+*10 |
| *5/*10 | + | ─ | 3 | 3 | 3 | 1 | *5/*36×2+*10 |
| *10/*10 | + | ─ | 3 | 3 | 3 | 2 | *10/*36+*10 |
| *10/*10 | + | ─ | 4+ | 4+ | 4+ | 2 | *10/*36×N+*10 |
| *10/*10 | + | + | 4 | 4 | 4 | 2 | *36/*36×2+*10 |
| *10/*17 | + | + | 2 | 2 | 2 | 2‡ | *17/*36 |
| *10/*41 | + | ─ | 3 | 3 | 3 | 2 | *41/*36+*10 |
†‘4+’ represents a total copy number of at least four by MLPA.
‡Representative CYP2D6*36 sample with normal ‘downstream’ region copy number by MLPA testing (see Discussion).
MLPA: Multiplex ligation-dependent probe amplification.
CYP2D6 duplication allele-specific genotyping
All samples with CYP2D6 copy number gains by Luminex and MLPA (with the exception of *10 allele carriers) were further subjected to duplication allele-specific multiplexed targeted genotyping (n = 26). Upstream and downstream copies were amplified for each sample and both amplicons were used independently as templates for Luminex genotyping. This strategy unambiguously identified the haplotype of each duplicated CYP2D6 copy among all tested samples, including 16 samples (61.5%) that were compound heterozygous for CYP2D6 alleles with different activity scores (e.g., CYP2D6*2/*4, Dup; Table 3).
Table 3. . CYP2D6 duplication allele-specific genotyping.
| Original diplotype | Copy-specific genotyping | MLPA copy number | Revised diplotype | Activity score | |
|---|---|---|---|---|---|
| Upstream | Downstream | ||||
| *1/*2, Dup | *1 | *1/*2 | 3 | *1×2/*2 | 3 |
| *4/*17, Dup | *4 | *4/*17 | 3 | *4×2/*17 | 0.5 |
| *2/*4, Dup | *4 | *2/*4 | 3 | *2/*4×2 | 1 |
| *2/*4, Dup | *2 | *2/*4 | 3 | *2×2/*4 | 2 |
| *2/*4, Dup | *4 | *2/*4 | 3 | *2/*4×2 | 1 |
| *1/*4, Dup | *4 | *1/*4 | 3 | *1/*4×2 | 1 |
| *1/*4, Dup | *4 | *1/*4 | 3 | *1/*4×2 | 1 |
| *4/*17, Dup | *4 | *4/*17 | 3 | *4×2/*17 | 0.5 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *2/*41, Dup | *2 | *2/*41 | 3 | *2×2/*41 | 2.5 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *1/*2, Dup | *2 | *1/*2 | 4 | *1/*2×3 | 4 |
| *2/*9, Dup | *2 | *2/*9 | 3 | *2×2/*9 | 2.5 |
| *1/*2, Dup | *1/*2 | *1/*2 | 4 | *1×2/*2×2 | 4 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *1/*4, Dup | *1 | *1/*4 | 3 | *1×2/*4 | 2 |
| *2/*41, Dup | *2 | *2/*41 | 3 | *2×2/*41 | 2.5 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *2/*4, Dup | *2 | *2/*4 | 3 | *2×2/*4 | 2 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *2/*35, Dup | *2 | *2/*35 | 3 | *2×2/*35 | 3 |
| *1/*4, Dup | *1 | *1/*4 | 3 | *1×2/*4 | 2 |
| *1/*2, Dup | *2 | *1/*2 | 3 | *1/*2×2 | 3 |
| *2/*17, Dup | *2 | *2/*17 | 3 | *2×2/*17 | 2.5 |
| *1/*4, Dup | *1 | *1/*4 | 3 | *1×2/*4 | 2 |
| *2/*41, Dup | *2 | *2/*41 | 3 | *2×2/*41 | 2.5 |
Revised CYP2D6 allele & genotype frequencies
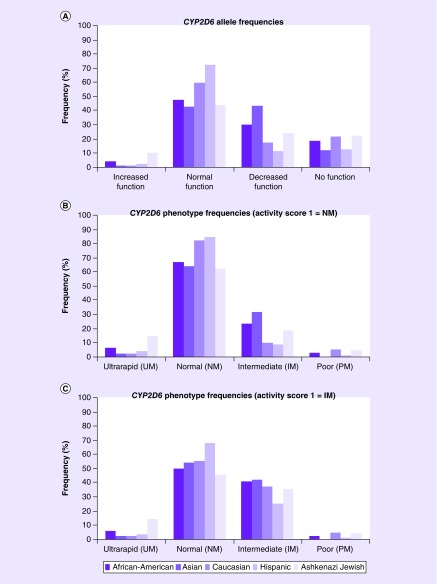
The results from the CYP2D6*36 exon 9 and duplication allele-specific genotyping were incorporated into the previously identified multiethnic CYP2D6 allele and genotype frequencies, which are summarized in Table 4 and Supplementary Table 2, respectively. The CYP2D6*7, *8, *11 and *15 alleles were not detected in any of the tested subjects. The combined frequencies of detected alleles in the AA, Asian, Caucasian, Hispanic and AJ populations were distributed as increased function (0.038, 0.011, 0.012, 0.024 and 0.104), normal function (0.475, 0.426, 0.596, 0.725 and 0.438), decreased function (0.300, 0.432, 0.175, 0.116 and 0.240) and no function (0.188, 0.120, 0.217, 0.128 and 0.219), respectively (Table 4 & Figure 2A).
Table 4. . Revised CYP2D6 allele frequencies.
| CYP2D6 allele | African–American (n = 160) | Asian (n = 174) | Caucasian (n = 166) | Hispanic (n = 162) | Ashkenazi Jewish (n = 192) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Freq. | 95% CI | Freq. | 95% CI | Freq. | 95% CI | Freq. | 95% CI | Freq. | 95% CI | |
| Increased function | ||||||||||
| *1×2 | 0.013 | 0.000–0.030 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.018 | 0.026 | 0.004–0.049 |
| *2×2 | 0.025 | 0.001–0.049 | 0.011 | 0.000–0.027 | 0.012 | 0.000–0.029 | 0.018 | 0.000–0.039 | 0.078 | 0.040–0.116 |
| Subtotal | 0.038 | 0.011 | 0.012 | 0.024 | 0.104 | |||||
| Normal function | ||||||||||
| *1 | 0.256 | 0.189–0.324 | 0.273 | 0.207–0.339 | 0.361 | 0.288–0.435 | 0.475 | 0.398–0.552 | 0.313 | 0.247–0.378 |
| *2 | 0.213 | 0.149–0.276 | 0.142 | 0.090–0.194 | 0.169 | 0.112–0.226 | 0.226 | 0.162–0.290 | 0.099 | 0.057–0.141 |
| *29×2 | 0.006 | 0.000–0.018 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *35 | 0.000 | 0.000–0.000 | 0.011 | 0.000–0.027 | 0.066 | 0.028–0.104 | 0.024 | 0.001–0.048 | 0.026 | 0.004–0.049 |
| Subtotal | 0.475 | 0.426 | 0.596 | 0.725 | 0.438 | |||||
| Decreased function | ||||||||||
| *9 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.017 | 0.012 | 0.000–0.029 | 0.024 | 0.001–0.048 | 0.005 | 0.000–0.015 |
| *10 | 0.044 | 0.012–0.075 | 0.136 | 0.086–0.187 | 0.030 | 0.004–0.056 | 0.000 | 0.000–0.000 | 0.078 | 0.040–0.116 |
| *17 | 0.125 | 0.074–0.176 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.018 | 0.010 | 0.000–0.025 |
| *29 | 0.106 | 0.059–0.154 | 0.006 | 0.000–0.017 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.018 | 0.000 | 0.000–0.000 |
| *36+*10 | 0.000 | 0.000–0.000 | 0.153 | 0.100–0.207 | 0.006 | 0.000–0.018 | 0.006 | 0.000–0.018 | 0.005 | 0.000–0.015 |
| *36×2+*10 | 0.000 | 0.000–0.000 | 0.057 | 0.023–0.092 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *41 | 0.025 | 0.001–0.049 | 0.074 | 0.035–0.113 | 0.127 | 0.076–0.177 | 0.073 | 0.033–0.113 | 0.141 | 0.091–0.190 |
| *41×2 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| Subtotal | 0.300 | 0.432 | 0.175 | 0.116 | 0.240 | |||||
| No function | ||||||||||
| *3 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.017 | 0.018 | 0.000–0.038 | 0.006 | 0.000–0.018 | 0.005 | 0.000–0.015 |
| *4 | 0.069 | 0.030–0.108 | 0.051 | 0.019–0.084 | 0.169 | 0.112–0.226 | 0.091 | 0.047–0.136 | 0.203 | 0.146–0.260 |
| *4×2 | 0.050 | 0.016–0.084 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.018 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *5 | 0.056 | 0.021–0.092 | 0.046 | 0.015–0.077 | 0.018 | 0.000–0.038 | 0.030 | 0.004–0.057 | 0.005 | 0.000–0.015 |
| *6 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.006 | 0.000–0.018 | 0.000 | 0.000–0.000 | 0.005 | 0.000–0.015 |
| *7 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *8 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *11 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *14 | 0.000 | 0.000–0.000 | 0.011 | 0.000–0.027 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *15 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| *36 | 0.013 | 0.000–0.030 | 0.006 | 0.000–0.017 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 | 0.000 | 0.000–0.000 |
| Subtotal | 0.188 | 0.120 | 0.217 | 0.128 | 0.219 | |||||
Freq.: Frequency.
Figure 2. . Graphical illustration of the identified multiethnic CYP2D6 allele frequencies (A), and metabolizer phenotype frequencies using two systems, activity score of 1 = normal metabolizers (B) and activity score of 1 = intermediate metabolizers (C).
Prediction of CYP2D6 phenotype from genotype data
There currently is no standardized protocol for the translation of CYP2D6 genotype to phenotype, which mostly affects diplotypes consisting of one functional and one no function, or two decreased function alleles (i.e., total activity score = 1). These diplotypes are often classified in the literature as either normal metabolizers (NMs) or intermediate metabolizers, which recently has been highlighted by the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen [22]. Classifying patients with an activity score of 1 as NMs results in predicted CYP2D6 metabolizer phenotypes in the AA, Asian, Caucasian, Hispanic and AJ of ultrarapid (6.3, 2.3, 2.4, 3.7 and 14.6%), normal (67.5, 64.8, 83.1, 85.4 and 62.5%), intermediate (23.8, 31.8, 9.6, 8.5 and 18.8%) and poor (2.5, 0.0, 4.8, 1.2 and 4.2%) metabolizers, respectively (Supplementary Table 2 & Figure 2B). However, classifying patients with an activity score of 1 as intermediate metabolizers results in modified CYP2D6 metabolizer phenotypes in the AA, Asian, Caucasian, Hispanic and AJ of normal (50.0, 54.5, 55.4, 68.3 and 45.8%) and intermediate (41.3, 42.1, 37.3, 25.6 and 35.4%) metabolizers, respectively (Figure 2C).
Discussion
The technical challenges with interrogating the polymorphic CYP2D6 gene prompted our development of a novel PCR assay for the exon 9 conversion that unequivocally allows us to detect the CYP2D6*36 allele, as well as a genotyping strategy that specifically characterizes CYP2D6 duplication alleles. In conjunction with multiplexed CYP2D6-targeted genotyping and MLPA analysis, this integrated testing approach was performed on a large cohort of AA (n = 80), Asian (n = 87), Caucasian (n = 83), Hispanic (n = 81) and AJ (n = 96) individuals from the New York City metropolitan area. Despite the polymorphic nature and structural complexity of the CYP2D6 gene, our comprehensive genotyping and copy number interrogation (requiring ∼400–500 ng of DNA) enabled refined allele and diplotype detection across all tested populations. Moreover, the inclusion of our exon 9 conversion PCR assay determined that almost half of all CYP2D6*10 alleles are actually unrecognized *36 or *36 tandem alleles, which are not directly interrogated by most commercial genotyping panels.
Despite the increasing availability of high-throughput short-read pharmacogenomic sequencing programs [23,24], the challenges with accurately interrogating the CYP2D6 locus and the uncertainty of interpreting both novel and rare variants [3,25] together suggest that targeted genotyping of functional CYP2D6 variants will continue to be used by both clinical and research laboratories. Similarly, the recent development of long-read CYP2D6 SMRT sequencing using the Pacific Biosciences platform [2] is currently only accessible to those with the necessary sequencing infrastructure and bioinformatics expertise. As such, at the center of this study was the targeted genotyping of 16 well-characterized variant CYP2D6 alleles (including gene deletion and duplication), coupled with quantitative copy number interrogation at four loci across the CYP2D6 gene by MLPA. These multiplexed commercial assays are robust and accurate, and their results complement each other; however, their inability to directly genotype the exon 9 conversion and identify the sequence of duplicated CYP2D6 alleles prompted our development of alternative strategies for more comprehensive characterization.
The 100C>T (p.P34S) transition was one of the first variant CYP2D6 alleles reported [26–28] and later cataloged among several human CYP2D6 haplotypes [6,29]. This common variant is located in exon 1 of the CYP2D6 gene (NM_000106.5:c.100C>T; rs1065852), and has a global minor allele frequency (MAF) of 20.5% in the Genome Aggregation Database (http://gnomad.broadinstitute.org/), which is highest among east Asians with a MAF of 57.8% (European: 24.9%; south Asian: 18.1%; African: 15.1% and Latino: 15.1%) [30]. When 100C>T is in cis with only common ‘benign’ CYP2D6 variants (i.e., 1661G>C,4180G>C), the haplotype is defined as CYP2D6*10 and classified as decreased function. Of note, an international working group has recently suggested revising the activity score of CYP2D6*10 from 0.5 to 0.25 (https://cpicpgx.org/resources/cyp2d6-genotype-to-phenotype-standardization-project/).
However, 100C>T (p.P34S) is also an important variant of the nonfunctional *4 haplotype (with 1846G>A), as well as several other nonfunctional (*14, *36, *47, *56, *57, *69, *99, *100, *101), decreased (*49, *54, *72) and uncertain (*37, *52, *65, *87, *94, *95) function haplotypes. The prevalence and functionality of 100C>T prompted its inclusion into most commercial CYP2D6 genotyping assays and laboratory-developed tests; however, it is not typically differentiated by these platforms beyond the CYP2D6*10 and *4 haplotypes. Although this is most likely due to the low or unknown population frequencies of the other star (*) allele variants that also harbor 100C>T [31], notable among them is the nonfunctional CYP2D6*36 that is principally defined by 100C>T and an exon 9 conversion [10]. This related allele (often found as a tandem *36+*10) is more prevalent among east Asians (∼27%) [31,32], but has also been found in Africans and AAs (∼1%) [31]; however, it is rarely included in commercial genotyping platforms.
Our multiethnic population screen detected 100C>T at a MAF of 24.2% across all studied populations (42.0% in Asians), and resulted in original CYP2D6*10 allele frequencies in the AA, Asian, Caucasian, Hispanic and AJ of 5.6, 35.6, 3.6, 0.6 and 8.3%, respectively. However, after applying our exon 9 conversion PCR assay to all subjects with a CYP2D6*10-containing diplotype, almost half of the *10 alleles were redefined to *36 (3.2%), *36+*10 (31.9%) or *36×N+*10 (10.6%). As such, the revised CYP2D6*10 allele frequencies in our multiethnic cohort were reduced to 4.4, 13.8, 3.0, 0 and 7.8%, respectively.
MLPA testing confirmed all the CYP2D6 deletion (*5) and duplication alleles identified by Luminex genotyping; however, 40 (9.4%) multiethnic samples had copy number gains by MLPA (exons 1, 4 and 6) without a gene duplication signal by Luminex. The absence of a copy number gain at the 3′ downstream region in these cases with concurrent 100C>T was supportive of a CYP2D6*36+*10 tandem allele as the exon 9 conversion likely interferes with MLPA probe hybridization in the ‘downstream’ copy. However, it is notable that 2 of 42 (4.8%) isolated CYP2D6*36 allele carriers did not have a 3′ downstream copy number loss by MLPA, which was attributed to variability in the extent of the downstream gene conversion and its capacity to influence MLPA probe hybridization. This is supported by the location of 3′ downstream MLPA probes and the exon 9 (*36; 597 bp amplicon) PCR primers, as the MLPA probes hybridize 480–503 bp downstream of exon 9, whereas the reverse PCR primer hybridizes only 434–458 bp downstream of exon 9. As such, integrating our exon 9 conversion PCR assay and MLPA analysis enabled the complementary detection of all complex CYP2D6*36 alleles, which occur in multiethnic populations (5.0% MAF in our cohort) and most frequently among Asians (21.8% MAF in our cohort).
All other samples with copy number gains detected by both Luminex and MLPA were subjected to duplication allele-specific genotyping using an amplicon that specifically amplified the ‘upstream’ CYP2D6 copy or copies and a modified Luminex protocol. This approach eliminated the ambiguity in characterizing duplications [13], which was not previously possible with only Luminex genotyping and/or MLPA testing. Assessing the Luminex median fluorescent intensity ratios of heterozygous alleles among duplication-positive samples can provide support for the haplotype of some ‘upstream’ copies [20]; however, only direct interrogation of ‘upstream’ amplicons can characterize these alleles with certainty. Importantly, the majority (61.5%) of duplication alleles in our multiethnic cohort were detected among individuals with compound heterozygous sequence variants that defined two haplotypes with different activity scores. This strategy is similar to the previously reported TaqMan genotyping of ‘fragment D’ duplication-specific amplicons [12]. The data presented in that study and those presented from this investigation underscore the importance and utility of CYP2D6 duplication allele-specific genotyping, as the direct characterization of ‘upstream’ copies in these compound heterozygotes enabled more precise activity score and metabolizer phenotype prediction.
Conclusion
As the frequencies of variant CYP2D6 alleles continue to be elucidated in diverse populations [31], it is increasingly apparent that comprehensive characterization is needed when studying this important polymorphic gene. Despite several clinically relevant examples of CYP2D6-mediated drug response [22,33–36], some CYP2D6 association studies have been inconsistent, which is likely due, at least in part, to discrepant and/or inadequate CYP2D6 genotyping [37]. As such, our integrated testing of CYP2D6 using multiple platforms, including novel assays, enabled the identification of alleles that are found in diverse populations, emphasizing the need to expand common genotyping approaches when interrogating non-Caucasian or admixed populations. Moreover, in the absence of phased long-read sequencing [2] and haplotype-specific [4] approaches, the addition of CYP2D6*36 and duplication allele-specific assays to multiplexed genotyping improves metabolizer phenotype prediction and may facilitate more consistent outcomes among CYP2D6 pharmacogenetic association studies.
Summary points.
CYP2D6 genotyping & copy number analysis in a multiethnic cohort
Multiplexed targeted genotyping and MLPA analysis of 427 African–American, Asian, Caucasian, Hispanic and Ashkenazi Jewish individuals identified the frequencies of 16 variant CYP2D6 alleles and the copy number of exons 1, 4, 6 and 3′ downstream region.
Detection of CYP2D6*36 alleles
A novel PCR strategy was developed to distinguish the CYP2D6*36 exon 9 conversion, which was applied to all subjects that carried *10 based on targeted genotyping.
The combined results of this PCR strategy redefined approximately 46% of all identified *10 alleles into *36 (3.2%), *36+*10 (31.9%) or *36×2+*10 (10.6%).
MLPA analysis across the CYP2D6 gene supported the results of the CYP2D6*36 exon 9 conversion PCR assay.
CYP2D6 duplication allele-specific genotyping
Duplication allele-specific multiplexed genotyping unambiguously identified the haplotype of each duplicated CYP2D6 copy among all tested samples, including samples that were compound heterozygous for CYP2D6 alleles with different activity scores.
Revised CYP2D6 allele & genotype frequencies
The combined allele frequencies after integrating all testing results in the African–American, Asian, Caucasian, Hispanic and Ashkenazi Jewish populations were distributed as increased function (0.038, 0.011, 0.012, 0.024 and 0.104), normal function (0.475, 0.426, 0.596, 0.725 and 0.438), decreased function (0.300, 0.432, 0.175, 0.116 and 0.240) and no function (0.188, 0.120, 0.217, 0.128 and 0.219), respectively.
CYP2D6 phenotype prediction
CYP2D6 metabolizer phenotypes were inferred based on identified diplotypes and classified using two systems: activity score of 1 as normal metabolizers, and activity score of 1 as intermediate metabolizers.
Conclusion
Integrated testing of CYP2D6 using novel genotyping assays and multiple platforms enabled the identification of allele frequencies across diverse populations.
The addition of CYP2D6*36 and duplication allele-specific assays to multiplexed genotyping improves metabolizer phenotype prediction and may facilitate more consistent outcomes among CYP2D6 pharmacogenetic association studies.
Supplementary Material
Footnotes
Financial & competing interests disclosure
The CYP450 multiplex ligation-dependent probe amplification kit reagents used in this tudy were generously provided by MRC-Holland (Amsterdam, The Netherlands) and the xTAG CYP2D6 v3 Kits by Luminex Corporation (TX, USA). R Vijzelaar is a paid employee of MRC-Holland, Amsterdam, The Netherlands; W Qiao, G Mendiratta, L Shi, L Edelmann, R Kornreich and SA Scott are paid employees of Sema4, Stamford, CT, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Owen RP, Sangkuhl K, Klein TE, Altman RB. Cytochrome P450 2D6. Pharmacogenet. Genomics. 2009;19(7):559–562. doi: 10.1097/FPC.0b013e32832e0e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao W, Yang Y, Sebra R, et al. Long-read single molecule real-time full gene sequencing of cytochrome P450-2D6. Hum. Mutat. 2016;37(3):315–323. doi: 10.1002/humu.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Botton MR, Scott ER, Scott SA. Sequencing the CYP2D6 gene: from variant allele discovery to clinical pharmacogenetic testing. Pharmacogenomics. 2017;18(7):673–685. doi: 10.2217/pgs-2017-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaedigk A, Riffel AK, Leeder JS. CYP2D6 haplotype determination using long range allele-specific amplification: resolution of a complex genotype and a discordant genotype involving the CYP2D6*59 allele. J. Mol. Diagn. 2015;17(6):740–748. doi: 10.1016/j.jmoldx.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nofziger C, Paulmichl M. Accurately genotyping CYP2D6: not for the faint of heart. Pharmacogenomics. 2018;19(13):999–1002. doi: 10.2217/pgs-2018-0105. [DOI] [PubMed] [Google Scholar]
- 6.Gaedigk A, Ingelman-Sundberg M, Miller NA, et al. The Pharmacogene Variation (PharmVar) consortium: incorporation of the Human Cytochrome P450 (CYP) Allele Nomenclature Database. Clin. Pharmacol. Ther. 2018;103(3):399–401. doi: 10.1002/cpt.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt VM, Zehnbauer B, Wilson JA, et al. Characterization of 107 genomic DNA reference materials for CYP2D6, CYP2C19, CYP2C9, VKORC1, and UGT1A1: a GeT-RM and Association for Molecular Pathology Collaborative Project. J. Mol. Diagn. 2010;12(6):835–846. doi: 10.2353/jmoldx.2010.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaedigk A. Complexities of CYP2D6 gene analysis and interpretation. Int. Rev. Psychiatry. 2013;25(5):534–553. doi: 10.3109/09540261.2013.825581. [DOI] [PubMed] [Google Scholar]
- 9.Pratt VM, Everts RE, Aggarwal P, et al. Characterization of 137 genomic DNA reference materials for 28 pharmacogenetic genes: a GeT-RM Collaborative Project. J. Mol. Diagn. 2016;18(1):109–123. doi: 10.1016/j.jmoldx.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaedigk A, Bradford LD, Alander SW, Leeder JS. CYP2D6*36 gene arrangements within the cyp2d6 locus: association of CYP2D6*36 with poor metabolizer status. Drug Metab. Dispos. 2006;34(4):563–569. doi: 10.1124/dmd.105.008292. [DOI] [PubMed] [Google Scholar]
- 11.Soyama A, Saito Y, Kubo T, et al. Sequence-based analysis of the CYP2D6*36-CYP2D6*10 tandem-type arrangement, a major CYP2D6*10 haplotype in the Japanese population. Drug Metab. Pharmacokinet. 2006;21(3):208–216. doi: 10.2133/dmpk.21.208. [DOI] [PubMed] [Google Scholar]
- 12.Gaedigk A, Ndjountche L, Divakaran K, et al. Cytochrome P4502D6 (CYP2D6) gene locus heterogeneity: characterization of gene duplication events. Clin. Pharmacol. Ther. 2007;81(2):242–251. doi: 10.1038/sj.clpt.6100033. [DOI] [PubMed] [Google Scholar]
- 13.Ramamoorthy A, Skaar TC. Gene copy number variations: it is important to determine which allele is affected. Pharmacogenomics. 2011;12(3):299–301. doi: 10.2217/pgs.11.5. [DOI] [PubMed] [Google Scholar]
- 14.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008;83(2):234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 15.Gaedigk A, Dinh JC, Jeong H, Prasad B, Leeder JS. Ten years’ experience with the CYP2D6 activity score: a perspective on future investigations to improve clinical predictions for precision therapeutics. J. Pers. Med. 2018;8(2) doi: 10.3390/jpm8020015. pii:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martis S, Peter I, Hulot JS, Kornreich R, Desnick RJ, Scott SA. Multi-ethnic distribution of clinically relevant CYP2C genotypes and haplotypes. Pharmacogenomics J. 2013;13(4):369–377. doi: 10.1038/tpj.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott SA, Edelmann L, Kornreich R, Desnick RJ. Warfarin pharmacogenetics: CYP2C9 and VKORC1 genotypes predict different sensitivity and resistance frequencies in the Ashkenazi and Sephardi Jewish populations. Am. J. Hum. Genet. 2008;82(2):495–500. doi: 10.1016/j.ajhg.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott SA, Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases. Hum. Mutat. 2010;31(11):1240–1250. doi: 10.1002/humu.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott SA, Martis S, Peter I, Kasai Y, Kornreich R, Desnick RJ. Identification of CYP2C19*4B: pharmacogenetic implications for drug metabolism including clopidogrel responsiveness. Pharmacogenomics J. 2012;12(4):297–305. doi: 10.1038/tpj.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott SA, Edelmann L, Kornreich R, Erazo M, Desnick RJ. CYP2C9, CYP2C19 and CYP2D6 allele frequencies in the Ashkenazi Jewish population. Pharmacogenomics. 2007;8(7):721–730. doi: 10.2217/14622416.8.7.721. [DOI] [PubMed] [Google Scholar]
- 21.Martis S, Mei H, Vijzelaar R, Edelmann L, Desnick RJ, Scott SA. Multi-ethnic cytochrome-P450 copy number profiling: novel pharmacogenetic alleles and mechanism of copy number variation formation. Pharmacogenomics J. 2013;13(6):558–566. doi: 10.1038/tpj.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz MP, Sangkuhl K, Guchelaar HJ, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 2018;103(5):770–777. doi: 10.1002/cpt.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush WS, Crosslin DR, Owusu-Obeng A, et al. Genetic variation among 82 pharmacogenes: the PGRNseq data from the eMERGE network. Clin. Pharmacol. Ther. 2016;100(2):160–169. doi: 10.1002/cpt.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng D, Hong CS, Singh LN, Johnston JJ, Mullikin JC, Biesecker LG. Assessing the capability of massively parallel sequencing for opportunistic pharmacogenetic screening. Genet. Med. 2017;19(3):357–361. doi: 10.1038/gim.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen-Torvik LJ, Almoguera B, Doheny KF, et al. Concordance between research sequencing and clinical pharmacogenetic genotyping in the eMERGE-PGx study. J. Mol. Diagn. 2017;19(4):561–566. doi: 10.1016/j.jmoldx.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson I, Oscarson M, Yue QY, Bertilsson L, Sjoqvist F, Ingelman-Sundberg M. Genetic analysis of the Chinese cytochrome P4502D locus: characterization of variant CYP2D6 genes present in subjects with diminished capacity for debrisoquine hydroxylation. Mol. Pharmacol. 1994;46(3):452–459. [PubMed] [Google Scholar]
- 27.Yokota H, Tamura S, Furuya H, et al. Evidence for a new variant CYP2D6 allele CYP2D6J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics. 1993;3(5):256–263. doi: 10.1097/00008571-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Gough AC, Miles JS, Spurr NK, et al. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990;347(6295):773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- 29.Sim SC, Ingelman-Sundberg M. Update on allele nomenclature for human cytochromes P450 and the Human Cytochrome P450 Allele (CYP-allele) Nomenclature Database. Methods Mol. Biol. 2013;987:251–259. doi: 10.1007/978-1-62703-321-3_21. [DOI] [PubMed] [Google Scholar]
- 30.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 2017;19(1):69–76. doi: 10.1038/gim.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwannasri P, Thongnoppakhun W, Pramyothin P, Assawamakin A, Limwongse C. Combination of multiplex PCR and DHPLC-based strategy for CYP2D6 genotyping scheme in Thais. Clin. Biochem. 2011;44(13):1144–1152. doi: 10.1016/j.clinbiochem.2011.06.985. [DOI] [PubMed] [Google Scholar]
- 33.Bell GC, Caudle KE, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin. Pharmacol. Ther. 2017;102(2):213–218. doi: 10.1002/cpt.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks JK, Sangkuhl K, Swen JJ, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 2017;102(1):37–44. doi: 10.1002/cpt.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015;98(2):127–134. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin. Pharmacol. Ther. 2014;95(4):376–382. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hertz DL, Mcleod HL, Irvin WJ., Jr Tamoxifen and CYP2D6: a contradiction of data. Oncologist. 2012;17(5):620–630. doi: 10.1634/theoncologist.2011-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.