Abstract
The Mechanistic or Mammalian Target of Rapamycin (mTOR) is a major signaling pathway in eukaryotic cells belonging to the P13K-related kinase family of the serine/threonine protein kinase. It has been established that mTOR plays a central role in cellular processes and implicated in various cancers, diabetes, and in the aging process with very poor prognosis. Inhibition of the mTOR pathway in the cells may improve the therapeutic index in cancer treatment. Photodynamic therapy (PDT) has been established to selectively eradicate neoplasia at clearly delineated malignant lesions. This review highlights recent advances in understanding the role or regulation of mTOR in cancer therapy. It also discusses how mTOR currently contributes to cancer as well as future perspectives on targeting mTOR therapeutically in cancer in vitro.
Keywords: cancers, mTOR, inhibitors, photodynamic therapy
1. Introduction
The Mechanistic or Mammalian Target of Rapamycin (mTOR) pathway incorporates both intra and extracellular signals, and functions as a key regulator of physiological processes including in the growth, metabolism, proliferation, metastasis and malignant transformation of various human tumors [1]. Based on statistics from the Cancer Genome Atlas Pan-Cancer effort, the mTOR signaling pathway was found to be one of the highest mutated genes in 12 cancers analyzed from 3281 tumors. Examples of these cancers include breast, colon, lung, uterine corpus endometrioid, head and neck as well as ovarian [2,3]. mTOR receives signals from its effectors to control the cell function and homeostasis in normal cells. However, in cancer cells, this function is lost. Somatic mutation and gene amplification encode key components leading to the activation of the pathway that enhances cell proliferation and tumor growth [4,5,6,7,8]. mTOR serves as the major growth and survival pathway for cancer pathogenesis and has been an attractive target development of anticancer therapies. mTOR functions in controlling the downstream processes of ribosomes, mRNA, protein synthesis as well as translation. To achieve these functions, they interfere with various signaling pathways including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), phosphatidylinositol-3-kinase (PI3K)/AKT, reticular activating system (RAS), and tuberous sclerosis complex (TSC). When deregulated, they may induce uncontrolled cell growth and proliferation [9]. Furthermore, growth factors such as tyrosine kinase receptors play an important role in the downstream processes within the pathway to enhance biological processes such as angiogenesis, proliferation, metabolism, survival and differentiation [4]. The pathway may therefore be very useful in cancer pathogenesis and disease progression if it is altered and further lead to the development of molecularly targeted treatments that could advance into successful clinical trials [10].
Various inhibitors and signaling components for downstream processes have shown promising results in clinical trials. Clinically, relevant inhibitors target different pathways that present high sensitivity and needs to be studied [11,12,13]. Second-generation mTOR inhibitors have shown improved antitumor activity both in animal models and in vitro. Some previously studied 1st generation inhibitors have shown very little sensitivity including 1st generation rapamycin derivatives (Rapalogs) which have not proven to be very efficient due to their pharmacodynamics. There is still ongoing preclinical and clinical trials to evaluate various targets [14]. Several cancers become resistant to conventional therapies leading to poor prognostics [2,3] and in the effort to enhance therapy and curb resistance, several combination therapies are been investigated [6,15,16].
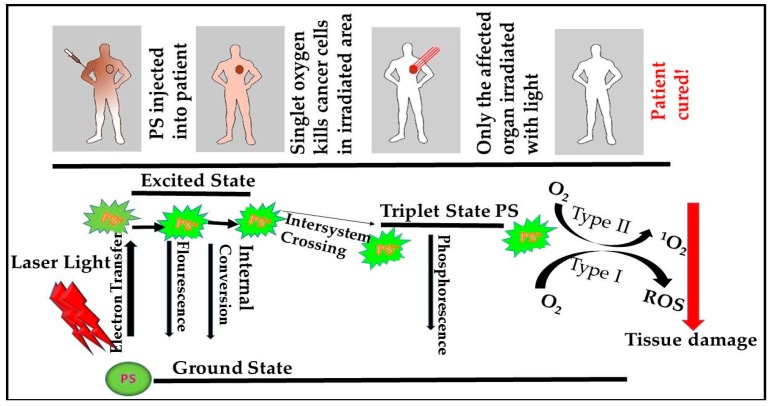
Photodynamic therapy (PDT) was originally developed about a hundred years ago for the treatment of various tumors and other non-malignant diseases [17]. The treatment mechanism involves the injection of a non-toxic photosensitizer (PS) locally, systemically or topically to a specific lesion accompanied by the absorption of visible light of a particular wavelength in the presence of oxygen from the singlet state to the triplet state as a means of generating cytotoxic reactions [18]. These reactions form reactive oxygen species (ROS) which result in tissue destruction, pathogenic microbes and cell death [19,20] (Figure 1). Photo activation may destroy cancer cells through apoptosis, necrosis or autophagy based on the organelle which the PS has accumulated [21]. PDT specifically targets malignant tumors and destroys the cell with minimal side effects [7]. Photoreactions release oxidant species which may alter the cell, its microenvironment, or even the whole organism. The process involves two types of reaction pathways namely type I (radicals and ROS) and type II (Singlet oxygen) [18] (Figure 1). More oxygen molecules are produced in the singlet state which makes type II more predominant [18]. The action of an ideal PS is based on various factors including PS concentration and localization, amount of energy released, the genetic profile, the dosage administered and wavelength [20]. mTOR has also been demonstrated as a target for PDT in vivo using the lysosomal-based phthalocyanine derivative. This was proven effective in treating 4-Nitroquinoline-1-Oxide (4-NQO) induced murine oral cancer. Velloso, et al. [22] found that the PI3K/Akt/mTOR pathway was inhibited in Human Oral Squamous Cell Carcinoma (OSCC) cells using Aluminum Phthalocyanine (AlPc)-based PDT. Furthermore, Fateye, et al. [23,24] found PI3K pathway inhibitors to significantly enhance the response of PDT [23,24]. Interactions between the mTOR signaling pathway and PDT is under research. This review focuses on targeting mTOR inhibitors in PDT of cancer cells.
Figure 1.
Schematic model of the Mechanism of Photodynamic Therapy (PDT), excitation and relaxation of a photosensitizer, and type I and type II photoreactions. Photosensitizers (PS) after an application as cream or injected become activated by light at specific wavelengths in the presence of oxygen (O2). When activated they become excited and move from the singlet state to the triple state generating cytotoxic reactions. Some of the phytophysical reactions include electron transfer, fluorescence, internal conversion, intersystem crossing, and phosphorescence. These reactions directly generate singlet oxygen (1O2) or indirectly, reactive oxygen species (ROS) resulting in tissue damage and cell death [18].
2. The mTOR Pathway
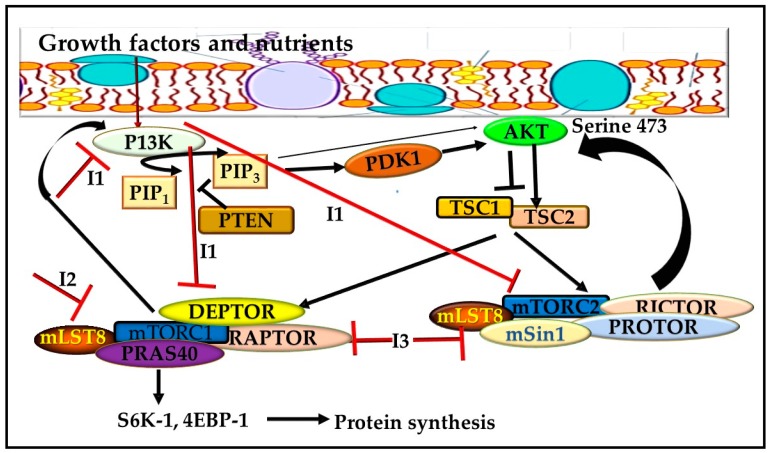
The mTOR pathway comprises a 289 kDa serine/threonine kinase situated downstream of the PI3K-AKT signaling pathway [25]. mTOR has been revealed to be a major regulator of cell growth, proliferation, migration, differentiation, and survival [25]. Studies have also shown that mTOR is deregulated in most human cancers both upstream via the PI3K-AKT pathway and downstream via the 4E-binding protein 1 (4E-BP1) and Ribosomal protein S6 kinase beta-1 (S6 kinase) pathway, all of which make it a target for tumor suppression [26]. Being the most distorted pathway in human cancers, thePI3K signaling pathway plays a very important role in tumor cell survival and progression. AKT and mTOR are further activated downstream mechanism through the conversion of phosphatidylinositol-4, 5-biphosphate (PIP2) to phosphatidylinositol-3, 4, 5-triphosphate (PIP3) in the cell membrane to induce a cascade of protein phosphorylation (Figure 2). Abnormal activation can enhance tumorigenesis making the pathway a highly attractive target for cancer therapy [27]. mTOR consists of various domains involved in the physiological process, namely the binding or HEAT domain composed of two N-terminals and involved in protein-protein interactions, the FRB (FKPB12-rapamycin binding domain) domain of mTOR which is the binding domain for rapamycin, the FAT and c-terminal FATC (FAT Carboxyterminal) domain present in P13K-related kinases as well as the catalytic kinase domain [28].
Figure 2.
mTOR Signaling pathway. Activation of P13K phosphorylates phosphatidylinositol 4,5-biphosphate (PIP2) to form phosphatidylinositol-3,4,5-triphosphate (PIP3). Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) regulates the function of PIP3. PIP3 prompts the activation of downstream processes such AKT, which transmits signals to effectors including mTOR complexes to enhance cellular processes. The mTORC1 is stimulated during cell activation whereby the T-cell receptor (TCR) stimulates the activation of P13K. mTORC1 comprises of three mTOR catalytic subunits, namely the regulatory associated protein of mTOR (RAPTOR), mammalian lethal with SEC13 protein 8 (MLST8), as well as the noncore components PRAS40 and DEP domain-containing mTOR-interacting protein (DEPTOR). mTORC2 comprises also of three proteins – the namely rapamycin-insensitive companion of mTOR (RICTOR), MLST8, and the mammalian stress-activated protein kinase interacting protein 1 (SIN1). Activation of mTORC2 occurs through the phosphorylation of AKT at serine-473 while that of mTORC1 when activated, phosphorylates the effectors which are major regulators of protein translation including translation-regulating factors ribosomal S6 kinase-1 (S6K-1) and eukaryote translation initiation factor 4E binding protein-1 (4EBP-1) to enhance protein synthesis.
Through interactions with nutrients, growth factors and energy stores, mTOR can directly affect cell proliferation and differentiation [29]. Furthermore, mTOR comprises a catalytic subunit of two unique protein complexes, namely mTOR complex 1 (mTORC1) and 2 (mTORC2) [30]. These complexes are unique in their function. mTORC1 is stimulated during cell activation whereby T-cell receptor (TCR) stimulates activation of P13K [31]. This activation is catalyzed by the pyruvate dehydrogenase kinase 1 enzyme (PDK1) [32]. mTORC1 comprises of three mTOR catalytic subunits, namely the regulatory associated protein of mTOR (RAPTOR), mammalian lethal with SEC13 protein 8 (MLST8), and the noncore components PRAS40 and DEP domain-containing mTOR-interacting protein (DEPTOR). When mTORC1 is activated, it phosphorylates the effectors which are major regulators of protein translation including translation-regulating factors ribosomal S6 kinase-1 (S6K-1) and eukaryote translation initiation factor 4E binding protein-1 (4EBP-1) to enhance protein synthesis [31,33,34] (Figure 2).
mTORC2, on the other hand, can be directly activated by P13K [35]. It phosphorylates and activates AKT and other related kinases [36]. Furthermore, through the PI3K-AKT signaling, co-stimulatory signals from cytokines and TCR can also activate the mTOR signaling pathway to further activate the T cells and attain energy supplies [37]. It comprises three proteins, namely the rapamycin-insensitive companion of mTOR (RICTOR), MLST8 and the mammalian stress-activated protein kinase interacting protein 1 (SIN1). Activation occurs through the phosphorylation of AKT at serine-473 [36,38]. Some cells have the same sensitivity to rapamycin [39] but rapamycin selectively inhibits mTOR with more sensitivity to mTORC1 compared to mTORC2 [40]. Studies have shown that mTORC2, as opposed to mTORC1, lacks sensitivity to rapamycin inhibition. Most cancer cells are resistant to the 1st generation mTOR inhibitors (Rapalogs) which particularly target mTORC1 which makes the insensitivity of mTORC2 a possible opening for drug discovery [41].
3. The Role of mTOR Inhibitors in Cancer
mTOR inhibitors can be classified into first and second generations depending on their mechanisms and targets. The first generation uses allosteric mechanisms to block the mTOR pathway while the second generation prevents kinase activity in both mTORC1 and 2 using their target ATP binding site. Examples of the 1st generation include the rapamycin and its analogs while the second generation includes AZD8055, Torin1, PP242 and PP30 [42]. Based on some clinical trials mTOR inhibitors are implicated in tumor cells with p53 and PTEN mutations [43]. Three generations of inhibitors has been developed namely Rapalogs (Rapamycin and derivatives), ATP-competitive inhibitors and the Rapalink [44].
Rapamycin also referred to as sirolimus was discovered as an antifungal, immunosuppressive and antitumor compound isolated from Streptomyces hygroscopicus a soil bacterium [45,46]. This drug was initially approved as an anti-host rejecter in 1997 by the food and drug administration (FDA) for kidney transplants [47]. It also functions in many human cancers mainly for the inhibition of signal transduction pathways by forming complexes with peptidyl-prolyl-isomerase FKBP12. These pathways are necessary for cell growth and proliferation [9]. According to Shafer, et al. [48], its anti-angiogenic and proliferative property can be seen in phase II preclinical studies on endometrial cancer cell lines whereby it has a synergistic effect on the paclitaxel. mTOR has been revealed to be the homolog of yeast TOR/DRR genes previously identified in genetic screens their resistance to rapamycin [49]. It has also been identified as a direct target of the complex of FKBP12-rapamycin (FRB domain) [50]. The mechanism of action for rapamycin is based on the binding of mTOR and rapamycin complex FKBP-12 with phosphatidic acid to block the function of mTOR kinase. It attaches to the FRB domain of the mTOR and finally destabilizes the mTOR–raptor–4EBP1/S6K-1 scaffold complex through the binding of mTOR and the complex FKBP-12–rapamycin. These result in dephosphorylation of 4EBP1 and S6K-1 [51,52]. The FRB domain is adjacent to the kinase domain and limits access to substrates to the kinase site [53,54]. However, rapamycin lacks sensitivity in some binding sites making them less sensitive [55].
The therapeutic development of mTOR inhibitors has improved due to their importance in cancer progression and development [56]. Several inhibitors have been approved by the FDA and are already being implemented in the treatment of various human cancers such as breast cancer (everolimus), metastatic renal cell cancer carcinoma (everolimus and temsirolimus), pancreatic neuroendocrine tumors (everolimus) and mantle cell lymphoma (temsirolimus) [57]. Temsirolimus (CCI-779), everolimus (RAD001), and ridaforolimus (MK-8669/AP23573) [6,58,59] have been improved due to their poor aqueous solubility and bioavailability. Studies have shown that rapamycin and its Rapalogs inhibit mTORC2 complex in a way that is independent on time, cell type and dose and based on interaction with newly synthesized molecules of complexes of rapamycin/Rapalogs-FKBP12 and mTOR molecules. This results in further interaction with RICTOR. Studies have shown that the inhibition of components such as RICTOR, RAPTOR, or mTOR significantly reduces the proliferation of cancer cells and offsets progression in the cell cycle [60,61,62]. Overexposure of cancer cells to rapamycin may encourage mTOR binding and inhibit AKT mediated signaling even before the mTORC2 complex is formed [63].
Rapalogs present antiproliferative characteristics in cells that have not been transformed and can efficiently inhibit T-cell proliferation in patients who have undergone transplants [64,65]. They have also shown antitumor responses in benign tumors of TSC [66,67] including lymphangiomyomatosis, renal angiomyolipoma, cardiac rhabdomyoma, facial angiofibroma and retinal astrocytic hamartoma [66]. Reduced efficacy was seen in sporadic cancers and when treatment was stopped [68,69]. Recently they have been approved for the treatment of various tumors including renal cell carcinoma [70,71], postmenopausal hormone receptor-positive advanced breast cancer in combination with exemestane [72], advanced pancreatic neuroendocrine tumors [73], advanced non-functional neuroendocrine tumors of the gastrointestinal tract or lung [74] and relapsed or refractory mantle cell lymphoma [75].
Gulhati, et al. [60] found that the knockdown of mTORC1and 2 mediated in colorectal cancer xenografts in vivo slows down the development of rapamycin sensitive and insensitive cell lines. In addition, the knockdown of mTORC2 increased apoptosis in colorectal cancer cells resistant to rapamycin. Guertin, et al. [76] also found that prostate cancer was mTORC2 dependent when induced in the prostate epithelium by phosphatase and tensin homolog deletion. mTOR is also vital in advanced cancer development and metastatic cancers. It alters the tumor environment to promote metastasis. The hyperactivation of mTOR by RICTOR enhanced cell proliferation in gliomas [77]. Inhibition of mTOR may also improve the way chemotherapeutic agents respond in advanced diseases. Patel, et al. [78] found that the inhibition of mTOR prevented the distribution of cancer cells to lymph nodes slowing down angiogenesis in head and neck cancer.
Everolimus was approved as an oral mTOR inhibitor for advanced renal cell cancer. It is also known for its anti-proliferative and angiogenic activity in human cancers [79,80]. This includes metastatic pancreatic neuroendocrine tumors, metastatic renal cell carcinoma, advanced estrogen receptor (ER)-positive [79] and human epidermal growth factor receptor-2 (HER2)-negative breast cancer [80]. Studies have recorded an improvement in cancer when rapamycin and its Rapalogs were used in combination with either standard chemotherapy, hormonal therapy, or alone. A current study found significant progression-free survival (PFS) when patients with HER-2 advanced stage of breast cancer, pre-treated with taxane and trastuzumab were administered both everolimus together with trastuzumab and vinorelbine [81]. Another breast cancer study by Hurvitz, et al. [82] found the combination of everolimus and paclitaxel and trastuzumab promising. Temsirolimus (Torisel®), was approved by the FDA as the 1st rapamycin analog to be used for the treatment of cancer cells. It is an intravenous injection which when injected in vivo becomes converted into rapamycin. Studies have shown an increase in progesterone mRNA and the inhibition of endoplasmic reticulum mRNA expressions when administered with bevacizumab or in combination with other chemotherapeutic agents for treating endometrial cancer cell lines [48,83]. In addition, Tinker, et al. [84] found positive results after using temsirolius for a preliminary phase II study in patients with metastatic cervical cancer. The drugs were effective when administered together with paclitaxel/carboplatin for treating stage II/IV patients with clear cell adenocarcinoma on a clinical phase II trial [85].
Not all studies have however proven positive outcomes with the drug. Behbakht, et al. [86] found decreased activity of temsirolimus with the drug efficacy failing in patients with primary peritoneal cancer or persistent/recurrent epithelial ovarian cancer. These results still need to be investigated by a phase III trial. Another inhibitor, Ridaforolimus (MK-8669/AP23573), a non-rapamycin pro-drug available in both intravenous and oral formulations has been evaluated in combination or as monotherapy on various cancers including breast, prostate, endometrial, sarcomas and non-small cell lung cancer [16]. It has had a 33% response rate when administered to patients with advanced endometrial cancer [87]. A phase two II study showed a partial response rate of 7.7% in advanced or recurrent [88]. Side effects of this drug include low toxicity with dose dependent skin rashes and mucositis [89] as well as hypertriglyceridemia, hypercholesterolemia, nausea, fatigue, anemia, and neutropenia [90]. In addition, sirolimus and temsirolimus present intense pulmonary toxicity. Other side effects include the risk of secondary lymphoma, interstitial lung disease, and the reactivation of latent infections. However, these are rare [91].
Even though Rapalogs are still been used in clinics as opposed to ATP-competitive inhibitors which have not yet been approved, and Rapalink still being developed and subject to experimentation. Several shortcomings of Rapalogs [55] have made the 2nd generation inhibitors better [92,93]. ATP-competitive inhibitors are the second-generation inhibitors. They inhibit both mTORC1 and 2 by blocking the kinase domain [94,95]. As opposed to the Rapalogs, inhibition is intense with blocking of the P13K from their kinase similarity [94]. Rapalink is the third-generation inhibitors designed to curb resistance mutations in both the rapalog and ATP-competitive inhibitors. These inhibitor crosslinks with kinase in the same molecule [96].
4. The Role of mTOR Pathway in Cancer Therapy
A major development has taken place in the last few years to understand the role of mTOR in cancer development and progression. mTOR and/or its components have been implicated in various genetic mutations of human malignant diseases [97,98,99]. Mutations of closely related pathways have enhanced mTOR signaling in cancers [59,95,100]. Currently, human cancer genome databases are being mined to aid identification of activated mTOR mutations [101]. Transmitted extracellular signals go through various pathways but P13K/AKT/RAS/RAF/MEK/MAPK are the most common and highly characterized. Using the same mechanism to activate PI3K/AKT/mTOR pathway has presented enhanced tumor progression and poor survival response to patients with different types of tumors [60,102]. Due to its vital function in cell growth and proliferation, its components have been increasingly used as potentials for therapeutic targets. Molecular approaches have been used to establish the role of the components of the mTOR pathway in cancer development. Components of the mTOR pathway have also been activated in various neuroendocrine tumors with a tendency of releasing bioactive products [103,104].
mTORC1 induces nucleotide and protein synthesis to regulate cellular growth via ribosome biogenesis, inhibits autophagy, protein, and nucleotide synthesis. When conditions are favorable, they sense environmental signals such as nutrients and growth factors to initiate cell growth but if conditions become unfavorable in cases of acidity and hypoxia, mTOR activity is inhibited [105,106]. When these pathways are activated, they inhibit mTORC1 through phosphorylation and inhibition of the protein complex, TSC 1 and 2. Mutation of the TSC genes causes TSC disease with benign tumors found in the brain, kidneys, heart, lungs and liver [107]. Activation could lead to the loss of phosphatase and tensin homolog (PTEN). This uncouples mTORC1 activation from growth factor signaling such as mutations of liver kinase B1/serine/threonine kinase 11 (LKB1/STK11) in nutrient-deprived vascular tumors but allows activation of mTORC1. The mutation of P53 inhibits bioenergetics processes and cell cycle arrest uncoupling DNA damage [95]. In addition, it could lead to hyperactivation of S6K-1, 4EBP1 and eukaryotic translation initiation factor 4E (eIF4E), as well as cancer growth through the activation of lipid and protein biosynthesis. Upon activation of S6K-1, 4EBP1 and other substrates are phosphorylated enhancing cell proliferation and growth from an anabolic cellular response [31,35,108]. Through the stimulation of the activity and expressions in small GTPases such as Rac1, cdc42 and Rho to control the activities of the actin cytoskeleton and motility [58,109]. Furthermore, S6K-1 and 4EBP1 mediated by mTORC1 extend vital roles in focal adhesion proteins phosphorylation including paxillion, p130 Cas and focal adhesion kinase as well as reorganization of F-actin [110].
Hyperactivation of mTORC1 results from mutations of mTOR or upstream effectors. This occurs in sporadic cancers [111,112,113]. Furthermore, in hamartoma syndromes, they are characterized by the growth of benign tumors and mutations in tumor suppressor genes [114]. The association of phosphorylated mTOR with AKT signaling and acquired cisplatin resistance affects primary platinum resistance and sensitivity to ovarian cancer cells [115]. Furthermore, these inhibitors restore chemosensitivity to platinum derivate both in vitro and in xenograft models [16,116]. Gulhati, Bowen, Liu, Stevens, Rychahou, Chen, Lee, Weiss, O’Connor and Gao [109] found that mTORC1 was associated with motility, metastasis, and epithelial-mesenchymal transition in colorectal cancer. mTORC1 activity has also been studied in breast cancer and gliomas [77,117]. Despite all these discoveries, more research needs to be conducted to understand how these components are regulated. Gulhati, Bowen, Liu, Stevens, Rychahou, Chen, Lee, Weiss, O’Connor and Gao [109] found that using oxaliplatin in colorectal cancer cells induced apoptosis as a result of the knockdown of mTORC1 and 2. mTORC1 is found to be associated with the transport hormone and, peptide-containing vesicles. They also regulate intestinal hormones which play a vital role in the gastrointestinal tract as well as other secreted neuroendocrine tumors to regulate neurotensin [104].
mTORC2, on the other hand, is activated via growth factors [118]. It phosphorylates and activates the AGC protein kinases including SGK1 (Ser422) and AKT (Ser473). Inhibiting mTORC2 activities will enhance the antitumor effect in several preclinical trials [61,69,76,119,120]. Varied molecular adjustments occur with this pathway which may suggest strategic therapy against cancer cells if targeted. The onset of cancer is provoked by enhanced cell growth and immune escape due to a build-up of genetic and epigenetic changes. Therefore an approach to cancer therapy would be to prevent these changes [121]. Tumor heterogeneity, as well as cellular resistance, are some of the hindrances to targeted cancer therapies. Activation of bypass mechanisms as well as making secondary reforms in the target are resistance mechanisms which have been identified [122]. Nonetheless, most of the targeted treatments have not been beneficial in the long run despite all the preclinical trials.
mTOR Signaling Pathway and PDT
The PS are composed of natural occurring macrocycles including hemoglobin, vitamin B12 and chlorophyll. These compounds consist of nitrogen, oxygen, or sulfur atoms locked in a hollow ring containing metals such as iron or magnesium. Currently, PDT makes use of plant extracts to complex synthetic macrocycles. These different agents can selectively target and accumulate in the tumor. The widely investigated PS includes tetrapyrroles such as bacteriochlorins, chlorins, porphyrins, and phthalocyanines [123]. To improve efficacy, clinical considerations have been given to other compounds such as synthetic dyes and targeted therapies which use various drug delivery systems to improve the penetration of light. The fate and effectiveness of PDT on tumors are based on the oxygen concentration, wavelength, types of photosensitizer and the genotype of the cell. This can affect certain organelles and specific target tissue [20]. Dual-specificity of the PS would depend on accumulation and localization of the PS in diseased tissue. The PS if hydrophobic accumulates in the mitochondria and endoplasmic reticulum, other polar compounds may Golgi apparatus, lysosomes and plasma membrane [124].
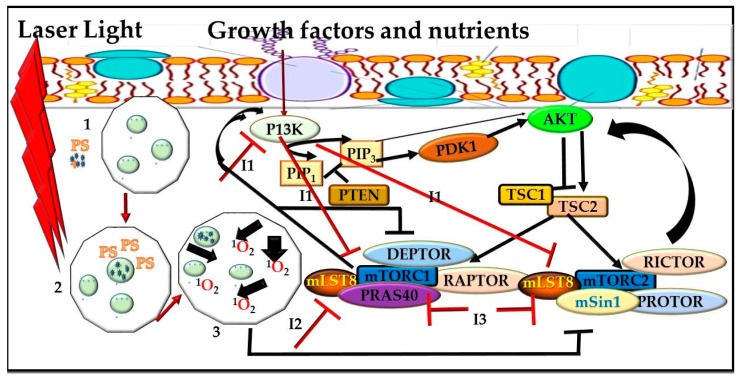
PDT down-regulates AKT-mTOR pathway because of ROS production (Figure 3). In modern oncology, a combination of different therapeutic modalities with non-overlapping toxic effects are strategies used to improve the therapeutic index of treatment. Combination therapies target different disease pathways, which represents an alternative approach that might offer potential advantages over a single therapy.
Figure 3.
Proposed mechanism between mTOR Signaling Pathway, Inhibitors and Photodynamic Therapy (PDT). PDT down-regulates AKT-mTOR pathway because of reactive oxygen species (ROS) production. 1. Photosensitizer is injected into a targeted tumor. 2. Laser light is emitted at a particular wavelength. 3. Cells become activated and release reactive oxygen species, which results to tissue destruction and cell death. Interaction with inhibitors phosphoinositide 3-kinase (I1), rapamycin (I2) and mTOR kinase (I3) to enhance cell death through the P13K/AKT-mTOR pathway.
Few studies have shown these combining effects on PDT. Kraus, et al. [125] found that combining P13K/mTOR inhibitors (BYL719, BKM120, and BEZ235) with verteporfin-PDT to synergistically enhance PDT response with BEZ235 presenting the strongest. Antiapoptotic inhibition of the Bcl-2 family protein Mcl-1 and P13K pathway was critical. Fateye, et al. [24] assessed the effect of combination of P13K/mTOR inhibitor (BEZ 235 (BEZ)) on PDT efficacy using prostate tumor (PC3) and SV40-transformed mouse endothelial cell lines (SVEC-40) and found that the sub-lethal PDT was enhanced in both cell lines. Combination of PDT with pan-PI3/ mTOR kinase inhibitor LY294002 (LY) also enhanced PDT effect with PC3. However, it produced a synergistic effect in SVEC-40. In contrast, Sasore and Kennedy [126] found that there are some combinations of PI3K/AKT/mTOR pathway inhibitors, which actually interrupt developmental angiogenesis due to their additive or synergistic effect. Tuo, et al. [127] used human SZ95 sebocytes to find out the potential pharmaceutical effect of combining ALA-PDT and rapamycin through the mTOR pathway and found that cell growth was suppressed, protein levels of P-mTOR, and P-Raptor were reduced as well as lipogenesis. Their study concluded that rapamycin enhanced aminolevulinic acid hydrochloride (ALA)-PDT in SZ95 cells. mTOR inhibition can induce autophagy in various ways: direct induction, pre-condition cells, or by stressor induction. A study by Weyergang, et al. [26] using colon adenocarcinoma cell line and amphiphilic endolysosome-localizing photosensitizer Al(II) phthalocyanine chloride disulfonic acid (AlPcS(2a)) showed that targeting mTOR signaling pathway in PDT caused partial loss of both total and phosphorylated mTOR in both tumor xenografts and cultured cells in vitro and in vivo. According to Weyergang, et al. [26] combining rapamycin potentiates cytotoxicity in vitro post-PDT. The interest in the combination of PDT and other therapeutic modulates in cancer treatment is to provide a platform for potential treatment options and limited adverse effects of chemotherapy since PDT does not have the inherent dose-limiting toxicity [128]. Combination therapies are aimed at increasing responses, improving patient tolerability, decreasing drug dosages and the emergence of drug resistance [129]. Combined effects of PI3K/AKT/mTOR and PDT as a treatment regimen for cancers still needs further investigation.
5. Perspective
Despite its promising minimal and non-toxic side effects, it is still unlikely that administering conventional chemotherapies and/or inhibitors alone will completely cure cancer. There are still challenges in cancer therapy including the activation of other proliferation signaling pathways, treatment-resistant mutations as well as the intramural heterogeneity of mTOR activities. Inhibitors alone have failed to induce tumor regression but are seen as cytostatic causing disease stability rather than death [130]. Another limitation might provide negative feedback loops in the mTOR pathway which have limited the efficiency of these Rapalogs. Taking into high consideration the level of toxicity, combined therapies would be the way forward. New generation inhibitors are being produced which can prevent the catalytic activity of both mTORC1 and mTORC2 complexes and enhance therapeutic indexes.
Author Contributions
Conceptualization, S.M.A.; writing—original draft preparation, S.M.A.; writing—review and editing, S.M.A., H.A.; supervision, H.A.
Funding
This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No. 98337), as well as grants received from the University of Johannesburg (URC), the African Laser Centre (ALC) (student scholarship), and Council for Scientific and Industrial Research (CSIR)—National Laser Centre (NLC) Laser Rental Pool Program. All lasers were supplied and set up the NLC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gomez-Pinillos A., Ferrari A.C. mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol. Oncol. N. Am. 2012;26:483–505. doi: 10.1016/j.hoc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomeusz C., Gonzalez-Angulo A.M. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:121–130. doi: 10.1517/14728222.2011.644788. [DOI] [PubMed] [Google Scholar]
- 3.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B., Lu C., Xie M., Zhang Q., McMichael J.F., Wyczalkowski M.A. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan T.L., Cantley L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willems L., Tamburini J., Chapuis N., Lacombe C., Mayeux P., Bouscary D. PI3K and mTOR signaling pathways in cancer: New data on targeted therapies. Curr. Oncol. Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 6.Zaytseva Y.Y., Valentino J.D., Gulhati P., Evers B.M. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H., Walls M., Baxi S., Yin M.-J. Targeting the mTOR pathway in tumor malignancy. Curr. Cancer Drug Targets. 2013;13:267–277. doi: 10.2174/1568009611313030005. [DOI] [PubMed] [Google Scholar]
- 8.Li T., Wang G. Computer-aided targeting of the PI3K/Akt/mTOR pathway: Toxicity reduction and therapeutic opportunities. Int. J. Mol. Sci. 2014;15:18856–18891. doi: 10.3390/ijms151018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Don A.S., Zheng X.F. Recent clinical trials of mTOR-targeted cancer therapies. Rev. Recent Clin. Trials. 2011;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- 11.Volanti C., Hendrickx N., Van Lint J., Matroule J.-Y., Agostinis P., Piette J. Distinct transduction mechanisms of cyclooxygenase 2 gene activation in tumour cells after photodynamic therapy. Oncogene. 2005;24:2981. doi: 10.1038/sj.onc.1208481. [DOI] [PubMed] [Google Scholar]
- 12.Bozkulak O., Wong S., Luna M., Ferrario A., Rucker N., Gulsoy M., Gomer C.J. Multiple components of photodynamic therapy can phosphorylate Akt. Photochem. Photobiol. 2007;83:1029–1033. doi: 10.1111/j.1751-1097.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 13.Koon H.K., Chan P.S., Wong R.N.S., Wu Z.G., Lung M.L., Chang C.K., Mak N.K. Targeted inhibition of the EGFR pathways enhances Zn-BC-AM PDT-induced apoptosis in well-differentiated nasopharyngeal carcinoma cells. J. Cell. Biochem. 2009;108:1356–1363. doi: 10.1002/jcb.22366. [DOI] [PubMed] [Google Scholar]
- 14.Rodon J., Dienstmann R., Serra V., Tabernero J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013;10:143. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 15.Meric-Bernstam F., Gonzalez-Angulo A.M. Targeting the mTOR signaling network for cancer therapy. J. Clin. Oncol. 2009;27:2278. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y.-J., Duan Y., Zheng X.F.S. Targeting the mTOR kinase domain: The second generation of mTOR inhibitors. Drug Discov. Today. 2011;16:325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Straten D., Mashayekhi V., de Bruijn H., Oliveira S., Robinson D. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers. 2017;9:19. doi: 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn J.-C., Biswas R., Chung P.-S. Synergistic effect of radachlorin mediated photodynamic therapy on propolis induced apoptosis in AMC-HN-4 cell lines via caspase dependent pathway. Photodiagn. Photodyn. Ther. 2013;10:236–243. doi: 10.1016/j.pdpdt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Allison R.R., Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clin. Endosc. 2013;46:24. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baptista M.S., Cadet J., Di Mascio P., Ghogare A.A., Greer A., Hamblin M.R., Lorente C., Nunez S.C., Ribeiro M.S., Thomas A.H. Type I and type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017;93:912–919. doi: 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velloso N.V., Muehlmann L.A., Longo J.P.F., Silva J.R., Zancanela D.C., Tedesco A.C., Azevedo R.B.D. Aluminum-phthalocyanine chloride-based photodynamic therapy inhibits PI3K/Akt/Mtor pathway in oral squamous cell carcinoma cells in vitro. Chemotherapy. 2012;1:5. [Google Scholar]
- 23.Fateye B., Li W., Wang C., Chen B. Combination of Phosphatidylinositol 3-Kinases Pathway Inhibitor and Photodynamic Therapy in Endothelial and Tumor Cells. Photochem. Photobiol. 2012;88:1265–1272. doi: 10.1111/j.1751-1097.2012.01160.x. [DOI] [PubMed] [Google Scholar]
- 24.Fateye B., Wan A., Yang X., Myers K., Chen B. Comparison between endothelial and tumor cells in the response to verteporfin-photodynamic therapy and a PI3K pathway inhibitor. Photodiagn. Photodyn. Ther. 2015;12:19–26. doi: 10.1016/j.pdpdt.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q., Chen X., Ma J., Peng H., Wang F., Zha X., Wang Y., Jing Y., Yang H., Chen R. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl Acad. Sci. USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weyergang A., Berg K., Kaalhus O., Peng Q., Selbo P.K. Photodynamic therapy targets the mTOR signaling network in vitro and in vivo. Mol. Pharm. 2008;6:255–264. doi: 10.1021/mp800156e. [DOI] [PubMed] [Google Scholar]
- 27.Thorpe L.M., Yuzugullu H., Zhao J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer. 2015;15:7. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weichhart T., Hengstschläger M., Linke M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blouet C., Ono H., Schwartz G.J. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Y., Kun-liang G. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 31.Laplante M., Sabatini D.M. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cantley L.C., Engelman J.A., Luo J. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 34.Pópulo H., Lopes J.M., Soares P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimobayashi M., Hall M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014;15:155. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 36.Russell R.C., Fang C., Guan K.-L. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12:325. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M.A., Hall A., Hall M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 39.Delgoffe G.M., Pollizzi K.N., Waickman A.T., Heikamp E., Meyers D.J., Horton M.R., Xiao B., Worley P.F., Powell J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011;12:295. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verbist K.C., Guy C.S., Milasta S., Liedmann S., Kamiński M.M., Wang R., Green D.R. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadlakonda L., Dash A., Pasupuleti M., Kotha A.K., Reddanna P. The paradox of Akt-mTOR interactions. Front. Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guertin D.A., Sabatini D.M. The pharmacology of mTOR inhibition. Sci. Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 43.Feng Z., Zhang H., Levine A.J., Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie J., Wang X., Proud C.G. mTOR inhibitors in cancer therapy. F1000Research. 2016;5:F1000. doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vezina C., Kudelski A., Sehgal S.N. Rapamycin (AY-22, 989), a new antifungal antibiotic. J. Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 46.Singh K., Sun S., Vezina C. Rapamycin (AY-22, 989), A New Antifungal Antibiotic. J. Antibiot. 1979;32:630–645. doi: 10.7164/antibiotics.32.630. [DOI] [PubMed] [Google Scholar]
- 47.Tsang C.K., Qi H., Liu L.F., Zheng X.F.S. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov. Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Shafer A., Zhou C., Gehrig P.A., Boggess J.F., Bae-Jump V.L. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int. J. Cancer. 2010;126:1144–1154. doi: 10.1002/ijc.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N.R., Hall M.N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-F. [DOI] [PubMed] [Google Scholar]
- 50.Sabers C.J., Martin M.M., Brunn G.J., Williams J.M., Dumont F.J., Wiederrecht G., Abraham R.T. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 51.Hara K., Maruki Y., Long X., Yoshino K.-i., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/S0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 52.Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 53.Aylett C.H.S., Sauer E., Imseng S., Boehringer D., Hall M.N., Ban N., Maier T. Architecture of human mTOR complex 1. Science. 2016;351:48–52. doi: 10.1126/science.aaa3870. [DOI] [PubMed] [Google Scholar]
- 54.Yuan H.-X., Guan K.-L. Structural insights of mTOR complex 1. Cell Res. 2016;26:267. doi: 10.1038/cr.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoreen C.C., Sabatini D.M. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 56.Calimeri T., Ferreri A.J.M. m-TOR inhibitors and their potential role in haematological malignancies. Br. J. Haematol. 2017;177:684–702. doi: 10.1111/bjh.14529. [DOI] [PubMed] [Google Scholar]
- 57.Pinto-Leite R., Arantes-Rodrigues R., Sousa N., Oliveira P.A., Santos L. mTOR inhibitors in urinary bladder cancer. Tumour Biol. 2016;37:11541–11551. doi: 10.1007/s13277-016-5083-1. [DOI] [PubMed] [Google Scholar]
- 58.Liu L., Luo Y., Chen L., Shen T., Xu B., Chen W., Zhou H., Han X., Huang S. Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. J. Biol. Chem. 2010;285:38362–38373. doi: 10.1074/jbc.M110.141168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarado Y., Mita M.M., Vemulapalli S., Mahalingam D., Mita A.C. Clinical activity of mammalian target of rapamycin inhibitors in solid tumors. Target. Oncol. 2011;6:69–94. doi: 10.1007/s11523-011-0178-5. [DOI] [PubMed] [Google Scholar]
- 60.Gulhati P., Cai Q., Li J., Liu J., Rychahou P.G., Qiu S., Lee E.Y., Silva S.R., Bowen K.A., Gao T. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin. Cancer Res. 2009;15:7207–7216. doi: 10.1158/1078-0432.CCR-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roulin D., Cerantola Y., Dormond-Meuwly A., Demartines N., Dormond O. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol. Cancer. 2010;9:57. doi: 10.1186/1476-4598-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu W.K.K., Lee C.W., Cho C.H., Chan F.K.L., Yu J., Sung J.J.Y. RNA interference targeting raptor inhibits proliferation of gastric cancer cells. Exp. Cell Res. 2011;317:1353–1358. doi: 10.1016/j.yexcr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.-H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 64.Thomson A.W., Turnquist H.R., Raimondi G. Immunoregulatory functions of mTOR inhibition. Nature Reviews Immunology. 2009;9:324. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waldner M., Fantus D., Solari M., Thomson A.W. New perspectives on mTOR inhibitors (rapamycin, rapalogs and TORKinibs) in transplantation. Br. J. Clin. Pharmacol. 2016;82:1158–1170. doi: 10.1111/bcp.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Habib S.L., Al-Obaidi N.Y., Nowacki M., Pietkun K., Zegarska B., Kloskowski T., Zegarski W., Drewa T., Medina E.A., Zhao Z. Is mTOR inhibitor good enough for treatment all tumors in TSC patients? J. Cancer. 2016;7:1621. doi: 10.7150/jca.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sasongko T.H., Ismail N.F.D., Zabidi-Hussin Z. Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD011272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiarini F., Evangelisti C., McCubrey J.A., Martelli A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol. Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Kim L.C., Cook R.S., Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191. doi: 10.1038/onc.2016.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., Staroslawska E., Sosman J., McDermott D., Bodrogi I. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 71.Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S., Grünwald V., Thompson J.A., Figlin R.A., Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 72.Baselga J., Campone M., Piccart M., Burris Iii H.A., Rugo H.S., Sahmoud T., Noguchi S., Gnant M., Pritchard K.I., Lebrun F. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N. Engl. J. Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E., Hobday T.J., Okusaka T., Capdevila J., De Vries E.G.E. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao J.C., Fazio N., Singh S., Buzzoni R., Carnaghi C., Wolin E., Tomasek J., Raderer M., Lahner H., Voi M. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hess G., Herbrecht R., Romaguera J., Verhoef G., Crump M., Gisselbrecht C., Laurell A., Offner F., Strahs A., Berkenblit A. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 76.Guertin D.A., Stevens D.M., Saitoh M., Kinkel S., Crosby K., Sheen J.-H., Mullholland D.J., Magnuson M.A., Wu H., Sabatini D.M. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–159. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masri J., Bernath A., Martin J., Jo O.D., Vartanian R., Funk A., Gera J. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 78.Patel V., Marsh C.A., Dorsam R.T., Mikelis C.M., Masedunskas A., Amornphimoltham P., Nathan C.A., Singh B., Weigert R., Molinolo A.A. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011;71:7103–7112. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavel M.E., Hainsworth J.D., Baudin E., Peeters M., Hörsch D., Winkler R.E., Klimovsky J., Lebwohl D., Jehl V., Wolin E.M. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 80.Burris Iii H.A., Lebrun F., Rugo H.S., Beck J.T., Piccart M., Neven P., Baselga J., Petrakova K., Hortobagyi G.N., Komorowski A. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer. 2013;119:1908–1915. doi: 10.1002/cncr.28010. [DOI] [PubMed] [Google Scholar]
- 81.André F., O’Regan R., Ozguroglu M., Toi M., Xu B., Jerusalem G., Masuda N., Wilks S., Arena F., Isaacs C. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. The lancet oncology. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 82.Hurvitz S.A., Dalenc F., Campone M., O’Regan R.M., Tjan-Heijnen V.C., Gligorov J., Llombart A., Jhangiani H., Mirshahidi H.R., Tan-Chiu E. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2013;141:437–446. doi: 10.1007/s10549-013-2689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Temkin S.M., Fleming G. Current treatment of metastatic endometrial cancer. Cancer Control. 2009;16:38–45. doi: 10.1177/107327480901600106. [DOI] [PubMed] [Google Scholar]
- 84.Tinker A.V., Ellard S., Welch S., Moens F., Allo G., Tsao M.S., Squire J., Tu D., Eisenhauer E.A., MacKay H. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199) Gynecol. Oncol. 2013;130:269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Takatori E., Shoji T., Miura Y., Takada A., Takeuchi S., Sugiyama T. Effective use of everolimus as salvage chemotherapy for ovarian clear cell carcinoma: A case report. Onco Targets Ther. 2014;7:165. doi: 10.2147/OTT.S54745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Behbakht K., Sill M.W., Darcy K.M., Rubin S.C., Mannel R.S., Waggoner S., Schilder R.J., Cai K.Q., Godwin A.K., Alpaugh R.K. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: A Gynecologic Oncology Group study. Gynecol. Oncol. 2011;123:19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colombo N., McMeekin S., Schwartz P., Kostka J., Sessa C., Gehrig P., Holloway R., Braly P., Matei D., Einstein M. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. J. Clin. Oncol. 2007;25:5516. [Google Scholar]
- 88.Mackay H., Welch S., Tsao M.S., Biagi J.J., Elit L., Ghatage P., Martin L.A., Tonkin K.S., Ellard S., Lau S.K. Phase II study of oral ridaforolimus in patients with metastatic and/or locally advanced recurrent endometrial cancer: NCIC CTG IND 192. J. Clin. Oncol. 2011;29:5013. doi: 10.1200/jco.2011.29.15_suppl.5013. [DOI] [Google Scholar]
- 89.Vignot S., Faivre S., Aguirre D., Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann. Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 90.Duran I., Siu L.L., Oza A.M., Chung T.B., Sturgeon J., Townsley C.A., Pond G.R., Seymour L., Niroumand M. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur. J. Cancer. 2006;42:1875–1880. doi: 10.1016/j.ejca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 91.Leary A., Auclin E., Pautier P., Lhommé C. The PI3K/Akt/mTOR pathway in ovarian cancer: Biological rationale and therapeutic opportunities. In: Díaz-Padilla I., editor. Ovarian Cancer-A Clinical and Translational Update. IntechOpen; London, UK: 2013. [DOI] [Google Scholar]
- 92.Cho D.C., Cohen M.B., Panka D.J., Collins M., Ghebremichael M., Atkins M.B., Signoretti S., Mier J.W. The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin. Cancer Res. 2010;16:3628–3638. doi: 10.1158/1078-0432.CCR-09-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blaser B., Waselle L., Dormond-Meuwly A., Dufour M., Roulin D., Demartines N., Dormond O. Antitumor activities of ATP-competitive inhibitors of mTOR in colon cancer cells. BMC Cancer. 2012;12:86. doi: 10.1186/1471-2407-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benjamin D., Colombi M., Moroni C., Hall M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10:868. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 95.Wander S.A., Hennessy B.T., Slingerland J.M. Next-generation mTOR inhibitors in clinical oncology: How pathway complexity informs therapeutic strategy. J. Clin. Investig. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rodrik-Outmezguine V.S., Okaniwa M., Yao Z., Novotny C.J., McWhirter C., Banaji A., Won H., Wong W., Berger M., de Stanchina E. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272. doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steuer-Vogt M.K., Bonkowsky V., Ambrosch P., Scholz M., Neiβ A., Strutz J., Hennig M., Lenarz T., Arnold W. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: A randomised controlled clinical trial. Eur. J. Cancer. 2001;37:23–31. doi: 10.1016/S0959-8049(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 98.Huang S., Houghton P.J. Inhibitors of mammalian target of rapamycin as novel antitumor agents: From bench to clinic. Curr. Opin. Investig. Drugs. 2002;3:295–304. [PubMed] [Google Scholar]
- 99.Dancey J.E. Clinical development of mammalian target of rapamycin inhibitors. Hematol./Oncol. Clin. 2002;16:1101–1114. doi: 10.1016/S0889-8588(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 100.Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardt M., Chantaravisoot N., Tamanoi F. Activating mutations of TOR (target of rapamycin) Genes Cells. 2011;16:141–151. doi: 10.1111/j.1365-2443.2010.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiang G.G., Abraham R.T. Targeting the mTOR signaling network in cancer. Trends Mol. Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Capdevila J., Salazar R., Halperín I., Abad A., Yao J.C. Innovations therapy: Mammalian target of rapamycin (mTOR) inhibitors for the treatment of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30:27–34. doi: 10.1007/s10555-011-9290-3. [DOI] [PubMed] [Google Scholar]
- 104.Li J., Liu J., Song J., Wang X., Weiss H.L., Townsend C.M., Jr., Gao T., Evers B.M. mTORC1 inhibition increases neurotensin secretion and gene expression through activation of the MEK/ERK/c-Jun pathway in the human endocrine cell line BON. Am. J. Phys.-Cell Phys. 2011;301:C213–C226. doi: 10.1152/ajpcell.00067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arsham A.M., Howell J.J., Simon M.C. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 106.Balgi A.D., Diering G.H., Donohue E., Lam K.K.Y., Fonseca B.D., Zimmerman C., Numata M., Roberge M. Regulation of mTORC1 signaling by pH. PLoS ONE. 2011;6:e21549. doi: 10.1371/journal.pone.0021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crino P.B., Nathanson K.L., Henske E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 108.Kennedy B.K., Lamming D.W. The mechanistic target of rapamycin: The grand conducTOR of metabolism and aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gulhati P., Bowen K.A., Liu J., Stevens P.D., Rychahou P.G., Chen M., Lee E.Y., Weiss H.L., O’Connor K.L., Gao T. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou H., Huang S. mTOR signaling in cancer cell motility and tumor metastasis. Crit. Rev. Eukaryot. Gene Expr. 2010;20:1–16. doi: 10.1615/CritRevEukarGeneExpr.v20.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Montalto G., Cervello M., Nicoletti F., Fagone P., Malaponte G., Mazzarino M.C. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954. doi: 10.18632/oncotarget.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grabiner B.C., Nardi V., Birsoy K., Possemato R., Shen K., Sinha S., Jordan A., Beck A.H., Sabatini D.M. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4:554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wagle N., Grabiner B.C., Van Allen E.M., Hodis E., Jacobus S., Supko J.G., Stewart M., Choueiri T.K., Gandhi L., Cleary J.M. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 2014;4:546–553. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inoki K., Corradetti M.N., Guan K.-L. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005;37:19. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 115.Mabuchi S., Kawase C., Altomare D.A., Morishige K., Sawada K., Hayashi M., Tsujimoto M., Yamoto M., Klein-Szanto A.J., Schilder R.J. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin. Cancer Res. 2009;15:5404–5413. doi: 10.1158/1078-0432.CCR-09-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng C.-L., Lai P.-S., Lin F.-H., Wu S.Y.-H., Shieh M.-J. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials. 2009;30:3614–3625. doi: 10.1016/j.biomaterials.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 117.Zhang P., Hu L., Yin Q., Zhang Z., Feng L., Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: Synthesis, preparation and in vivo evaluation. J. Control. Release. 2012;159:429–434. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 118.Oh W.J., Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tanaka K., Babic I., Nathanson D., Akhavan D., Guo D., Gini B., Dang J., Zhu S., Yang H., De Jesus J. Oncogenic EGFR signaling activates an mTORC2–NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1:524–538. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Driscoll D.R., Karim S.A., Sano M., Gay D.M., Jacob W., Yu J., Mizukami Y., Gopinathan A., Jodrell D.I., Evans T.R.J. mTORC2 signaling drives the development and progression of pancreatic cancer. Cancer Res. 2016;76:6911–6923. doi: 10.1158/0008-5472.CAN-16-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weinstein I.B., Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 122.Garraway L.A., Jänne P.A. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- 123.Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Almeida R.D., Manadas B.J., Carvalho A.P., Duarte C.B. Intracellular signaling mechanisms in photodynamic therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Kraus D., Palasuberniam P., Chen B. Targeting phosphatidylinositol 3-kinase signaling pathway for therapeutic enhancement of vascular-targeted photodynamic therapy. Mol. Cancer Ther. 2017;16:2422–2431. doi: 10.1158/1535-7163.MCT-17-0326. [DOI] [PubMed] [Google Scholar]
- 126.Sasore T., Kennedy B. Deciphering Combinations of PI3K/AKT/mTOR Pathway Drugs Augmenting Anti-Angiogenic Efficacy In Vivo. PLoS ONE. 2014;9:e105280. doi: 10.1371/journal.pone.0105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tuo J., Wang Q., Zouboulis C.C., Liu Y., Ma Y., Ma L., Ying J., Zhang C., Xiang L. ALA-PDT suppressing the cell growth and reducing the lipogenesis in human SZ95 sebocytes by mTOR signaling pathway in vitro. Photodiagn. Photodyn. Ther. 2017;18:295–301. doi: 10.1016/j.pdpdt.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Brodin N.P., Guha C., Tomé W.A. Photodynamic therapy and its role in combined modality anticancer treatment. Technol. Cancer Res. Treat. 2015;14:355–368. doi: 10.1177/1533034614556192. [DOI] [PubMed] [Google Scholar]
- 129.Chou T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 130.Sadowski K., Kotulska K., Jozwiak S. Management of side effects of mTOR inhibitors in tuberous sclerosis patients. Pharmacol. Rep. 2016;68:536–542. doi: 10.1016/j.pharep.2016.01.005. [DOI] [PubMed] [Google Scholar]